Abstract
Decorin, an archetypal member of the small leucine-rich proteoglycan gene family, has a broad binding repertoire that encompasses matrix structural components, such as collagens, and growth factors, particularly those that belong to the transforming growth factor–β ligand superfamily. Within the tumor microenvironment, stromal decorin has an inherent proclivity to directly bind and down-regulate several receptor tyrosine kinases, which are often overexpressed in cancer cells. The decorin interactome commands a powerful antitumorigenic signal by potently repressing and attenuating tumor cell proliferation, survival, migration, and angiogenesis. This collection of interacting molecules also regulates key downstream signaling processes indirectly via the sequestration of growth factors or directly via the antagonism of receptor tyrosine kinases. We propose that decorin can be considered a “guardian from the matrix” because of its innate ability to oppose pro-tumorigenic cues.
Neoplastic growth has long been viewed in the paradigm of activating mutations in oncogenes and silencing of tumor suppressor genes that confer, collectively over time, selective advantages to fundamental cellular processes such as cell proliferation, survival, migration, and metastasis. However, relatively recently, the profound importance of the surrounding tumor stroma, encompassing that of abnormal synthesis and deposition of several proteoglycans, has emerged as an active participant in coordinating many aspects of tumor growth and progression. Indeed, an early defining histopathological feature of certain carcinomas is the presence of a strong desmoplastic reaction surrounding the tumor proper that is inherently enriched in various proteoglycan species.1,2
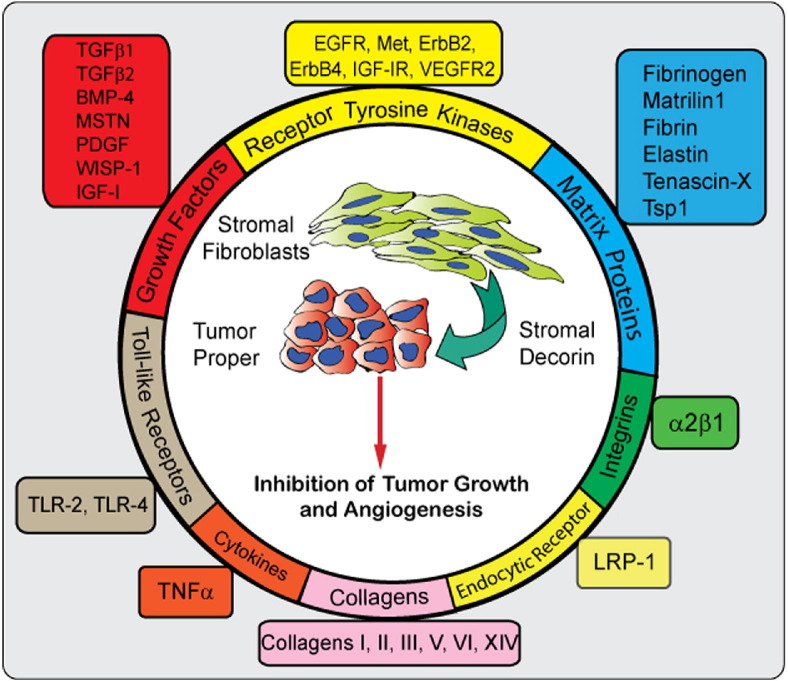
Decorin represents a prototypical member of the small leucine-rich proteoglycan (SLRP) gene family that houses 18 distinct members segregating into five discrete classes with sequence conservation across multiple species.3 Decorin contains a single glycosaminoglycan (GAG) chain composed of either dermatan or chondroitin sulfate, and 12 leucine-rich repeats comprising the protein core. Decorin is a stromal proteoglycan synthesized chiefly by fibroblasts, stressed vascular endothelial cells, and smooth muscle cells. Initially, decorin was named for and characterized by its high-affinity interactions with collagen fibers and for the subsequent regulation of collagen fibrillogenesis.4–7 It was subsequently discovered that decorin sequesters multiple growth factors, such as transforming growth factor (TGF)–β1, and directly antagonizes several members of the receptor tyrosine kinase (RTK) family, including the epidermal growth factor receptor (EGFR), the insulin-like growth factor receptor I (IGF-IR), and the hepatocyte growth factor receptor (Met)1 (Figure 1). Consequently, these latter bioactivities have been attributed to evoke potent tumor repression. The unique nature of this repressive activity is that it functions wholly within the extracellular matrix to attenuate, in an integrated and protracted fashion, key pro-survival, migratory, proliferative, and angiogenic signaling pathways.2 In a novel discovery, decorin has been implicated in modulating inflammatory responses as they pertain to cancer progression via engagement of Toll-like receptors (TLRs).8 Moreover, reduced decorin within the tumor stroma has been established as a poor prognosticator of invasive breast cancer and in murine models of spontaneous breast cancer with mammary gland carcinogenesis.2 Endogenously, certain neoplasms have a proclivity to hypermethylate the decorin promoter, effectively silencing expression and allowing tumor progression.2,9 Thus, loss of decorin expression may also favor tumor growth. In this review, we propose the concept of decorin as a “guardian from the matrix”—that is, a powerful endogenous tumor repressor acting in a paracrine fashion to limit tumor growth and angiogenesis.
Figure 1.
Decorin interactome, encompassing growth factors, receptors, and putative extracellular matrix components to which decorin physically binds through high-affinity interactions and regulates either negatively or positively. Additional details are provided in the text.
Decorin Modulates Tumor Inflammatory Properties of the Stroma
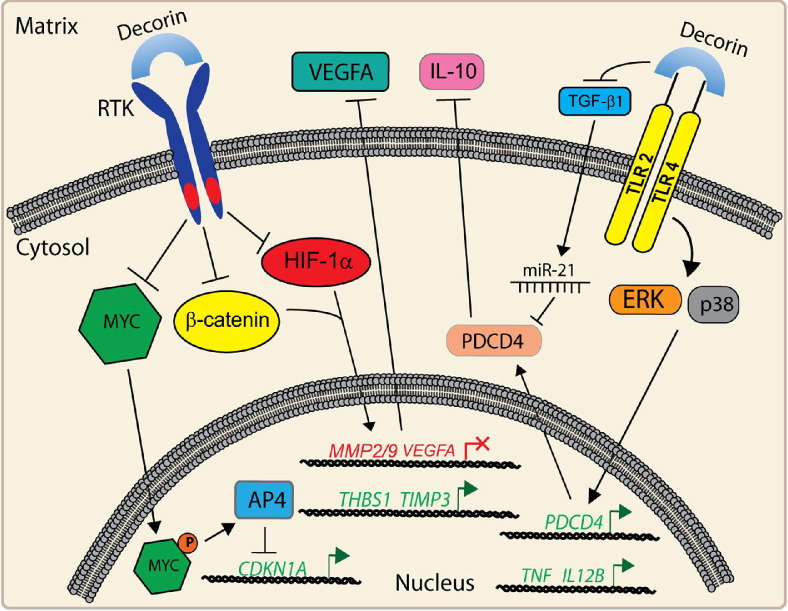
A rapidly emerging hallmark of cancer involves inflammatory processes as active and critical participants in tumorigenesis.10 Several articles have been published to indicate an immunomodulatory role of decorin to recruit monocytes to injury sites by induction of MCP-1,11 inhibiting apoptotic death of macrophages,12 and modulating allergen-induced asthma.13 A recently identified mechanism was elucidated, linking decorin, inflammation, and tumor growth.8 This process entails direct binding of soluble decorin to TLR 2 and 4 on macrophages, leading to enhanced production of the pro-inflammatory protein programed cell death 4 (PDCD4) and the oncomir miR-21, thus causing stabilization and increased translation of PDCD4. The increased abundance of PDCD4 concurrently causes a decrease of anti-inflammatory cytokines such as interleukin (IL)–10 (Figure 2). As an endogenous inhibitor of TGFβ1 by sequestration (Figure 1), decorin also attenuates TGFβ1 signaling pathways, thereby further curbing tumor growth and inflammation.8 Thus, by antagonizing TGFβ1, decorin circumvents PDCD4 translational repression to generate a pro-inflammatory tumor microenvironment. It also induces the synthesis of pro-inflammatory modulators (eg, TNFα, and IL-12b) for the suppression of tumorigenic growth. It is important to note that TNFα is a binding partner for decorin14 (Figure 1) and shows a moderate affinity for this ligand, thus highlighting an important regulatory function to further modify TNFα activities.
Figure 2.
Broad receptor antagonism and attenuation of downstream signaling cascades mediated by decorin in tumor cells. Antiproliferative, immunomodulatory, and antiangiogenic properties are regulated after engagement of decorin to cell surface receptors. A detailed discussion of pathway modulation is provided in the text.
Recent experiments using an animal model of delayed-type hypersensitivity in decorin-null mice are also supportive of a role of decorin for stimulating a more pro-inflammatory environment.15 In this setting, lack of decorin is associated with reduced TNFα levels, and increased expression of leukocyte adhesion molecules coincides with an increased adherence of leukocytes to the endothelium.15 Finally, the closest relative of decorin, biglycan, has been previously shown to modulate immune responses16,17 by acting as an endogenous ligand for TLR-2/4 to increase pro-inflammatory signals. Thus, these studies offer a new functional paradigm for SLRPs and inflammation.
Decorin Curbs the Lethality of the Tumor Niche
Decorin has exquisite binding affinities for members of the TGFβ superfamily (Figure 1), including TGFβ1, TGFβ2, and myostatin,1,2 and through this interaction can effectively trap ligands within the matrix and indirectly attenuate downstream signaling pathways mediated by the TGFβ receptor complex, as recently shown in a murine model of hepatic fibrosis.18 However, bone-derived decorin seems to enhance TGFβ activity.19 This finding has broad implications on tumorigenicity, as it can advocate tumor immunosuppression and growth retardation.20 However, it is not known whether decorin influences the assembly of the large latent TGFβ1 complex on collagen fibers. In the case of myostatin, decorin sequestration quenches myostatin growth inhibitory effects and thus promotes myoblastic growth in vitro.21 In an analogous antagonistic activity, direct sequestration of platelet-derived growth factor (PDGF) results in a potent decrease of PDGF-dependent phosphorylation of the PDGF receptor and attenuation of PDGF-evoked cellular migration.22
Decorin binds to low-density lipoprotein receptor–related protein 1 (LRP-1) and causes endocytosis of the complex, leading to PI3K activation and indirect cross-talk and modulation of TGFβ signaling via Smad 2/3/7.23 Although the interaction with LRP-1 has not yet been evaluated in cancer models, it could have implications for tumor cell bioenergetics.
Another binding partner of decorin is Wnt-1–induced secreted protein 1 (WISP-1), a protein mainly confined to the stroma of colon tumors.24 Decorin binding to WISP-1 results in an inhibition of WISP-1 interactions with other partners, thus suggesting a regulatory function of decorin in Wnt signaling.24 It is not known whether WISP-1 activity is further modulated or antagonized in either a Wnt-1–dependent or –independent manner by decorin in colon tumors. Notably, decorin down-regulates β-catenin in a non-canonical way after binding to Met,25 and decorin-null mice have increased β-catenin levels in the intestinal epithelium.26
In addition to the aforementioned interactions and attenuation of growth factors by direct binding, decorin also binds a multitude of structural components within the extracellular matrix, particularly numerous collagen molecules, tenascin X27, and elastin28 (Figure 1). Aside from binding fibrillar collagens I, II, and III, decorin also binds saturably to collagen XIV. Thus, decorin may mediate binding to fibril-forming collagens while simultaneously affecting collagen XIV biology. Analyses of the supramolecular composition of collagen VI complexes have determined that decorin and matrilin directly bind to link collagen VI to either aggrecan or collagen type II fibrils.29 Taking these findings together, it seems plausible that decorin can certainly participate in regulating desmoplastic reactions and higher-order matrix structure formation in the tumor stroma. The abundant presence of decorin in the tumor stroma of solid tumors has been proposed to represent a negative-feedback loop on the activity of adjacent RTKs expressed by growing malignant cells.1
Decorin Is an Endogenous Pan-Receptor Tyrosine Kinase Inhibitor
The initial finding that decorin bound with avid affinity to RTKs (Figure 1) heralded a paradigm shift in the study of SLRPs and their contributions to cancer biology. EGFR was the first RTK discovered to bind decorin30 and was considered the primary target of decorin in various types of cancer cells,2 as it triggered dimerization, internalization via caveosomes, and ultimately the degradation of the receptor complex within lysosomes,31 presumably terminating EGFR signaling. This finding is in contrast to EGF–EGFR complexes that are competent to signal from endosomes after internalization where EGF is still capable of maximally stimulating tyrosine phosphorylation. Furthermore, this stimulation engages several signaling pathways, such as PLC-γ1, that are required for cell motility.32 However, it is currently unknown whether decorin is proficient to stimulate signaling postinternalization in a fashion analogous to that of EGF.
Systemic delivery of decorin, or viral vectors expressing decorin, affects the growth of several types of solid cancers in which RTKs play key roles.33–36 Paramount to this feature is the ability of decorin to compete with EGF and to exert a potent physiological down-regulation of the receptor at the cell surface of tumor cells.2 However, in 2009, a new target of decorin was discovered via the use of an RTK phosphotyrosine array, which clearly demonstrated a rapid burst of phosphorylation of Met.37 It was further shown that Met is the main target of decorin in HeLa cells, as inhibition of the kinase domain of EGFR with the small molecule inhibitor AG1478 or with the monoclonal blocking antibody mAb425 did not block the down-regulation of Met evoked by decorin. Moreover, Met exhibits a higher binding affinity for decorin when compared with that of EGFR.37
It is a curious biological feature of decorin to evoke differential phosphorylation signatures in EGFR and Met on binding. This observation might reflect altered structural conformations of the receptors postbinding relative to the active conformation that the receptor adopts after binding natural ligands. EGFR, for example, undergoes rapid phosphorylation to stimulate the MAPK signaling cascade, which paradoxically aids in cell cycle arrest and caspase-3 activation38 despite total EGFR levels decreasing up to 50%.2 Further proof-of-concept for this phenomenon involves Met. A rapid burst of phosphorylation of the tyrosine residues of the intracellular tail of Met ensues and lasts up to 30 minutes, whereas increased phospho-Y1003 recruits the E3 ubiquitin ligase c-Cbl for receptor internalization and degradation.37 It is possible that these signatures, particularly that of Met, has biological significance to convey the properties of decorin bioactivity. However, at this time, experimental evidence to evaluate these differential phosphorylation patterns is lacking but would be essential to gain a more mechanistic understanding of the biology of decorin. This is of critical importance, considering the lack of endogenous ligands for Met, which, at present, include only hepatocyte growth factor (HGF), internalin B, and decorin.37,39
Along these same lines, engagement of HGF with Met induces association of the receptor complex with clathrin-coated pits40 in HeLa cells for endosomal sorting and eventual recycling to the plasma membrane. However, this is not the case for decorin, as it drives caveolin-1 to associate with Met40 and concomitant lysosomal degradation in the same fashion as EGFR. Clearly, the differential phosphorylation patterns are encoding messages pertinent for decorin activity to selectively dictate finite biological outcomes.
Additional members of the Erb family of RTKs are also affected by decorin via degradation and signal suppression, including ErbB2 and ErbB4, presumably via titration of EGFR away from functional signaling complexes composed of ErbB2 or ErbB4 heterodimers. However, new findings indicate a direct antagonism of ErbB4 signaling by decorin.41 During scar tissue repair in the central nervous system, employment of small molecule inhibitors and siRNAs specific for ErbB4 also blocks the decorin-mediated down-regulation of semaphorin 3A, a target of ErbB4 activity.41
Opposing roles of decorin in the signaling cascades of endothelial cells have also been documented. Because decorin binds collagen with nanomolar affinity, it has been reported that an interaction among α2β1 integrin, collagen type I, and decorin increases endothelial cell migration by enhancing integrin–collagen interactions.42 However, VEGF-R2 has been reported to be antagonized by decorin and, more specifically, by an engineered fragment encoding LRR5 via downstream attenuation of ERK1/2 signaling in human extravillous trophoblastic (EVT) cells.43
A recurring theme concerning this form of receptor antagonism is the concept of receptor internalization and degradation evoked by decorin for most of the RTKs studied so far. However, this is not the case for IGF-IR, in which decorin binds to and attenuates the downstream signaling of IRS-1 and blunts IGF-I activation of Akt, ERK, and p70S6K, culminating in a migratory block.44 In this case, decorin does not promote association of IGF-IR with caveolin-1 but is capable of preventing IGF-I from localizing IGF-IR to caveosomes. Consequently, this receptor complex is not internalized or degraded in cellular models of urinary bladder carcinoma.44 In contrast, in other cellular systems using non-transformed cells, decorin seems to be an agonist of IGF-IR.45,46
Collectively, decorin can be considered an endogenous matrix-centric pan-RTK inhibitor that exhibits hierarchical binding affinities for various RTKs expressed at a given time by a tumor cell. This might aid in cross-talk signal integration, as decorin would be able to bind multiple receptors with varying kinetics as a mechanism to subdue growth signals for tumor growth arrest. Furthermore, a common thread uniting these receptors, with the exception of α2β1 integrin, is the inclusion of Ig-like modules in their ectodomain. It is possible that decorin has an inherent ability to bind all receptors harboring this particular domain. However, these binding events and potential biological outputs have not yet been experimentally evaluated.
Decorin Antagonizes Tumorigenesis by Attenuating Multiple Signaling Pathways
Robust and high-affinity binding concurrent with rapid receptor internalization and degradation underlie the initial events for decorin-mediated tumor growth repression in relation to broad RTK antagonism (Figure 2). Subsequent to this binding, a potent attenuation of multiple pathways coordinating proliferation, survival, and angiogenesis ensues. Decorin-mediated antagonism of Met specifically leads to a non-canonical repression of β-catenin and Myc.40 HGF, signaling via Met, enhances β-catenin stability by two distinct modes, including direct phosphorylation of β-catenin47 and concomitant repression of glycogen synthase kinase 3β (GSK3β) via phosphorylation. This is an example of RTK-mediated stabilization of β-catenin independent of traditional Wnt signaling. Collectively, this cascade promotes nuclear translocation of β-catenin, which drives transcription of prosurvival and protumorigenic genes.10 Myc, a target of β-catenin and downstream of HGF/Met,1 coordinates large networks conducive to growth and proliferation. Interestingly, among the repertoire of Myc targets is AP4, a transcriptional repressor of p21WAF148, a cyclin-dependent kinase inhibitor that is specifically induced by decorin treatment of various cancer cells.1 Conversely, addition of exogenous decorin induces a profound and maintained suppression of β-catenin and Myc, leading to inhibition of several key Met-mediated pathways, including migration, proliferation, survival, and scattering. This is mediated in part by relieving RTK inhibition of GSK3β, which in turn phosphorylates Myc at specific sites.40 Phosphorylation of Thr58 on Myc by GSK3β is known to evoke Myc degradation via the proteasome. Notably, phosphorylation at this site is markedly induced by the soluble decorin protein core, coincident with nuclear translocation, and 26S proteasomal degradation.40 It is tempting to speculate that protein phosphatase 2A, the serine–threonine phosphatase directly opposing GSK3β, might also be attenuated, thereby allowing enhanced β-catenin and Myc degradation. This would lead to de-repression and subsequent induction of the p21 locus while providing a mechanism for growth arrest (Figure 2). In addition, β-catenin drives cyclin D1 expression, and this pathway is also antagonized by decorin.40
Recently, a physical interaction between the armadillo repeats 10–11 of β-catenin and the forkhead domain of FoxM1 was identified as crucial for Wnt3a-directed β-catenin nuclear translocation and assembly of the ternary complex on promoters of target genes.49 Hyperactivation of FoxM1 occurs primarily in various gastrointestinal tumors,50 where it is now believed to facilitate aberrant β-catenin activation. This is intriguing insofar as decorin-null mice exhibit abnormal intestinal tumor formation when exposed to a Western diet, showing increased levels of β-catenin.26 It is thus plausible that decorin in the intestine represents a safeguard against the improper activity of the FoxM1/β-catenin signaling axis.
A connection might exist between decorin and a genomically imprinted tumor suppressor known as paternally expressed gene 3 (Peg3). First, morpholino-induced depletion of decorin in a zebrafish model results in stunted head-to-tail growth, strongly suggesting a role for decorin in regulating convergent extension,51 a fundamental developmental process mediated primarily by β-catenin signaling via the planar cell polarity pathway. Interestingly, Peg3 decreases β-catenin expression, thereby inhibiting zebrafish tail development in a Wnt-dependent manner52 and indicating a possible relationship between decorin and Peg3. This connection is further reinforced by the observation that the molecular interaction between the Peg3 N-terminal domain and β-catenin is the basis for the GSK3β-independent antagonism of β-catenin signaling.52 This is very analogous to the GSK3β- and Wnt-independent antagonism of β-catenin protein orchestrated by decorin via Met in various tumor cell lines. In the context of tumor progression, this potential link between decorin and non-canonical β-catenin attenuation might prove crucial, as there is a strong inverse association between decreased Peg3 mRNA expression and glioblastoma progression and grade.52
A new study has evaluated the role of Wnt signaling in promoting a supportive hematopoietic niche for stem cell and progenitor development.53 Importantly, Wnt3a was found to potently and consistently induce decorin expression in co-culture models. Furthermore, it was demonstrated that mesenchymal stem cells, derived from the bone marrow, act as decorin-producing cells.53 Surprisingly, decorin seems to phenocopy almost all of the same biological effects of Wnt3a, including stimulation of c-Kit expression, blockade of B-cell lymphopoiesis, and maintenance of undifferentiated hematopoietic stem/progenitor cells.53 These data indicate an intricate role of decorin in modulating the hematopoietic microenvironment by mimicking, instead of opposing, in a non-canonical fashion, downstream effects promoted by canonical Wnt signaling.
Decorin Affects the Angiogenic Network
A key step in malignant tumor progression is neo-vascularization, beginning with activation of the angiogenic switch. The molecular components and mechanisms involved are beginning to come into clear focus. However, the role of decorin in tumor angiogenesis is quite controversial, with studies indicating a pro-angiogenic role such as enhanced endothelial cell migration via increased α2β1 integrin interaction with collagen type I42. However, a recent study has clearly shown an inhibitory and high-affinity binding of decorin to the VEGF receptor 2 (VEGFR2).43 The intricacies of decorin in regulating angiogenesis are even more complex in the cornea, where pro-54 and antiangiogenic roles55 exist. Again, we stress the fact that these studies reporting pro-angiogenic activity of decorin are typically seen in normal, non-tumorigenic settings. In contrast, as it pertains to tumor angiogenesis, decorin, applied as an exogenous or endogenous agent, exerts powerful angiostatic activities to curtail vascularization in a variety of tumor cell lines56 and inversely correlates with the extent of vascularization.57
Stromal decorin is able to directly abrogate the HGF/Met signaling axis to inhibit VEGF-mediated angiogenesis58 by transcriptionally repressing hypoxia-inducible factor1α (HIF-1α), β-catenin, Myc, and SP1 under normoxia, and non-canonically suppressing HIF-1α protein.58 This net repression of critical transcription factors impairs HGF/Met-driven VEGFA. Moreover, Sp1 requires p42/44 mitogen-activated protein kinase (MAPK)–dependent phosphorylation for competent localization and activation of VEGF transcription, which is presumably attenuated via RTK antagonism.40,58 Further repression of VEGF occurs through the attenuation of matrix metalloproteinase (MMP) 2 and 9 transcription, which also depends on β-catenin. This disallows matrix-bound VEGFA from engaging VEGFR2 on endothelial cell surfaces.58
Abrogation of HGF/Met in vivo provides mechanistic evidence for the action of decorin. In stark contrast to HGF, which promotes angiogenesis through positive VEGF and negative thrombospondin regulation,59 decorin retards angiogenesis by negative VEGFA and positive thrombospondin regulation. Indeed, in vivo studies demonstrate that decorin can subvert HGF signaling through Met to achieve reduced tumor vascularization and vessel density.58
Through degradation of β-catenin and HIF-1α, several intertwined feedback loops are interrupted. Potent loss of HIF-1α results in reduced expression of Met,58,60 thus compromising the ability of tumor cells to respond to HGF signaling. This is further enhanced by the well-documented notion that HGF/Met potentiates β-catenin signaling and Myc expression.1 Thus, decorin silences this important feedback loop for sustained cellular growth while mitigating the overall migratory, proliferative, and angiogenic capacity of malignant cells. Collectively, these findings indicate a tripartite attack on Met, including ectodomain shedding,37 despite TIMP3 induction, caveolin-mediated endocytosis and degradation,40 and disruption of positive-feedback loops.58 This is of paramount importance because decorin is one of only two known mammalian ligands for Met, the third being internalin B, a bacterial protein.39
Decorin can simultaneously induce endogenous angiogenic inhibitors, such as TIMP3 and thrombospondin-1, which act to enhance the blockade of VEGF signaling via HGF/Met (Figure 2), consistent with suppressing Met activation to alleviate thrombospondin-1.59 The ramifications and implications of normoxic attenuation of HIF-1α open multiple possibilities of modulating pertinent pathways that are active in the early stages of tumor development by circumventing the engagement of the angiogenic switch. An intriguing possibility relates to HIF-1α in orchestrating a metabolic adaptation that drives tumor vascularization, thus linking decorin to possibly modulate tumor metabolism before the onset of angiogenesis at early normoxic stages.
Relatives of Decorin in Tumorigenesis: Lumican and Biglycan
Related members of the SLRP family include lumican (class II) and biglycan (class I), which share 26% and 57% homology with decorin, respectively. Lumican is a keratan sulfate proteoglycan that is normally found within the cornea. It was recently discovered that lumican is highly expressed within the stroma of high-grade breast cancers, while exhibiting an altered expression pattern in various other tumor types.1 Interestingly, the biological and prognostic correlates of lumican, classified as either a pro- or antioncogenic agent, within the varied tumors is certainly diversified, given that specific tumors (eg, colorectal, pancreatic, and pulmonary) exhibit poor prognostic outcomes as lumican expression increases within the stroma, which is typically indicative of advanced tumors. However, in the case of osteosarcoma and melanoma, decreased lumican expression correlates with increased tumor progression,1 suggesting that lumican is antioncogenic. This apparent discrepancy for lumican function among different tumor types is reminiscent of the context-dependent function of decorin in angiogenesis, thus highlighting the complex intricacies of SLRPs in tumorigenesis.
Lumican induces cell cycle arrest by inducing the cyclin-dependent kinase inhibitor p21, decreasing the activity and abundance of multiple cyclins, including cyclin-D1, and activating pro-apoptotic pathways61 akin to decorin. Furthermore, lumican evokes antimetastatic properties via high-affinity binding to the α2 I domain of the α2β1 integrin, thus suggesting a mechanism of action. In parallel, decorin binds to and antagonizes Met, resulting in decreased migratory capacity and impaired cellular proliferation and survival. Finally, lumican and decorin are both capable of inhibiting metastasis.35,36,62
Biglycan, the most closely related SLRP family member to decorin, has a very limited involvement in cancer progression. Primarily pro-inflammatory via TLR2/4 signaling, a study has implicated biglycan overexpression within human pancreatic cancer tissue as accompanied by a concomitant induction of p27 and decrease in cyclin A and PCNA,63 thereby resulting in an inhibition of tumor growth. Despite the relatively high degree of homology, it is interesting that decorin and biglycan do not have more extensive overlapping functions in tumorigenesis.
Conclusion and Perspectives
Decorin, an archetypal small leucine-rich proteoglycan, possesses intrinsic and potent antitumorigenic capabilities. Initially characterized as an avid binding partner of collagen and an inherent regulator of fibrillogenesis, soluble decorin is now emerging as a pan-RTK inhibitor coincident with powerful downstream signaling attenuation. Because of its highly promiscuous and broad binding repertoire with extracellular matrix constituents, growth factors, and cell surface receptors, decorin can be considered a “guardian from the matrix.” Decorin functions as a guardian in the context of constraining the activity of multiple growth factors, receptor tyrosine kinases, and extracellular matrix components. The source of decorin bioactivity lies within the unique attenuation of potent growth signals and cues that would otherwise facilitate malignant transformation. Furthermore, in most cases, these direct protein interactions act to ameliorate and counteract the overall tumorigenicity of the surrounding tumor microenvironment that would also otherwise foster and promote malignant transformation and tumor progression. Thus, decorin and perhaps other structurally related SLRPs act at a crossroad between inflammation and cancer, and could be determinant players in combatting many forms of solid tumors in which RTKs are deregulated.64
In addition, we can draw a comparison between decorin action outside cells with that of transcription factors inside cells. Several transcription factors, such as p53 and Myc, coordinately regulate the expression of large subsets of genes that are crucial for the viability of cellular processes, whereas decorin regulates growth factor bioavailability and receptor modulation within the extracellular milieu. Notably, the genetic cooperation between decorin and the tumor suppressor p53, which is widely regarded as the “guardian of the genome,” has already been established insofar as germline-null mutations in the decorin and p53 genes cause enhanced lymphoma tumorigenesis.65 Thus, it is plausible to connect stromal decorin to the intracellular regulation of the expression of a large genetic network.
One of the challenges of future research is finding and isolating the leucine-rich repeats of decorin that harbor distinct bioactivities. This could be of great clinical interest in regard to adjuvant peptide therapies. Further engineering of these smaller domains to specifically target cognate receptors could underlie advanced therapeutic drug designs that could serve as potent additions to the growing armamentarium of matrix-derived cancer modalities.
Acknowledgments
We thank all of the members of the Iozzo laboratory, and we apologize for not citing, because of space limitations, original articles from many laboratories that have contributed to the decorin field.
Footnotes
Supported by NIH grants RO1 CA39481, RO1 CA47282, and RO1 CA120975 (R.V.I.) and by NIH training grant T32 AA07463 (T.N.).
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University was involved in the peer review process or final disposition of this article.
CME Disclosure: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
References
- 1.Iozzo R.V., Sanderson R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldoni S., Iozzo R.V. Tumor microenvironment: modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 3.Iozzo R.V., Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–3875. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danielson K.G., Baribault H., Holmes D.F., Graham H., Kadler K.E., Iozzo R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keene D.R., San Antonio J.D., Mayne R., McQuillan D.J., Sarris G., Santoro S.A., Iozzo R.V. Decorin binds near the C terminus of type I collagen. J Biol Chem. 2000;275:21801–21804. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G., Ezura Y., Chervoneva I., Robinson P.S., Beason D.P., Carine E.T., Soslowsky L.J., Iozzo R.V., Birk D.E. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 7.Zhang G., Chen S., Goldoni S., Calder B.W., Simpson H.C., Owens R.T., McQuillan D.J., Young M.F., Iozzo R.V., Birk D.E. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merline R., Moreth K., Beckmann J., Nastase M.V., Zeng-Brouwers J., Tralhão J.G., Lemarchand P., Pfeilschifter J., Schaefer R.M., Iozzo R.V., Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Sci Signal. 2011;4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adany R., Heimer R., Caterson B., Sorrell J.M., Iozzo R.V. Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma: Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem. 1990;265:11389–11396. [PubMed] [Google Scholar]
- 10.Hanahan D., Winberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Köninger J., Giese N.A., Bartel M., di Mola F.F., Berberat P.O., di Sebastiano P., Giese T., Büchler M.W., Friess H. The ECM proteoglycan decorin links desmoplasia and inflammation in chronic pancreatitis. J Clin Pathol. 2012;59:21–27. doi: 10.1136/jcp.2004.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xaus J., Comalada M., Cardó M., Valledor A.F., Celada A. Decorin inhibits macrophage colony-stimulating factor proliferation of macrophages and enhances cell survival through induction of p27Kip1 and p21Waf1. Blood. 2001;98:2124–2133. doi: 10.1182/blood.v98.7.2124. [DOI] [PubMed] [Google Scholar]
- 13.Marchica C.L., Pinelli V., Borges M., Zummer J., Narayanan V., Iozzo R.V., Ludwig M.S. A role for decorin in a murine model of allergen-induced asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:863–873. doi: 10.1152/ajplung.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tufvesson E., Westergren-Thorsson G. Tumor necrosis factor-α interacts with biglycan and decorin. FEBS Lett. 2002;530:124–128. doi: 10.1016/s0014-5793(02)03439-7. [DOI] [PubMed] [Google Scholar]
- 15.Seidler D.G., Mohamed N.A., Bocian C., Stadtmann A., Hermann S., Schäfers K., Schäfers M., Iozzo R.V., Zarbock A., Götte M. The role for decorin in delayed-type hypersensitivity. J Immunol. 2011;187:6108–6199. doi: 10.4049/jimmunol.1100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer L., Babelova A., Kiss E., Hausser H.-J., Baliova M., Krzyzankova M., Marsche G., Young M.F., Mihalik D., Götte M., Malle E., Schaefer R.M., Gröne H.-J. The matrix component biglycan is proinflammatory and signals through toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreth K., Brodbeck R., Babelova A., Gretz N., Spieker T., Zeng-Brouwers J., Pfeilschifter J., Young M.F., Schaefer R.M., Schaefer L. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest. 2010;120:4251–4272. doi: 10.1172/JCI42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baghy K., Dezsó K., László V., Fullár A., Péterfia B., Paku S., Nagy P., Schaff Z., Iozzo R.V., Kovalszky I. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab Invest. 2011;91:439–451. doi: 10.1038/labinvest.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuchi Y., Kodama Y., Matsumoto T. Bone matrix decorin binds transforming growth factor-β and enhances its bioactivity. J Biol Chem. 1994;269:32634–32638. [PubMed] [Google Scholar]
- 20.Schaefer L., Iozzo R.V. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura T., Kishioka Y., Wakamatsu J., Hattori A., Hennebry A., Berry C.J., Sharma M., Kambadur R., Nishimura T. Decorin binds myostatin and modulates its activity to muscle cells. Biochem Biophys Res Commun. 2006;340:675–680. doi: 10.1016/j.bbrc.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 22.Nili N., Cheema A.N., Giordano F.J., Barolet A.W., Babaei S., Hickey R., Eskandarian M.R., Smeets M., Butany J., Pasterkamp G., Strauss B.H. Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: Potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am J Pathol. 2003;163:869–878. doi: 10.1016/S0002-9440(10)63447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabello-Verrugio C., Brandan E. A novel modulatory mechanism of transforming growth factor-β signaling through decorin and LRP-1. J Biol Chem. 2007;282:18842–18850. doi: 10.1074/jbc.M700243200. [DOI] [PubMed] [Google Scholar]
- 24.Desnoyers L., Arnott D., Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- 25.Young C.S., Kitamura M., Hardy S., Kitajewski J. Wnt-1 induces growth, cytosolic β-Catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol Cell Biol. 1998;18:2474–2485. doi: 10.1128/mcb.18.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi X., Tong C., Dokendorff A., Banroft L., Gallagher L., Guzman-Hartman G., Iozzo R.V., Augenlicht L.H., Yang W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29:1435–1440. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elefteriou F., Exposito J.-Y., Garrone R., Lethias C. Binding of tenascin-X to decorin. FEBS Lett. 2001;495:44–47. doi: 10.1016/s0014-5793(01)02361-4. [DOI] [PubMed] [Google Scholar]
- 28.Reinboth B., Hanssen E., Cleary E.G., Gibson M.A. Molecular interactions of biglycan and decorin with elastic fiber components: biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J Biol Chem. 2002;277:3950–3957. doi: 10.1074/jbc.M109540200. [DOI] [PubMed] [Google Scholar]
- 29.Wiberg C., Klatt A.R., Wagener R., Paulsson M., Bateman J.F., Heinegård D., Mörgelin M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278:37698–37704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- 30.Iozzo R.V., Moscatello D., McQuillan D.J., Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J.-X., Goldoni S., Bix G., Owens R.A., McQuillan D., Reed C.C., Iozzo R.V. Decorin evokes protracted internalization and degradation of the EGF receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 32.Haugh J.M., Schooler K., Wells A., Wiley H.S., Lauffenburger D.A. Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-γ1 signaling pathway. J Biol Chem. 1999;274:8958–8965. doi: 10.1074/jbc.274.13.8958. [DOI] [PubMed] [Google Scholar]
- 33.Reed C.C., Gauldie J., Iozzo R.V. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–3695. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 34.Tralhão J.G., Schaefer L., Micegova M., Evaristo C., Schönherr E., Kayal S., Veiga-Fernandes H., Danel C., Iozzo R.V., Kresse H., Lemarchand P. In vivo selective and distant killing of cancer cells using adenovirus-mediated decorin gene transfer. FASEB J. 2003;17:464–466. doi: 10.1096/fj.02-0534fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed C.C., Waterhouse A., Kirby S., Kay P., Owens R.A., McQuillan D.J., Iozzo R.V. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 36.Goldoni S., Seidler D.G., Heath J., Fassan M., Baffa R., Thakur M.L., Owens R.A., McQuillan D.J., Iozzo R.V. An anti-metastatic role for decorin in breast cancer. Am J Pathol. 2008;173:844–855. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldoni S., Humphries A., Nyström A., Sattar S., Owens R.T., McQuillan D.J., Ireton K., Iozzo R.V. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seidler D.G., Goldoni S., Agnew C., Cardi C., Thakur M.L., Owens R.A., McQuillan D.J., Iozzo R.V. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 39.Niemann H.H. Structural insights into Met receptor activation. Eur J Cell Biol. 2011;90:972–981. doi: 10.1016/j.ejcb.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Buraschi S., Pal N., Tyler-Rubinstein N., Owens R.T., Neill T., Iozzo R.V. Decorin antagonizes Met receptor activity and downregulates β-catenin and Myc levels. J Biol Chem. 2010;285:42075–42085. doi: 10.1074/jbc.M110.172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minor K.H., Bournat J.C., Toscano N., Giger R.J., Davies S.J.A. Decorin, erythroblastic leukaemia viral oncogene homologue B4 and signal transducer and activator of transcription 3 regulation of semaphorin 3A in central nervous system scar tissue. Brain. 2011;134:1140–1155. doi: 10.1093/brain/awq304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiedler L.R., Schönherr E., Waddington R., Niland S., Seidler D.G., Aeschlimann D., Eble J.A. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of α2β1 integrin activity. J Biol Chem. 2008;283:17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 43.Khan G.A., Girish G.V., Lala N., DiGuglielmo G.M., Lala P.K. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol. 2011;25:1431–1443. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iozzo R.V., Buraschi S., Genua M., Xu S.-Q., Solomides C.C., Peiper S.C., Gomella L.G., Owens R.T., Morrione A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J Biol Chem. 2011;286:34712–34721. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schönherr E., Sunderkötter C., Iozzo R.V., Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 46.Schaefer L., Tsalastra W., Babelova A., Baliova M., Minnerup J., Sorokin L., Gröne H.-J., Reinhardt D.P., Pfeilschifter J., Iozzo R.V., Schaefer R.M. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-1 receptor and mammalian target of rapamycin. Am J Pathol. 2007;170:301–315. doi: 10.2353/ajpath.2007.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herynk M.H., Tsan R., Radinsky R., Gallick G.E. Activation of c-Met in colorectal carcinoma cells leads to constitutive association of tyrosine-phosphorylated β-catenin. Clin Exp Metastasis. 2003;20:291–300. doi: 10.1023/a:1024024218529. [DOI] [PubMed] [Google Scholar]
- 48.Jung P., Menssen A., Mayr D., Hermeking H. AP4 encodes a c-Myc-inducible reperessor of p21. Proc Natl Acad Sci USA. 2008;105:15046–15051. doi: 10.1073/pnas.0801773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang N., Wei P., Gong A., Chiu W.-T., Lee H.-T., Colman H., Huang H., Jianfei X., Liu M., Wang Y., Sawaya R., Xie K., Yung W.K.A., Medema R.H., He X., Huang S. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427–442. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida Y., Wang I.-C., Yoder H.M., Davidson N.O., Costa R.H. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology. 2007;132:1420–1431. doi: 10.1053/j.gastro.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 51.Zoeller J.J., Pimtong W., Corby H., Goldoni S., Iozzo A.E., Owens R.T., Ho S.-Y., Iozzo R.V. A central role for decorin during vertebrate convergent extension. J Biol Chem. 2009;284:11728–11737. doi: 10.1074/jbc.M808991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang X., Yu Y., Yang H.W., Agar N.Y.R., Frado L., Johnson M.D. The imprinted gene PEG3 inhibits Wnt signaling and regulates glioma growth. J Biol Chem. 2010;285:8472–8480. doi: 10.1074/jbc.M109.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ichii M., Frank M.B., Iozzo R.V., Kincade P.W. The canonical Wnt pathway shapes niches supportive for hematopoietic stem/progenitor cells. Blood. 2012;119:1683–1692. doi: 10.1182/blood-2011-07-369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schönherr E., Sunderkotter C., Schaefer L., Thanos S., Grässel S., Oldberg Å.A., Iozzo R.V., Young M.F., Kresse H. Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J Vasc Res. 2004;41:499–508. doi: 10.1159/000081806. [DOI] [PubMed] [Google Scholar]
- 55.Mohan R.R., Tovey J.C.K., Sharma A., Schultz G., Cowden J.W., Tandon A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS ONE. 2011;6:e26432. doi: 10.1371/journal.pone.0026432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grant D.S., Yenisey C., Rose R.W., Tootell M., Santra M., Iozzo R.V. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 57.Salomäki H.H., Sainio A.O., Söderström M., Pakkanen S., Laine J., Järveläinen H.T. Differential expression of decorin by human malignant and benign vascular tumors. J Histochem Cytochem. 2008;56:639–646. doi: 10.1369/jhc.2008.950287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neill T., Painter H., Buraschi S., Owens R.T., Lisanti M.P., Schaefer L., Iozzo R.V. Decorin antagonizes the angiogenic network: Concurrent inhibition of Met, hypoxia inducible factor-1α and vascular endothelial growth factor A and induction of thrombospondin-1 and tissue inhibitor of metalloproteinase 3. J Biol Chem. 2012;287:5492–5506. doi: 10.1074/jbc.M111.283499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y.-W., Su Y., Volpert O.V., Vande Woude G.F. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci USA. 2003;100:12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckerich C., Zapf S., Fillbrandt R., Loges S., Westphal M., Lamszus K. Hypoxia can induce c-Met expression in glioma cells and enhance SF/HGF-induced cell migration. Int J Cancer. 2007;121:276–283. doi: 10.1002/ijc.22679. [DOI] [PubMed] [Google Scholar]
- 61.Vij N., Roberts L., Joyce S., Chakravarti S. Lumican suppresses cell proliferation and aids Fas-Fas ligand mediated apoptosis: implications in the cornea. Exp Eye Res. 2004;78:957–971. doi: 10.1016/j.exer.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Brézillon S., Zeltz C., Schneider L., Terryn C., Vuillermoz B., Ramont L., Perreau C., Pluot M., Diebold M.D., Radwanska A., Malicka-Blaszkiewicz M., Maquart F.-X., Wegrowski Y. Lumican inhibits B16F1 melanoma cell lung metastasis. J Physiol Pharmacol. 2009;60(Suppl 4):15–22. [PubMed] [Google Scholar]
- 63.Weber C.K., Sommer G., Michl P., Fensterer H., Weimer M., Gansauge F., Leder G., Adler G., Gress T.A. Biglycan is overexpressed in pancreatic cancer and induces G1-arrest in pancreatic cancer cell lines. Gastroenterology. 2001;121:657–667. doi: 10.1053/gast.2001.27222. [DOI] [PubMed] [Google Scholar]
- 64.Schaefer L., Iozzo R.V. Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr Opin Genet Dev. 2012;22:56–57. doi: 10.1016/j.gde.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Iozzo R.V., Chakrani F., Perrotti D., McQuillan D.J., Skorski T., Calabretta B., Eichstetter I. Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci USA. 1999;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]