Abstract
Pancreatic disease has onset in utero in humans with cystic fibrosis (CF), and progresses over time to complete destruction of the organ. The exact mechanisms leading to pancreatic damage in CF are incompletely understood. Inflammatory cells are present in the pancreas of newborn pigs with CF (CF pigs) and humans, which suggests that inflammation may have a role in the destructive process. We wondered whether tissue inflammation and genes associated with inflammatory pathways were increased in the pancreas of fetal CF pigs [83 to 90 days gestation (normal pig gestation is ∼114 days)] and newborn pigs. Compared with fetal pigs without CF (non-CF pigs), in fetal CF pigs, the pancreas exhibited patchy inflammation and acinar atrophy, with progression in distribution and severity in neonatal CF pigs. Large-scale transcript profiling revealed that the pancreas in fetal and newborn CF pigs exhibited significantly increased expression of proinflammatory, complement cascade, and profibrotic genes when compared with fetal and newborn non-CF pigs. Acinar cells exhibited increased apoptosis in the pancreas of fetal and newborn CF pigs. α-Smooth muscle actin and transforming growth factor β1 were increased in both fetal and newborn CF pig pancreas, suggesting activation of profibrotic pathways. Cell proliferation and mucous cell metaplasia were detected in newborn, but not fetal, CF pigs, indicating that they were not an initiator of pathogenesis but a response. Proinflammatory, complement cascade, proapoptotic, and profibrotic pathways are activated in CF pig pancreas, and likely contribute to the destructive process.
Cystic fibrosis (CF) is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel.1 This life-threatening disease involves multiple organ systems (eg, sweat ducts, airway, pancreatic duct, intestines, biliary tree, and vas deferens). The greatest morbidity and mortality in CF stem from lung disease.2–4
Because pancreatic function studies in humans with CF showed the presence of acidic, concentrated, and dehydrated pancreatic secretions,5–9 and autopsy findings were consistent with plugging of acinar and ductal lumens,10–18 it is generally agreed that CFTR dysfunction leads to defective pancreatic fluid and bicarbonate secretion and plugging. As the disease progresses, the exocrine pancreas is replaced by fatty infiltration and fibrosis10,12–14,16,17 and completely loses its function in ∼85% of patients with CF.19–24 Although pancreatic disease is nearly universal in CF, the time of disease onset and the stages between lumen-occluding secretions and pancreatic damage are not well understood. Pancreatic insufficiency has a major effect on growth, nutrition, and lung disease,19,25,26 and currently there are no treatments to halt the progression of pancreatic disease in CF. Designing therapies that can halt the progression of pancreatic involvement can be accomplished only with better understanding of the disease mechanisms that evolve in utero and immediately after birth.
Because the pancreas is not easily accessible in humans, CFTR gene knockout mouse models have been developed to study the mechanisms of pancreatic disease in CF.27,28 Inflammation,29 defective acinar cell endocytosis,30–33 and an imbalance in membrane essential fatty acids34 have all been suggested as potential mechanisms of pancreatic disease in the mouse model of CF. However, pathologic changes in the pancreas are mild in mice with CF, and do not progress to fibrosis and organ failure as typically observed in humans with CF.11–16
In the present study, we investigated the mechanisms of pancreatic damage in newborn and 83- to 90-day-old fetal pigs (pig gestation is ∼114 days) with CFTR null35 and homozygous ΔF508 mutations.36 We found that the pancreatic disease progressed over time in the pigs with CF (CF pigs) and that proinflammatory, complement cascade, proapoptotic, and profibrotic pathways were activated in CF compared with non-CF at both time points. Tissue remodeling (eg, mucous cell metaplasia and duct cell proliferation) was found in the newborn CF pig pancreas but not in fetuses, suggesting that these processes do not drive early disease pathogenesis but are a late response to ongoing tissue damage. These findings suggest that the pathogenesis of progressive pancreatic damage in CF may be, in part, a consequence of the activation and progression of the proinflammatory, proapoptotic, profibrotic, and complement cascade pathways.
Materials and Methods
Animals
All studies were approved by the University of Iowa Animal Care and Use Committee. CF (CFTR−/− and CFTRΔF508/ΔF508) and non-CF (CFTR+/+, CFTR+/−, and CFTR+/ΔF508) piglets were obtained from Exemplar Genetics (Sioux Center, IA) and studied within 24 hours after birth. Fetuses (83 to 90 days' gestation [pig gestation is ∼114 days]) were collected via cesarean section. The 83- to 90-day gestation point was chosen because that is when the pancreas has a well-developed architecture and is able to produce pancreatic enzymes.37
Total RNA Extraction
Pancreas was collected immediately after euthanasia, sectioned into 1- to 2-mm pieces, immersed in a stabilizer reagent (RNAlater; Ambion, Inc., Austin, TX), and snap frozen in liquid nitrogen. Samples were used immediately or stored at −80°C until use. Tissues were homogenized briefly using a rotor/stator-type tissue homogenizer (model 780CL-04; Bio Spec Products, Inc., Bartlesville, OK) at 18,000 to 22,000 rpm at 4°C. The pancreatic homogenate was centrifuged at 2000 rpm for 10 minutes, and the supernatant was collected. Total RNA was extracted from the supernatant using the PerfectPure RNA Fibrous Tissue Kit (catalog No. 2900303; 5 PRIME GmbH, Hamburg, Germany). RNA concentration was measured via optical density at a 260-nm wavelength using a NanoDrop 1000 Spectrophotometer (ThermoFisher Scientific, Inc., Wilmington, DE). The RNA quality was assessed via electrophoresis using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA).
Microarray and Analysis Protocol
The sample population included four non-CF (one CFTR+/+ and three CFTR+/ΔF508) and four CF (CFTRΔF508/ΔF508) pancreata from 83- to 90-day-old fetuses, and eight non-CF (four CFTR+/+ and four CFTR+/−) and four CF (CFTR−/−) pancreata from newborn pigs. Only samples with an RNA integrity number higher than 7.0 on the Agilent Bioanalyzer (Agilent Technologies) were processed further. Two micrograms total RNA was used to generate biotinylated complementary RNA using the GeneChip one-cycle target labeling kit (Affymetrix, Inc., Santa Clara, CA) according to the manufacturer's recommended protocols, and was hybridized to the Affymetrix Porcine GeneChip (23,937 probe sets that interrogate ∼23,256 transcripts from 20,201 Sus scrofa genes). The arrays were washed, stained, and scanned using a Fluidics Station (model 450) and scanner (model 3000) (both from Affymetrix), and data were collected using GeneChip operating software (version 1.4; Affymetrix) using the manufacturer's recommended protocols. The following tools were used to analyze the microarray data: Gene Pattern38 (unsupervised hierarchical clustering), Partek Genomics Suite (2008, revision 6.3; Partek, Inc., St. Louis, MO) (one-way analysis of variance; ANOVA), GSEA (Gene Set Enrichment Analysis),39 and DAVID (Database for Annotation, Visualization and Integrated Discovery; National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD).40 Use of these tools to analyze microarray data has been previously described.41
Quantitative RT-PCR
RNA from eight non-CF (CFTR+/+ and CFTR+/ΔF508) and seven CF (CFTRΔF508/ΔF508) fetal pancreata, and eight non-CF (CFTR+/+) and seven CF (one CFTRΔF508/ΔF508 and six CFTR−/−) newborn pancreata were treated using a DNase1 kit (New England BioLabs, Inc., Ipswich, MA). One microgram RNA was used to generate first-strand cDNA using SuperScript II (Invitrogen Corp., Carlsbad, CA), and oligo-dT and random hexamer primers. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a control. Primer-probe sequences were ordered from Integrated DNA Technologies, Inc. (IDT, Coralville IA). All reactions were set up using TaqMan Fast Universal PCR Master Mix (Applied Biosystems, Inc., Foster City, CA) and run on the 7900HT Real-Time PCR system (Applied Biosystems). All experiments were performed in quadruplicate using 56-FAM (single isomer of 6-carboxyfluorescein), and 3IABlkFQ (Iowa Black FQ dark quencher). Primer and probe sequences were as follows: TGFB1, forward 5′-AGGGCTACCATGCCAATTTCT-3′, reverse 5′-CGGGTTGTGCTGGTTGTACA-3′, probe 5′-/56-FAM/CCTAGACACTCAGTACAGCAAGGTCCTGGC/3IABlk-FQ/-3′; IL-8, forward 5′-CCGTGTCAACATGACTTCCAA-3′, reverse 5′-GCCTCACAGAGAGCTGCAGAA-3′, probe 5′-/56-FAM/CTGTTGCCTTCTTGGCAGTTTTCCTGC/3IABlk-FQ/-3′; NFKB1, forward 5′-CTGGCAGCTCTCCTCAAAGC-3′, reverse 5′-CACGAGTCATCCAGGTCATACAG-3′, probe 5′-/56-FAM/CTCAAAGTTCTCCACCAGGGGATCTGCTC/3IABlk-FQ/-3′; C3, forward 5′-CAAGAAATGATTGGTGGCTTCAA-3′, reverse 5′-GACCTGTGGTTCACAGATGTCTTT-3′, probe 5′-/56-FAM/AGCCTTTGTTCTCATCGCGCTGCA/3IABlk-FQ/-3′; CFB, forward 5′-TGATCAGGCTCTCGAAAAAGC-3′, reverse 5′-GGTTGTCAGAGGAAGCCTCAGA-3′, probe 5′-/56-FAM/ATCAGGCCCATTTGTCTCCCCTGC/3IABlk-FQ/-3′; C1QB, forward 5′-CCGGCCTTTACTACTTCACCTATC-3′, reverse 5′-CGGCCACGCATGAGGTT-3′, probe 5′-/56-FAM/CGCACAGGTTCCCTCGAGAGCTG/3IABlk-FQ/-3′; GAPDH, forward 5′-GGCATGGCCTTCCGTGT-3′, reverse 5′-GCCCAGGATGCCCTTGAG-3′, probe 5′-/56-FAM/CCTGCTTCACCACCTTCTTGATGTCATCAT/3IABlk-FQ/-3′.
IHC Staining
Pancreata were harvested immediately after necropsy and placed in 10% neutral buffered formalin for 48 to 96 hours, and were routinely processed, embedded, sectioned, and stained with H&E or amylase-pretreated PAS.36
Ki-67
Ki-67 IHC was performed as previously described.42 In brief, between sequential incubations, sections were intermittently rinsed with primary antibody (rabbit anti-Ki-67 monoclonal antibody, 1:800 dilution, overnight at 4°C), a secondary antibody (Rabbit Envision HRP System), and chromogen diaminobenzidine (DAB Plus for 5 minutes and enhancer for 3 minutes) (all agents from Dako, Carpinteria, CA). Morphometric analysis was performed on 5 to 10 random sections of pancreas. Cells of ductuloacinar units were counted (mean, ∼200 cells per pancreas), and each nucleus was noted as stained or unstained for enumeration of the cellular percentage of Ki-67 immunostaining.
Caspase-3
Caspase-3 IHC was performed as previously described.43 In brief, sections were intermittently rinsed between sequential incubations with primary antibody (rabbit monoclonal antibody against activated caspase-3, No. 9664L; Cell Signaling Technologies, Inc., Beverly, MA) and a secondary antibody (Rabbit Envision HRP System), and chromogen diaminobenzidine (DAB Plus for 5 minutes and enhancer for 3 minutes) (both from Dako). Morphometric analysis was performed on 5 to 10 random sections of pancreas. Ductuloacinar units (200 to 250 cells per pancreas) were counted along with positive ductuloacinar immunostaining. Particular attention was given to exclude caspase-3 immunostaining, commonly associated with infiltrative leukocytes,44 from the morphometric analysis. Total ductuloacinar units, along with immunostained ductuloacinar units, were enumerated to give the caspase-3 immunostaining index (percentage of stained cells to total cells counted).
α-SMA
Paraffin-embedded tissue sections were cut into 4-μm pieces, hydrated through a series of alcohol baths, and rinsed (1× Dako Buffer). Endogenous peroxidase was quenched with 3% H2O2 for 8 minutes, followed by rinsing. Tissues were incubated with primary antibody (mouse monoclonal antibody, anti-α-smooth muscle actin [α-SMA] antibody, catalog No. A2547; Sigma-Aldrich, St. Louis, MO), and rinsed, and then with secondary antibody (Mouse Envision HRP System, Dako), and rinsed, and stained with chromogen diaminobenzidine (DAB Plus for 5 minutes and enhancer for 3 minutes; Dako). Slides were counterstained with hematoxylin for 1 minute (Surgipath Medical Industries, Richmond, IL), rinsed, dehydrated through a series of alcohol and xylene baths, coverslipped, and stored.
Statistical Analyses
Data are given as the mean (SD) of individual data points. Statistical significance between groups was determined using Student's t-test or one-way ANOVA. The P values generated for the microarray analyses were not corrected for multiple comparisons. For the microarray studies, a highly stringent cutoff of P < 0.01 was considered significant because the list of differentially expressed genes with P < 0.05 was large.
Results
CF Pancreatic Lesions Progress From 83 to 90 Days Gestation to Birth (∼114 Days)
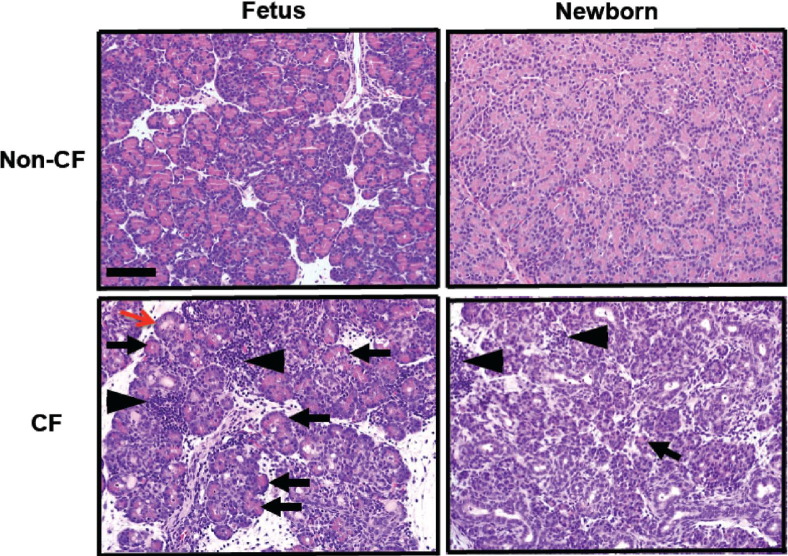
To determine how pancreatic lesions progress over time in CF pigs, we compared the pancreatic histologic findings in the newborn and 83- to 90-day-old fetal CF and non-CF pigs. The fetal CF pancreata exhibited a patchy loss of zymogen-filled acini (Figure 1), often with concurrent inflammation and tissue destruction. Inflammation was composed of loose lymphoid (lymphocytes and plasma cells) interstitial aggregates, and neutrophils and macrophages were scattered in the lumens of affected acini and ducts. At birth, CF pig pancreata had lost most zymogen-filled acini; however, remnants of degenerative pancreas were detected in most of the sections (Figure 1). The newborn CF pig pancreas exhibited duct proliferation, expansion of interlobular connective tissue, and scattered inflammatory cell aggregates. These findings confirm that, as in humans, pancreatic lesions began in utero in the CF pigs, and progressed over time to near destruction of the organ,10,14,15 with mixed inflammatory infiltrates detected in both fetal and neonatal pigs.
Figure 1.
CF and non-CF pancreata at 83 to 90 days gestation and at birth. Eosinophilic zymogen material is abundant in all pancreatic acini in non-CF animals (eosinophilic staining). At 83 to 90 days gestation, CF pancreata exhibit reduced zymogen-filled mature acini (black arrows), which was associated with increased acini plugging and degeneration by luminal eosinophilic zymogen secretions (red arrow). This eventually results in further loss of normal zymogen-filled acini by birth (arrow). While less extensive in fetal versus neonatal CF pancreas, discrete patchy aggregates of lymphoid inflammation (arrowheads) expand the interstitium, with scattered neutrophils and macrophages occasionally seen in the lumen of adjacent ducts and acini, similar to the localization pattern of inflammation previously described in CF pig pancreas.36,47 H&E. Scale bar = 100 μm.
Loss of CFTR Alters Global Transcriptome of Pancreas in CF
We next examined the transcriptional profile to identify the genes that were differentially expressed between the pancreata of CF and non-CF fetal and newborn pigs. Unsupervised hierarchical clustering38,41 of 12 newborn and 8 fetal samples revealed segregation of the gene expression profiles of the newborn and fetal samples by genotype (Figure 2A). Furthermore, within each developmental stage, the samples also clustered by genotypes (Figure 2A). By one-way ANOVA (Partek Genomics Suite),41 there were many differentially expressed genes between the CF and non-CF pancreas samples. Using as significance P < 0.01, 2586 genes were differentially expressed in the fetal CF pancreas, and 4411 genes were differentially expressed in the newborn CF pancreas (Figure 2B). Analysis of the differentially expressed genes using DAVID40,41 identified 27 pathways and 18 pathways with significant genotype-dependent differences in the fetal and newborn pancreata, respectively (P < 0.05) (Table 1). To further elucidate the gene pathways that were altered in the CF pancreas, the data were analyzed using GSEA.39,41,45 With a false discovery rate of <0.25, 1327 pathways were differentially expressed in the fetal CF samples, and 1576 pathways were differentially expressed in the newborn CF samples (Table 1). The large number of differentially expressed pathways using a low stringency significance level (DAVID, P < 0.05; GSEA, false discovery rate <0.25) reflects the high number of genes exhibiting significant changes in expression, as observed in the ANOVA data sets. Together, these data indicate a global change in the pancreas transcriptome brought about by the absence of functional CFTR.
Figure 2.
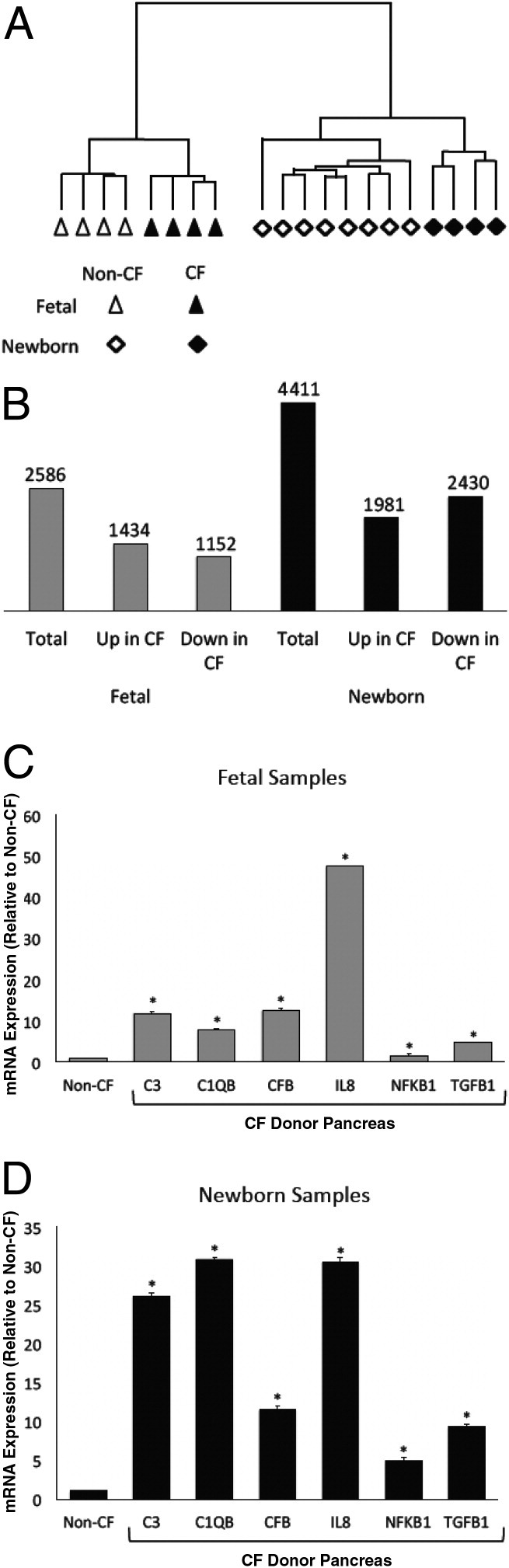
Loss of CFTR function alters the transcriptome in the CF pancreas. A: Unsupervised hierarchical clustering in 8 fetal and 12 newborn CF samples reveals significant differences in the transcriptome profile of CF and non-CF samples at fetal and newborn stages. B: One-way ANOVA reveals differentially expressed genes in the fetal and newborn CF samples (P < 0.01). C and D: Quantitative RT-PCR of the fetal and newborn pancreas samples shows the relative abundance of genes. Each bar represents data from eight non-CF and seven CF samples tested in quadruplicate. Error bars indicate mean (SD). *P < 0.01, CF versus non-CF.
Table 1.
Pathway Analysis (GSEA) of Differentially Expressed Genes (DAVID) in Fetal and Newborn Pig Pancreas Samples
| Pancreas | GSEA | DAVID gene sets |
GSEA gene sets |
||
|---|---|---|---|---|---|
| P < 0.01 | P < 0.05 | FDR < 0.05 | FDR < 0.25 | ||
| Fetal | Enriched in CF | 18 | 27 | 0 | 1327 |
| Enriched in Non-CF | 0 | 0 | 0 | 0 | |
| Newborn | Enriched in CF | 6 | 18 | 6 | 1576 |
| Enriched in Non-CF | 0 | 0 | 0 | 0 | |
DAVID, Database for Annotation, Visualization, and Integrated Discovery; FDR, false discovery rate; GSEA, Gene Set Enrichment Analysis.
Inflammation Is an Early Event in CF Pig Pancreatic Disease
Similar to histopathologic observations35,36,46 and flow cytometry studies47 that showed increased inflammation of mixed cell type in the CF pig pancreas, GSEA revealed enrichment of inflammatory gene sets in the CF samples at both developmental stages. To further identify the nature of inflammatory response in the CF samples, we prepared custom gene sets that addressed the following biological processes: i) response to injury, ii) inflammatory response, iii) host-defense response, and iv) innate immunity (see Supplemental Table S1 at http://ajp.amjpathol.org). Significant enrichment of these gene sets was observed in both fetal and newborn CF pancreata (Table 2),48 with greater fold increases in the newborn samples compared with the fetal samples. To further substantiate these findings, we assessed the mRNA abundance of the proinflammatory markers IL-8 and the nuclear factor NF-κB p105 subunit using quantitative RT-PCR. We found a significant increase in both of these mRNAs in the fetal and newborn CF samples (Figure 2, C and D). These results further support the activation of proinflammatory genes in the CF pig pancreas in utero. In addition, the number of activated proinflammatory genes increased over time as the lesions progressed.
Table 2.
Gene Set Enrichment Analysis of Fetal and Newborn Pancreas Samples48
| Custom gene sets | No. of Genes⁎ | Fetal pancreas samples |
Newborn pancreas samples |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of enriched genes† | NES | FDR | Description of change | No. of enriched genes† | NES | FDR | Description of change | ||
| Response to injury | 688 | 310 | 1.54 | 0.02 | Enriched in CF | 476 | 1.91 | 0.09 | Enriched in CF |
| Inflammatory response | 251 | 125 | 1.44 | 0.03 | Enriched in CF | 84 | 1.88 | 0.147 | Enriched in CF |
| Host defense response | 283 | 136 | 1.51 | 0.02 | Enriched in CF | 137 | 1.94 | 0.13 | Enriched in CF |
| Innate immunity | 99 | 46 | 1.55 | 0.03 | Enriched in CF | 45 | 1.86 | 0.025 | Enriched in CF |
| Complement activation | 37 | 25 | 1.7 | 0.02 | Enriched in CF | 17 | 1.9 | 0.005 | Enriched in CF |
| Classical complement pathway | 27 | 19 | 1.68 | 0.02 | Enriched in CF | 13 | 1.76 | 0.004 | Enriched in CF |
| Alternative complement pathway | 32 | 21 | 1.75 | 0.02 | Enriched in CF | 17 | 1.77 | 0.007 | Enriched in CF |
| Induction of apoptosis | 268 | 104 | 1.31 | 0.06 | Enriched in CF | 144 | 1.57 | 0.01 | Enriched in CF |
| Tissue remodeling | 72 | 29 | 1.37 | 0.07 | Enriched in CF | 29 | 1.84 | 0.004 | Enriched in CF |
| Fibroblast proliferation | 45 | 24 | 1.34 | 0.06 | Enriched in CF | 21 | 1.55 | 0.03 | Enriched in CF |
| Endothelial cell proliferation | 47 | 22 | 1.37 | 0.13 | Enriched in CF | 18 | 1.57 | 0.05 | Enriched in CF |
| Adipose tissue development | 76 | 28 | 1.4 | 0.03 | Enriched in CF | 27 | 1.77 | 0.04 | Enriched in CF |
FDR, false discovery rate (FDR <0.25 is considered significant); NES, normalized enrichment score.
Number of genes represents the genes present in the gene set annotated in the porcine array.
Number of enriched genes-represents genes from the total list that were enriched in that gene set.
Complement Cascade Pathways Are Activated in CF Pig Pancreas Starting in Utero
An intriguing observation was the significant increase in complement pathways at stringent significance levels in both the fetal and neonatal CF pig pancreata, as analyzed using DAVID (P < 0.01) and GSEA (false discovery rate <0.05) (Table 1). To better understand the involvement of complement pathways in the CF pig pancreas, we built custom gene sets for i) complement activation, ii) classical complement pathway, iii) alternative complement pathway, and iv) induction of apoptosis (Table 2; see also Supplemental Table S1 at http://ajp.amjpathol.org). We investigated apoptosis because this process often accompanies complement-mediated cell destruction.49 All four gene sets were significantly enriched in the CF pig pancreas at both time points. Furthermore, these pathways were significantly more enriched in the newborn CF samples than in fetal samples. Using quantitative RT-PCR, we confirmed a significant increase in the following complement genes in both fetal and newborn CF samples: complement factor 1 (C1QB), complement factor 3 (C3), and complement factor B (CFB) (Figure 2, C and D). These data show that key genes in the complement pathway are overexpressed in the CF pig pancreas starting in utero.
Apoptosis Is Increased in Fetal and Newborn CF Pig Pancreas
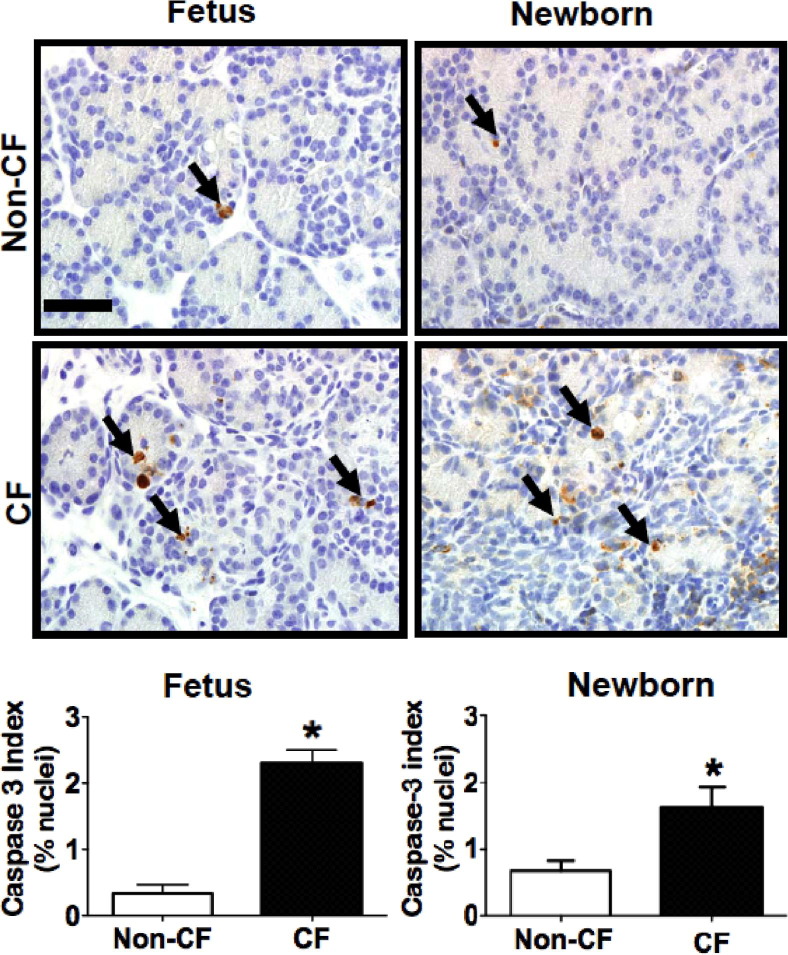
To further investigate the activation of apoptotic pathways in the CF pig pancreas, we immunostained fetal and newborn CF and non-CF pig pancreata for activated caspase-3, a key enzyme in apoptotic cell death. Scattered acini exhibited immunoreactive acinar cells, and these affected acini were sometimes associated with leukocytes near or infiltrative into the acinus or by luminal filling or obstruction by zymogen material. We observed a significant increase in activated caspase-3 immunostaining of acinar cells in the CF pig pancreas samples at both fetal and newborn stages (Figure 3). Infiltrative neutrophils also commonly exhibited activated caspase-3 staining, as has been described,42 and these were excluded from the morphometric analysis. This finding suggests that apoptotic pathways are activated in the CF pig pancreas starting in utero.
Figure 3.
Apoptosis is increased in both fetal and newborn CF pig pancreas. Activated caspase-3 staining (arrows) of non-CF and CF pig pancreas at 83 days gestation and in newborn pigs (IHC). Caspase-3 index was measured in 6 non-CF and 3 CF fetuses (left graph) and 10 non-CF and 18 CF newborn pigs (right graph), which show increased immunostaining in the CF pig pancreas at both time points. *P < 0.05. Scale bar = 35 μm.
Tissue Remodeling Is a Late Event in CF Pig Pancreatic Disease
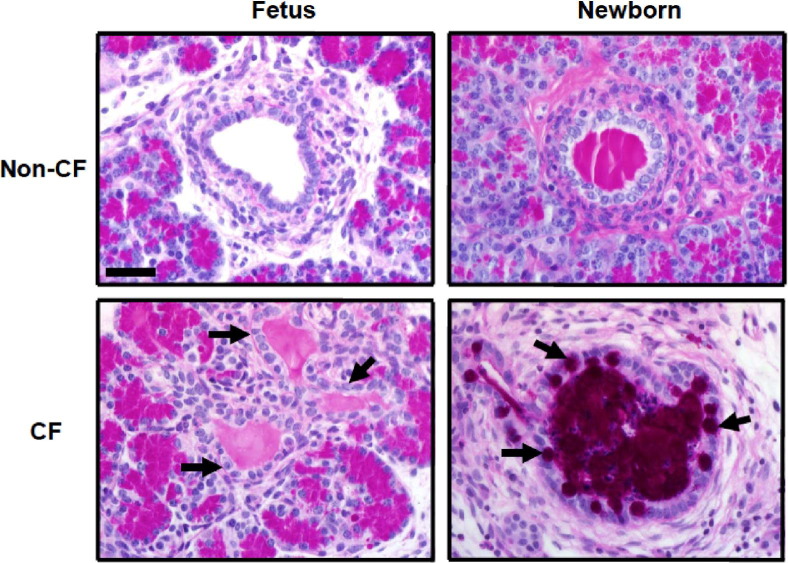
Intralobular duct mucous cell metaplasia has been described in the pancreas in children and adults with CF,15,17 possibly as a late event. We examined fetal and newborn CF and non-CF pig pancreata for the presence of mucous cell metaplasia in obstructed ducts; however, this was found only in the pancreata of CF newborn pigs, not fetuses (Figure 4), which suggests that mucous cell metaplasia is a late event in CF pancreatic disease.
Figure 4.
Top panels: Fetal and newborn Non-CF pig pancreata showing a normal duct that is empty and one filled by normal secretions, respectively. Mucous cell metaplasia is a late event in pancreatic disease in CF. Left panels: Mucous cell change in the duct epithelium is lacking in non-CF and CF pancreata at 90-days gestation (arrows). Bottom right panel: In the newborn pigs with CF, obstructed ducts were often distended with mucus (magenta area), and the mucous cells were prominent in the epithelium (arrows). Mucous cell metaplasia was not detected in non-CF neonatal pig pancreas. dPAS stain. Scale bar = 45 μm.
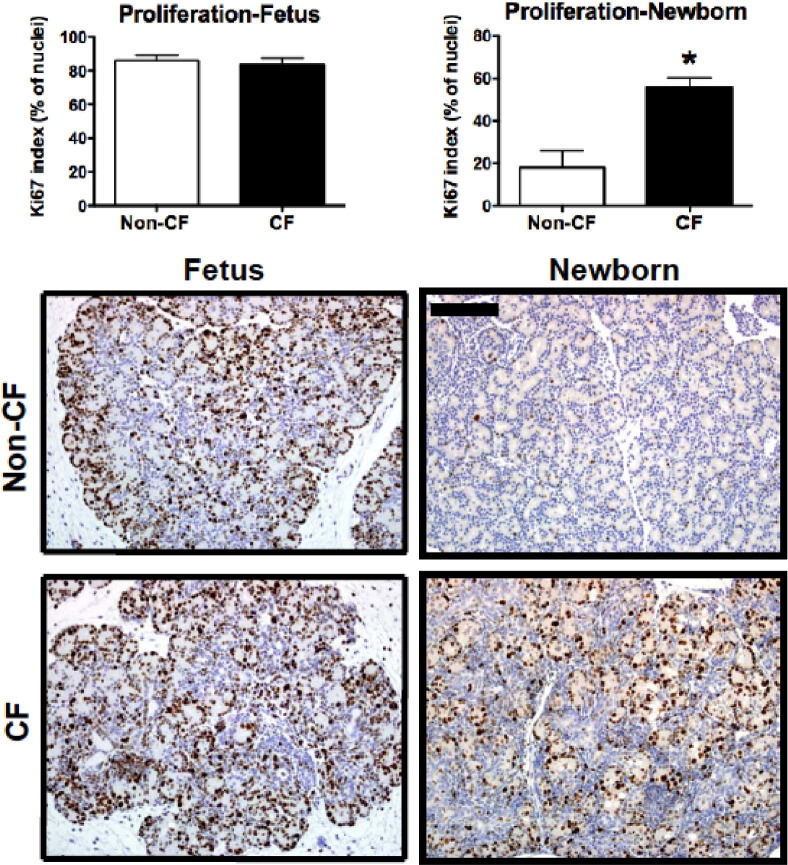
We then examined whether the myofibroblastic changes and cell proliferation typically found in the pancreas of humans with CF14,16 were present in the newborn and fetal pigs with CF. We used IHC staining to detect differences in cell proliferation (Ki-67 antigen), along with morphometric analysis. Fetal pancreata in both groups similarly exhibited high levels of cellular proliferation, consistent with a growing fetus. In contrast, cellular proliferation in neonatal pancreata was generally reduced; however, the newborn pancreata were significantly more proliferative in the CF versus the non-CF group (Figure 5). This finding was also supported by the custom GSEA data that showed significant enrichment of pathways associated with proliferation in the newborn CF pig pancreas but not in the fetal samples (Table 2).
Figure 5.
Increased cell proliferation in the newborn CF pig pancreas. Ki-67 immunostaining is increased in newborn CF pig pancreas (n = 20) compared with non-CF pancreas (n = 10) (*P < 0.001) but is similar between fetal pig groups (CF, n = 4; non-CF, n = 6). Ki-67-positive cells are often associated with proliferative ductuloacinar units. Scale bar = 100 μm.
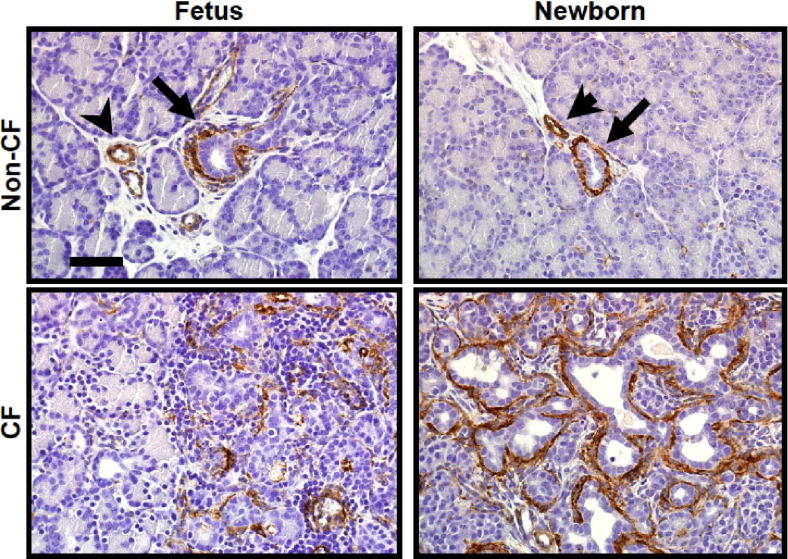
We then stained CF and non-CF fetal and newborn pig pancreata for α-SMA to detect early fibroplasia (Figure 6). In the non-CF animals, immunoreactive cells were uncommon, and were restricted to the normal perivascular and periductular cells. In the fetal CF pig pancreas, there was enhanced and patchy immunostaining in expanded interacinar interstitial spaces. In the newborn CF pigs, α-SMA staining was prominent in the myofibroblasts around the proliferative ducts. Consistent with these findings, transforming growth factor β1 mRNA levels were significantly higher in the pancreata of newborn CF pigs compared with CF fetuses (Figure 2, C and D). These findings are consistent with the development of tissue remodeling from the fetal to the neonatal period, and confirm the progressive nature of pancreatic disease in CF.
Figure 6.
CF pig pancreas exhibit increased α-SMA immunostaining in fetal pancreas at 90 days gestation and in newborn pigs. Top panels: In non-CF pig pancreas, α-SMA staining is detected, as normally expected, surrounding the ducts (arrows) and vessels (arrowheads). Bottom panels: At 90-days gestation, CF pancreas exhibits patchy mild to moderate staining adjacent to relatively unaffected exocrine tissue, whereas neonatal CF pancreas demonstrate widespread α-SMA staining surrounding proliferative and dilated ducts. Scale bar = 350 μm.
Discussion
In the present study, pancreatic lesions started in utero in CF pigs and progressed over time, in concordance with previous autopsy studies in humans with CF.10,14,15 We detected activation of proinflammatory, complement cascade, and apoptotic pathways early in development, whereas components of tissue remodeling (mucous cell metaplasia, duct proliferation, and increased myofibroblast activity) were detected at birth and constituted later events. Our observations suggest that mucous cell metaplasia and duct cell proliferation do not have a primary role in the pathogenesis of pancreatic disease in CF but are a late response to inflammation and tissue destruction.
A major advantage of the present study was the availability of the fetal and newborn pancreas samples, which enabled us to study the timing of onset and the mechanisms that contribute to inflammation, tissue destruction, and injury in a pristine environment lacking microbial and environmental insults. Because the pancreas cannot be easily obtained from human fetuses or infants and young children with CF at early stages of pancreatic disease, such a study is not possible in humans. CF mice have limited applicability for such studies because the pancreas is mildly involved.27,28 With pancreatic involvement similar to pancreatic disease in humans, the CF pig model enables access to tissues at all stages of the disease and provides the opportunity for mechanistic studies of the pathogenesis of pancreatic disease.
We observed a significant difference in the global gene expression profile between CF and non-CF pancreata in both fetal and neonatal pigs. The number of activated proinflammatory, complement cascade, apoptotic, and profibrotic pathway genes were higher in the newborn CF pancreata compared with fetal pancreata, consistent with the disease progression. These findings support the hypothesis of early activation and contribution of these pathways in pancreatic disease in CF.
We found that many proinflammatory pathways were activated in the pancreas of CF pigs starting in the fetal period. These findings are consistent with histopathologic observations35,36,46 and flow cytometry studies47 in the newborn CF pig pancreas. Together with the presence of inflammatory cell infiltrates in the human CF pancreas10,13,15 and heightened inflammatory response to cerulein hyperstimulation in CF mice,29 our data suggest that inflammation may have a significant role in the disease process. Although inflammatory cell infiltrates are well-known features of pancreatic disease in humans with CF,10,13,15 the earliest studies in humans suggest that the ductuloacinar plugging may be the first histopathologic finding in the CF pancreas.11,18 Plugging and obstruction may trigger an inflammatory response in the CF pancreas that leads to tissue destruction and organ failure. Analysis of earlier time points in utero may be helpful in identifying the initiating pathways that are activated during the early phases.
The increased activation of caspase-3 in the CF pig pancreas suggests that apoptosis is a component of pancreatic injury in CF; however, its role as a primary cause or secondary mediator is unclear. Apoptosis is a prominent component of the acute inflammatory phase of injury, and correlates with the degree of damage and injury in several models.50
The presence of activated classical and alternative complement pathways in the fetal and newborn CF pancreas is intriguing and not previously described in humans with CF. Complement pathways may be activated in acute pancreatitis, which then generate biologically active peptides that mediate tissue injury.51–53 While complement activation results in the massive amplification and activation of the cell-killing membrane attack complexes, it is interesting that the inhibitory stimuli (ie, CD59 [protectin] or C1 inhibitor) failed to control the activated complement pathways. It is not known whether inhibition of the complement cascade might modify the progressive tissue damage associated with pancreatic disease in CF.
We found that mucous cell metaplasia, cell proliferation, and increased α-SMA staining were observed in the newborn CF pig pancreas but was lacking in fetuses. Mucus is produced only by the epithelial cells of the interlobular and main pancreatic ducts54,55; thus, intralobular duct mucous cell metaplasia is pathognomonic for pancreatic disease in CF.15,17 The presence of mucous cell metaplasia in the pancreas of newborns CF pigs, but not fetuses, suggests that mucous cell metaplasia was a response to chronic destruction and inflammation of the pancreas, rather than an initiating event. Likewise, we observed prominent cell proliferation in the newborn CF pig pancreas, which was lacking in the fetuses. Duct cell proliferation is a common response to chronic injury or obstruction,56–59 and the presence of increased cellular proliferation in the CF pancreas at birth would be consistent with this premise. α-SMA, a marker of myofibroblast and activation of early pathways of fibrosis,44 was present in both fetal and newborn CF pig pancreas; however, overt fibrosis in the CF pig pancreas was not observed until weeks to months after birth.46,60 Considered together, these data highlight the progressive nature of pancreatic disease in CF and support our hypothesis that the tissue remodeling processes are late events in CF pathogenesis.
In summary, pancreatic inflammation and damage starts in utero in CF pigs, and advances over time to severe destruction of the organ. Inflammation and degenerative changes to acini are characteristic in both fetal and newborn pig pancreas. Mucous cell metaplasia and duct cell proliferation were present in newborn but not fetal pancreas, which suggests that they do not have a major role in the pathogenesis of the disease but represent a late response to ongoing tissue inflammation and damage. The pathways described in the present study may contribute to the pathogenesis of pancreatic disease in CF and may represent possible targets for modifying the disease course in future studies.
Footnotes
Supported by the NIH (grants DK084049 to A.U.; P01 HL51670 to P.B.M.; and PPG HL091842 to M.J.W.; and T32 Immunology grant 21703687-10 to M.A.), the Cystic Fibrosis Foundation (fellowship grant 8125000 to M.A.), the Children's Miracle Network (grant 86023157-30 to M.A.), the Roy J. Carver Charitable Trust (P.B.M. and M.J.W.), and the Iowa Cystic Fibrosis Foundation Research Development Program (grant R458-CR07 to M.J.W.). The Cell Morphology Core was supported by NIH (P30 DK54759).
M.A. and S.R. contributed equally to this work.
Disclosure: M.J.W. holds equity in Exemplar Genetics (Sioux Center, IA), which is licensing materials and technology related to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.04.024.
Supplementary data
References
- 1.Welsh M.J., Ramsey B.W., Accurso F.J., Cutting G.R. Cystic fibrosis. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic and Molecular Bases of Inherited Disease. ed 8. McGraw-Hill; New York: 2001. pp. 5121–5188. [Google Scholar]
- 2.Borowitz D., Durie P.R., Clarke L.L., Werlin S.L., Taylor C.J., Semler J., De Lisle R.C., Lewindon P., Lichtman S.M., Sinaasappel M., Baker R.D., Baker S.S., Verkade H.J., Lowe M.E., Stallings V.A., Janghorbani M., Butler R., Heubi J. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2005;41:273–285. doi: 10.1097/01.mpg.0000178439.64675.8d. [DOI] [PubMed] [Google Scholar]
- 3.Ooi C.Y., Dorfman R., Cipolli M., Gonska T., Castellani C., Keenan K., Freedman S.D., Zielenski J., Berthiaume Y., Corey M., Schibli S., Tullis E., Durie P.R. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011;140:153–161. doi: 10.1053/j.gastro.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 4.Dodge J.A., Lewis P.A., Stanton M., Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J. 2007;29:522–526. doi: 10.1183/09031936.00099506. [DOI] [PubMed] [Google Scholar]
- 5.Kopelman H., Durie P., Gaskin K., Weizman Z., Forstner G. Pancreatic fluid secretion and protein hyperconcentration in cystic fibrosis. N Engl J Med. 1985;312:329–334. doi: 10.1056/NEJM198502073120601. [DOI] [PubMed] [Google Scholar]
- 6.Kopelman H., Forstner G., Durie P., Corey M. Origins of chloride and bicarbonate secretory defects in the cystic fibrosis pancreas, as suggested by pancreatic function studies on control and CF subjects with preserved pancreatic function. Clin Invest Med. 1989;12:207–211. [PubMed] [Google Scholar]
- 7.Gaskin K.J., Durie P.R., Corey M., Wei P., Forstner G.G. Evidence for a primary defect of pancreatic HCO3-secretion in cystic fibrosis. Pediatr Res. 1982;16:554–557. doi: 10.1203/00006450-198207000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Hadorn B., Zoppi G., Shmerling D.H., Prader A., McIntyre I., Anderson C.M. Quantitative assessment of exocrine pancreatic function in infants and children. J Pediatr. 1968;73:39–50. doi: 10.1016/s0022-3476(68)80037-x. [DOI] [PubMed] [Google Scholar]
- 9.Johansen P.G., Anderson C.M., Hadorn B. Cystic fibrosis of the pancreas. a generalised disturbance of water and electrolyte movement in exocrine tissues. Lancet. 1968;1:455–460. [PubMed] [Google Scholar]
- 10.Andersen D.H. Cystic fibrosis of the pancreas and its relation to celiac disease: clinical and pathological study. Am J Dis Child. 1938;56:344–399. –399. [Google Scholar]
- 11.Boue A., Muller F., Nezelof C., Oury J.F., Duchatel F., Dumez Y., Aubry M.C., Boue J. Prenatal diagnosis in 200 pregnancies with a 1-in-4 risk of cystic fibrosis. Hum Genet. 1986;74:288–297. doi: 10.1007/BF00282551. [DOI] [PubMed] [Google Scholar]
- 12.Imrie J.R., Fagan D.G., Sturgess J.M. Quantitative evaluation of the development of the exocrine pancreas in cystic fibrosis and control infants. Am J Pathol. 1979;95:697–707. [PMC free article] [PubMed] [Google Scholar]
- 13.Oppenheimer E.H., Esterly J.R. Cystic fibrosis of the pancreas: morphologic findings in infants with and without diagnostic pancreatic lesions. Arch Pathol. 1973;96:149–154. [PubMed] [Google Scholar]
- 14.Oppenheimer E.H., Esterly J.R. Pathology of cystic fibrosis: review of the literature and comparison with 146 autopsied cases. Perspect Pediatr Pathol. 1975;2:241–278. [PubMed] [Google Scholar]
- 15.Porta E.A., Stein A.A., Patterson P. Ultrastructural changes of the pancreas and liver in cystic fibrosis. Am J Clin Pathol. 1964;42:451–465. doi: 10.1093/ajcp/42.5.451. [DOI] [PubMed] [Google Scholar]
- 16.Sturgess J.M. Structural and developmental abnormalities of the exocrine pancreas in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1984;3(Suppl 1):S55–S66. doi: 10.1097/00005176-198400031-00011. [DOI] [PubMed] [Google Scholar]
- 17.Tucker J.A., Spock A., Spicer S.S., Shelburne J.D., Bradford W. Inspissation of pancreatic zymogen material in cystic fibrosis. Ultrastruct Pathol. 2003;27:323–335. [PubMed] [Google Scholar]
- 18.Ornoy A., Arnon J., Katznelson D., Granat M., Caspi B., Chemke J. Pathological confirmation of cystic fibrosis in the fetus following prenatal diagnosis. Am J Med Genet. 1987;28:935–947. doi: 10.1002/ajmg.1320280420. [DOI] [PubMed] [Google Scholar]
- 19.Bronstein M.N., Sokol R.J., Abman S.H., Chatfield B.A., Hammond K.B., Hambidge K.M., Stall C.D., Accurso F.J. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr. 1992;120:533–540. doi: 10.1016/s0022-3476(05)82478-3. [DOI] [PubMed] [Google Scholar]
- 20.Couper R.T., Corey M., Moore D.J., Fisher L.J., Forstner G.G., Durie P.R. Decline of exocrine pancreatic function in cystic fibrosis patients with pancreatic sufficiency. Pediatr Res. 1992;32:179–182. doi: 10.1203/00006450-199208000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Dooley R.R., Guilmette F., Leubner H., Patterson P.R., Shwachman H., Weil C. Cystic fibrosis of the pancreas with varying degrees of pancreatic insufficiency. AMA J Dis Child. 1956;92:347–368. doi: 10.1001/archpedi.1956.02060030341004. [DOI] [PubMed] [Google Scholar]
- 22.Gaskin K., Waters D., Dorney S., Gruca M., O'Halloran M., Wilcken B. Assessment of pancreatic function in screened infants with cystic fibrosis. Pediatr Pulmonol Suppl. 1991;7:69–71. doi: 10.1002/ppul.1950110714. [DOI] [PubMed] [Google Scholar]
- 23.Waters D.L., Dorney S.F., Gaskin K.J., Gruca M.A., O'Halloran M., Wilcken B. Pancreatic function in infants identified as having cystic fibrosis in a neonatal screening program. N Engl J Med. 1990;322:303–308. doi: 10.1056/NEJM199002013220505. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs G.E., Bostick W.L., Smith P.M. Incomplete pancreatic deficiency in cystic fibrosis of the pancreas. J Pediatr. 1950;37:320–325. doi: 10.1016/s0022-3476(50)80150-6. [DOI] [PubMed] [Google Scholar]
- 25.Gaskin K., Gurwitz D., Durie P., Corey M., Levison H., Forstner G. Improved respiratory prognosis in patients with cystic fibrosis with normal fat absorption. J Pediatr. 1982;100:857–862. doi: 10.1016/s0022-3476(82)80501-5. [DOI] [PubMed] [Google Scholar]
- 26.Kraemer R., Rudeberg A., Hadorn B., Rossi E. Relative underweight in cystic fibrosis and its prognostic value. Acta Paediatr Scand. 1978;67:33–37. doi: 10.1111/j.1651-2227.1978.tb16273.x. [DOI] [PubMed] [Google Scholar]
- 27.Snouwaert J.N., Brigman K.K., Latour A.M., Malouf N.N., Boucher R.C., Smithies O., Koller B.H. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 28.Clarke L.L., Grubb B.R., Gabriel S.E., Smithies O., Koller B.H., Boucher R.C. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 1992;257:1125–1128. doi: 10.1126/science.257.5073.1125. [DOI] [PubMed] [Google Scholar]
- 29.DiMagno M.J., Lee S.H., Hao Y., Zhou S.Y., McKenna B.J., Owyang C. A proinflammatory, antiapoptotic phenotype underlies the susceptibility to acute pancreatitis in cystic fibrosis transmembrane regulator (−/−) mice. Gastroenterology. 2005;129:665–681. doi: 10.1016/j.gastro.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 30.Freedman S.D., Kern H.F., Scheele G.A. Pancreatic acinar cell dysfunction in CFTR(-/-) mice is associated with impairments in luminal pH and endocytosis. Gastroenterology. 2001;121:950–957. doi: 10.1053/gast.2001.27992. [DOI] [PubMed] [Google Scholar]
- 31.Freedman S.D., Kern H.F., Scheele G.A. Cleavage of GPI-anchored proteins from the plasma membrane activates apical endocytosis in pancreatic acinar cells. Eur J Cell Biol. 1998;75:163–173. doi: 10.1016/S0171-9335(98)80058-7. [DOI] [PubMed] [Google Scholar]
- 32.Freedman S.D., Sakamoto K., Venu R.P. GP2, the homologue to the renal cast protein uromodulin, is a major component of intraductal plugs in chronic pancreatitis. J Clin Invest. 1993;92:83–90. doi: 10.1172/JCI116602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman S.D., Sakamoto K., Scheele G.A. Nonparallel secretion of GP-2 from exocrine pancreas implies luminal coupling between acinar and duct cells. Am J Physiol. 1994;267:G40–G51. doi: 10.1152/ajpgi.1994.267.1.G40. [DOI] [PubMed] [Google Scholar]
- 34.Freedman S.D., Katz M.H., Parker E.M., Laposata M., Urman M.Y., Alvarez J.G. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr(-/-) mice. Proc Natl Acad Sci U S A. 1999;96:13995–14000. doi: 10.1073/pnas.96.24.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers C.S., Stoltz D.A., Meyerholz D.K., Ostedgaard L.S., Rokhlina T., Taft P.J., Rogan M.P., Pezzulo A.A., Karp P.H., Itani O.A., Kabel A.C., Wohlford-Lenane C.L., Davis G.J., Hanfland R.A., Smith T.L., Samuel M., Wax D., Murphy C.N., Rieke A., Whitworth K., Uc A., Starner T.D., Brogden K.A., Shilyansky J., McCray P.B., Jr, Zabner J., Prather R.S., Welsh M.J. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostedgaard L.S., Meyerholz D.K., Chen J.H., Pezzulo A.A., Karp P.H., Rokhlina T., Ernst S.E., Hanfland R.A., Reznikov L.R., Ludwig P.S., Rogan M.P., Davis G.J., Dohrn C.L., Wohlford-Lenane C., Taft P.J., Rector M.V., Hornick E., Nassar B.S., Samuel M., Zhang Y., Richter S.S., Uc A., Shilyansky J., Prather R.S., McCray P.B., Jr, Zabner J., Welsh M.J., Stoltz D.A. The DeltaF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001868. 74ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki N., Yoneda K., Bigger C., Brown J., Mullen Y. Fetal pancreas transplantation in miniature swine: I: Developmental characteristics of fetal pig pancreases. Transplantation. 1984;38:335–340. doi: 10.1097/00007890-198410000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Reich M., Liefeld T., Gould J., Lerner J., Tamayo P., Mesirov J.P. GenePattern 2.0. Nat Genetics. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang da W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramachandran S., Clarke L.A., Scheetz T.E., Amaral M.D., McCray P.B., Jr Microarray mRNA expression profiling to study cystic fibrosis. Methods Mol Biol. 2011;742:193–212. doi: 10.1007/978-1-61779-120-8_12. [DOI] [PubMed] [Google Scholar]
- 42.Meyerholz D.K., Stoltz D.A., Namati E., Ramachandran S., Pezzulo A.A., Smith A.R., Rector M.V., Suter M.J., Kao S., McLennan G., Tearney G.J., Zabner J., McCray P.B., Jr, Welsh M.J. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010;182:1251–1261. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunninghake G.W., Doerschug K.C., Nymon A.B., Schmidt G.A., Meyerholz D.K., Ashare A. Insulin-like growth factor-1 levels contribute to the development of bacterial translocation in sepsis. Am J Respir Crit Care Med. 2010;182:517–525. doi: 10.1164/rccm.200911-1757OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daigle I., Simon H.U. Critical role for caspases 3 and 8 in neutrophil but not eosinophil apoptosis. Int Arch Allergy Immunol. 2001;126:147–156. doi: 10.1159/000049506. [DOI] [PubMed] [Google Scholar]
- 45.Subramanian A., Kuehn H., Gould J., Tamayo P., Mesirov J.P. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics. 2007;23:3251–3253. doi: 10.1093/bioinformatics/btm369. [DOI] [PubMed] [Google Scholar]
- 46.Meyerholz D.K., Stoltz D.A., Pezzulo A.A., Welsh M.J. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176:1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu-El-Haija M., Sinkora M., Meyerholz D.K., Welsh M.J., McCray P.B., Jr, Butler J., Uc A. An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis. Pancreatology. 2011;11:506–515. doi: 10.1159/000332582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Harris M.A., Hill D.P., Issel-Tarver L., Kasarskis A., Lewis S., Matese J.C., Richardson J.E., Ringwald M., Rubin G.M., Sherlock G. Gene ontology: tool for the unification of biology: the Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fishelson Z., Attali G., Mevorach D. Complement and apoptosis. Mol Immunol. 2001;38:207–219. doi: 10.1016/s0161-5890(01)00055-4. [DOI] [PubMed] [Google Scholar]
- 50.Mantell L.L., Kazzaz J.A., Xu J., Palaia T.A., Piedboeuf B., Hall S., Rhodes G.C., Niu G., Fein A.F., Horowitz S. Unscheduled apoptosis during acute inflammatory lung injury. Cell Death Differ. 1997;4:600–607. doi: 10.1038/sj.cdd.4400278. [DOI] [PubMed] [Google Scholar]
- 51.Seelig R., Ehemann V., Tschahargane C., Seelig H.P. The serum complement system: a mediator of acute pancreatitis. Virchows Arch A Pathol Anat Histol. 1975;365:193–199. doi: 10.1007/BF00434038. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein I.M., Cala D., Radin A., Kaplan H.B., Horn J., Ranson J. Evidence of complement catabolism in acute pancreatitis. Am J Med Sci. 1978;275:257–264. doi: 10.1097/00000441-197805000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Horn J.K., Ranson J.H., Goldstein I.M., Weissler J., Curatola D., Taylor R., Perez H.D. Evidence of complement catabolism in experimental acute pancreatitis. Am J Pathol. 1980;101:205–216. [PMC free article] [PubMed] [Google Scholar]
- 54.Lev R., Spicer S.S. A histochemical comparison of human epithelial mucins in normal and in hypersecretory states including pancreatic cystic fibrosis. Am J Pathol. 1965;46:23–47. [PMC free article] [PubMed] [Google Scholar]
- 55.Shackleford J.M., Bentley H.P., Jr Carbohydrate histochemistry of the salivary glands and pancreas in cystic fibrosis. J Histochem Cytochem. 1964;12:512–521. doi: 10.1177/12.7.512. [DOI] [PubMed] [Google Scholar]
- 56.Boerma D., Straatsburg I.H., Offerhaus G.J., Gouma D.J., van Gulik T.M. Experimental model of obstructive, chronic pancreatitis in pigs. Dig Surg. 2003;20:520–526. doi: 10.1159/000073688. [DOI] [PubMed] [Google Scholar]
- 57.Hamamoto N., Ashizawa N., Niigaki M., Kaji T., Katsube T., Endoh H., Watanabe M., Sumi S., Kinoshita Y. Morphological changes in the rat exocrine pancreas after pancreatic duct ligation. Histol Histopathol. 2002;17:1033–1041. doi: 10.14670/HH-17.1033. [DOI] [PubMed] [Google Scholar]
- 58.Pitkaranta P., Kivisaari L., Nordling S., Saari A., Schroder T. Experimental chronic pancreatitis in the pig. Scand J Gastroenterol. 1989;24:987–992. doi: 10.3109/00365528909089245. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe S., Abe K., Anbo Y., Katoh H. Changes in the mouse exocrine pancreas after pancreatic duct ligation: a qualitative and quantitative histological study. Arch Histol Cytol. 1995;58:365–374. doi: 10.1679/aohc.58.365. [DOI] [PubMed] [Google Scholar]
- 60.Stoltz D.A., Meyerholz D.K., Pezzulo A.A., Ramachandran S., Rogan M.P., Davis G.J., Hanfland R.A., Wohlford-Lenane C., Dohrn C.L., Bartlett J.A., Nelson G.A., Chang E.H., Taft P.J., Ludwig P.S., Estin M., Hornick E.E., Launspach J.L., Samuel M., Rokhlina T., Karp P.H., Ostedgaard L.S., Uc A., Starner T.D., Horswill A.R., Brogden K.A., Prather R.S., Richter S.S., Shilyansky J., McCray P.B., Jr, Zabner J., Welsh M.J. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000928. 29ra312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.