Abstract
Objective
Controlled inpatient studies on the effects of food, physical activity (PA), and insulin dosing on glucose excursions exist, but such outpatient data are limited. We report here outpatient data on glucose excursions and its key determinants over 5 days in 30 adolescents with type 1 diabetes (T1D) as a proof-of-principle pilot study.
Subjects and Methods
Subjects (20 on insulin pumps, 10 receiving multiple daily injections; 15±2 years old; diabetes duration, 8±4 years; hemoglobin A1c, 8.1±1.0%) wore a continuous glucose monitor (CGM) and an accelerometer for 5 days. Subjects continued their existing insulin regimens, and time-stamped insulin dosing data were obtained from insulin pump downloads or insulin pen digital logs. Time-stamped cell phone photographs of food pre- and post-consumption and food logs were used to augment 24-h dietary recalls for Days 1 and 3. These variables were incorporated into regression models to predict glucose excursions at 1–4 h post-breakfast.
Results
CGM data on both Days 1 and 3 were obtained in 57 of the possible 60 subject-days with an average of 125 daily CGM readings (out of a possible 144). PA and dietary recall data were obtained in 100% and 93% of subjects on Day 1 and 90% and 100% of subjects on Day 3, respectively. All of these variables influenced glucose excursions at 1–4 h after waking, and 56 of the 60 subject-days contributed to the modeling analysis.
Conclusions
Outpatient high-resolution time-stamped data on the main inputs of glucose variability in adolescents with T1D are feasible and can be modeled. Future applications include using these data for in silico modeling and for monitoring outpatient iterations of closed-loop studies, as well as to improve clinical advice regarding insulin dosing to match diet and PA behaviors.
Introduction
The importance of dietary intake and physical activity (PA) on glucose excursions for the care of type 1 diabetes (T1D) is well accepted,1–3 yet these key human behaviors are poorly integrated into current decision-making regarding insulin dosing. At best, insulin dosing is given as a function of total carbohydrate (CHO), combined with “rules of thumb” based on clinical consensus and individual experience plus consideration for anticipated PA.4
Numerous carefully controlled inpatient studies have been performed to establish the interrelationship of glucose excursions with insulin dose timing,5,6 type of bolus delivery,7 diet,5,8,9 and PA.10 These studies and others have provided important data on the effects of these variables on glucose excursions for the treatment of T1D; however, as with any inpatient study, the translation of these data to the outpatient environment is uncertain.
Given advances in technology, we posited that such data could be obtained in an outpatient setting, capturing the real-life experiences of youth. We used current technologies to concurrently measure factors that determine glucose excursions by use of the following time-stamped data: (1) the outcome variable glucose excursions obtained with continuous glucose monitoring (CGM); (2) insulin dosing obtained via insulin pump or insulin pen with a digital log; (3) dietary intake obtained with diet records and 24-h dietary recall and confirmed with digital camera phone technology; and (4) PA obtained via accelerometry, plus self-report integrated with the 24-h dietary recall process. Herein, we present these integrated pilot data obtained in an outpatient setting in adolescents and establish a proof-of-principle that such high-resolution data acquisition is possible and can be used in regression models to predict outpatient glucose excursions.
Research Design and Methods
The study population were adolescents seen at the Barbara Davis Center for Childhood Diabetes 12–18 years old with T1D for >1 year who were using insulin/CHO ratios and using either insulin pump therapy (Medtronic [Northridge, CA] or Animas [West Chester, PA]) or on multiple daily injections (MDI). Subjects with celiac disease, T1D with <1 year in duration, hemoglobin A1c (HbA1c) >12%, or not on an insulin pump or using MDI insulin therapy were excluded. The study was approved by the Colorado Multiple Institutional Review Board, and all participants provided informed assent or consent (if ≥18 years).
Study visits
Basic demographics, clinical and anthropometric data, and medical history were collected along with nutrition education received (amount, type) and CHO counting practices (when subjects CHO count, what resources are used, insulin bolusing behavior, etc.). A standard questionnaire based on those used in the SEARCH for Diabetes in Youth study11 was administered by study staff and used to obtain past medical and family history, demographics such as date of birth and ethnicity, insulin dosing, and CHO counting questions. Height, weight, and blood pressure were measured using standard methods,11 and body mass index was calculated. HbA1c values were obtained from the patient's most recent clinic visit (DCA Vantage™ analyzer, Siemens Healthcare Diagnostics, Tarrytown, NY).
The FreeStyle® Navigator® (Abbott Diabetes Care, Alameda, CA) CGM system was inserted, and subjects were instructed on its use. Subjects were shown how to wear the Actical™ accelerometer (Phillips Respironics, Bend, OR) and when it was acceptable to remove the device (sleeping and bathing). Instructions on how to fill out the food and PA records (on Days 1 and 3) were reviewed. As part of the initial study visit, subjects were asked about their usual insulin/CHO ratio and the modifications to the ratio for exercise or glucose levels that are made regularly. On days assigned for diet record keeping, subjects were asked to record times of meals, snacks, and PA. The timing of insulin boluses was captured in either the insulin pump download or the Lilly (Indianapolis, IN) Memoir™ pen digital log.
Participants filled out food and activity records (at home) for Days 1 and 3 during the 5-day study period, and these records were transmitted electronically for use on the following day to facilitate administration of a 24-h dietary and PA recall, which took place on Day 2 (regarding Day 1) and Day 4 (regarding Day 3). Recalls were conducted by phone by trained staff at the University of North Carolina Diet Assessment, Body Composition and Physical Activity Core of the National Institute of Diabetes and Digestive and Kidney Diseases–funded Nutrition Obesity Research Center. The participant then returned on Day 5 to have the CGM device removed and downloaded along with the insulin pump or Lilly Memoir pen data, cell phone, and accelerometer. Specific details of data acquisition for each variable follow.
Data acquisition and analysis—outcome: glucose excursion data
CGM was performed using the FreeStyle Navigator system, which provided a glucose reading every 10 min for a 5-day period. Subjects were blinded to the data.12 The primary outcome variable was glucose data from the CGM system, but glucose meter data were obtained and required to calibrate the CGM device.
Predictors of glucose excursions
Insulin dosing data
Data from the insulin pump or the Lilly Memoir pen, which included time of bolus deliver, were compiled at the completion of the 5-day CGM period. If subjects were using the Lilly Memoir pen, then they were instructed to go through the digital memory on the pen daily and record date, time, and units of insulin given on a log given to them at the initial study visit. This log was faxed/scanned to the study coordinator along with the food and activity logs on Days 2 and 4. The pen's memory was also reviewed by study staff on Day 5. Data on insulin/CHO ratios used, basal insulin rates (glargine or detemir), and correction factors for hyperglycemia were recorded. In addition, logs were provided for the study participants to record comments on any decisions to override bolusing decisions based on insulin/CHO ratio or on correction factor boluses that commonly occur due to impending exercise or other factors. Adjustment of insulin for impending exercise is currently a formidable challenge for closed-loop technology in an outpatient setting, and we used these forms to capture insulin dosing adjustments made for exercise or other factors.
Dietary data
Diet was assessed using an integration of standardized food records, camera cell phone-based time-stamped pictures of food to be consumed and after consumption, and detailed 24-h dietary recalls for Days 1 and 3. Traditional food records were done on Days 1 and 3. The food logs were supplemented by use of camera cell phone pictures. In addition to providing the time-stamp, a standard measure (e.g., a ruler or coin) was used to provide an embedded scale to facilitate portion size estimation, as we have done previously and used by numerous studies.13–18 Finally, using the food logs and photos to prompt the subject, nutritionists at the University of North Carolina administered a detailed 24-h dietary recall using standardized methods supported by Nutrition Data System for Research. Specifically, the day following each day of recorded intake, a nutritionist reviewed the food record with the subject following the rigorous protocol of a multiple-pass 24-h dietary recall, with particular attention to estimation of portion size, review of possible omissions to the record, and review of timing of meals and snacks. The method of combined use of food records and 24-h dietary recalls has recently been shown to provide good validity compared with directly observed dietary intake in youth.19
PA data
Two complementary methodologies were used to obtain data on PA. First, PA was collected from the PDPAR, which is an interviewer-administered PA record. Second, each subject wore an Actical accelerometer that provided time-stamped data on each subjects' activity. Accelerometers provided data on physical exertion in real time. This approach provides both the activities of the adolescents (PDPAR) and an objective (accelerometry) measure of exercise intensity. Both instruments provided information on the pattern of activity, which allowed for the evaluation of timing of activity with respect to meals, insulin, and glucose excursions. The PDPAR divides the day into half-hour time blocks and requires the respondent to report the dominant activity and the approximate intensity of that activity for that period, categorized as “light,” “medium,” “hard,” or “very hard.” The PDPAR was administered in the same session as the 24-h dietary recall. The interview was used to cue the previous day's activities and to determine what activities these adolescents were performing.
The Actical has been validated against continuous measurements of energy expenditure in a respiratory room calorimeter and measurements in an exercise laboratory using a portable calorimeter and treadmill in 7–18-year-old children.20 The Actical also compared favorably with total energy expenditure measured from the doubly labeled water method in 10 normal-weight premenopausal women over 14 days, and the Actical21 better estimated total energy expenditure compared with the ActiGraph™ (Pensacola, FL) device by more than 12%. Accelerometer data were recorded in 15-s epochs, and four consecutive records were added to get an activity count per minute. Participants were instructed to wear the accelerometer continuously and remove it only for sleeping and bathing. Participants also recorded activity in the same 30-min blocks queried in the PDPAR. The objectively time-stamped accelerometry data were used in statistical modeling of the predictors of glucose excursions, and the PDPAR data were used in interpretation of findings relative to the actual activities in which the youth engaged. When analyzing the accelerometry data we excluded periods of >1 h with no activity right before midnight and sleep time, which is defined as having >2 h with no activity and less than 10 min awake between midnight and 6 a.m. (0600 h). Activity below 10 counts/min was considered as no activity. To determine minutes at various PA levels the 15-s epoch were converted to minute epochs by adding four nearby epochs. Then the following classifications of accelerometry data were made: sedentary, ≤50 counts/min; light, 51–2,000 counts/min; moderate, 2,001–2,900 counts/min; and vigorous, >2,900 counts/min.22
Statistical methods
Descriptive baseline data are stratified by pump/MDI status of the subjects; no statistical tests were performed as there were no a priori hypotheses tested based on insulin delivery modality, but rather subjects using both types of insulin delivery were included to demonstrate feasibility of data acquisition. Mean (±SD) CGM, physical activity, and dietary data on Days 1 and 3 were computed. The following indices were calculated using CGM data: mean±SD of daily recordings, number of daily glucose recordings, and percentage of time spent <70 mg/dL, 70–180 mg/dL, 180–300 mg/dL, and >300 mg/dL.23,24 Only Day 1 and Day 3 data were used in the analysis because, by design, those were the days in which complete nutrition data (24-h recall) were performed.
Linear regression models were performed using an autoregression and moving average correlation matrix to account for within-person correlation to predict CGM between 1 h before and 4 h after the first meal of the day. This type of analysis allowed us to use all complete days of data, even though some data (e.g., either insulin, glucose, PA, or diet) were missing from some subjects for either day. Other correlation matrices were considered, but the autoregression and moving average had the smallest (best) Akaike criterion index. The following time varying variables were considered as predictors of CGM: time (per 10-min increments), square term of time, first CGM reading (1 h prior to first meal), body weight, daily basal insulin and cumulative bolus insulin, cumulative PA, and cumulative CHO and fiber intake. PROC MIXED in SAS version 9.2 (SAS Institute, Cary, NC) was used for the regression analysis.
Results
Participant characteristics stratified by insulin regimen (pump or MDI) are shown in Table 1 and overall had the following characteristics: 63% male, 90% non-Hispanic white, 15.2±2.0 years old, diabetes duration 7.3±4.5 years, body mass index z-score 0.3±0.8, and HbA1c 8.1±1.0%. Both groups had similar total daily doses of insulin and distribution as basal/bolus, but those on pumps had more daily boluses (6.2±2.9 vs. 2.9±1.3).
Table 1.
Participant Characteristics
| |
Mean percentage or (SD) [range]a |
|
|---|---|---|
| Characteristic | Pump (n=20) | MDI (n=10) |
| Male | 75.0% | 40.0% |
| Race | ||
| White | 90.0% | 90.0% |
| Hispanic/Asian Indian | 10.0% | 10.0% |
| Age (years) | 15.1 (1.8) [12–18] | 15.4 (2.3) [12–18] |
| Weight (kg) | 64.8 (16.8) [41.9–111] | 62.4 (8.5) [52.8–75.4] |
| Height (cm) | 172 (11.2) [151–192] | 168 (12.4) [154–188] |
| BMI (kg/m2) | 21.6 (3.8) [17.3–32.5] | 22.2 (3.0) [18.4–27.3] |
| BMI z-scoreb | 0.3 (0.9) [−1.4 to 2.1] | 0.4 (0.6) [−0.5 to 1.2] |
| T1DM duration (years) | 7.8 (4.4) [1.7–14.8] | 6.2 (4.5) [1.1–15.5] |
| HbA1c (%) | 8.0 (0.8) [6.9–10.1] | 8.3 (1.3) [6.0–10.1] |
| Daily insulin (Days 1 and 3) | ||
| Total units (basal+bolus)/day | 57.5 (25.1) [18.6–146] | 59.9 (32.9) [20.0–180] |
| Basal (units) | 26.8 (13.5) [7.5–87.0] | 30.0 (12.9) [11.0–60.0] |
| Bolus (units) | 30.2 (16.0) [3.4–71.4] | 29.2 (22.5) [3.0–120] |
| Number of boluses/day | 6.2 (2.9) [1.0–17.0] | 2.9 (1.3) [1.0–6.0] |
| % basal | 48.9% | 53.0% |
| % bolus | 51.1% | 47.0% |
SD not adjusted for within-person correlation for insulin doses.
Using 2000 Centers for Disease Control and Prevention growth charts.
BMI, body mass index; HbA1c, hemoglobin A1c; MDI, multiple daily injections; T1DM, type 1 diabetes mellitus.
Descriptive data on CGM by day are listed in Table 2 Data were similar by pump and MDI, on Days 1 and 3, and when examined in those in whom greater than 75% compared with less than 75% of CGM recordings were obtained (data not shown). CGM data on both Days 1 and 3 were obtained in 57 of the possible 60 subject-days with an average of 125 daily CGM readings (out of a possible 144), with a mean glucose of 200±68 mg/dL. Among these readings 3% were<70 mg/dL, 48% were between 70 and 180 mg/dL, 32% were between 181 and 300 mg/dL, and 17% were >300 mg/dL. Results were very similar in the subset of 46 days in which ≥75% of CGM recordings were obtained on both Days 1 and 3.
Table 2.
Continuous Glucose Monitoring, Physical Activity, and Nutritional Intake
| Day 1 | Day 3 | |
|---|---|---|
| CGM | ||
| Number of patients | 30 | 27 |
| CGM average (mg/dL) | 195 (57) | 205 (80) |
| Number of daily recordings | 124 (29) | 126 (31) |
| % <70 mg/dL | 3.5 (5.7) | 3.1 (5.4) |
| % 70–180 mg/dL | 50.9 (25.5) | 44.5 (27.2) |
| % 181–300 mg/dL | 30.8 (18.8) | 34.0 (20.0) |
| % >300 mg/dL | 14.8 (19.9) | 18.5 (26.2) |
| Physical activity (n)a | 30 | 27 |
| Non-sleep mean (counts/min) | 213 (151) | 186 (127) |
| Sedentary (min/day) | 592 (41.1%) | 632 (43.9%) |
| Light (min/day) | 278 (19.3%) | 259 (18.0%) |
| Moderate (min/day) | 11 (0.7%) | 9 (0.6%) |
| Vigorous (min/day) | 9 (0.6%) | 6 (0.4%) |
| Nutritional intake | ||
| Days with diet recall | 29 | 30 |
| Number of meals and snacks/day | 7 (3) | 8 (3) |
| Total energy (Kcal) | 2,187 (1,101) | 2,513 (1,028) |
| Total CHO (g) | 263 (113) | 294 (150) |
| Dietary fiber (g) | 17 (7) | 20 (10) |
Data are mean (SD) values. The SD was not adjusted for within-person correlation. Pump and MDI subjects were combined.
Sleeping time is defined as having >2 h with no activity and less than 10 min woken up, or >1 h no activity right before midnight. Sedentary is defined as not sleeping and ≤50 counts/min, light activity as 51–2,000 counts/min, moderate as 2,001–2,900 counts/min, and vigorous as >2,900 counts/min, measured as accelerometer counts.
CGM, continuous glucose monitoring; CHO, carbohydrate.
Descriptive data on non-sleep accelerometer data are displayed in Table 2 by day, with 100% of subjects with data on Day 1 and 90% on Day 3 (14–16 h/day of accelerometry data). Activity during non-sleep averaged 200±139 counts/minute. Subjects spent approximately 2% of their time (15–20 min/day) in moderate or vigorous activity and 28–31% (approximately 4.5 h/day) in light activity, with 67–70% (>10 h/day) of their time during these 2 days being sedentary.
Results of the 24-h dietary recall are given in Table 2 for Days 1 and 3 (97% and 100% complete, respectively). Subjects averaged 2,353 kcal in 7±3 meals and snacks daily. Forty-seven percent of calories were from CHOs, 17% from protein, and 37% from fat, and dietary fiber intake was 18±9 g/day.
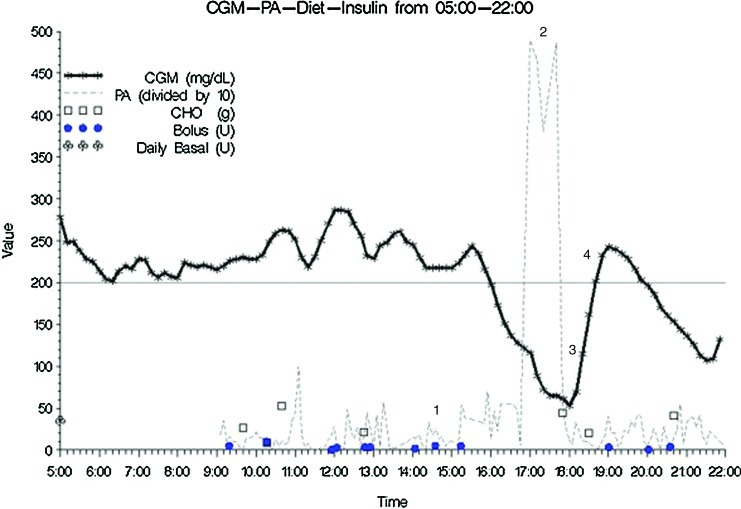
One example of the temporal effects of insulin (basal and bolus), CHO intake, and PA on glucose excursions as measured by CGM is depicted in Figure 1. The following points were evident: (1) multiple boluses of insulin given 1–2 h prior to, (2) vigorous exercise with, (3) resultant hypoglycemia, cessation of exercise, and (4) ingestion of excess CHO with subsequent rebound hyperglycemia.
FIG. 1.
Example of temporal relationship of insulin doses, carbohydrate (CHO), and physical activity (PA) with continuous glucose monitoring (CGM): region 1, multiple boluses for hyperglycemia; region 2, vigorous PA; region 3, hypoglycemia with CHO treatment and cessation of PA; and region 4, rebound hyperglycemia. Color images available online at www.liebertonline.com/dia
Regression analyses predicting CGM at 1, 2, 3, and 4 h after the first meal of the day are presented in Table 3. Of the 60 subject-days, 56 had data that contributed to these analyses. As expected, the first CGM value significantly predicted CGM at 1, 2, 3, and 4 h. Among dietary data, cumulative CHO (increased) and fiber (decreased) significantly predicted CGM. For PA, cumulative light PA significantly predicted increased CGM at all time points, and moderate-to-vigorous PA (which was recorded only approximately 1% of the time) significantly predicted decreased CGM at 1 and 2 h and predicted decreased CGM at 3 and 4 h. Cumulative basal insulin was not a significant predictor of CGM, but cumulative bolus significantly predicted decreased CGM at all time points.
Table 3.
Regression Models to Predict Continuous Glucose Monitoring Between 1 and 4 H After the First Meal
| |
Estimate (95% CI) |
|||
|---|---|---|---|---|
| Variable | 1 h | 2 h | 3 h | 4 h |
| Intercept | 50.4 (−2.5, 103.2) | 55.6 (−14.6, 125.9) | 40.6 (−65.3, 146.5) | 56.3 (−50.8, 163.5) |
| Time (10-min) | 3.77 (2.86, 4.68)a | 1.93 (0.70, 3.15)a | 1.34 (0.07, 2.61)a | 1.29 (0.11, 2.47)a |
| (Time)2 | 0.65 (0.44, 0.87)a | 0.11 (−0.04, 0.27) | −0.02 (−0.11, 0.07) | −0.08 (−0.14, −0.01)a |
| First CGM (mg/dL) | 0.79 (0.72, 0.85)a | 0.82 (0.74, 0.91)a | 0.90 (0.82, 0.99)a | 0.74 (0.67, 0.82)a |
| Cumulative | ||||
| CHO (g) | 0.27 (0.16, 0.39)a | 0.41 (0.28, 0.55)a | 0.28 (0.16, 0.40)a | 0.24 (0.14, 0.33)a |
| Fiber (g) | −4.02 (−5.73, −2.30)a | −6.51 (−8.63, −4.38)a | −2.53 (−4.35, −0.70)a | −2.41 (−3.80, −1.02)a |
| Bolus (U) | −1.17 (−1.58, −0.75)a | −1.71 (−2.24, −1.18)a | −1.92 (−2.40, −1.44)a | −1.27 (−1.65, −0.90)a |
| PA (min) | ||||
| Light | 1.00 (0.72, 1.28)a | 0.71 (0.44, 0.97)a | 0.88 (0.68, 1.09)a | 0.87 (0.73, 1.01)a |
| Moderate-vigorous | 1.84 (0.11, 3.56)a | 4.68 (3.23, 6.12)a | −2.46 (−3.67, −1.25)a | −3.36 (−4.11, −2.60)a |
| Body weight (kg) | −0.34 (−1.31, 0.63) | −0.20 (−1.49, 1.10) | −0.88 (−2.81, 1.05) | −0.51 (−2.44, 1.42) |
| Daily basal (U) | −0.11 (−1.19, 0.97) | −0.46 (−1.90, 0.98) | 0.98 (−1.02, 2.99) | 0.69 (−1.22, 2.60) |
Statistically significant.
CGM, continuous glucose monitoring; CI, confidence interval; CHO, carbohydrate; PA, physical activity.
Conclusions
In this study we demonstrate the feasibility of obtaining high-resolution time-stamped data on the main inputs of glucose variability in adolescents with T1D in an outpatient setting. Previous studies have performed such detailed data collection in an inpatient setting to carefully control research variables, but also due to previous technological limitations. We found adolescents willing and able to follow this research protocol to gather these data for a 5-day study period, and these methods present opportunities for future research and clinical care.
These data are consistent with previous studies in adolescents with T1D that report suboptimal glucose control,25 poor dietary habits,26 and limited PA.27,28 For example, the mean HbA1c was 8.1±1.0%, above the target of 7.5%, with only 48% of time spent in relative euglcyemia of 70–180 mg/dL. Additionally, as is not uncommon in adolescents, subjects reported 7±3 meals or snacks daily (but only 6.2 [pump] or 2.9 [MDI] boluses daily), with 37% of calories as fat and only 18±9 g of fiber daily. Finally, subjects spent only 1% of their day (17 min) in moderate-to-vigorous PA and 19% (4.5 hours) in light PA.
These data highlight the current challenges to achieving tight glucose control in adolescents with T1D. For example, if seven meals or snacks are consumed daily then optimally this requires seven insulin boluses (either via pump or injection) to limit glucose excursions. These data are consistent with previous studies showing missed boluses for meals29 or snacks30 are common in adolescents and associated with poorer glucose control. Given the current onset of action of rapid-acting insulin analogs, it is desirable to give boluses up to 20 min prior to eating, depending on blood glucose at that time, which adds another layer of complexity to diabetes care.5 Similarly, the prolonged duration of action (approximately 4 h) of rapid-acting insulin results in persistent effects of insulin on glycemia that may overlap with PA and result in hypoglycemia. Such an example is depicted in Figure 1, in which multiple boluses were given to correct for hyperglycemia 1–2 h prior to vigorous exercise. Combined, these resulted in hypoglycemia and cessation of exercise to overtreat the hypoglycemia with CHO with subsequent rebound hyperglycemia. Unfortunately, moderate-to-vigorous PA was rare in this cohort, and concern for hypoglycemia is one barrier that can prevent or limit PA and its health benefits.31 PA-related hyperglycemia can be a result of the effects of the sympathetic nervous system during PA,32 a pre-activity strategy to avoid anticipated PA-induced hypoglycemia, or a result of overtreatment of a low blood sugar; all of these can lead to poorer glucose control and present a challenge to people with T1D to achieve recommended daily PA goals.
Refinement of insulin dosing algorithms for either a closed- or hybrid-closed loop artificial pancreas or for current diabetes management is a topic of intensive investigation.33 To date, data informing such efforts have been based on inpatient studies in small groups of subjects in which variables are carefully controlled. Outpatient data such as those we present here could provide a complementary dataset on which to develop such dosing decisions or a methodology by which outpatient implementation of such insulin dosing algorithms (either patient or device driven) could be monitored. Results of the regression analyses show the expected associations of insulin dosing, diet, and PA on resultant CGM values 1–4 h post-waking. Future articles will include more detailed analyses of these associations as well as the accuracy of CHO counting, insulin-to-CHO ratios, and the effect of diet on glucose excursions.
Given advances in diabetes technology, it is not difficult to envision enhanced dietary programs on future insulin pumps via smartphones or other platforms. Similarly, accelerometry devices could also be incorporated to enhance adjustments for PA. Challenges will exist in precisely and accurately obtaining such data, but perhaps even more so with processing and translating such data into a format that is usable for both the patient and the diabetes care team. Possible future applications using data such as those obtained in this study include the following: (1) Can accelerometry provide a “rule” or advice on how to adjust insulin doses for periods of exercise and to predict and avoid hypoglycemia post-exercise, especially nocturnally? (2) Can real-time data for food intake be used for insulin dosing, and if so, how can this be presented in a “user-friendly” format? (3) Can these methods and data be used to identify a pattern of inputs that can predict hypo- or hyperglycemia and alert patients so that preemptive actions can be taken? (4) How does dysglycemia (either hypo- or hyperglycemia) predict future dysglycemia, and how might these be avoided?
Strengths of our study include its methodologic novelty in acquiring these time-stamped data in an outpatient setting in adolescents for whom compliance with a detailed research protocol can be a challenge. On the other hand, adolescents are the population of people with T1D who typically have the highest HbA1c but also the greatest willingness and aptitude to adopt technology, and this offers the possibility that novel technology could have the greatest benefit in this age group. Subjects tolerated wearing a CGM device and accelerometer well for this 5-day study period, although previous studies have highlighted that improvements in such technologies must be made to increase their use for extended period of time.34 A recent outpatient study combined CGM and accelerometry in 16 adolescents with T1D for 3 days but did not include dietary or insulin dosing data.35 Limitations of our study include uncertainties about generalization of these data to all adolescents with T1D. We also did not obtain complete data on all subjects, although this is also the case with inpatient studies because of imperfections in applying technology to human use (i.e., CGM failure). It is also possible that not all boluses via the Memoir pen were recorded by the subjects, although daily recording was performed, and the lower number of boluses in the MDI population may be due to the increased effort to bolus with MDI as compared with a pump. Finally, these 5 days may not be representative of these adolescents' typical days, but it is our opinion that this methodology is more likely to capture typical behavior than inpatient studies.
In conclusion, these data demonstrate the feasibility of outpatient acquisition of high-resolution time-stamped data on the main inputs of glucose variability in adolescents with T1D. Future applications include using these data for in silico modeling and for monitoring outpatient iterations of closed-loop studies, as well as clinically to advise insulin dosing and refine current diabetes care. Advances in diabetes technology will continue as will the challenge to adapt these technologies into “user-friendly” formats for both patients and diabetes care teams.
Acknowledgments
We would like to thank the study participants and their families as well as the staff of the Barbara Davis Center for Childhood Diabetes. D.M.M. was supported by a K23 grant (DK075360) from the National Institute for Diabetes and Digestive and Kidney Diseases. This study was supported by investigator-initiated grants from Eli Lilly and Abbott Diabetes Care. This research was supported in part by the University of North Carolina Diet, Physical Activity and Body Composition core with grant DK56350 from the National Institutes of Health.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverstein J. Klingensmith G. Copeland K. Plotnick L. Kaufman F. Laffel L. Deeb L. Grey M. Anderson B. Holzmeister LA. Clark N. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 3.Chase HP. Maahs DM. 12th. Denver: Children's Diabetes Foundation at Denver; 2011. Understanding Diabetes: A Handbook for People Who Are Living with Diabetes. [Google Scholar]
- 4.Maahs DM. Higgins J. Is carbohydrate counting enough? Towards perfection or unwanted complexity? Diabetes Technol Ther. 2012;14:3–5. doi: 10.1089/dia.2011.0234. [DOI] [PubMed] [Google Scholar]
- 5.Cobry E. McFann K. Messer L. Gage V. VanderWel B. Horton L. Chase HP. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with type 1 diabetes. Diabetes Technol Ther. 2010;12:173–177. doi: 10.1089/dia.2009.0112. [DOI] [PubMed] [Google Scholar]
- 6.Weinzimer SA. Steil GM. Swan KL. Dziura J. Kurtz N. Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 7.Chase HP. Saib SZ. Mackenzie T. Hansen MM. Garg SK. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med. 2002;19:317–321. doi: 10.1046/j.1464-5491.2002.00685.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones SM. Quarry JL. Caldwell-McMillan M. Mauger DT. Gabbay RA. Optimal insulin pump dosing and postprandial glycemia following a pizza meal using the continuous glucose monitoring system. Diabetes Technol Ther. 2005;7:233–240. doi: 10.1089/dia.2005.7.233. [DOI] [PubMed] [Google Scholar]
- 9.Wolever TM. Mullan YM. Sugars and fat have different effects on postprandial glucose responses in normal and type 1 diabetic subjects. Nutr Metab Cardiovasc Dis. 2011;21:719–725. doi: 10.1016/j.numecd.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Tsalikian E. Mauras N. Beck RW. Tamborlane WV. Janz KF. Chase HP. Wysocki T. Weinzimer SA. Buckingham BA. Kollman C. Xing D. Ruedy KJ. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147:528–534. doi: 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SEARCH Study Group. SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Kovatchev B. Anderson S. Heinemann L. Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31:1160–1164. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson DA. Allen HR. Martin PD. Alfonso AJ. Gerald B. Hunt A. Comparison of digital photography to weighed and visual estimation of portion sizes. J Am Diet Assoc. 2003;103:1139–1145. doi: 10.1016/s0002-8223(03)00974-x. [DOI] [PubMed] [Google Scholar]
- 14.Kikunaga S. Tin T. Ishibashi G. Wang DH. Kira S. The application of a handheld personal digital assistant with camera and mobile phone card (Wellnavi) to the general population in a dietary survey. J Nutr Sci Vitaminol (Tokyo) 2007;53:109–116. doi: 10.3177/jnsv.53.109. [DOI] [PubMed] [Google Scholar]
- 15.Wang DH. Kogashiwa M. Kira S. Development of a new instrument for evaluating individuals' dietary intakes. J Am Diet Assoc. 2006;106:1588–1593. doi: 10.1016/j.jada.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang DH. Kogashiwa M. Ohta S. Kira S. Validity and reliability of a dietary assessment method: the application of a digital camera with a mobile phone card attachment. J Nutr Sci Vitaminol (Tokyo) 2002;48:498–504. doi: 10.3177/jnsv.48.498. [DOI] [PubMed] [Google Scholar]
- 17.Six BL. Schap TE. Zhu FM. Mariappan A. Bosch M. Delp EJ. Ebert DS. Kerr DA. Boushey CJ. Evidence-based development of a mobile telephone food record. J Am Diet Assoc. 2010;110:74–79. doi: 10.1016/j.jada.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiel R. Kaps A. Bieber G. Kramer G. Seebach H. Hoffmeyer A. Identification of determinants for weight reduction in overweight and obese children and adolescents. J Telemed Telecare. 2010;16:368–373. doi: 10.1258/jtt.2010.091005. [DOI] [PubMed] [Google Scholar]
- 19.Weber JL. Lytle L. Gittelsohn J. Cunningham-Sabo L. Heller K. Anliker JA. Stevens J. Hurley J. Ring K. Validity of self-reported dietary intake at school meals by American Indian children: the Pathways Study. J Am Diet Assoc. 2004;104:746–752. doi: 10.1016/j.jada.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Puyau MR. Adolph AL. Vohra FA. Zakeri I. Butte NF. Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exer. 2004;36:1625–1631. [PubMed] [Google Scholar]
- 21.Blanton CA. Kretsch MJ. Baer DJ. Staples RC. Measuring physical activity energy expenditure in normal-weight premenopausal women [abstract] FASEB J. 2005;19:A468. [Google Scholar]
- 22.Evenson KR. Catellier DJ. Gill K. Ondrak KS. McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26:1557–1565. doi: 10.1080/02640410802334196. [DOI] [PubMed] [Google Scholar]
- 23.Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther. 2009;11:551–565. doi: 10.1089/dia.2009.0015. [DOI] [PubMed] [Google Scholar]
- 24.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability, quality of glycemic control. Diabetes Technol Ther. 2009;11(Suppl 1):S-55–S-67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 25.Petitti DB. Klingensmith GJ. Bell RA. Andrews JS. Dabelea D. Imperatore G. Marcovina S. Pihoker C. Standiford D. Waitzfelder B. Mayer-Davis E. Glycemic control in youth with diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155:668–672. doi: 10.1016/j.jpeds.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer-Davis EJ. Nichols M. Liese AD. Bell RA. Dabelea DM. Johansen JM. Pihoker C. Rodriguez BL. Thomas J. Williams D. Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Am Diet Assoc. 2006;106:689–697. doi: 10.1016/j.jada.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Lobelo F. Liese AD. Liu J. Mayer-Davis EJ. D'Agostino RB., Jr Pate RR. Hamman RF. Dabelea D. Physical activity and electronic media use in the SEARCH for Diabetes in Youth case-control study. Pediatrics. 2010;125:e1364–e1371. doi: 10.1542/peds.2009-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Øverby NC. Margeirsdottir HD. Brunborg C. Anderssen SA. Andersen LF. Dahl-Jørgensen K. Norwegian Study Group for Childhood Diabetes: Physical activity and overweight in children and adolescents using intensified insulin treatment. Pediatr Diabetes. 2009;10:135–141. doi: 10.1111/j.1399-5448.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 29.Burdick J. Chase HP. Slover RH. Knievel K. Scrimgeour L. Maniatis AK. Klingensmith GJ. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113:e221–e224. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 30.Vanderwel BW. Messer LH. Horton LA. McNair B. Cobry EC. McFann KK. Chase HP. Missed insulin boluses for snacks in youth with type 1 diabetes. Diabetes Care. 2010;33:507–508. doi: 10.2337/dc09-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dube MC. Valois P. Prud'homme D. Weisnagel SJ. Lavoie C. Physical activity barriers in diabetes: development and validation of a new scale. Diabetes Res Clin Pract. 2006;72:20–27. doi: 10.1016/j.diabres.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Bussau VA. Ferreira LD. Jones TW. Fournier PA. The 10-s maximal sprint: a novel approach to counter an exercise-mediated fall in glycemia in individuals with type 1 diabetes. Diabetes Care. 2006;29:601–606. doi: 10.2337/diacare.29.03.06.dc05-1764. [DOI] [PubMed] [Google Scholar]
- 33.Tamborlane W. Closed-loop insulin delivery: we're “virtually” there. Diabetes Technol Ther. 2012;14:203–204. doi: 10.1089/dia.2011.0261. [DOI] [PubMed] [Google Scholar]
- 34.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV. Beck RW. Bode BW. Buckingham B. Chase HP. Clemons R. Fiallo-Scharer R. Fox LA. Gilliam LK. Hirsch IB. Huang ES. Kollman C. Kowalski AJ. Laffel L. Lawrence JM. Lee J. Mauras N. O'Grady M. Ruedy KJ. Tansey M. Tsalikian E. Weinzimer S. Wilson DM. Wolpert H. Wysocki T. Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 35.Schiel R. Thomas A. Kaps A. Bieber G. An innovative telemedical support system to measure physical activity in children and adolescents with type 1 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119:565–568. doi: 10.1055/s-0031-1273747. [DOI] [PubMed] [Google Scholar]