Abstract
Background
Most patients who use insulin do not achieve optimal glycemic control and become susceptible to complications. Numerous clinical trials have shown that frequent insulin dosage titration is imperative to achieve glycemic control. Unfortunately, implementation of such a paradigm is often impractical. We hypothesized that the Diabetes Insulin Guidance System (DIGS™) (Hygieia, Inc., Ann Arbor, MI) software, which automatically advises patients on adjustment of insulin dosage, would provide safe and effective weekly insulin dosage adjustments.
Subjects and Methods
In a feasibility study we enrolled patients with type 1 and type 2 diabetes, treated with a variety of insulin regimens and having suboptimal glycemic control. The 12-week intervention period followed a 4-week baseline run-in period. During the intervention, DIGS processed patients' glucose readings and provided insulin dosage adjustments on a weekly basis. If approved by the study team, the adjusted insulin dosage was communicated to the patients. Insulin formulations were not changed during the study. The primary outcome was the fraction of DIGS dosage adjustments approved by the study team, and the secondary outcome was improved glycemic control.
Results
Forty-six patients were recruited, and eight withdrew. The DIGS software recommended 1,734 insulin dosage adjustments, of which 1,731 (99.83%) were approved. During the run-in period the weekly average glucose was stable at 174.2±36.7 mg/dL (9.7±2.0 mmol/L). During the following 12 weeks, DIGS dosage adjustments resulted in progressive improvement in average glucose to 163.3±35.1 mg/dL (9.1±1.9 mmol/L) (P<0.03). Mean glycosylated hemoglobin decreased from 8.4±0.8% to 7.9±0.9% (P<0.05). Concomitantly, the frequency of hypoglycemia decreased by 25.2%.
Conclusions
The DIGS software provided patients with safe and effective weekly insulin dosage adjustments. Widespread implementation of DIGS may improve the outcome and reduce the cost of implementing effective insulin therapy.
Introduction
Insulin therapy is mandatory for all patients with type 1 diabetes and ultimately needed in most patients with type 2 diabetes, as endogenous insulin secretion diminishes.1 Despite the availability of an array of insulin formulations with different time–action profiles and absence of an upper dosage limitation, most insulin users do not achieve optimal glycemic targets (e.g., glycosylated hemoglobin [HbA1c] <7%) and are at increased risk of developing complications.2,3
Multiple clinical trials have shown that frequent insulin dosage titration is a key element for successful outcome. In these somewhat artificial and laborious clinical trial conditions, study teams contacted patients every few days or weeks to titrate insulin dosage, and thus good glycemic control was achieved and maintained.4–7 This beneficial effect only lasted as long as periodic adjustments were made by the medical staff, evidenced by deterioration of glycemic control within a few months after the study ended, very likely as insulin titrations became more sporadic with less health professional contact.7 Unfortunately, implementation of the frequent insulin titration paradigm in day-to-day clinical practice has been hindered by a lack of medical expertise, limited reimbursement, and time.8
The majority of insulin users are managed by primary care physicians and not necessarily by endocrinologists or diabetes educators specifically trained and experienced in insulin management.9 Consequently, insulin is underprescribed and underdosed in many cases to avoid the most feared side effect for patients and care providers alike, namely, hypoglycemia.8,10 Hypoglycemia is often referred to as the primary barrier to obtaining optimal glycemic control in both type 1 and type 2 diabetes.11,12
The process of insulin titration is complex and governed by two contradicting forces: the achievement of near-normal glucose readings and the avoidance of hypoglycemia. When glucose levels get close to normal, the frequency of hypoglycemia typically increases, and vice versa. Successful maneuvering of these two contradicting forces to realize effective and safe glycemic control necessitates special expertise and time, neither of which is widely available.
To address this unmet clinical need, Hygieia, Inc. (Ann Arbor, MI) developed the Diabetes Insulin Guidance System (DIGS™), which automatically adjusts a patient's insulin dosage between clinic appointments.13 If successful, such a tool could improve diabetes management without escalating its economic burden. In this article, we report a feasibility study in which insulin therapy of patients with suboptimally controlled type 1 and type 2 diabetes was adjusted weekly by the DIGS software supervised by an expert diabetes study team. The ability to apply the DIGS in an unsupervised manner was assessed by monitoring events in which the study team intervened in the process and decided to override the software recommendations.
Subjects and Methods
Study design
This 16-week feasibility study was designed as a prospective, open-label, uncontrolled, single-arm, single-center study. It aimed to predict the capacity of the DIGS software to provide safe and effective, unsupervised, weekly insulin dosage adjustments.
Settings
The study was conducted at the International Diabetes Center at Park Nicollet, Minneapolis, MN, in accordance with all applicable guidelines for the protection of human patients for research as outlined in 21 CFR Part 50. All patients provided informed consent before enrollment. The protocol, its amendments, and informed consent form were reviewed and approved by the Park Nicollet Institutional Review Board prior to being initiated.
Participants
All patients were receiving insulin therapy prior to enrollment but had suboptimal metabolic control. Patients were eligible to participate if they were 21–70 years of age and had had a clinical diagnosis of type 1 or type 2 diabetes for at least 1 year. Patients were excluded from the trial if they had a body mass index of ≥45 kg/m2, severe impairment of cardiac, hepatic, or renal functions, psychological or cognitive impairment, more than two episodes of severe hypoglycemia in the past year, or a history of hypoglycemia unawareness. Eligible patients were enrolled into one of three treatment groups: patients with suboptimally controlled type 1 diabetes (HbA1c ≥7.4%) treated with basal-bolus insulin therapy and incorporating carbohydrate counting (Group I), patients with suboptimally controlled type 2 diabetes (HbA1c ≥7.4%) treated with basal-bolus insulin therapy (without carbohydrate counting) (Group II), and patients with suboptimally controlled type 2 diabetes (HbA1c ≥7.8%) treated with twice-daily biphasic insulin (Group III). The higher threshold HbA1c in the latter group was chosen to reinforce its statistical power, in view of the likely inferiority of biphasic insulin therapy compared with basal-bolus regimens.14
Intervention
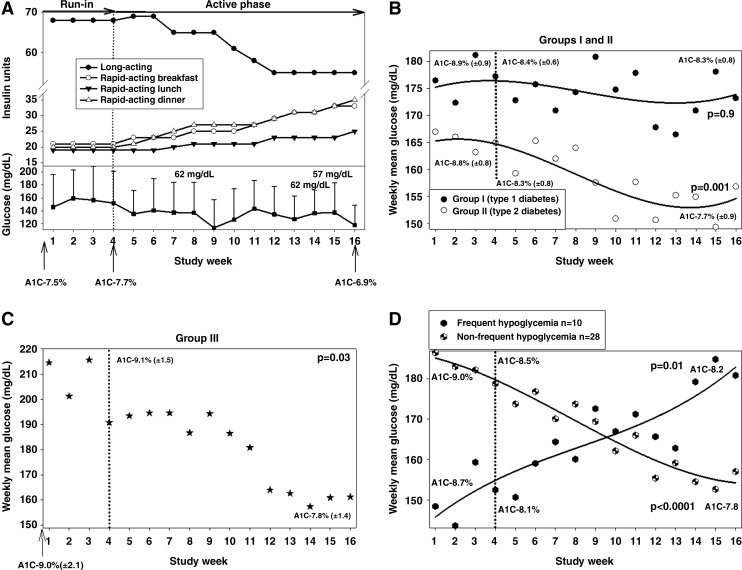
During the first 4 weeks patients continued their pre-enrollment regimens without intervention. During this period patients were acquainted with weekly submission of a diabetes diary, but their insulin dosage was unaltered. During the remaining 12 weeks, DIGS adjusted patients' insulin dosage on a weekly basis, based on self-measured blood glucose readings reported on patients' diaries (an example is given in Fig. 1A). Upon review and approval by the study team, the DIGS software insulin dosage recommendations were communicated to the patients. Although generally encouraged to follow dosage recommendations, patients deviated from the prescribed dosage during certain situations (e.g., anticipated physical activity). Patients in Groups I and II were asked to test and record their capillary glucose four times a day before meals and before bedtime, and patients in Group III were asked to test twice a day, before breakfast and dinner. All patients were asked to measure capillary glucose during the night every 5–9 days. Information captured in diaries included time-stamped scheduled and unscheduled glucose readings, insulin doses, and carbohydrate quantities (Group I only).
FIG. 1.
Capillary glucose levels during the study. (A) An example of a patient managed with the Diabetes Insulin Guidance System (DIGS) software in Group II. This was a 58-year-old woman with a 13-year history of type 2 diabetes, complicated with neuropathy and nephropathy. (Upper panel) Insulin dosage. (Lower panel) Weekly mean glucose and events of hypoglycemia. During the first 4 weeks the subject continued to follow her current insulin dosage. During the active phase of the study (Weeks 4–16), the DIGS software adjusted each component of the basal-bolus regimen. Her total daily insulin dosage increased by 19% (from 123 to 151 units/day), but its distribution considerably changed, that is, different components in the regimen were diametrically redirected according to glucose patterns. (B) Weekly mean glucose (and regression lines) in Groups I and II. (C) Weekly mean glucose in Group III (because of fewer data points, a regression line was not plotted). (D) Weekly mean glucose (and regression line) of patients with and without frequent hypoglycemia. During the active 12 weeks weekly mean glucose improved when possible. A1C, glycosylated hemoglobin.
Main outcome measures
The primary study outcome was the fraction of dosage adjustments made by DIGS and conveyed to the patients without intervention from the study team. Events in which the study team decided to override the software insulin dosage adjustments were thoroughly documented to enable full investigation of each incident. The efficacy of the weekly adjustments was documented by improvement in weekly mean of self-measured glucose. The secondary efficacy outcome was defined as at least a 10% decrease in weekly mean blood glucose between Weeks 4 and 16 (when insulin dosage adjustments were made). Additional efficacy analyses included reduction in HbA1c and fructosamine between Weeks 4 and 16. The latter values were not used as primary efficacy end points because of their inherent inability to assess glycemic attenuations over a relatively short period of 12 weeks.15 The safety of the weekly adjustments was documented by the frequency of hypoglycemia. Hypoglycemia was defined as a blood glucose level of <65 mg/dL (<3.6 mmol/L), severe hypoglycemia was defined as a hypoglycemic episode requiring assistance of another person, and a severe adverse event was defined as an event resulting in death, life-threatening experience, hospitalization, or significant disability.
Software description and utilization
The DIGS software incorporates algorithms that process time-tagged glucose readings and adjust insulin dosage while ignoring any additional parameters. The software was developed by E.B. and I.H. prior to the study. The algorithms embedded in the software are based on guidelines for insulin management16 and on the following four principles:
a. Time-tagged (e.g., pre-breakfast, bedtime) glucose readings were the only input used to adjust the current dosage and create recommendations for the next insulin dosage.
b. Insulin dosage (for all insulin types) was increased for glucose level above target and decreased for glucose level below target.
c. The intensity of adjustments decreases as glucose readings get closer to target to prevent unstable oscillations of dosage.
d. The ability to detect “outliers.” The software utilized higher-order statistics to detect outliers and treats them separately from the remainder of the data.
Statistical methods
The study was designed as intention-to-treat. Because each group was analyzed independently unrelated to other groups, no statistical comparison was done for demographic and clinical characteristics. Normality was assessed by the Shapiro–Wilk test. Attenuations in weekly mean glucose were assessed for statistical significance by a mixed model repeated-measurements analysis. Patients' HbA1c and fructosamine were compared by paired two-tailed Student's t test or, if non-normally distributed, by the Wilcoxon test. The frequency and severity of hypoglycemia were compared by unpaired Student's t test or, if non-normally distributed, by the Mann–Whitney test. Results are presented as mean±SD values, and a value of P<0.05 was defined as statistically significant.
Results
Study population
In total, 46 patients were enrolled: 38 completed, and eight were withdrawn (Supplementary Table S1 [Supplementary Data are available online at www.liebertonline.com/dia]). Six of the eight patients were withdrawn during the run-in period, before intervention was initiated. Of these patients, one failed to meet the inclusion criteria, and two patients withdrew consent during the first week of the run-in period. One patient in Group I withdrew consent 9 weeks after the beginning of the study in order to start a pump therapy, and one patient from this group was withdrawn 5 weeks after the beginning of the study because he was unable to be consistent in carbohydrate counting techniques. Available data from patients who withdrew from the study were used for analysis as part of the intention-to-treat design. The entire study population was cumulatively followed up for 12.2 patient-years. Patients' demographics, comorbidities, and diabetes medications are outlined in Table 1. Mean baseline HbA1c across all three groups was 8.9±1.1%. (Average daily insulin dosage and basal to bolus ratios are given in Supplementary Table S2.) As expected, patients with type 1 diabetes required more attention from the study team. This reinforced consistency and accuracy of carbohydrate counting and improved adherence to recommended insulin dosage.
Table 1.
Baseline Demographics and Clinical Characteristics
| Total (n=46) | Group I (n=20) | Group II (n=20) | Group III (n=6) | |
|---|---|---|---|---|
| Sex | ||||
| Male | 27 (59%) | 13 (65%) | 10 (50%) | 4 (66.7%) |
| Female | 19 (41%) | 7 (35%) | 10 (50%) | 2 (33.3%) |
| Age (years) | 52 (12) | 44 (12) | 60 (7) | 55 (7) |
| Race | ||||
| White | 45 (98%) | 20 (100%) | 20 (100%) | 5 (83%) |
| African American | 1 (2%) | — | — | 1 (17%) |
| Ethnicity | ||||
| Non-Hispanic/Latino | 46 (100%) | 20 (100%) | 20 (100%) | 6 (100%) |
| Insulin typesa | ||||
| Glargine | 40 (89.9%) | 20 (100%) | 20 (100%) | — |
| Lispro | 22 (55%) | 13 (65%) | 9 (45%) | — |
| Aspart | 18 (45%) | 7 (35%) | 11 (55%) | — |
| Aspart-mix70/30 | 5 (10.9%) | — | — | 5 (83%) |
| Humulin-mix70/30 | 1 (2.2%) | — | — | 1 (17%) |
| Other medications | ||||
| Glucophage | 17 (37%) | — | 12 (60%) | 5 (83%) |
| Glipizide | 1 (2%) | — | 1 (5%) | — |
| Diabetes complications | ||||
| Retinopathy | 11 (24%) | 6 (30%) | 5 (25%) | — |
| Nephropathy | 11 (24%) | 3 (15%) | 4 (20%) | 4 (67%) |
| Neuropathy | 18 (39%) | 4 (20%) | 10 (50%) | 4 (67%) |
| Comorbidities | ||||
| Hypertension | 24 (52%) | 2 (10%) | 17 (85%) | 5 (83%) |
| Dyslipidemia | 41 (89%) | 16 (80%) | 19 (95%) | 6 (100%) |
| Coronary artery disease | 6 (13%) | 1 (5%) | 4 (20%) | 1 (17%) |
| Congestive heart failure | 1 (2%) | — | 1 (5%) | — |
| Cerebral vascular disease | 1 (2%) | — | 1 (5%) | — |
| Smoking (current) | 4 (8.7%) | 2 (10%) | 2 (10%) | — |
| BMI (kg/m2) | 33 (7) | 28 (5) | 37 (6) | 35 (4) |
| Duration of diabetes (years) | 18 (10) | 25 (11) | 14 (5) | 11 (3) |
| Baseline HbA1c (%) | 8.9 (1.1) | 8.9 (0.9) | 8.8 (0.8) | 9.0 (2.1) |
Data are number (%) or mean (SD).
Glargine® (sanofi-aventis, Bridgewater, NJ), Lispro® (Eli Lilly, Indianapolis, IN), Aspart® (Novo-Nordisk, Princeton, NJ), Aspart-mix70/30® (Novo-Nordisk), or Humulin-mix70/30® (NPH/regular) (Eli Lilly).
BMI, body mass index; HbA1c, glycosylated hemoglobin.
Run-in period
During the first 4-week run-in period, patients became acquainted with the study protocol while continuing their pre-enrollment insulin dosage and submitting their diaries weekly. During this period patients measured capillary glucose tests more than requested. Likely because of trends preceding the study and the Hawthorne effect, mean HbA1c improved by 0.5% (P<0.0001) during the run-in period. Glucose testing frequency was stable during the active intervention phase (roughly four per day for Groups I and II and two per day for Group III).
Study team overrides
The DIGS software recommended 1,734 insulin dosage adjustments, of which 1,731 (99.83%) were approved by the study team. Three nonconsecutive recommendations in different patients were overruled. In the first case for a Group III patient, a software “bug” yielded an erroneous recommendation. The study team was advised of the situation, prior to reporting the recommendation to the patient, by the sponsor who recognized the case. This “bug” was subsequently corrected. In the second case for a Group I patient in Week 10, the DIGS software recommended changing dinner insulin-to-carbohydrate ratio from 1:2 (i.e., 1 unit of insulin for every 2 g of carbohydrates) to 1:1. This patient consumed about 38 g of carbohydrates at dinner, and his daily total insulin was about 80 units. The study team instead recommended a ratio of 1:1.7. On Week 11, it was thought the patient required an additional increase of prandial insulin dosage, and the regimen for this patient was changed to a basal-bolus without carbohydrate counting. In the third case for a Group I patient in Week 12, the study team preferred to keep a patient's breakfast insulin-to-carbohydrate ratio at 1:3 and rejected the DIGS recommendation to increase the ratio to 1:2. This patient consumed about 40 g of carbohydrates at breakfast, and his daily total insulin was about 105 units.
Insulin dosage titrations made by DIGS and overseen by the study team improved glycemia
Over a cumulative 8.9 patient-years (the 12-week intervention period), patients were provided with 1,734 (1,731 uninterrupted) weekly insulin dosage adjustments. These included four recommendations per week for Groups I and II (three mealtime boluses and one basal; a correction factor was also used but not counted as a separate recommendation as it is calculated based on the other four dosage recommendations) and two recommendations per week for Group III (breakfast and dinner). Weekly mean glucose progressively improved during the active 12 weeks across all groups. Among patients in Group II (Fig. 1B), weekly mean glucose improved by 5.2%, and among patients in Group III (Fig. 1C), weekly mean glucose improved by 18.3%. Cumulatively for all patients with type 2 diabetes (Groups II and III), weekly mean glucose improved by 7.4%. Among patients in Group I (patients with type 1 diabetes), whose frequency of hypoglycemia was significantly higher, weekly mean glucose did not significantly improved (Fig. 1B). There was a significant reduction over the entire study population in HbA1c and fructosamine (Supplementary Table S2), although the efficacy end point (>10% improvement in weekly mean glucose) was not attained. Generally, patients with type 2 diabetes experienced greater reduction in these parameters.
Insulin dosage titrations made by DIGS and overseen by the study team reduced hypoglycemia
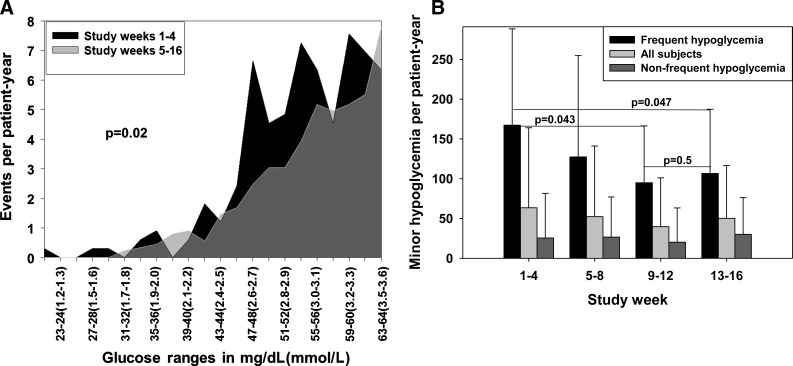
Thirty-eight of the 46 patients enrolled reported 632 episodes of minor hypoglycemia during the study. No episodes of severe hypoglycemia were documented. As shown in Figure 2B, during the 4-week run-in period patients reported 63.5±100.6 episodes of minor hypoglycemia per patient-year. The frequency of hypoglycemia did not significantly change during the 12-week active phase (weeks 5–16) when patients reported 47.5±73.3 episodes per patient-year (P=0.8). Yet, as shown in Figure 2A, glucose levels falling below the hypoglycemic threshold (glucose<65 mg/dL [<3.6 mmol/L]) during the 12-week active phase were significantly milder than the ones reported during the 4-week run-in period (P=0.02).
FIG. 2.
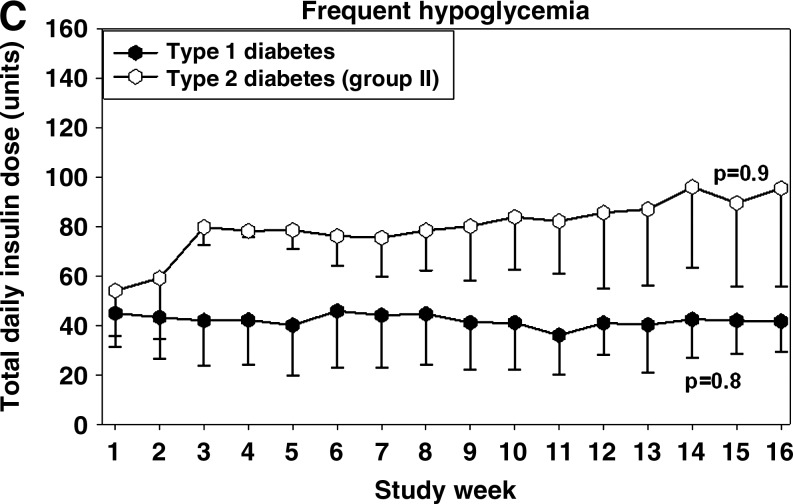
Hypoglycemia during the study. (A) Histogram depicting frequency of hypoglycemic glucose readings per glucose value during the 12-week active phase and the 4-week run-in period. The histogram shows that glucose readings below 65 mg/dL (<3.6 mmol/L) during the active phase had different distribution than during the run-in period and were generally milder. (B) Frequency of minor hypoglycemia (glucose <65 mg/dL [<3.6 mmol/L]) during each quartile for all patients and patients with or without frequent hypoglycemia (>85 events per patient-year). (C) Total daily insulin in patients with frequent minor hypoglycemia. During the active 12-week period, the frequency and severity of hypoglycemia decreased.
Performance according to individual glycemic balance (post hoc analysis)
Post hoc analysis suggests that the study population could fit into two glycemic profiles. The hypothesis generated by this post hoc analysis is presented herein because of its potential clinical relevance and because of the authors' intent to validate it in another study. About one-quarter of the patients exhibited a high frequency of hypoglycemia, which may preclude safe reduction of average glucose and HbA1c, particularly among patients managed in primary care clinics.11 We sought to separate the study population based on their hypoglycemic frequency. To refrain from partiality, it was essential to identify an exogenous hypoglycemia frequency threshold, unrelated to the study results. A frequency of 85 events of minor hypoglycemia per patient-year was chosen as a cutoff point because it was double the frequency among patients with type 1 diabetes found in a community-based survey.17 It was thought that such frequency might be considered unsafe or at least excessive by primary care teams for their insulin-treated patients. Based on this threshold, patients were separated into two groups:
i. Patients with frequent hypoglycemia (an example is given in Supplementary Fig. S1A). Ten of the 20 patients enrolled with type 1 diabetes and two of the 26 patients enrolled with type 2 diabetes demonstrated a high rate of hypoglycemia. Ten of these patients completed the study (eight with type 1 diabetes and two with type 2 diabetes). As shown in Figure 2C, during the 12-week active phase reduction of hypoglycemia was achieved by changing the distribution of insulin formulations (i.e., fast-acting insulin for each meal and long-acting insulin) while maintaining stable average daily total insulin dose. In these patients DIGS insulin dosage adjustments resulted in an increase of mean weekly glucose by 18.7%, from 152.5±23.3 mg/dL (8.5±1.3 mmol/L) to 180.9±32.9 mg/dL (10±1.8 mmol/L) (Fig. 1D), while the frequency of hypoglycemia significantly decreased by 36.3% (P=0.047), from 167.3 to 106.6 events per patient-year (Fig. 2B). For these patients, HbA1c did not significantly change (8.1% to 8.2%; P=0.5).
ii. Patients without frequent hypoglycemia (an example is given in Supplementary Fig. S1B). Ten of the 20 patients with type 1 diabetes and 24 of the 26 with type 2 diabetes (18 in Group II and six in Group III) did not demonstrate a high rate of hypoglycemia. Clearly, in these cases it was possible to safely increase insulin dosage. Of the 34 patients composing this category, 28 completed the study (six with type 1 diabetes and 22 with type 2 diabetes [18 in Group II and four in Group III]). As shown in Figure 1D, in this group weekly mean glucose improved by 12.2%, from 178.8±36.0 mg/dL (9.9±2 mmol/L) to 157±34.2 mg/dL (8.7±1.9 mmol/L), meeting the study efficacy end point (>10% reduction in weekly mean glucose), while maintaining a low and stable rate of hypoglycemia of approximately 25 events per patient-year (Fig. 2B). During the 12-week active phase, daily total insulin dosage was increased, and HbA1c decreased from 8.5% to 7.8% (P=0.0005).
Overall, this post hoc analysis suggests that the DIGS software was able to provide effective and safe insulin dosage adjustments to improve glycemic control for all subjects, as defined by reducing either the average glucose or hypoglycemia frequency or both, by tailoring each individual's therapy.
Discussion
This feasibility study tested the autonomy of the DIGS software in its ability to provide safe and effective weekly insulin dosage adjustments. This novel approach was intended to facilitate frequent insulin dosage adjustments between clinic appointments to achieve and maintain improved glycemic control. The utility of frequent insulin dosage adjustments has been widely demonstrated in clinical studies4–7 but has not been implemented in day-to-day clinical practice because of insufficiency of medical expertise, limited time and reimbursement, and fear of hypoglycemia.8 It has been previously demonstrated that glucose readings are sufficient to adjust insulin dosage, provided that insulin adjustments are modest and frequent.13 Moreover, because of the dynamic nature of insulin therapy, frequent insulin dosage adjustments are needed not only to achieve the therapy goal but also to maintain it.18
During a cumulative period of 8.9 patient-years, the DIGS software provided 1,731 effective and safe weekly insulin dosage adjustments for patients with type 2 and type 1 diabetes, using a variety of insulin regimens. In only two cases did the study team disagree with the software recommendations (excluding the software “bug”) to increase insulin-to-carbohydrate ratios. Consequently, the DIGS software was changed, after the study, to refrain from recommending high insulin-to-carbohydrate ratios.
The weekly dosage adjustments were shown to improve glycemia, evidenced by a lower rate of hypoglycemia, reduced weekly mean glucose, or both (defined as improved glycemic balance or improved glycemic composite index). Weekly mean glucose significantly improved in patients in Groups II and III with type 2 diabetes. It is notable that all patients in these groups, excluding two patients, did not have frequent hypoglycemia. Conversely, half of the patients in Group I with type 1 diabetes had frequent hypoglycemia—nearly three times the rate reported by others among patients with type 1 diabetes.11,17 It is surprising that these patients were not exceptionally insulin sensitive (according to their daily total insulin [Fig. 2C]). Thus in these patients, during the study period the DIGS software primarily decreased the frequency of hypoglycemia and did not achieve a significant reduction in weekly mean glucose or HbA1c. The relatively large group of patients with frequent hypoglycemia may have been the result of the study being conducted in a referral center focused on diabetes management and education. Moreover, the average duration of diabetes in our study population was fairly long compared with many clinical trials (Table 1), and it was demonstrated that the propensity to develop hypoglycemia in insulin-treated patients with type 2 diabetes increases several years after insulin initiation.17,19,20
In a post hoc analysis we identified two different glycemic profiles that may prompt different approaches in insulin titrations. The hypothesis generated by this post hoc analysis suggests that during the study period each individual patient's requirements were diverse, yet the DIGS software tailored the therapy to match the unique patient's needs. Specifically, patients with frequent hypoglycemia were able to reduce its frequency without substantial deterioration in glycemic control, and patients without frequent hypoglycemia had significantly improved average glucose. Accordingly, the efficacy end point of reduced mean glucose could only be safely achieved by patients having moderate or low frequency of hypoglycemia (about three-quarters of the patients). This hypothesis will be tested in a larger clinical trial.
Hypoglycemia is the main limiting factor in obtaining optimal glycemic control in patients on insulin (in both type 1 and type 2 diabetes)11 and has been shown to be inversely related to average glucose and HbA1c.7 It has been demonstrated that the main risk factor for severe hypoglycemia is previous hypoglycemic events.11,17 Because in day-to-day life hypoglycemic events are often reviewed in a retrospective manner, during infrequent clinic appointments, it is not surprising they are hard to prevent. As we demonstrated, routine insulin dosage adjustments between appointments can reduce the frequency and severity of hypoglycemia and thus enable optimization of average glucose. The incidence of minor hypoglycemia in community-based circumstances has not been often studied, and no consensus regarding what constitutes an acceptable amount of hypoglycemia20 has been established. We elected to use 85 events per patient-year as a threshold in our sorting process based on a large community-based study.17 This number was independent of our study results, and using a lower threshold would have yielded a more significant difference between groups. Over time, with more sophisticated technology like continuous glucose monitoring21,22 included in clinical practice, it may be possible to more accurately sort out the usual rates of mild, moderate, and severe hypoglycemia and to determine a more precise composite index or balance of improved glucose control (HbA1c or average glucose) and rate of hypoglycemia.
The study was limited by lack of a control group. Thus, we could not have unequivocally excluded the possibility that improved glycemia resulted from participation in the study. During the run-in period HbA1c improved, likely because of trends preceding the study and the Hawthorne effect, evidenced by transient increase in glucose readings during this period. The study duration was relatively short; therefore it is possible that different HbA1c levels would have been recorded during a longer follow-up. Because weekly mean glucose levels were stable during the run-in period and improved particularly toward the middle of the active phase, it is our belief that HbA1c would have further improved.
The DIGS concept relies on its capability to properly assist healthcare providers in the process of insulin management between appointments without the healthcare provider's constant involvement. Essentially, this is a virtual extension of the physician's availability to follow patients and provides simple and safe instructions to modify treatment without increased effort. We believe, that if our findings are confirmed in a randomized controlled trial, then implementation of such a solution on a large scale can potentially improve outcomes of insulin therapy, reduce costs, and increase consistency. Ultimately it may make insulin therapy more suitable for the majority of people with type 2 diabetes (most are treated in primary care clinics) who fail to successfully control glucose level with other medications. Because the fear of hypoglycemia is one of the main limitations in insulin therapy, it was important to show that the DIGS software does not increase its rate.
A clinical implementation of DIGS is planned to be tested in a prospective, multicenter, randomized controlled study. Additional studies are also needed to focus on improved glycemic balance among the minority of patients who experience frequent hypoglycemia. The DIGS software may serve as a bridge from the currently ineffective standard practice of sparse evaluation of glucose patterns and insulin dosage adjustments to the desired and steadily evolving closed loop or artificial pancreas technology.23–25
Supplementary Material
Acknowledgments
The authors thank the study coordinators, Margaret Gehrman, R.N., and Sue List, R.N., C.D.E., for coordination of all aspects of the primary data collection; Janet Davidson, R.N., C.D.E., for her review of the DIGS software-generated dosage recommendations; Marcia Madden for her review of the diaries and communication with patients; Innovative Analytics for providing data management support; and the patients involved in the trial. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (award number R41DK085974). R.M.B., the Principal Investigator, reviewed/edited the manuscript, E.B. performed data analysis and reviewed/edited the manuscript, M.M. performed data collection and reviewed/edited the manuscript; M.J. provided research coordination and reviewed/edited the manuscript, and I.H. performed data analysis and wrote/edited the manuscript.
Author Disclosure Statement
I.H., E.B., and M.M. are affiliated with Hygieia, Inc., a medical devices company that developed DIGS. M.J. has no personal financial interest in Hygieia. R.M.B. is a medical advisor to Hygieia with a contract through his employer, Park Nicollet Health Services, and received no personal financial compensation. R.M.B. also has served on an advisory board, consulted, or conducted clinical research with Abbott Diabetes Care, Johnson & Johnson, Roche, Bayer, Medtronic, DexCom, Eli Lilly, Novo Nordisk, and Sanofi, all under contract with Park Nicollet, and received no personal compensation.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. Erratum in Lancet 1999;354:602. [PubMed] [Google Scholar]
- 2.Koro CE. Bowlin SJ. Bourgeois N. Fedder DO. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17–20. doi: 10.2337/diacare.27.1.17. [DOI] [PubMed] [Google Scholar]
- 3.Chew EY. Ambrosius WT. Update of the ACCORD Eye Study. N Engl J Med. 2010;364:188–189. doi: 10.1056/NEJMc1011499. [DOI] [PubMed] [Google Scholar]
- 4.Herman WH. Ilag LL. Johnson SL. Martin CL. Sinding J. Al Harthi A. Plunkett CD. LaPorte FB. Burke R. Brown MB. Halter JB. Raskin P. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28:1568–1573. doi: 10.2337/diacare.28.7.1568. [DOI] [PubMed] [Google Scholar]
- 5.Bergenstal RM. Johnson M. Powers MA. Wynne A. Vlajnic A. Hollander P. Rendell M. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care. 2008;31:1305–1310. doi: 10.2337/dc07-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oyer DS. Shepherd MD. Coulter FC. Bhargava A. Brett J. Chu PL. Trippe BS INITIATEplus Study Group. A1c control in a primary care setting: self-titrating an insulin analog pre-mix (INITIATEplus trial) Am J Med. 2009;122:1043–1049. doi: 10.1016/j.amjmed.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes RP. Fitzgerald JT. Jacober SJ. Primary care physician beliefs about insulin initiation in patients with type 2 diabetes. Int J Clin Pract. 2008;62:860–868. doi: 10.1111/j.1742-1241.2008.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeWitt DE. Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254–2264. doi: 10.1001/jama.289.17.2254. [DOI] [PubMed] [Google Scholar]
- 10.Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev. 2002;18(Suppl 3):S42–S49. doi: 10.1002/dmrr.277. [DOI] [PubMed] [Google Scholar]
- 11.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 13.Bashan E. Herman WH. Hodish I. Are glucose readings sufficient to adjust insulin dosage? Diabetes Technol Ther. 2011;13:85–92. doi: 10.1089/dia.2010.0112. [DOI] [PubMed] [Google Scholar]
- 14.Holman RR. Farmer AJ. Davies MJ. Levy JC. Darbyshire JL. Keenan JF. Paul SK 4-T Study Group. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–1747. doi: 10.1056/NEJMoa0905479. Erratum in N Engl J Med 2010;363:2078. [DOI] [PubMed] [Google Scholar]
- 15.Koenig RJ. Peterson CM. Jones RL. Saudek C. Lehrman M. Cerami A. Correlation of glucose regulation and hemoglobin A1c in diabetes mellitus. N Engl J Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 16.Nathan DM. Buse JB. Davidson MB. Ferrannini E. Holman RR. Sherwin R. Zinman B American Diabetes Association. European Association for Study of Diabetes: Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly LA. Morris AD. Frier BM. Ellis JD. Donnan PT. Durrant R. Band MM. Reekie G. Leese GP. DARTS/MEMO Collaboration: Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med. 2005;22:749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal EB. Bashan E. Herman WH. Hodish I. The effort required to achieve and maintain optimal glycemic control. J Diabetes Complications. 2011;25:283–288. doi: 10.1016/j.jdiacomp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Leese GP. Wang J. Broomhall J. Kelly P. Marsden A. Morrison W. Frier BM. Morris AD. DARTS/MEMO Collaboration: Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 20.UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 21.Wolpert HA. Use of continuous glucose monitoring in the detection and prevention of hypoglycemia. J Diabetes Sci Technol. 2007;1:146–150. doi: 10.1177/193229680700100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bode BW. Schwartz S. Stubbs HA. Block JE. Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care. 2005;28:2361–2366. doi: 10.2337/diacare.28.10.2361. [DOI] [PubMed] [Google Scholar]
- 23.Weinzimer SA. Steil GM. Swan KL. Dziura J. Kurtz N. Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 24.Kowalski AJ. Can we really close the loop, how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther. 2009;11(Suppl 1):S-113–S-119. doi: 10.1089/dia.2009.0031. [DOI] [PubMed] [Google Scholar]
- 25.Hovorka R. Allen JM. Elleri D. Chassin LJ. Harris J. Xing D. Kollman C. Hovorka T. Larsen AM. Nodale M. De Palma A. Wilinska ME. Acerini CL. Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375:743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.