Abstract
The incidence of type 2 diabetes mellitus (T2DM) has reached epidemic levels, and current trends indicate that its prevalence will continue to rise. The development of T2DM can be delayed by several years, and may even be prevented, by identifying individuals at risk for T2DM and treating them with lifestyle modification and/or pharmacological therapies. There are a number of methods available for assessing the insulin resistance (IR) that characterizes, and is the precursor to, T2DM. However, current clinical methods for assessing IR, based on measures of plasma glucose and/or insulin are either laborious and time-consuming or show a low specificity. IR manifests its earliest measurable abnormalities through changes in lipoproteins, and thus we propose that by examining lipoprotein subclass profile, it may be possible to alert physicians and patients to a heightened risk of developing diabetes. This will allow us to institute appropriate lifestyle changes and treatment potentially to delay the onset or possibly prevent the progression to diabetes.
Introduction
The estimate for the worldwide prevalence of diabetes mellitus (DM) is estimated to be 220 million, rising to 300 million in 2025.1,2 Data from the National Health and Nutrition Examination Survey (NHANES) estimates that 8.2% of the U.S. population have DM,3 90%–95% of which is accounted for by type 2 diabetes (or non-insulin dependent; T2DM), according to the American Diabetes Association (ADA).4 T2DM is associated with metabolic dysfunction, characterized by insulin resistance (IR), hyperglycemia, and dyslipoproteinemia.5 The end results are chronic microvascular, neuropathic, nephropathic, and/or macrovascular disease. The principal element in preventing the complications of diabetes is early identification of those patients at risk, prompt diagnosis, and effective treatment. Several studies have demonstrated that the development of T2DM can be delayed for several years by identifying individuals with prediabetes and treating them with lifestyle modification and/or pharmacological therapies.6–8 For instance, the Diabetes Prevention Program, a large prevention study, showed that lifestyle intervention reduced diabetes development by 58% during a 3-year period.8 In spite of this, approximately 25% of citizens in the United States with T2DM remain undiagnosed.9 For that reason, the difficult task is to identify an appropriate marker that can detect IR and the risk of developing T2DM early in the disease process to institute interventions that can delay or prevent progression to clinical diabetes.
There are number of methods commercially available for assessing IR and risk of T2DM. However, current clinical methods for assessing IR are based on plasma glucose and/or insulin levels, which require steady-state conditions for accuracy. Thus, identifying IR accurately is laborious and time-consuming.10 An alternative is the lipoprotein subfraction concentration distribution profile (reflected as the average diameter for each fraction), measured by nuclear magnetic resonance spectroscopy (NMR), which a number of studies have revealed can provide both early assessment of IR11–13 as well as identification of those, patients with increased risk for developing diabetes.14,15 This article considers NMR in the light of existing technologies aimed at identifying those with IR, and proposes NMR as an alternative method that can offer potential clinical utility. After reviewing existing methods, this perspective will focus on the NMR method and clinical studies that evaluate the utility of six lipoprotein parameters, including the concentrations of small low-density lipoprotein particle (LDL-P), large very-low-density lipoprotein particle (VLDL-P), and large high-density lipoprotein particle (HDL-P) subfractions, and the average size of these three lipoprotein fractions in assessing IR and the risk of developing diabetes.
Prediabetic States
Diabetes costs have increased by 32% since 2002, and the disease claimed over 284,000 lives in 2007.16 Seventy-five percent of complications in diabetes are associated with cardiovascular events.17 Data from the U.K. Prospective Diabetes Study suggest that β-cell function declines an average of 12 years before the diagnosis of T2DM is made; by the time of diagnosis, approximately 50% of β-cell function may be irreversibly lost.18 The poor clinical outcomes associated with diabetes have led to increased efforts to identify those in the “prediabetic” state to administer early lifestyle and pharmacological interventions.
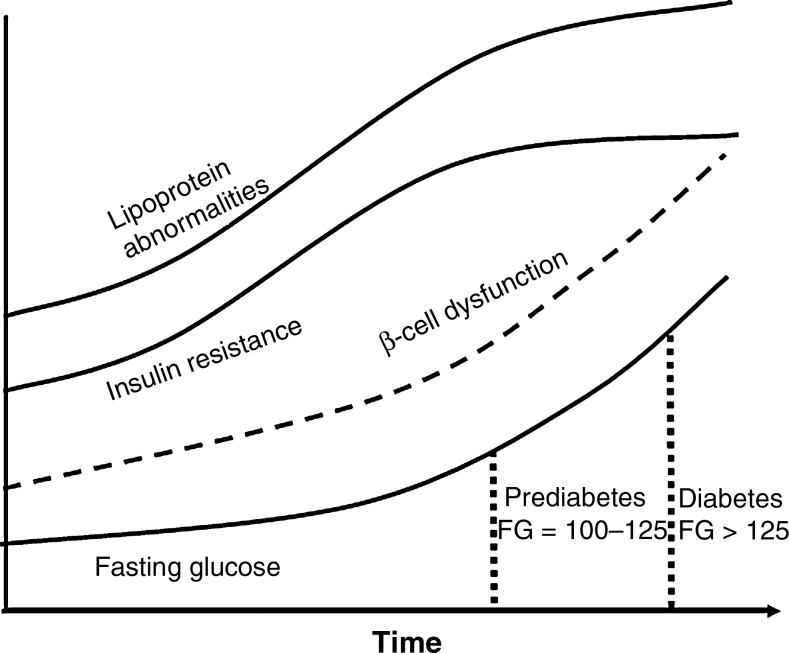
As illustrated in Fig. 1, metabolic changes in T2DM occur over time. The natural course of T2DM can be long and consists of normoglycemia, intermediate hyperglycemia, and then overt hyperglycemia, which results from cellular resistance to glucose stimulated uptake. The hepatic stress of the ensuing hyperinsulinemia leads to the progressive loss of β-cell function.18 There are two prediabetic conditions that identify individuals at high future risk for development of T2DM, as well as for cardiovascular events.
FIG. 1.
Time course of the pathogenesis of type 2 diabetes. FG, fasting glucose.
The first clinical assessment for T2DM risk is for a condition termed prediabetes, which encompasses those with increased glucose levels that are higher than the normal range but do not yet meet criteria for diabetes. Prediabetes can be diagnosed on the basis of impaired fasting glucose (IFG), impaired glucose tolerance (IGT) defined by the 2-h post-oral glucose tolerance test (OGTT) glucose value, or by an elevation in glycosylated hemoglobin (HbA1c), as shown in Table 1.
Table 1.
Glucose Levels Meeting Diagnostic Criteria for Categories of Glucose Tolerance
| Fasting glucose | 2-h glucose during OGTT | HbA1c | Random glucose | |
|---|---|---|---|---|
| Diabetes | ≥126 mg/dL | ≥200 mg/dL | ≥6.5% | ≥200 mg/dL |
| (7.0 mmol/L) | (11.1 mmol/L) | (11.1 mmol/L) with symptoms | ||
| Prediabetes | 100–125 mg/dL | 140–199 mg/dL | 5.7%–6.4% | |
| (5.6–6.9 mmol/L) | (7.8–11.0 mmol/L) | |||
| Normoglycemic | <100 mg/dL | <140 mg/dL | <5.7% | |
| (5.6 mmpl/L) | (7.8 mmol/L) |
OGTT, oral glucose tolerance test; HbA1c, glycosylated hemoglobin.
The second clinical assessment for T2DM risk is for a condition known as the metabolic syndrome, which is defined by a cluster of risk factors mechanistically related to IR, including abdominal obesity, raised fasting glucose and triglycerides, lowered HDL cholesterol (HDL-C), and hypertension (Table 2). Metabolic syndrome is characterized not only by glucose abnormalities but also by atherogenic dyslipidemia. Therefore, it is not surprising that this condition is associated with age-, gender-, and risk factor–adjusted hazard ratios for cardiovascular disease mortality of 1.82 [95% confidence interval (CI) 1.40–2.37] in those free of the cardiovascular disease at diagnosis.19 Before the loss of β-cell function, hepatic IR manifests its earliest measurable abnormalities with changes in lipoprotein metabolism alongside changes in fasting glucose levels. Specifically, large VLDL and small LDL subclass particle concentrations are higher and large HDL subclass particle concentrations are lower in IR. Hence, VLDL size tends to be greater and LDL and HDL sizes smaller when a patient is in an IR state.11–13,20,21 Because lipoprotein abnormalities are evident early, prior to the development of T2DM, we argue these abnormalities provide powerful information for identifying patients with IR at increased risk for T2DM.
Table 2.
Diagnostic Criteria for Metabolic Syndrome
| Risk factor/trait | ATP III criteria Any 3 out of the 5 risk factors | IDF criteria Abnormal waist+2 risk factors | WHO criteria IFG or IGT+2 risk factors |
|---|---|---|---|
| Waist circumference | ≥40 in (102 cm) in males ≥35 in (85 cm) in females |
Region specific, e.g., ≥90 cm in males and ≥80 cm in females among south Asians. | BMI >30 Waist/hip >0.90 men and >0.85 women |
| Fasting triglycerides | ≥150 mg/dL (>1.7 mmol/L) |
≥150 mg/dL (>1.7 mmol/L) |
≥150 mg/dL (>1.7 mmol/L) |
| HDL-C | <40 mg/dL in males (<1.04 mmol/L); <50 mg/dL in females (<1.29 mmol/L) |
<40 mg/dL in males (<1.04 mmol/L); <50 mg/dL in females (<1.29 mmol/L) |
<35 mg/dL in males (<0.9 mmol/L); <39 mg/dl in females (<1.0 mmol/L) |
| Blood pressure | Systolic ≥130 and/or Diastolic ≥85 mmHg and/or use of medication for hypertension | Systolic ≥130 and/or diastolic ≥85 mmHg and/or use of medication for hypertension | Systolic ≥140 and/or Diastolic ≥90 mmHg and/or use of medication for hypertension |
| Fasting glucose | ≥100 mg/dL (5.6 mmol/L) and/or use of medication for hyperglycemia | ≥100 mg/dL (5.6 mmol/L) and/or use of medication for hyperglycemia | IFG: fasting ≥100 mg/dL (5.6 mmol/L) and/or IGT: 2-h 140–199 mg/dL (7.8–11.0 mmol/L) |
| Microalbuminuria | ≥20 μg/min ≥30 mg/g creatinine |
ATP III, Adult Treatment Panel III; IDF, International Diabetes Federation; WHO, World Health Organization; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol.
Current Clinical Guidelines for Assessing Risk of T2DM
There are a number of methods commercially available for assessing IR and the risk of T2DM (Table 3). The ADA type 2 diabetes risk test (available at www.diabetes.org/diabetes-basics/prevention/diabetes-risk-test/) is the quickest and least invasive because it is a simple questionnaire that is available online. This method of assessment is unsatisfactory for assessing type 2 diabetes risk because it is unvalidated in clinical samples and based only on the aggregation of a few risk factors—those of age, gender, ethnicity, body mass index (BMI), and activity level. Therefore, it shows poor sensitivity and specificity and is suitable only for the crudest indication of a need for clinical follow-up.
Table 3.
Description, Strengths, and Limitations of Current Methods in Assessing Insulin Resistance and Diabetes Predictions
| Fasting plasma glucose (mg/dL) | 2-h oral glucose tolerance test (mg/dL) | Hyperinsulinemic–euglycemic clamp (μU/mL) | Homeostasis model assessment of IR index ln(HOMA) | HbA1c (%) | ADA type 2 diabetes risk test | |
|---|---|---|---|---|---|---|
| Description | Simple, noninvasive blood test. Person must not eat for at least 8 h | After fasting, blood is drawn to establish a fasting glucose level and a patient is given a glucose-rich beverage (typically the beverage contains 75 grams of carbohydrates), and blood is drawn at various intervals to measure glucose levels (usually 2 h). | This test requires a steady intravenous infusion of insulin to be administered in patient's arm. The serum glucose level is clamped at a normal fasting concentration by administering a variable intravenous glucose infusion in the other arm of the patient. | The product of the fasting values of glucose (expressed as mg/dL) and insulin (expressed as μU/mL) is divided by a constant; (HOMA is calculated as insulin [mU/L]×glucose [mg/dL]×0.055/22.5). | HbA1c is a minor component of hemoglobin to which glucose is bound, also referred to as glycosylated hemoglobin. HbA1c gives the physician an idea of how much sugar has been around for the preceding 3 months. | An online computer monitoring test, it uses information on age, race, BMI, and activity levels. |
| Clinical values | 70–99 mg/dL, normal; 100–126 mg/dL, prediabetes; >126 mg/dL, diabetes | <40 mg/dL, normal; 140–200 mg/dL, prediabetes; >200 mg/dL, diabetes | A fasting level of 30 μU/mL indicates greater IR. | Less than HOMA-IR of 4.65 of negative IR; more than tHOMA-IR of 4.65, positive IR | The ADA currently recommends an HbA1c of less than 7.0%. The American Association of Clinical Endocrinologists recommends a goal of less than 6.5%. |
None |
| Strengths | Simple, noninvasive, and inexpensive | More accurate test than FPG test | Gold standard for evaluating insulin sensitivity | Simple, inexpensive, and reliable surrogate measure of IR | Simple, noninvasive, and inexpensive | Simple, noninvasive, free and online |
| Limitations | Specificity is poor and glucose can be “falsely low” if too much time passes between the blood drawn and lab analysis | Unpleasant for the patient and it requires 2 h to perform in physician's office. Certain medications may affect test results. | The technique is expensive, time-consuming, labor-intensive, and not practical in a clinical office setting | Lack of use by the health-care providers and additional validation around diabetes prediction cut-points | HbA1c is not influenced by daily fluctuations in blood glucose levels; therefore, it cannot be used to monitor day-to-day blood glucose. | Very low specificity and sensitivity. No clinical value for follow up value for follow-up |
IR, insulin resistance; HOMA, homeostasis model assessment; HbA1c, glycosylated hemoglobin; ADA, American Diabetes Association; BMI, body mass index; FPG, fasting plasma glucose.
The hyperinsulinemic–euglycemic clamp technique is the gold standard for evaluating insulin sensitivity. It requires a steady intravenous infusion of insulin administered via the patient's arm and the serum glucose level clamped at a normal fasting concentration by administering a variable intravenous glucose infusion in the other arm of the patient. Fasting levels of 30 μU/mL indicate greater IR. Although this is an accurate diagnostic test, the validity of glucose clamp measurements of insulin sensitivity depend on achieving steady-state conditions; the insulin infusion rate aimed at achieving this, if based on normoglycemic individuals, may not fully suppress hepatic glucose production in IR patients.10 In addition, the clamp technique is expensive, time-consuming, labor-intensive, and therefore is impractical in a clinical office setting.
As a result, more commonly used clinical methods are fasting plasma glucose (FPG), 2-h OGTT, homeostasis model assessment of IR index (HOMA-IR), and HbA1c. The FPG test is performed after an individual has fasted for at least 8 h. In the FPG test, 70–99 mg/dL is considered within the normal range, a reading of 100–126 mg/dL suggests prediabetes, and a reading above 126 mg/dL is the threshold for the diagnosis of diabetes. Although FPG is a simple, noninvasive, and inexpensive test, the specificity is poor and the absolute risk of conversion to diabetes is only 5%–10% per year.22
OGTT measures the body's ability to metabolize glucose or remove it from the bloodstream. After fasting, blood is drawn to establish a fasting glucose level; the patient is given a glucose-rich beverage (typically the beverage contains 75 grams of carbohydrates). Following consumption, blood is drawn at various intervals to measure glucose levels (usually 2 h). At 2 h, expected blood glucose levels are less than 140 mg/dL. A blood glucose level between 140 mg/dL and 200 mg/dL indicates impaired glucose tolerance (prediabetes) and a level greater than 200 mg/dL indicates diabetes. OGTT is a more accurate test than FPG for risk assessment, but it is inconvenient for the patient and it requires 2 h to perform. Furthermore, taking certain medicines, such as corticosteroids, diuretics, birth control pills, and nonsteroidal antiinflammatory drugs may affect the test results.10
Newer Alternatives Proposed for Clinical Assessment
HOMA-IR has also been used to assess insulin sensitivity and β-cell function in clinical and epidemiologic studies and could be a viable candidate for clinical assessment.23 HOMA-IR is calculated as insulin [mU/L]×glucose [mg/dL]×0.055/22.5. An individual with HOMA-IR greater than 4.56 or BMI greater than 27.5 kg/m2 and HOMA-IR greater than 3.60 is positive for IR.24 Although HOMA-IR values correlates well with euglycemic clamp techniques,25,26 the coefficient of variation for HOMA-IR varies considerably depending upon the number of fasting samples obtained and the type of insulin assay used.10,26 Other limitations of the HOMA-IR test may include its lack of correlation with euglycemic clamp measures observed in T2DM patients who may have lower BMI, lower β-cell function, and high fasting glucose levels.27
The results of the Diabetes Control and Complications Trial (DCCT) and the U.K. Prospective Diabetes Study established the relationship between HbA1c levels and the risk for diabetic complications in patients with T2DM.18,28 HbA1c is a minor component of hemoglobin to which glucose is bound, also referred to as glycosylated hemoglobin. HbA1c gives the physician an idea of how much glucose has been circulating for the preceding 3 months. According to a 2010 statement from the ADA, elevated HbA1c levels at or beyond the threshold of 6.5% are enough to make a diagnosis of diabetes, whereas levels from 5.7% to 6.4% point to high risk for developing both diabetes and cardiovascular disease and are a marker of prediabetes.29,30 HbA1c may be increased falsely in certain medical conditions, such as uremia, excessive alcohol consumption, and hypertriglyceridemia or cases of hemolysis and/or low red blood cell (RBC) survival. HbA1c is also an average of glucose over 3 months time. Therefore, in the early stages of insulin resistance, compensatory hyperinsulinemia occurs, which may lower glucose and/or even cause hypoglycemia. In this setting, HbA1c may not accurately predict a patient at risk for developing diabetes until the disease has progressed further.
In summary, the challenges with the current methods for assessing IR and T2DM risk include time, cost, inconvenience and discomfort to the patient, and poor specificity related to FPG. By the time glucose or insulin abnormalities are identified, the underlying disease has already progressed for many years.
Lipoprotein Measures in T2DM Risk Assessment
The dyslipidemia accompanying, and possibly preceding, the development of IR offers hope for earlier detection and treatment. Although elevated triglyceride (TG) concentrations and reduced HDL-C form part of the diagnosis for the metabolic syndrome (Table 2), these measures lack specificity. Lipoproteins particles are spherical containers with a core of lipid molecules, predominantly cholesterol esters and TGs, surrounded by a shell of phospholipids and proteins. Seminal work in the 1980s demonstrated that lipoprotein fractions were not polydispersed, but rather discrete entities with each fraction having a different composition; this allows for acceptance of the differential associations of lipoprotein subfractions within each fraction of VLDL, LDL, and HDL in cardiometabolic diseases.31,32 The division of HDL into three subfractions (HDL1–3) has led to the observation that the different subfractions exhibit differential relationships to the development of coronary heart disease.33 The relative amounts of cholesterol and TG carried in the particle core can differ among individuals, giving rise to heterogeneity in the size of the particles within a given lipoprotein fraction. Another key component of IR is the dyslipoproteinemia, manifested by elevated small LDL-P and large VLDL-P and reduced large HDL-P concentrations, which accompanies low HDL-C and high TG levels.
Clinical Data Supporting the Relationship of Lipoprotein Subclass and Size to IR and Diabetes
Clinical data supporting the relationships of lipoprotein size and subclass quantification were initially reported using lipoprotein data from gradient gel electrophoresis.34,35 This method of measuring lipoprotein size and subfraction concentration involves separation and quantification and can take several hours to several days to complete. Precision is limited by many sources of analytical variability inherent to separation techniques.36,37
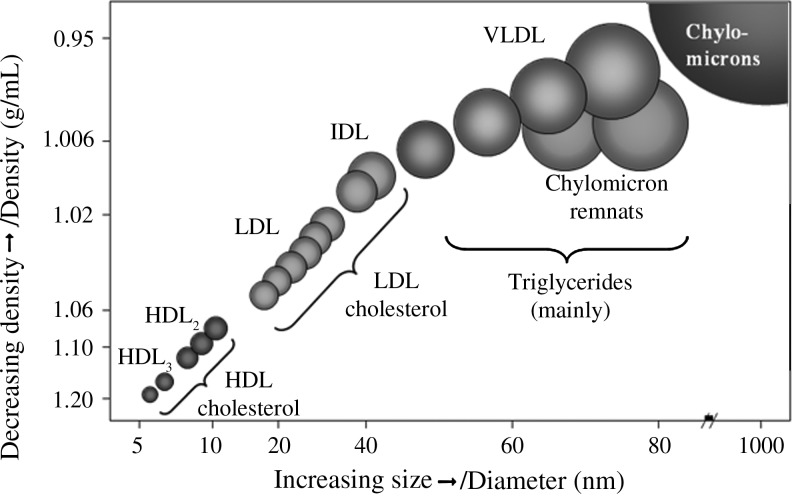
NMR spectroscopy is used to measure lipoprotein concentration and size for the three fractions of lipoprotein (VLDL, HDL, and LDL), with intermediate-density lipoproteins grouped as a subclass of LDL in serum and plasma samples. It is used as an alternative to separation-based methods of lipoprotein analysis (Fig. 2). The process uses the natural proton NMR spectroscopic signatures of lipoprotein particles of different sizes, and the measured amplitudes of these signals provide an efficient means for quantifying lipoprotein subclasses. Hence, even in the face of significant variation in the cholesterol composition, the constancy of the relationship between subclass signal amplitude and particle concentration is what gives NMR its unique ability to quantify lipoprotein particles number without separation techniques.36
FIG. 2.
Diameter ranges of lipoprotein subclasses measured by nuclear magnetic resonance. HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
Numbers of studies have reported an inverse association between insulin sensitivity and an increased preponderance of smaller LDL particles and larger VLDL particles, accompanied by a decrease in the number of large HDL particles. This is reflected as an overall shift to smaller LDL and HDL, but larger VLDL diameters (measured in nanometers).11–15,20,34,35 The first published study using NMR data was in a single-site study of 148 subjects (46 with untreated diabetes; mean age 37 years; 43% male; 66% Caucasian).12 IR was measured using the gold-standard euglycemic clamp method, and NMR LipoProfile analyses were conducted on frozen fasting serum specimens. Subjects were characterized as either insulin sensitive (n=56), insulin resistant (n=46), or having T2DM (n=46). Garvey et al. noted that IR, manifested by a decreasing glucose disposal rate, was associated with a progressive increase in small LDL-P and large VLDL-P and a shift toward lower concentration of large HDL-P (Table 4). These relationships with particle concentration and size were highly significant regardless of whether adjusted for sex, age, race, and BMI, but the conventional lipid panel of LDL cholesterol (LDL-C) did not associate with IR at this early stage.
Table 4.
Means (±SD), for NMR Lipoprotein Particle Concentrations in Insulin Sensitive, Insulin Resistance, and Type 2 Diabetic Subjectsa
| Insulin sensitive | IR | Diabetes | IR vs. IS P values | DM vs. IS P values | |
|---|---|---|---|---|---|
| Small LDL-P (nmol/L) | 365.6 (±397) | 668.4 (±599) | 805.0 (±711) | 0.008 | 0.0002 |
| Large VLDL-P (nmol/L) | 1.7 (±2.7) | 3.4 (±4) | 4.8 (±5) | 0.033 | 0.0002 |
| Large HDL-P (μmol/L) | 6.9 (±3.5) | 5.3 (±3.8) | 4.7 (±3.7) | 0.029 | 0.1162 |
Adapted from Garvey et al.12
SD, standard deviation; NMR, nuclear magnetic resonance; IR, insulin resistance; IS, insulin sensitivity; DM, diabetes mellitus; LDL-P, low-density lipoprotein particles; VLDL-P, very-low-density lipoprotein particles; HDL-P, high-density lipoprotein particles.
The Insulin Resistance Atherosclerosis Study (IRAS) was the first large multicenter study to examine whether NMR lipoprotein subclass profile predicts T2DM.15,20 IRAS was a cohort study of middle-aged men and women (n=1,371) with 437 having T2DM, 301 with impaired glucose tolerance, and 633 with normal glucose tolerance. IR was measured by the frequently sampled intravenous glucose tolerance test. NMR-derived lipoprotein analyses were conducted on frozen plasma samples from the participants. Very similar to the previous study, there was a lack of association between LDL-C concentration and measure of insulin sensitivity. However, after adjusting for age, sex, and ethnicity, insulin sensitivity was associated with high concentrations of small LDL-P and large VLDL-P and a low concentration of large HDL-P (Table 5).15 Of the 830 subjects who were nondiabetic at baseline, 130 (15.75) developed diabetes after a mean follow-up of 5.2 years. Lipoprotein subclass concentrations were positively associated with 5-year diabetes incidence, independent of TG and HDL-C concentrations.15
Table 5.
Means (±SEM) for NMR Lipoprotein Particle Concentrations In Normal Glucose Tolerance, Impaired Glucose Tolerance, and Diabetes Subjectsa
| Normal glucose tolerance | Impaired glucose tolerance | Diabetes | P valuesb | |
|---|---|---|---|---|
| Small LDL-P (nmol/L) | 433.1±14.5 | 550.8±22.7 | 636.3±19.1 | 0.0001 |
| LDL-P size (nm) | 21.4±0.03 | 21.2±0.05 | 20.9±0.04 | 0.0001 |
| Large VLDL-P (nmol/L) | 2.6±0.1 | 3.5±0.1 | 4.7±0.1 | 0.0001 |
| VLDL-P size (nm) | 48.1±0.4 | 51.3±0.6 | 55.2±0.6 | 0.0001 |
| Large HDL-P (μmol/L) | 5.1±0.1 | 4.5±0.1 | 3.9±0.1 | 0.0001 |
| HDL-P size (nm) | 9.0±0.02 | 8.9±0.02 | 8.8±0.02 | 0.0001 |
Adapted from Goff et al.20
Adjusted for age, sex, and ethnicity.
SEM, standard error of the mean; NMR, nuclear magnetic resonance; LDL-P, low-density lipoprotein particles; VLDL-P, very-low-density lipoprotein particles; HDL-P, high-density lipoprotein particles.
Support for the predictive ability of lipoprotein subclasses was reported by the Melbourne Collaborative Cohort (MCCS), which was established to prospectively study cancer and other lifestyle-related diseases.14 Subjects included 813 male and female participants, aged 40–69 years at baseline, with a fasting plasma glucose <7.0 mmol/L; incident T2DM was self-reported and confirmed by doctors. A total of 59 cases of diabetes and 754 noncases were selected with NMR data available. Concentrations of large VLDL-P and HDL-P predicted diabetes incidence, supporting a relationship between lipoprotein subclass profile changes and the prediction of T2DM.
Consistent with prior studies in individuals with insulin resistance, the Women's Health Study (WHS) also reported that NMR-measured lipoprotein profiles were associated with increased incidence of diabetes, independent of TG and HDL-C levels.13 A cohort of 26,836 study participants was followed for a median of 13.3 years, and a total of 1,687 incident cases of clinical T2DM were documented (using ADA diagnostic criteria). In fully adjusted models, neither LDL-C nor total cholesterol showed association with diabetes. As depicted in Table 6, the adjusted hazard ratios for incident diabetes associated with extreme quintiles of small LDL-P, large VLDL-P, and large HDL-P were 4.04, 3.11, and 4.51, respectively.
Table 6.
Adjusted Hazard Ratios for the Association of Lipoprotein Measure with Incident Type 2 Diabetes in Quintiles 1 through 5a
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for trend | |
|---|---|---|---|---|---|---|
| Small LDL-P (nmol/L) | Ref. | 1.54 (1.18–2.02) | 2.09 (1.63–2.68) | 2.62 (2.06–3.32) | 4.04 (3.21–5.09) | <0.001 |
| LDL-P size (nm) | 4.16 (3.30–5.24) | 3.04 (2.4–3.86) | 2.21 (1.74–2.81) | 1.63 (1.25–2.13) | Ref. | <0.001 |
| Large VLDL-P (nmol/L) | Ref. | 1.49 (1.09–2.05) | 2.54 (1.91–3.39) | 2.98 (2.24–3.96) | 3.11 (2.35–4.11) | <0.001 |
| VLDL-P size (nm) | Ref. | 1.25 (0.97–1.62) | 1.67 (1.32–2.10) | 2.22 (1.78–2.76) | 2.80 (2.27–3.46) | <0.001 |
| Large HDL-P (μmol/L) | 4.51 (3.68–5.52) | 3.19 (2.58–3.94) | 2.54 (2.04–3.15) | 1.72 (1.36–2.17) | Ref. | <0.001 |
| HDL-P (nm) | 4.56 (3.50–5.93) | 3.97 (3.03–5.21) | 3.08 (2.35–4.03) | 1.72 (1.29–2.29) | Ref. | <0.001 |
Adapted from Mora et al.13
Ranges (minimum–maximum) and hazard ratios with 95% confidence intervals are given for each quintile. P for trend obtained from using median quintile as a dependent variable in Cox regression models. Data represented are adjusted for age, race, randomized treatment assignment, smoking, exercise, education, menopausal status, hormone use, blood pressure, body mass index, family history of diabetes, HbA1c, and high-sensitivity C-reactive protein.
LDL-P, low-density lipoprotein particles; VLDL-P, very-low-density lipoprotein particles; HDL-P, high-density lipoprotein particles; HBA1c, glycosylated hemoglobin.
As described in the above studies, the NMR-derived lipoprotein associations with IR are remarkably consistent among the four studies, despite differences in the populations studied and the use of different methods to assess insulin resistance.
Perspectives and Future Directions
A simple and reliable test for assessing IR would enable more effective diabetes prevention and treatment by identifying patients at risk for diabetes when blood glucose levels are still in the normal range and β-cell function has not yet deteriorated. The current methods for assessing IR are costly and time-consuming, limiting their use in the primary care settings. NMR spectroscopy offers a convenient serum test that measures lipoprotein subclass particle concentration and size, can show a strong association with IR, and may be detectable before the occurrence of abnormal fasting glucose levels. On the basis of the observed IR association of the six lipoprotein parameters from two forms of measure (small LDL-P, large VLDL-P, and large HDL-P concentrations in conjunction with LDL-P, VLDL-P, and HDL-P size in nanometers), a single parameter employing all six measures has been developed to provide a Lipoprotein IR Index (LP-IR; LipoScience Inc.) score.
Additional prospective trials to validate the predictive value of LP-IR score are underway, and the outcome of these analyses will help confirm the results of the previous studies using longitudinal data and validate the clinical utility of predicting T2DM and disease progression from this simple serum test. Future research needs to focus on quantifying the additional predictive value of the LP-IR score over and above current lipid measures in prediabetes screening, but as it stands, the LP-IR may play a role in reducing the increasing economic and clinical burden that is associated with the dramatic increase in the rate of incident diabetes.
Acknowledgments
The authors acknowledge research support from the National Institutes of Health (NIH; DK-038764, DK-083562) and the Merit Review program of the Department of Veterans Affairs. We also gratefully acknowledge the support of the UAB Diabetes Research and Training Center (P60-DK079626). Dr. Frazier-Wood is supported by NIH National Heart, Lung, and Blood Institute grant U01HL072524-04.
Author Disclosure Statment
Drs. Wood, Garvey, and Dall report no competing financial interests. Dr. Pourfarzib is the vice president of Medical Affairs at LipoScience. Dr. Honigberg is the vice president and chief medical officer at LipoScience.
References
- 1.Amos AF. McCarty DJ. Zimmet P. The rising global burden of diabetes and its complications: Estimates and projections to the year 2010. Diabetic Med. 1997;14(S5):S7–S85. [PubMed] [Google Scholar]
- 2.King H. Aubert RE. Herman WH. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen QM. Srinivasan SR. Xu J-H, et al. Changes in risk variables of metabolic syndrome since childhood in pre-diabetic and type 2 diabetic subjects. Diabetes Care. 31:2044–2049. doi: 10.2337/dc08-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunzell JD. Davidson M. Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811–822. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 6.Knowler WC. Fowler SE. Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerstein HC. Yusuf S. Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 8.Orchard TJ. Temprosa M. Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: The Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blonde L. State of diabetes care in the United States. Am J Managed Care. 2007;13(Suppl 2):S36–S40. [PubMed] [Google Scholar]
- 10.Muniyappa R. Lee S. Chen H, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 11.Wood AC. Glasser SP. Garvey WT, et al. A clustering analysis of lipoproteins diameters in the metabolic syndrome. Lipids Health Dis. 2011;10:237. doi: 10.1186/1476-511X-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvey WT. Kwon S. Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 13.Mora S. Otvos JD. Rosenson RS, et al. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. 2010;59:1153–1160. doi: 10.2337/db09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodge AM. Jenkins AJ. English DR, et al. NMR-determined lipoprotein subclass profile predicts type 2 diabetes. Diabetes Res Clin Pract. 2009;83:132–139. doi: 10.1016/j.diabres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Festa A. Williams K. Hanley AJ, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC. Dysglycemia and cardiovascular risk in the general population. Circulation. 2009;119:773–775. doi: 10.1161/CIRCULATIONAHA.108.834408. [DOI] [PubMed] [Google Scholar]
- 18.U.K. Prospective Diabetes Study 16. Overview of 6 years' therapy of type II diabetes: A progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 19.Malik S. Wong ND. Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 20.Goff DC., Jr D'Agostino RB., Jr. Haffner SM, et al. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metabolism. 2005;54:264–270. doi: 10.1016/j.metabol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Mora S. Szklo M. Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Wong TY. Liew G. Tapp RJ, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: Three population-based cross-sectional studies. Lancet. 2008;371:736–743. doi: 10.1016/S0140-6736(08)60343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Y. Manson JE. Tinker L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: The Women's Health Initiative Observational Study. Diabetes Care. 2007;30:1747–1752. doi: 10.2337/dc07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stern SE. Williams K. Ferrannini E, et al. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2004;54:333–339. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- 25.Katsuki A. Sumida Y. Gabazza EC, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda Y. Suehiro T. Nakamura T, et al. Clinical significance of the insulin resistance index as assessed by homeostasis model assessment. Endocr J. 2001;48:81–86. doi: 10.1507/endocrj.48.81. [DOI] [PubMed] [Google Scholar]
- 27.Kang ES. Yun YS. Park SW, et al. Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism. 2005;54:206–211. doi: 10.1016/j.metabol.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 28.The Diabetes Control And Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The International Expert Committee. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher WR. Heterogeneity of plasma low density lipoproteins manifestations of the physiologic phenomenon in man. Metabolism. 1983;32:283–291. doi: 10.1016/0026-0495(83)90194-4. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd J. Packard CJ. Metabolic heterogeneity in very low-density lipoproteins. Am Heart J. 1987;113(2 Pt 2):503–508. doi: 10.1016/0002-8703(87)90621-1. [DOI] [PubMed] [Google Scholar]
- 33.Miller NE. Hammett F. Saltissi S, et al. Relation of angiographically defined coronary artery disease to plasma lipoprotein subfractions and apolipoproteins. Br Med J (Clin Res Ed) 1981;282:1741–1744. doi: 10.1136/bmj.282.6278.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reaven GM. Chen YD. Jeppesen J, et al. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest. 1993;92:141–146. doi: 10.1172/JCI116541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin MA. Selby JV. LDL subclass phenotypes and the risk factors of the insulin resistance syndrome. Int J Obes Related Metabol Disord. 1995;19(Suppl 1):S22–S26. [PubMed] [Google Scholar]
- 36.Jeyarajah EJ. Cromwell WC. Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Ip S. Lichtenstein AH. Chung M, et al. Systematic review: Association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med. 2009;150:474–484. doi: 10.7326/0003-4819-150-7-200904070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]