Abstract
Outcome prediction following severe traumatic brain injury (sTBI) is a widely investigated field of research. A major breakthrough is represented by the IMPACT prognostic calculator based on admission data of more than 8500 patients. A growing body of scientific evidence has shown that clinically meaningful biomarkers, including glial fibrillary acidic protein (GFAP), ubiquitin C-terminal hydrolase-L1 (UCH-L1), and αII-spectrin breakdown product (SBDP145), could also contribute to outcome prediction. The present study was initiated to assess whether the addition of biomarkers to the IMPACT prognostic calculator could improve its predictive power. Forty-five sTBI patients (GCS score≤8) from four different sites were investigated. We utilized the core model of the IMPACT calculator (age, GCS motor score, and reaction of pupils), and measured the level of GFAP, UCH-L1, and SBDP145 in serum and cerebrospinal fluid (CSF). The forecast and actual 6-month outcomes were compared by logistic regression analysis. The results of the core model itself, as well as serum values of GFAP and CSF levels of SBDP145, showed a significant correlation with the 6-month mortality using a univariate analysis. In the core model, the Nagelkerke R2 value was 0.214. With multivariate analysis we were able to increase this predictive power with one additional biomarker (GFAP in CSF) to R2=0.476, while the application of three biomarker levels (GFAP in CSF, GFAP in serum, and SBDP145 in CSF) increased the Nagelkerke R2 to 0.700. Our preliminary results underline the importance of biomarkers in outcome prediction, and encourage further investigation to expand the predictive power of contemporary outcome calculators and prognostic models in TBI.
Key words: biomarkers, IMPACT calculator, outcome, prognostic models, traumatic brain injury
Introduction
According to the World Health Organization (WHO) in high-income countries traumatic brain injury (TBI) is the leading cause of death under the age of 40, and approximately 10 million head-injured persons are hospitalized annually worldwide. Based on the prognosis of the WHO, in two decades, head injury will be the third most frequent cause of death in the world (Murray and Lopez, 1997).
Based on a nationwide survey initiated by the Centers for Disease Control and Prevention (CDC) in the United States in 2006, at least 1.4 million people sustained TBI. Of them, approximately 50,000 died, 235,000 were hospitalized, and 1.1 million were treated and released from an emergency department (ED; Langlois et al., 2006).
The socioeconomic consequences of TBI have ignited widespread research aimed at decreasing the burden of the disease. A major field to be explored is the establishment and construction of reliable prognostic tools that will facilitate more efficient design of clinical trials and improve individualized patient management (Menon and Zahed, 2009).
To date, there are three different approaches to outcome prediction following severe TBI (sTBI). The first is based on admission characteristics such as age, the reaction of pupils, Glasgow Coma Scale (GCS) score, GCS motor score, body temperature, blood glucose level, and significant non-cranial injuries, in addition to other factors (Hukkelhoven et al., 2005; Mushkudiani et al., 2008). The second approach is based on the pathological findings seen on the first available CT scan, and is represented by the Marshall CT classification (Marshall et al., 1992), and the primarily prognostically-oriented Rotterdam score (Maas et al., 2005). The third utilizes blood and/or cerebrospinal fluid (CSF) levels of biomarkers of brain injury (Kovesdi et al., 2010; Svetlov et al., 2009). The methods used in the first two approaches have recently been synthesized during detailed analysis of data derived from the IMPACT (Steyerberg et al., 2008) and the CRASH (Perel et al., 2008) trials, and led to the development of prognostic calculators that are available for online analysis.
The International Mission for Prognosis and Clinical Trial (IMPACT) database was developed by Andrew I.R. Maas and his colleges in 2003. They collected and analyzed the data for 9205 patients from eight randomized controlled trials and three epidemiological studies (Marmarou et al., 2007). In 2008, after detailed statistical analysis of the admission data of 8509 patients with logistic regression, and after an internal and external validation process (using data from 6681 patients with a GCS score≤12 from the MRC CRASH trial), they created a prognostic calculator that is available online (Steyerberg et al., 2008). These workers decided to consider the 6-month outcome as the prognostic end-point based on the Glasgow Outcome Scale (GOS; Murray and Lopez, 1997), applying two different forms of dichotomy: mortality (GOS 1) versus survival (GOS 2–5), and unfavorable (GOS 1–3) versus favorable (GOS 4–5) outcomes. Twenty-six parameters have been assessed that are available at or soon after admission, and may have predictive value. With the utilization of logistic regression, the 10 strongest predictive parameters were identified, leading to the definition of three prognostic models that are superimposed on each other. Constituents of the core model are the age, motor score component of the GCS, and reaction of the pupils. The extended model utilizes hypoxia, hypotension, CT characteristics (Marshall CT classification), and the presence of epidural hematoma (EDH) or traumatic subarachnoid hemorrhage (tSAH) on the first CT scan, which are added to the parameters of the core model. The third, a laboratory model, includes all parameters of the extended model plus blood glucose and hemoglobin levels. The core model alone has been associated with considerable predictive value, as defined by an area under the receiver-operating characteristic (ROC) curve values between 0.66 and 0.84 during the internal and external validation processes. The IMPACT group attained slightly better values with the extended and lab models (Steyerberg et al., 2008).
Ideally a biomarker should be an easily and reliably measurable molecule with serum levels that display a close correlation with a biological or pathogenic process and/or a pharmacological intervention. Currently, biomarkers that can be used to predict clinical outcome are considered surrogate biomarkers or surrogate end-points (Biomarkers Definitions Working Group, 2001). During the last few decades a rapidly growing number of molecules have been tested as potential biomarkers of TBI. However, so far no single molecule has proven specific and sensitive enough to be employed as a comprehensive clinical diagnostic tool to predict the extent of neural tissue damage, or to aid in monitoring care and forecasting outcome. Nevertheless, there are a handful of molecules that are potential candidates for a complex biomarker panel, including neuron-specific enolase (NSE), glial fibrillary acid protein (GFAP), S-100β protein, myelin basic protein, cleaved tau protein, spectrin breakdown products (SBDPs), and ubiquitin C-terminal hydrolase-L1 (UCH-L1; Hergenroeder et al., 2008; Papa et al., 2010; Svetlov et al., 2009).
In this study we investigated the serum and CSF levels of GFAP and UCH-L1, as well as CSF levels of a 145-kDa SBDP (SBDP145).
GFAP is an intermediate filament monomer found only in the cytoskeleton of astroglial cells, and thus is specific for the central nervous system. After damage to the astroglia, GFAP is released into the peripheral blood circulation in neurodegenerative disorders (Middeldorp and Hol, 2011), and after stroke (Herrmann et al., 2000) and TBI (Lumpkins et al., 2008; Vos et al., 2010). In a recent study, serum GFAP levels displayed greater sensitivity and specificity after TBI than serum S-100β or NSE (Honda et al., 2010). It was also proven that serum GFAP levels possess significant predictive power of mortality and outcome (Pelinka et al., 2004).
The ubiquitin C-terminal hydrolases (UCH-L1, UCH-L3, UCH37, and BAP1) form a subfamily among the deubiquitinating enzymes that are capable of removing ubiquitin from their protein substrates. Among them UCH-L1, also known as neuronal specific gene product 9.5 (PGP9.5), is the most strongly associated with the central nervous system, where it is predominantly detectable in neuronal cell bodies. A role of mutations and polymorphisms of UCH-L1 was found in Parkinson's disease (Schulz 2008), and other neurodegenerative disorders (Gong and Leznik, 2007). Recent studies have demonstrated significant increases in CSF and serum levels of UCH-L1 after controlled cortical impact TBI in rats (Liu et al., 2010), and have associated CSF levels of UCH-L1 with injury severity (Papa et al., 2010), as well as CSF and serum levels of UCH-L1 with outcome and 3-month mortality (Brophy et al., 2011).
Non-erythroid αII-spectrin is a well known component of the cytoskeleton of all non-erythroid tissues. Neurons contain the highest concentrations in the subaxolemmal compartment and presynaptic terminals. After TBI, SBDPs are cleaved from the intact brain spectrin. The 145-kDa fragment is specific for calpain, while the 120-kDa SBDP is associated with caspase-3-mediated cleavage. The former is mainly associated with necrotic and the latter with apoptotic processes. To date our findings are primarily based on the analysis of CSF levels of SBDPs. Nevertheless the results indicate that both calpain and caspase-derived spectrin fragments may serve as potential biomarkers of TBI (Brophy et al., 2009; Farkas et al., 2005; Mondello et al., 2010; Pineda et al., 2007).
In light of these findings, the aim of our study was to examine whether biomarkers of TBI can improve outcome prediction when combined with the IMPACT core prediction model.
Methods
Patient enrollment, sample, and clinical data collection
This study is part of the Biomarker Assessment For Neurotrauma Diagnosis and Improved Triage System (BANDITS) Study, which is a project involving the clinical, biochemical, and neuroimaging evaluation of patients with TBI. In this study, we focused on a cohort of 45 patients with severe TBI for whom biomarkers of brain damage in the CSF and serum were available. Severe TBI patients from four different centers (University of Pecs, Hungary: 21 patients; University of Szeged, Hungary: 12 patients; University of Maryland, U.S.: 6 patients; and University of Florida, U.S.: 6 patients) were selected for the study.
The main inclusion criterion was GCS score≤8 years on admission caused by sTBI less than 24 h before enrollment. Exclusion criteria were age<18 years, known autoimmune disease, and pregnancy. Because of the comatose state of these patients, informed consent was obtained from their legally-authorized representative (LAR). Lack of informed consent by the LAR was an exclusion criterion.
Initial serum and CSF sample collection was carried out on enrollment, and every 6 h up to 24 h post-injury. All blood and CSF samples were centrifuged (4000 rpm for 8 min), stored at −80°C, and shipped in dry ice to Banyan Biomarkers Inc. (Alachua, FL), where the biomarker levels were measured.
Outcome was measured by GOS scores recorded 1, 2, 3, and 4 weeks, and 3 and 6 months post-injury. GOS scores were dichotomized two ways: lethal outcome (GOS 1) versus survival (GOS 2–5), and unfavorable (GOS 1–3) versus favorable (GOS 4–5) outcomes.
All procedures were carried out with the permission and under the rigorous control of the local institutional review boards of each site, the Western Institutional Review Board, and the Human Research Protection Office.
Measurement of biomarker levels in the serum and CSF samples
Measurement of GFAP and UCH-L1 levels in serum and CSF samples and SBDP145 levels in CSF were carried out using sandwich ELISA protocols, which were previously described in detail by members of our research team (Mondello et al., 2010,2011).
For GFAP and UCH-L1, reaction wells were coated with capture antibody (500 ng/well of purified anti-rabbit UCH-L1, made in-house) in 0.1 M sodium bicarbonate (pH 9), and were incubated overnight at 4°C. Then they were emptied out and 300 μL/well of blocking buffer (Startingblock T20-TBS; Thermo Fisher Scientific, Rockford, IL) was added and incubated for 30 min at room temperature, followed by the addition of antigen standard (UCH-L1 standard curve: 0.05–50 ng/well), unknown samples (3–10 μL CSF), or assay internal control samples. The plates were incubated for 2 h at room temperature, then washed using an automatic plate washer (each well was rinsed with 5×300 μL with wash buffer [TBST]). Detection antibody (50 μg/mL of anti-rabbit UCH-L1-HRP conjugate, made in-house) in blocking buffer was next placed in the wells (100 μL/well), and the plates were incubated for 1.5 h at room temperature. More automatic washing was followed by 15 min of incubation in biotinyl-tyramide solution (Elast Amplification Kit; PerkinElmer, Waltham, MA) at room temperature, followed by automatic washing. The addition of streptavidin-HRP (1:500; 100 μL/well) in PBS with 0.02% Tween-20 and 1% BSA with 30 min incubation at room temperature was followed by automatic washing. Then the wells were developed with substrate solution: ultra-TMB ELISA 100 μL/well (Pierce no. 34028; Pierce Protein Research Products, Rockford, IL), incubated for 5–30 min, and read at 652 nm with a 96-well spectrophotometer (Spectramax 190; Molecular Devices, Sunnyvale, CA). GFAP protein was analyzed using a commercially available (catalog no. rd192072200; BioVendor, Candler, NC) polyclonal two-sided immunoluminometric assay according to the manufacturer's instructions. A standard curve was constructed by plotting absorbance values versus GFAP concentrations of calibrators.
To detect SBDP145 another sandwich ELISA was used. First, 96-well plates were coated with 100 μL/well of capture antibody (1000 ng/well of affinity-purified rabbit polyclonal anti-SBDP145 fragment-specific antibody) overnight at 4°C. The blocking buffer (Fisher 37539 Startingblock T20-PBS; Thermo Fisher Scientific), was followed by an antigen standard (recombinant GST-fusion-αII-spectrin, repeats 13–18[ +145]) cleaved with calpain (1:50 ratio for 10 min at 4°C, or with caspase-3 for 4 h at room temperature) to establish a standard curve. Stock solution of 0.5–5000 ng/mL of prepared SBDP145 protein (0.005–50 ng in 10 mL) were diluted 1:10 with sample diluent to a final incubation volume of 100 μL/well. Thus the standard curve range was 0.05–500 ng/ml in the wells. Each sample was evaluated in duplicate. The target (10 μL CSF/well and 90 μL PBST blocking buffer) and capture antibody were incubated for 2 h at 27°C with gentle shaking. The plate was washed with TBST washing buffer 5× with an automatic plate washer. Then a 1:3000–1:4000 dilution of HRP-labeled detection antibody (alpha fodrin; Biomol International, Plymouth Meeting, PA) was added to each well (100 μL/well), and was incubated at room temperature for 1.5 h with gentle shaking. If amplification was needed, biotinyl-tyramide solution (Elast Amplification Kit; PerkinElmer) was added for 15 min at room temperature, then washed and followed by 100 μL/well streptavidin-HRP (1:3000) in PBS with 0.02% Tween-20 and 1% BSA for 30 min, followed by 5× washing in the automatic plate washer. As a final step, the wells were developed with 100 μL/well of chemiluminescent substrate solution (SuperSignal ELISA Femto #37075; Thermo Fisher Scientific) and incubated for 1 min. The signal was read by a 96-well chemiluminescence microplate reader (GloRunner DXL Luminometer; Turner BioSystems, Inc., Sunnyvale, CA).
GFAP and UCH-L1 levels were measured in each CSF and serum sample, and SBDP145 in each CSF sample up to 24 h post-injury for the statistical analysis, and we defined the following parameters: GFAP level in the first serum sample (GFAP_1S), GFAP level in the first CSF sample (GFAP_1C), UCH-L1 level in the first serum sample (UCH-L1_1S), UCH-L1 level in the first CSF sample (UCH-L1_1C), SBDP145 level in the first CSF sample (SBDP145_1C), the average of the GFAP levels of all the serum samples taken in the first 24 h (GFAP_24S), the average of all of the GFAP levels of the CSF samples taken in the first 24 h (GFAP_24C), the average of all of the UCH-L1 levels in the serum samples taken in the first 24 h (UCH-L1_24S), the average of all of the UCH-L1 levels in the CSF samples taken in the first 24 h (UCH-L1_24C), and the average of the SBDP145 levels in all of the CSF samples taken in the first 24 h (SBDP145_24C).
Statistical analysis
Receiver-operating characteristic (ROC) curves have been constructed for the biomarkers to describe their values in predicting 6-month lethal and unfavorable outcomes. The area under the curve (AUC) has been computed for each, and its difference from the non-discriminative 0.5 value has been tested. The optimal thresholds that maximize the predictive abilities of the biomarkers have been determined. The sensitivity and specificity for these cut-points have been calculated as well.
The probability of a 6-month lethal outcome has been calculated by the core model of the IMPACT calculator utilizing clinical data of the enrolled patients.
The relationship between observed and biomarker-based predicted outcomes have been analyzed by univariate logistic regression. The strength of the association between observed and IMPACT core model-based predicted outcomes have been tested by logistic regression analysis as well.
The potential additive value of biomarker data to the predictive power has been investigated by quantifying the increase in predictive power in logistic regression models by inserting one or more biomarkers into the basic logistic regression model, and assessing the actual outcome and the outcome predicted by the IMPACT core model. The predictive power was described by Nagelkerke R2 values.
Statistical test results are considered significant at p<0.05. All statistical procedures were carried out using SPSS 19.0 for Windows software.
Results
Descriptive statistics
The mean age of the patients was 49.78±19.99 years, ranging between 19 and 88 years (median 53 years, interquartile range 30–63 years). Our statistical analysis showed that the demographic characteristics and the etiology of the injuries in our patients were consistent with the findings of recent epidemiological studies conducted in high-income countries (Langlois et al., 2006; Tagliaferri et al., 2006). Table 1 shows demographic characteristics, relevant admission parameters, and 6-month outcomes of our patient sample.
Table 1.
Summary of Demographic Characteristics, Admission Parameters, and 6-Month Outcomes of the Subjects
| Severe TBI (n=45) | ||
|---|---|---|
| Age, years | 49.78±19.99 | |
| Range, years | 19–88 | |
| Gender, n (%) | Female | 12 (26.67) |
| Male | 33 (73.33) | |
| Race, n (%) | Caucasian | 44 (97.78) |
| African-American | 1 (2.22) | |
| Injury mechanism, n (%) | Motor vehicle accident | 14 (31.11) |
| Motorcycle accident | 1 (2.22) | |
| Gunshot wound | 2 (4.44) | |
| Fall | 22 (48.89) | |
| Assault | 1 (2.22) | |
| Other | 5 (11.11) | |
| GCS score, median (range) | 5 (3–8) | |
| GCS motor score, median (range) | 3 (1–6) | |
| Reaction of pupils, n (%) | Both | 17 (37.78) |
| One | 3 (6.67) | |
| None | 23 (51.11) | |
| NA | 2 (4.44) | |
| Marshall CT score, n (%) | Diffuse injury I | 1 (2.86) |
| Diffuse injury II | 8 (22.86) | |
| Diffuse injury III | 4 (11.43) | |
| Diffuse injury IV | 4 (11.43) | |
| Evacuated focal mass lesion V | 4 (11.43) | |
| Focal mass lesion VI | 14 (40) | |
| NA | 10 | |
| 6-Month survival, n (%) | Deceased | 31 (68.89) |
| Alive | 14 (31.11) |
NA, not applicable; GCS, Glasgow Coma Scale; CT, computed tomography.
Of the 45 patients 18 died within the first week, representing 40% mortality, and this increased to 68.9% by the 6-month end-point (in all, 31 patients died). We had unfavorable outcomes in 35 patients (77.8%) at the end of the first week, and 34 patients (75.6%) at the end of the 6-month period, which represents a dramatic decrease in the number of patients in a vegetative state or with severe disability during the first 6 months post-injury.
A detailed summary of the results of the descriptive statistical analysis of the measured biomarker levels is provided in Table 2. It is of note that despite the relatively small number of patients enrolled in this study, the standard deviations are small, while the amount of missing data is minimal, and was caused by unmeasurable samples, but this did not hinder the statistical analysis.
Table 2.
Serum (S) and Cerebrospinal Fluid (C) Biomarker Levels in Patients with Severe Traumatic Brain Injury
| Biomarker | n (valid) | n (missing) | Mean | Median | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| GFAP_24S | 44 | 1 | 2.823 | 0.203 | 6.760 | 0.002 | 29.028 |
| UCH-L1_24S | 40 | 5 | 1.698 | 0.653 | 2.623 | 0.025 | 11.370 |
| GFAP_1S | 42 | 3 | 3.456 | 0.291 | 8.428 | 0.009 | 40.865 |
| UCH-L1_1S | 41 | 4 | 2.551 | 0.765 | 4.488 | 0.026 | 19.773 |
| GFAP_24C | 36 | 9 | 4.641 | 0.646 | 9.978 | 0.015 | 49.614 |
| UCH-L1_24C | 41 | 4 | 137.051 | 78.188 | 143.654 | 7.900 | 611.411 |
| GFAP_1C | 34 | 11 | 4.114 | 0.807 | 8.403 | 0.036 | 38.069 |
| UCH-L1_1C | 41 | 4 | 151.133 | 80.940 | 153.953 | 7.900 | 597.932 |
| SBDP145_24C | 41 | 4 | 58.319 | 31.858 | 82.515 | 4.867 | 471.120 |
| SBDP145_1C | 41 | 4 | 45.829 | 29.915 | 57.345 | 5.501 | 298.570 |
All biomarker levels are expressed in ng/mL.
GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin C-terminal hydrolase-L1; SBDP145, 145-kDa αII-spectrin breakdown product; 1S, first measured level in serum; 1C, first measured level in cerebrospinal fluid; 24S, average of all measured serum levels within 24 h post-injury; 24C, average of all measured cerebrospinal fluid levels within 24 h post-injury; n (valid), number of cases with measurable biomarker levels; n (missing), number of cases with unmeasurable biomarker levels; SD, standard deviation.
Results of ROC analysis
AUCs from ROC curves of lethal outcome proved to be significant for GFAP_1S, UCH-L1_1S, GFAP_24S, UCH-L1_24S, and SBDP145_1C for at least one investigated time point (Table 3). In case of the 6-month outcome, which was the most important aspect of our comparative analysis, we observed four significant AUCs: GFAP_1S: 0.845 (p=0.005), GFAP_24S: 0.851 (p=0.004), UCH-L1_24S: 0.768 (p=0.028), and SBDP145_1C: 0.744 (p=0.045).
Table 3.
Area Under the Curve (AUC) from Receiver-Operating Characteristic Curve Analysis of the Predictive Potential of Biomarkers for 6-Month Unfavorable Outcome
| |
AUC mortality (p value) |
AUC unfavorable outcome (p value) |
||||
|---|---|---|---|---|---|---|
| Biomarker | 1 week | 4 weeks | 6 months | 1 week | 4 weeks | 6 months |
| GFAP_1C | 0.697 (0.072) | 0.662 (0.150) | 0.667 (0.172) | 0.558 (0.667) | 0.633 (0.356) | 0.608 (0.453) |
| GFAP_1S | 0.889 (<0.001) | 0.833 (0.003) | 0.845 (0.005) | 0.826 (0.015) | 0.850 (0.015) | 0.867 (0.011) |
| GFAP_24C | 0.707 (0.059) | 0.672 (0.126) | 0.661 (0.188) | 0.601 (0.451) | 0.667 (0.248) | 0.642 (0.326) |
| GFAP_24S | 0.861 (0.001) | 0.838 (0.003) | 0.851 (0.004) | 0.848 (0.010) | 0.875 (0.009) | 0.883 (0.008) |
| UCH-L1_1C | 0.683 (0.096) | 0.535 (0.753) | 0.542 (0.733) | 0.725 (0.095) | 0.708 (0.149) | 0.675 (0.225) |
| UCH-L1_1S | 0.841 (0.002) | 0.788 (0.010) | 0.720 (0.071) | 0.790 (0.031) | 0.858 (0.013) | 0.725 (0.119) |
| UCH-L1_24C | 0.697 (0.072) | 0.576 (0.500) | 0.560 (0.626) | 0.768 (0.046) | 0.783 (0.050) | 0.708 (0.149) |
| UCH-L1_24S | 0.880 (0.001) | 0.833 (0.003) | 0.768 (0.028) | 0.833 (0.013) | 0.892 (0.007) | 0.767 (0.065) |
| SBDP145_1C | 0.615 (0.293) | 0.631 (0.243) | 0.744 (0.045) | 0.667 (0.216) | 0.575 (0.603) | 0.717 (0.133) |
| SBDP145_24C | 0.625 (0.254) | 0.626 (0.261) | 0.702 (0.097) | 0.710 (0.118) | 0.742 (0.094) | 0.725 (0.119) |
GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin C-terminal hydrolase-L1; SBDP145, 145-kDa αII-spectrin breakdown product; 1S, first measured level in serum; 1C, first measured level in cerebrospinal fluid; 24S, average of all measured serum levels within 24 h post-injury; 24C, average of all measured cerebrospinal fluid levels within 24 h post-injury.
When considering unfavorable outcome as the end-point we found significant AUCs for GFAP_1S, UCH-L1_1S, GFAP_24S, UCH-L1_24S, and UCH-L1_24C (Table 3). We observed significant AUCs for only two biomarkers with regard to 6-month unfavorable outcome: GFAP_1S: 0.867 (p=0.011) and GFAP_24S: 0.883 (p=0.008).
Optimal threshold levels were calculated for each biomarker that showed significant AUC. Table 4 shows the threshold levels of the biomarkers related to the 6-month outcome and their related specificity and sensitivity.
Table 4.
Threshold Levels for Biomarkers with Significant Area Under the Curve and the Relationship of the Core Model of the IMPACT Calculator and Biomarker-Based Outcomes to the Observed 6-Month Mortality and Unfavorable Outcomes as Assessed by Univariate Logistic Regression Analysis
| Biomarker | Threshold | Sensitivity | Specificity | OR (95% CI) | p | R2 |
|---|---|---|---|---|---|---|
| 6-Month mortality | ||||||
| GFAP_1S | 0.546 | 0.619 | 0.875 | 3.571 (0.812–15.714) | 0.092 | 0.102 |
| GFAP_24S | 0.529 | 0.667 | 1.000 | 12.800* (1.479–110.789) | 0.021 | 0.255 |
| UCH-L1_24S | 1.279 | 0.619 | 0.875 | nc | nc | Nc |
| SBDP145_1C | 20.905 | 0.571 | 0.750 | 4.444* (1.058–18.667) | 0.042 | 0.146 |
| Core model | 2.303* (1.178–4.506) | 0.015 | 0.214 | |||
| 6-Month unfavorable outcome | ||||||
| GFAP_1S | 0.220 | 0.583 | 1.000 | 2.111 (0.433–10.284) | 0.355 | 0.033 |
| GFAP_24S | 0.195 | 0.583 | 1.000 | 11.000* (1.219–99.258) | 0.033 | 0.233 |
| Core model | 1.895 (0.969–3.706) | 0.062 | 0.138 | |||
OR, odds ratio (significant ORs indicated by asterisks); CI, confidence interval; nc, not calculable; GFAP: glial fibrillary acidic protein; UCH-L1, ubiquitin C-terminal hydrolase-L1; SBDP145, 145-kDa αII-spectrin breakdown product; 1S, first measured level in serum; 1C, first measured level in cerebrospinal fluid; 24S, average of all measured serum levels within 24 h post-injury.
Univariate logistic regression
The predictive value for 6-month lethal and unfavorable outcomes of those biomarkers with significant AUCs were tested using univariate logistic regression. For the prediction of 6-month lethality two biomarkers (OR=12.800, p=0.021 for GFAP_24S; OR=4.444, p=0.042 for SBDP145_1C) showed significant predictive power. GFAP_1S was not significant (OR=3.571, p=0.092), and in the case of UCH-L1_24S, the OR was not calculable. However, GFAP_24S proved to be the only biomarker with a significant relationship with 6-month unfavorable outcome. The association between the 6-month outcomes calculated using the IMPACT core model alone and the observed outcomes were significant for mortality, and nearly significant for unfavorable outcome (OR=2.303, p=0.015 for lethality, and OR=1.895, p=0.062 for unfavorable outcome; Table 4).
Combined predictive models
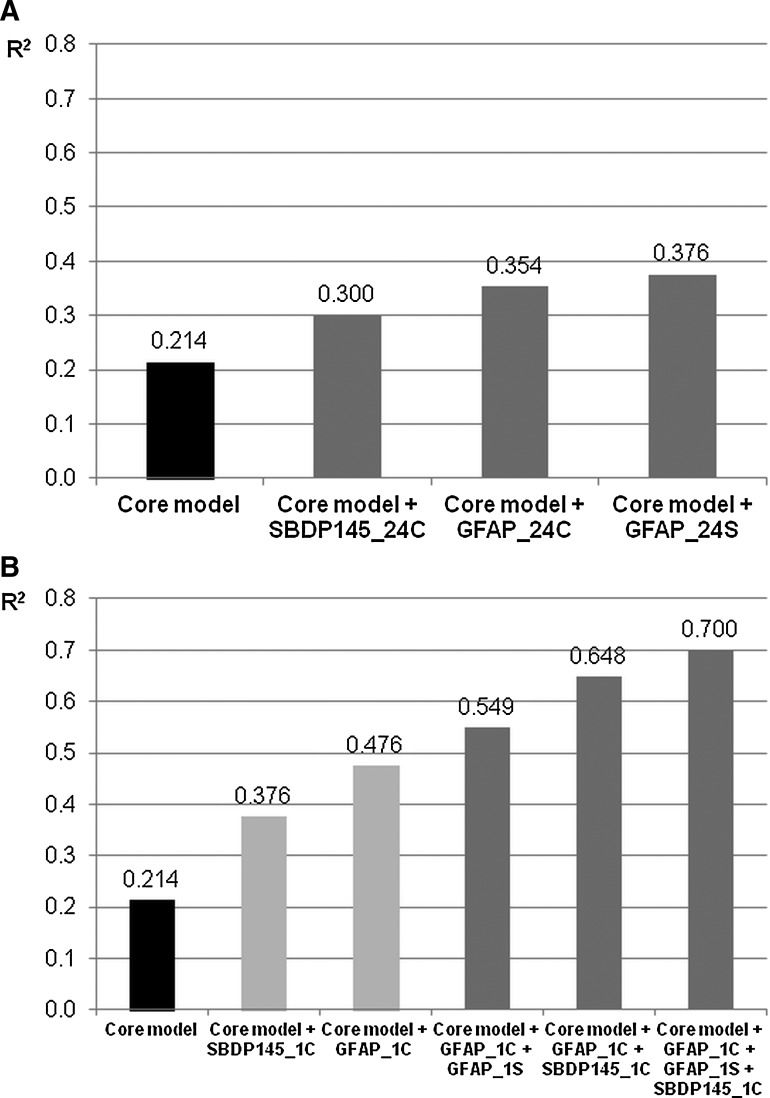
Upon univariate analysis the core model of the prognostic calculator reached an R2 value of 0.214 for predicting 6-month mortality (Table 4). In an attempt to increase the predictive power in terms of R2 values, multivariate logistic regression models were utilized with the addition of one or more biomarker (Fig. 1A and B). Although some of the investigated biomarkers (GFAP_24S, GFAP_24C, SBDP145_24C, GFAP_1C, and SBDP145_1C) were able to increase the predictive power in bivariate models, GFAP_1C was found to be the most effective, increasing R2 from 0.214 to 0.476. Upon multivariate analysis, the IMPACT core model+GFAP_1C+GFAP_1S+SBDP145_1C performed the best, with an R2 level of 0.70.
FIG. 1.
Predictive power of combined models for 6-month lethal outcome by multivariate logistic regression analysis. (A) Core model+the average level of a biomarker over the first 24 h post-injury. (B) Core model+one or more first available biomarker levels (GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin C-terminal hydrolase-L1; SBDP145, 145-kDa αII-spectrin breakdown product; 1S, first measured level in serum; 1C, first measured level in cerebrospinal fluid; 24S, average of all measured serum levels within 24 h post-injury; 24C: average of all measured cerebrospinal fluid levels within 24 h post-injury).
In the case of unfavorable outcome, we only observed a slight increase in R2 values with the application of GFAP_24S in a bivariate model, increasing the level from 0.138 to 0.233.
Discussion
Our results corroborate previous findings on the predictive power of the IMPACT calculator, defining it as a useful clinical tool in the prediction of outcomes in the head injured. Specifically, we have demonstrated that the assumptions made by the core model of the IMPACT calculator were significantly associated with 6-month mortality, and were closely related to unfavorable outcome. Similarly, our findings reinforce recent observations regarding the predictive power of our three biomarker candidates (GFAP, UCH-L1, SBDP145), demonstrating that their levels and the outcomes of patients with sTBI are statistically linked.
The most important and novel finding of our work highlights that in a multivariate logistic regression analysis, the addition of biomarker levels can improve the predictive power of the IMPACT calculator. To the best of our knowledge this is the first report demonstrating the feasibility and sensitivity of this combined approach to outcome prediction to aid in the care of those with TBI.
Our observations provide further evidence about the significant independent predictive power of the biomarkers we have been analyzing in recent collaborative efforts (Brophy et al., 2011; Mondello et al., 2011). Intriguingly, when threshold values of these biomarkers are compared with the minimum and mean values of the biomarkers summarized in Table 2, it is impressive that they are able to provide accurate outcome prediction at low levels. It is of note that two of the biomarkers (GFAP and UCH-L1) that we have statistically linked both via AUC and logistic regression analysis to the outcomes in severe TBI were measured in serum samples, indicating that these two proteins may be clinically useful surrogate markers of brain injury of traumatic origin (Mondello et al., 2010; Papa et al., 2010; Svetlov et al., 2009). As current practice guidelines and protocols in severe TBI favor CSF sampling via ventricular catheters (Bratton et al., 2007), similar clinical importance could be attributed to the finding that levels of SBDP145 in the first available CSF sample had significant predictive power of 6-month mortality in both statistical models.
Outcome prediction is crucial to effective patient care in TBI. The information provided to the relatives should be based on solid clinical as well as scientific evidence. This will not only help them prepare for the future, but also facilitates understanding of the indications for the risky and potentially painful interventions required in these patients. Predictive systems may also aid with quality assurance, providing a useful means of assessing patient care, which can facilitate comparisons of the care provided by different institutions, or of differing departments in a single institution.
Prognostic calculators can also play a major role in the design of clinical trials, and will aid in the construction of successful clinical studies to identify novel treatment strategies to improve the care of TBI patients. As ongoing clinical trials and translational research may lead to the construction of rapid, point-of-care biomarker assays, prognostic models including biomarker panels may serve as a future enrollment tool. This is particularly important, as many recent studies have highlighted our failure to identify novel therapeutic agents due to a lack of pathophysiologically accurate and reliable assessment tools (Perel et al., 2008; Steyerberg et al., 2008). Indeed, our most important enrollment tool, the GCS motor score, does not provide any information on the pathobiology or the morphological alterations in the injured brain, and thus many different conditions may have the same GCS motor score (Saatman et al., 2008). Further, state-of-the-art treatment protocols include early sedation and the use of muscle-relaxants in the prehospital setting, which makes reliable post-resuscitation GCS scores difficult to obtain. Effective prognostic calculators could also identify patients whose inclusion in clinical trials would be of little benefit, as they would likely end up at the two extremes of the outcome scale regardless of treatment (Menon and Zahed 2009).
A set of recent articles underscore that automated outcome calculation is feasible and should be a useful tool in clinical practice (Lingsma et al., 2010; Maas et al., 2010). There are two online outcome calculators available, and both of them are based on admission characteristics and they have been cross-validated. One is derived from the IMPACT database (Marmarou et al., 2007), and contains three models: the core model (age, GCS motor score, and reaction of pupils), the extended model (core+hypoxia, hypotension, CT characteristics [Marshall CT classification], tSAH, and EDH), and the lab model (extended+glucose and hemoglobin levels), superimposed upon each other (Steyerberg et al., 2008). The other, which is based on the MRC CRASH trial, involves country, age, GCS score, the reaction of pupils, the presence of major extra-cranial injuries, and some CT characteristics depending on the availability of a CT scan (Perel et al., 2008). The collection of clinical data in the centers participating in this work was not designed to allow assessment of outcome calculators, and thus we only were able to utilize the core model of the IMPACT calculator. Nevertheless, our results are in accord with previous evidence indicating that outcome prediction is feasible and reliable with the IMPACT calculator, and that biomarkers may make this tool even more accurate.
The future role biomarkers may play in the care of TBI patients has been detailed in various recent articles (Dash et al., 2010; Hergenroeder et al., 2008). Although S-100β, NSE, and other candidate biomarkers have failed to lead to a breakthrough in this field (Pelinka et al., 2005; Piazza et al., 2007; Svetlov et al., 2009), we believe that our most recent investigations have identified a new set of biomarkers that can be linked to pathobiological processes (calpain- and caspase-mediated spectrin breakdown) and/or brain constituents (UCHL-1 and GFAP), that are indicative of traumatically-evoked brain damage (Brophy et al., 2011; Mondello et al., 2010,2011). The results detailed here of how these biomarkers aided in outcome prediction should spur further research to explore the potential of these substances.
Although our results clearly indicate the important role biomarkers may play in models and calculators used to establish the prognosis of TBI patients, we must proceed with caution in the interpretation of our data. The first limiting factor is the relatively low number of patients enrolled, which forced us to make compromises during our statistical analysis. To this end we had to dichotomize the biomarker values with the application of post-hoc-determined threshold values instead of utilizing them as a continuous variable, an approach that precludes any reliable internal validation. The relatively low proportion of patients with favorable 6-month outcomes may also exaggerate the predictive power of the models applied.
Second, while here we aimed to demonstrate the potential prognostic power of biomarker levels in addition to the use of admission criteria, the technology and the methodology utilized here to assess biomarker levels cannot be considered an admission parameter, which is another limitation of our results.
Third, although the high percentage of patients with less favorable outcomes according to classical dichotomization for those assessed at 6 months could have benefitted from utilization of the sliding dichotomy model described by Murray and associates (Murray et al., 2005), we decided to assess outcomes on the basis of conventional dichotomization. This approach was selected to be consistent with the analyses provided by the other outcome calculators.
On the basis of the clinical data on hand, we decided to analyze our data exclusively in light of the results obtainable via the IMPACT calculator. Nevertheless, our candidate biomarkers should soon be tested in conjunction with the CRASH outcome calculator, to assess whether they can improve the prediction of 2-week mortality as well. To this end, on the basis of the similarity between the results and efficacy of these two outcome calculators, we foresee that the results achieved in this study will be reproduced in other experiments.
It is also of note that biomarkers and their use with outcome calculators may also be subject to other limitations, such as the false-positive (non-TBI-related) detection of biomarkers associated with neurodegenerative diseases and/or stroke (Gong and Leznik 2007; Herrmann et al., 2000; Middeldorp and Hol, 2011), as well as the increasing complexity of these calculators, which may be difficult to handle in the acute clinical setting.
While the above limitations underscore the preliminary nature of our findings, we hope that this work will highlight the importance of combining state-of-the-art outcome prediction models with biomarker analysis, and will set the stage for the application of this approach in future large-scale clinical trials. We believe that our results will pave the way for tools that connect basic science and clinical research with pathobiology-driven and clinical data-based decision making, which will ultimately improve the care of the head-injured patient.
Acknowledgments
The authors would like to thank the staff personnel at the study sites who participated in the enrollment of the patients, and facilitated serum and CSF sampling as well as clinical data collection, for their valuable assistance.
This study was primarily sponsored by Department of Defense Award numbers DAMD17-03-1-0772 and DAMD17-03-1-0066; we also acknowledge additional funding support from the National Institutes of Health (R01-NS049175-01, R01-NS052831-01, and R01-NS051431-01), Navy grant no. N00014-06-1-1029 (University of Florida), and Developing Competitiveness of Universities in the South Transdanubian Region (SROP-4.2.1.B-10/2/KONV-2010-0002), as well as the MTA-PTE Clinical Neuroscience MR Research Group and ETT grant no. 269/2009.
Author Disclosure Statement
Drs. Czeiter, Mondello, Kovacs, Gabrielli, Barzo, Ezer, and Buki are consultants for Banyan Biomarkers, Inc., and received consulting fees. Drs. Wang and Hayes own stock, receive royalties from, and are officers of Banyan Biomarkers Inc., and as such may benefit financially as a result of the outcomes of this research. No competing financial interests exist for Drs. Sandor, Schmid, Tortella, and Doczi.
References
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Bratton S.L. Chestnut R.M. Ghajar J. McConnell Hammond F.F. Harris O.A. Hartl R. Manley G.T. Nemecek A. Newell D.W. Rosenthal G. Schouten J. Shutter L. Timmons S.D. Ullman J.S. Videtta W. Wilberger J.E. Wright D.W. Guidelines for the management of severe traumatic brain injury. VII. Intracranial pressure monitoring technology. J. Neurotrauma. 2007;24(Suppl. 1):S45–S54. doi: 10.1089/neu.2007.9989. [DOI] [PubMed] [Google Scholar]
- Brophy G.M. Mondello S. Papa L. Robicsek S.A. Gabrielli A. Tepas J. Buki A. Robertson C. Tortella F.C. Hayes R.L. Wang K.K. Biokinetic analysis of ubiquitin c-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. J. Neurotrauma. 2011;28:861–870. doi: 10.1089/neu.2010.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy G.M. Pineda J.A. Papa L. Lewis S.B. Valadka A.B. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Tepas J.J., 3rd Gabrielli A. Robicsek S. Wang K.K. Robertson C.S. Hayes R.L. alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J. Neurotrauma. 2009;26:471–479. doi: 10.1089/neu.2008.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P.K. Zhao J. Hergenroeder G. Moore A.N. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics. 2010;7:100–114. doi: 10.1016/j.nurt.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas O. Polgar B. Szekeres-Bartho J. Doczi T. Povlishock J.T. Buki A. Spectrin breakdown products in the cerebrospinal fluid in severe head injury—preliminary observations. Acta Neurochir. (Wien.) 2005;147:855–861. doi: 10.1007/s00701-005-0559-6. [DOI] [PubMed] [Google Scholar]
- Gong B. Leznik E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 2007;20:365–370. doi: 10.1358/dnp.2007.20.6.1138160. [DOI] [PubMed] [Google Scholar]
- Hergenroeder G.W. Redell J.B. Moore A.N. Dash P.K. Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol. Diagn. Ther. 2008;12:345–358. doi: 10.1007/BF03256301. [DOI] [PubMed] [Google Scholar]
- Herrmann M. Vos P. Wunderlich M.T. de Bruijn C.H. Lamers K.J. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31:2670–2677. doi: 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- Honda M. Tsuruta R. Kaneko T. Kasaoka S. Yagi T. Todani M. Fujita M. Izumi T. Maekawa T. Serum glial fibrillary acidic protein is a highly specific biomarker for traumatic brain injury in humans compared with S-100B and neuron-specific enolase. J. Trauma. 2010;69:104–109. doi: 10.1097/TA.0b013e3181bbd485. [DOI] [PubMed] [Google Scholar]
- Hukkelhoven C.W. Steyerberg E.W. Habbema J.D. Farace E. Marmarou A. Murray G.D. Marshall L.F. Maas A.I. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J. Neurotrauma. 2005;22:1025–1039. doi: 10.1089/neu.2005.22.1025. [DOI] [PubMed] [Google Scholar]
- Kovesdi E. Luckl J. Bukovics P. Farkas O. Pal J. Czeiter E. Szellar D. Doczi T. Komoly S. Buki A. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir. (Wien.) 2010;152:1–17. doi: 10.1007/s00701-009-0463-6. [DOI] [PubMed] [Google Scholar]
- Langlois J. Rutland-Brown W. Thomas K. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta: 2006. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. [Google Scholar]
- Lingsma H.F. Roozenbeek B. Steyerberg E.W. Murray G.D. Maas A.I. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9:543–554. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- Liu M.C. Akinyi L. Scharf D. Mo J. Larner S.F. Muller U. Oli M.W. Zheng W. Kobeissy F. Papa L. Lu X.C. Dave J.R. Tortella F.C. Hayes R.L. Wang K.K. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 2010;31:722–732. doi: 10.1111/j.1460-9568.2010.07097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkins K.M. Bochicchio G.V. Keledjian K. Simard J.M. McCunn M. Scalea T. Glial fibrillary acidic protein is highly correlated with brain injury. J. Trauma. 2008;65:778–782. doi: 10.1097/TA.0b013e318185db2d. discussion 782–774. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Hukkelhoven C.W. Marshall L.F. Steyerberg E.W. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–1182. doi: 10.1227/01.neu.0000186013.63046.6b. discussion 1173–1182. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Steyerberg E.W. Marmarou A. McHugh G.S. Lingsma H.F. Butcher I. Lu J. Weir J. Roozenbeek B. Murray G.D. IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics. 2010;7:127–134. doi: 10.1016/j.nurt.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmarou A. Lu J. Butcher I. McHugh G.S. Mushkudiani N.A. Murray G.D. Steyerberg E.W. Maas A.I. IMPACT database of traumatic brain injury: design and description. J. Neurotrauma. 2007;24:239–250. doi: 10.1089/neu.2006.0036. [DOI] [PubMed] [Google Scholar]
- Marshall L.F. Marshall S.B. Klauber M.R. Van Berkum Clark M. Eisenberg H. Jane J.A. Luerssen T.G. Marmarou A. Foulkes M.A. The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma. 1992;9(Suppl. 1):S287–S292. [PubMed] [Google Scholar]
- Menon D.K. Zahed C. Prediction of outcome in severe traumatic brain injury. Curr. Opin. Crit. Care. 2009;15:437–441. doi: 10.1097/MCC.0b013e3283307a26. [DOI] [PubMed] [Google Scholar]
- Middeldorp J. Hol E.M. GFAP in health and disease. Prog. Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Mondello S. Papa L. Buki A. Bullock R. Czeiter E. Tortella F. Wang K.K. Hayes R.L. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit. Care. 2011;15:R156. doi: 10.1186/cc10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S. Robicsek S.A. Gabrielli A. Brophy G.M. Papa L. Tepas J. Robertson C. Buki A. Scharf D. Jixiang M. Akinyi L. Muller U. Wang K.K. Hayes R.L. alphaII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J. Neurotrauma. 2010;27:1203–1213. doi: 10.1089/neu.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J. Lopez A.D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Murray G.D. Barer D. Choi S. Fernandes H. Gregson B. Lees K.R. Maas AI. Marmarou A. Mendelow A.D. Steyerberg E.W. Taylor G.S. Teasdale G.M. Weir C.J. Design and analysis of phase III trials with ordered outcome scales: the concept of the sliding dichotomy. J. Neurotrauma. 2005;22:511–517. doi: 10.1089/neu.2005.22.511. [DOI] [PubMed] [Google Scholar]
- Mushkudiani N.A. Hukkelhoven C.W. Hernandez A.V. Murray G.D. Choi S.C. Maas A.I. Steyerberg E.W. A systematic review finds methodological improvements necessary for prognostic models in determining traumatic brain injury outcomes. J. Clin. Epidemiol. 2008;61:331–343. doi: 10.1016/j.jclinepi.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Papa L. Akinyi L. Liu M.C. Pineda J.A. Tepas J.J., 3rd Oli M.W. Zheng W. Robinson G. Robicsek S.A. Gabrielli A. Heaton S.C. Hannay H.J. Demery J.A. Brophy G.M. Layon J. Robertson C.S. Hayes R.L. Wang K.K. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 2010;38:138–144. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelinka L.E. Hertz H. Mauritz W. Harada N. Jafarmadar M. Albrecht M. Redl H. Bahrami S. Nonspecific increase of systemic neuron-specific enolase after trauma: clinical and experimental findings. Shock. 2005;24:119–123. doi: 10.1097/01.shk.0000168876.68154.43. [DOI] [PubMed] [Google Scholar]
- Pelinka L.E. Kroepfl A. Leixnering M. Buchinger W. Raabe A. Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma. 2004;21:1553–1561. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- Perel P. Arango M. Clayton T. Edwards P. Komolafe E. Poccock S. Roberts I. Shakur H. Steyerberg E. Yutthakasemsunt S. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza O. Storti M.P. Cotena S. Stoppa F. Perrotta D. Esposito G. Pirozzi N. Tufano R. S100B is not a reliable prognostic index in paediatric TBI. Pediatr. Neurosurg. 2007;43:258–264. doi: 10.1159/000103304. [DOI] [PubMed] [Google Scholar]
- Pineda J.A. Lewis S.B. Valadka A.B. Papa L. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Aikman J.M. Akle V. Brophy G.M. Tepas J.J. Wang K.K. Robertson C.S. Hayes R.L. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J.B. Update on the pathogenesis of Parkinson's disease. J. Neurol. 2008;255(Suppl. 5):3–7. doi: 10.1007/s00415-008-5011-4. [DOI] [PubMed] [Google Scholar]
- Steyerberg E.W. Mushkudiani N. Perel P. Butcher I. Lu J. McHugh G.S. Murray G.D. Marmarou A. Roberts I. Habbema J.D. Maas A.I. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165. doi: 10.1371/journal.pmed.0050165. discussion e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlov S.I. Larner S.F. Kirk D.R. Atkinson J. Hayes R.L. Wang K.K. Biomarkers of blast-induced neurotrauma: profiling molecular and cellular mechanisms of blast brain injury. J. Neurotrauma. 2009;26:913–921. doi: 10.1089/neu.2008.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferri F. Compagnone C. Korsic M. Servadei F. Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. (Wien.) 2006;148:255–268. doi: 10.1007/s00701-005-0651-y. discussion 268. [DOI] [PubMed] [Google Scholar]
- Vos P.E. Jacobs B. Andriessen T.M. Lamers K.J. Borm G.F. Beems T. Edwards M. Rosmalen C.F. Vissers J.L. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]