Abstract
Background
The purpose of this multicenter study was to investigate the accuracy of a real-time continuous glucose monitoring sensor in Chinese diabetes patients.
Subjects and Methods
In total, 48 patients with type 1 or 2 diabetes from three centers in China were included in the study. The MiniMed Paradigm® 722 insulin pump (Medtronic, Northridge, CA) was used to monitor the real-time continuous changes of blood glucose levels for three successive days. Venous blood of the subjects was randomly collected every 15 min for seven consecutive hours on the day when the subjects were wearing the sensor. Reference values were provided by the YSI® 2300 STAT PLUS™ glucose and lactate analyzer (YSI Life Sciences, Yellow Springs, OH).
Results
In total, 1,317 paired YSI–sensor values were collected from the 48 patients. Of the sensor readings, 88.3% (95% confidence interval, 0.84–0.92) were within±20% of the YSI values, and 95.7% were within±30% of the YSI values. Clarke and consensus error grid analyses showed that the ratios of the YSI–sensor values in Zone A to the values in Zone B were 99.1% and 99.9%, respectively. Continuous error grid analysis showed that the ratios of the YSI–sensor values in the region of accurate reading, benign errors, and erroneous reading were 96.4%, 1.8%, and 1.8%, respectively. The mean absolute relative difference (ARD) for all subjects was 10.4%, and the median ARD was 7.8%. Bland–Altman analysis detected a mean blood glucose level of 3.84 mg/dL. Trend analysis revealed that 86.1% of the difference of the rates of change between the YSI values and the sensor readings occurred within the range of 1 mg/dL/min.
Conclusions
The Paradigm insulin pump has high accuracy in both monitoring the real-time continuous changes and predicting the trend of changes in blood glucose level. However, actual clinical manifestations should be taken into account for diagnosis of hypoglycemia.
Introduction
Blood glucose monitoring is essential to assess glucose metabolism disorder and the efficacy of associated treatments.1 In humans, because blood glucose levels continuously fluctuate, routine determination of blood glucose levels at different time points does not reflect the fluctuation of blood glucose levels over the course of the day. As a result, continuous glucose monitoring (CGM) is extremely advantageous. CGM is a novel technique that only recently has been gradually applied in clinical practice to monitor blood glucose levels. By using a subcutaneously implanted glucose sensor to measure the glucose level in the interstitial fluid, the technique allows assessment of fluctuating blood glucose levels and occult hypoglycemia and hyperglycemia in addition to routine determination.
Currently, the CGM technique is categorized as either professional CGM (retrospective CGM system) or individual CGM (real-time [RT] CGM system). The RT-CGM system provides instant information about the blood glucose level. In addition, it has alert and forecasting functions that help to instantly regulate blood glucose levels.2 Previous studies of evidence-based medicine have supported the usefulness of the RT-CGM system in clinical practice.3,4 One report showed that the hemoglobin A1c levels were markedly reduced in patients 25 years of age or older who received intensive therapy for type 1 diabetes, and patients favored CGM over performing home monitoring with a blood glucose meter.5 Bergenstal et al.6 found that sensor-augmented pump therapy for the treatment of type 1 diabetes mellitus resulted in a significant decrease in hemoglobin A1c levels and a greater proportion of patients who reached the hemoglobin A1c target compared with those on a regimen of multiple daily insulin injections. Ehrhardt et al.7 found that RT-CGM significantly improved hemoglobin A1c compared with the self-monitoring of blood glucose in patients with type 2 diabetes mellitus.
In these studies, the accuracy of sensor readings is a critical factor that impacts the benefit to the patients. Only sensor readings that accurately reflect the blood glucose level can instruct clinicians and patients for better control of blood glucose levels. The venous blood glucose level is the gold standard for assessing the accuracy of CGM.8–10 However, the current standard for assessing the accuracy of CGM data in the Chinese population is mainly based on self-monitoring of blood glucose.11 Few studies have performed systematic evaluation of blood glucose levels using frequent venous blood collection in patients with diabetes mellitus. In particular, the accuracy of RT-CGM in Chinese populations remains unclear. Therefore, the current multicenter study was conducted to investigate the accuracy and safety of RT-CGM in Chinese diabetes subjects.
Subjects and Methods
Subjects
The present study was a multicenter, prospective, single-sample study. In total, 48 participants were collected from three hospitals in China, and the study period was between August and October 2010. The sample size of each center was as follows: 15 participants from the Shanghai Jiao Tong University Affiliated Sixth People's Hospital, 18 participants from the General Hospital of Beijing Military Area, and 15 participants from the Chinese PLA General Hospital. Those patients who met the following criteria were included in the present study: (1) 18–75 years old, (2) diagnosed as having diabetes who were willing to perform the study and complete the data collection (they complied with the specifications of instruments, including the MiniMed Paradigm® 722 insulin pump [Medtronic, Northridge, CA], MiniLink signal transmitter [Medtronic], and glucose sensor; (3) accepted capillary blood glucose tests at least four times a day; and (4) in stable condition prior to the study. Those who met the following criteria were excluded: (1) pregnant or planning to become pregnant; (2) a history of adhesive tape allergy; (3) skin abnormalities, like psoriasis, rash, or Staphylococcus aureus infections, that hindered the sensor-wearing; and (4) patients whose conditions were extremely severe and not stable. This study was independently approved by the ethics committee of each participating hospital. All subjects gave and signed written informed consent before study initiation.
Experimental procedure
All subjects received RT-CGM for three successive days. The Paradigm 722 insulin pump, subcutaneous glucose sensor, and MiniLink signal transmitter were connected to the participants for 3 days. The glucose sensor was implanted into the abdominal subcutaneous tissues of the participants and connected to the MiniLink signal transmitter to transmit the RT data to the pump. However, the infusion of the insulin pump was not enabled. During the period of monitoring, fingertip blood glucose levels were tested using the OneTouch® blood glucose meter (LifeScan Inc., a Johnson & Johnson Company, Milpitas, CA) at least four times daily and then were inputted into the insulin pump for calibration. The venous blood of the subjects was randomly collected every 15 min for seven consecutive hours on the day when subjects wore the sensor. It was tested using the YSI® 2300 STAT PLUS™ glucose and lactate analyzer (YSI Life Sciences, Yellow Springs, OH). Any adverse events were noted during the period of blood glucose monitoring.
Data collection
All data from the study were collected using Oracle Remote Data Capture (Oracle Corp., Redwood, CA). The electronic data stored in the Paradigm 722 insulin pump were exported using CareLink software (Medtronic).
Accuracy analysis of sensor
Statistical analysis of the accuracy of the sensor included primary analysis, secondary analysis, and trend analysis. The YSI's plasma glucose level (YSI value) was selected as the reference blood glucose level, and each YSI value was paired with the corresponding glucose sensor reading (sensor reading).
Primary analysis indicated the agreement between the sensor readings and YSI values at a deviation of 20%, which was calculated using the following formula: Rate of agreement (%)=(number of the paired sensors within±20% agreement of the YSI values/total number of the paired sensors)×100%. The statistical critical value of the agreement at the deviation of 20% was 59.52%.
Secondary analysis included the following statistical methods: (1) Error grid analysis. The confidence interval of Zone A plus Zone B was calculated following the report published by Clarke et al.12 in 1987. In addition, consensus error grid analysis and continuous error grid analysis were used in the current study. (2) Absolute relative difference (ARD) is the ratio of the absolute difference between the sensor reading and the YSI value to the YSI value. It was calculated each day during the study period, and the ARD for the whole study period was also analyzed. (3) Correlation analysis, a descriptive parameter, was used to assess the correlation between the sensor reading and YSI value. (4) Linear models were used to assess the bias between the sensor reading and the YSI value. When the slope of the linear curve was 1 and the intercept was 0, there was no bias between the sensor reading and YSI value. (5) Bland–Altman analysis. The Bland–Altman scatter plot based on the mean value of the YSI value and sensor reading (horizontal coordinate) and the difference between paired YSI–sensor values (vertical coordinate) was used to assess the relationship between the bias and blood glucose level.
Trend analysis was used to observe the changes in YSI value and sensor reading at different initial concentrations of glucose. The rate of change (ROC) of reference YSI values was defined as the change of YSI value per unit time (less than 20 min). The ROC of the sensor reading was defined as the change of sensor reading per unit time (less than 20 min). In the present study, we investigated the ROC of YSI value and sensor reading at different initial concentrations of glucose. Moreover, we observed the absolute difference of ROC of YSI value and sensor reading and the frequency of the various differences.
The sample size selected was based on the primary analysis of the primary effectiveness endpoint. A sample of approximately 42 adult subjects provided a total of approximately 392 paired measures for each of the three testing days, making a total of 1,176 paired measurements. A simulation was performed 1,000 times, and the lower boundary of the intercept of the null model was tested against the critical value of 0.5952. The results of the simulation indicated that a sample size of 42 yielded a power of 90% to demonstrate that the overall agreement rate was not inferior to 0.5952.
Safety analysis
The descriptive statistics were used to describe the security events. Adverse events were monitored each day. All moderate to serious adverse events associated with the instruments and operating instructions were reported to the sponsors via the electronic case report form. All serious adverse events and non-expected instrument-related adverse events were also reported to the sponsors via the electronic case report form. In addition, the skin of the subjects who used sensors was evaluated.
Statistical analysis
All data were entered in Excel (Microsoft Corp., Redmond, WA), and all statistical analyses were performed using the statistical software SAS version 9.2 (SAS Institute, Cary, NC).
Results
In total, 48 patients with diabetes completed the whole study, including four patients with type 1 diabetes and 44 patients with type 2 diabetes. Females represented 35.4% and males represented 64.6% of total subjects. The patients' mean age was 48.5±12.2 years old, and the mean body mass index was 25.4±3.5 kg/m2.
Agreement of the paired value
In total, 1,317 paired YSI–sensor values were collected from the 48 patients. On Days 1–3, there were 454, 440, and 423 values, respectively. Of the sensor readings, 88.3% (95% confidence interval 0.84–0.92) were within±20% of the YSI values (when the blood glucose level was within the range of hypoglycemia, the deviation was defined as less than 20 mg/dL), which reached the goal of preset accuracy. The rates of agreement were 84.8%, 87.5%, and 92.9%, respectively, on Days 1–3 (Table 1). When 30% deviation (when the blood glucose level was within the range of hypoglycemia, the deviation was defined as less than 30 mg/dL) was selected as the standard, the accuracy of the sensor was further improved, and 95.7% of the sensor readings were within±30% of the YSI values. If the range of blood glucose levels was partitioned, the deviation between the sensor reading and YSI value decreased, and the rate of agreement increased with the increment of the blood glucose level within the deviation of±20% or 30%. The highest rate of agreement occurred in the 240–400 mg/dL range, where 97.3% of sensor readings were within±20% of YSI values and 100% of sensor readings were within±30% of YSI values.
Table 1.
Agreement of Paired YSI–Sensor Values Within 20% (or 20 mg/dL in the 40–80 mg/dL Range) by Sensor Day, Real-Time Algorithm, and Percentage
| |
Number in agreement per total readings [% (n/N)] |
|||
|---|---|---|---|---|
| Reference YSI value range | All ranges | Day 1 | Day 2 | Day 3 |
| 40–80 mg/dL | 50.0 (13/26) | 0.0 (0/7) | 50.0 (4/8) | 81.8 (9/11) |
| >80–120 mg/dL | 82.3 (219/266) | 76.1 (54/71) | 86.5 (96/111) | 82.1 (69/84) |
| >120–240 mg/dL | 89.7 (785/875) | 86.3 (270/313) | 87.9 (246/280) | 95.4 (269/282) |
| >240–400 mg/dl | 97.3 (146/150) | 96.8 (61/63) | 95.1 (39/41) | 100.0 (46/46) |
| Overall | 88.3 (1,163/1,317) | 84.8 (385/454) | 87.5 (385/440) | 92.9 (393/423) |
n/N, number of paired values/total paired values.
Clarke error grid analysis
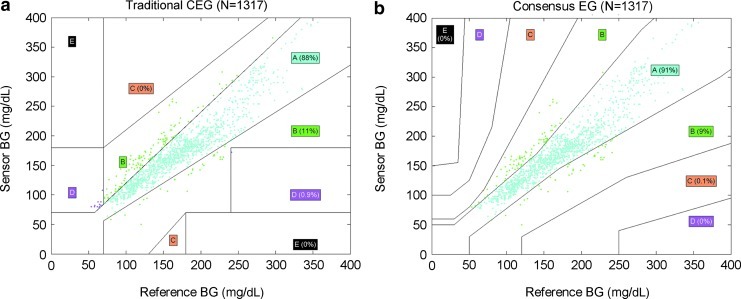
Clarke error grid analysis was conducted in 1,317 paired values. The scatter plot was created based on the YSI values (horizontal coordinate) and sensor readings (vertical coordinate), and the Clarke error grid was created. The results showed that 99.1% of the paired YSI–sensor values fell within Zones A and B (87.8% within Zone A and 11.3% within Zone B) (Fig. 1a). The accuracy of the sensor changed with the range of blood glucose level. When the blood glucose level was more than 120 mg/dL, more than 89.7% of the paired values fell within Zone A. Additionally, 23.1% and 82.3% of the paired values fell within Zone A in the 40–80 mg/dL and 80–120 mg/dL ranges, respectively (Table 2).
FIG. 1.
(a) Clark Error Grid (CEG) analysis of blood glucose (BG) measurements. Each grid shows the comparison of sensor values on the y-axis with BG values obtained with reference method on the x-axis. (b) Consensus Error Grid (EG) analysis of BG measurements. Each grid shows the comparison of sensor values on the y-axis with BG values obtained with reference method on the x-axis.
Table 2.
Clarke Error Grid Analysis of Paired YSI–Sensor Glucose Values at Different Blood Glucose Levels by Real-Time Algorithm
| |
Reference blood glucose level range (%) |
||||
|---|---|---|---|---|---|
| Clarke error grid zone | Total range (mg/dL) | 40–80 (mg/dL) | 80–120 (mg/dl) | 120–240 (mg/dL) | 240–400 (mg/dL) |
| A+B | 1,305 (99.1%) | 15 (57.7%) | 266 (100.0%) | 875 (100.0%) | 149 (99.3%) |
| A | 1,156 (87.8%) | 6 (23.1%) | 219 (82.3%) | 785 (89.7%) | 146 (97.3%) |
| B | 149 (11.3%) | 9 (34.6%) | 47 (17.7%) | 90 (10.3%) | 3 (2.0%) |
| C | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| D | 12 (0.9%) | 11 (42.3%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) |
| E | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Total | 1,317 (100.0%) | 26 (2.0%) | 266 (20.2%) | 875 (66.4%) | 150 (11.4%) |
Percentages represent number of paired values/total paired values.
Consensus error grid analysis
Consensus error grid analysis showed that 99.9% of the paired YSI–sensor values fell within Zones A and B (90.9% within Zone A and 9% within Zone B) (Fig. 1b). The accuracy of the sensor changed with the range of blood glucose level. When the blood glucose level was more than 120 mg/dL, over 91.1% of the paired values fell within Zone A. Moreover, 65.4% and 88.7% of the paired values fell within Zone A in the 40–80 mg/dL and 80–120 mg/dL ranges, respectively.
Continuous error grid analysis
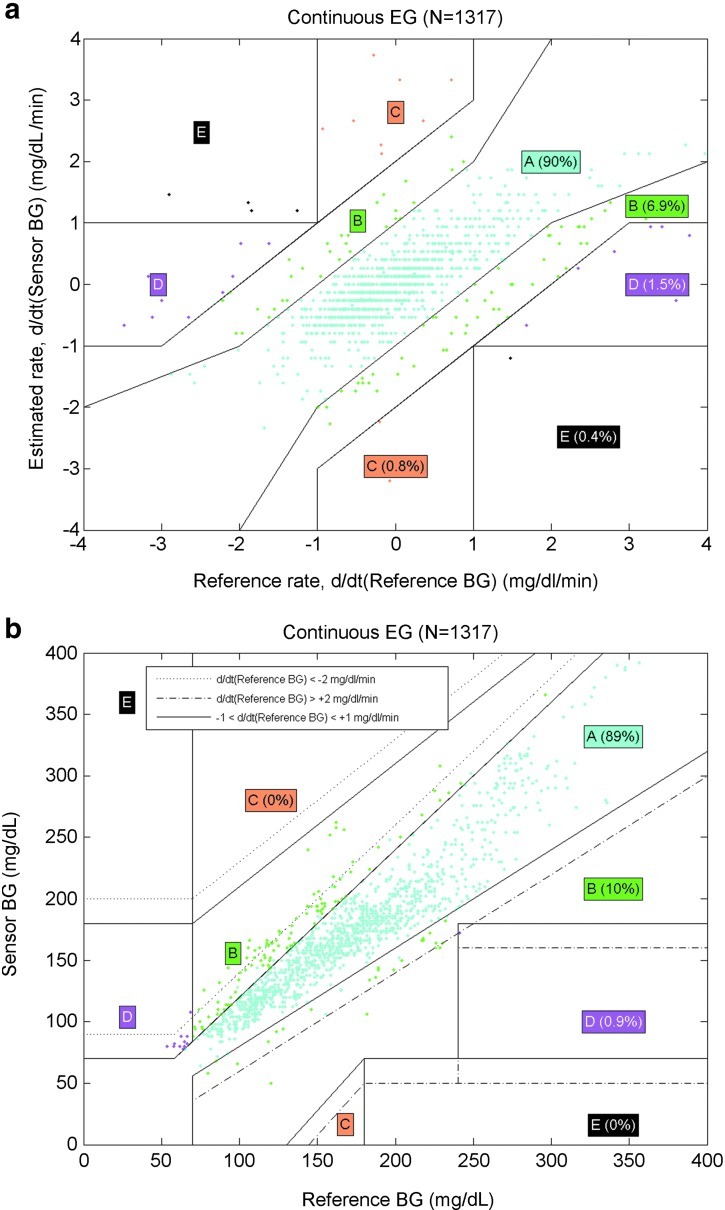
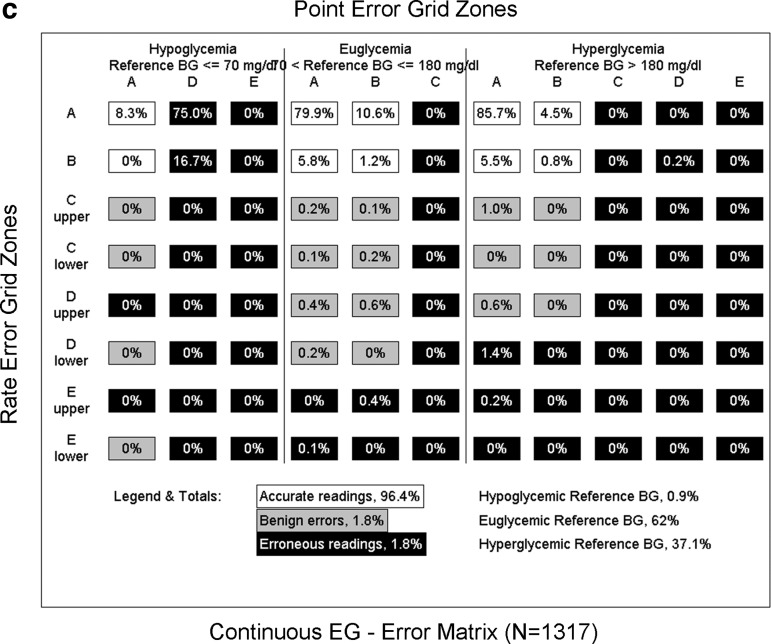
The continuous error grid analysis was performed in three steps: (1) Rate-error grid analysis showed that the ratio of the YSI–sensor values in Zones A and B was 96.9% (90% in Zone A and 6.9% in Zone B; Fig. 2a). (2) Point-error grid analysis showed that ratio of the YSI–sensor values in Zones A and B was 99% (89% in Zone A and 10% in Zone B; Fig. 2b). (3) The combined continuous error grid matrix of the rate error grid analysis and the point error grid analysis showed that ratios of the YSI–sensor values in zones of accurate reading, benign errors, and erroneous reading were 96.4%, 1.8%, and 1.8%, respectively (Fig. 2c).
FIG. 2.
(a) Continuous rate error grid (EG) of paired YSI–sensor values by the REAL-Time algorithm. Each grid shows the comparison of sensor values on the y-axis with blood glucose (BG) values obtained with reference method on the x-axis. (b) Continuous EG of paired YSI–sensor values by the REAL-Time algorithm. (c) Continuous EG matrix of paired YSI–sensor values by the REAL-Time algorithm.
ARD
The mean ARD for all subjects was 10.4%, and the median ARD was 7.8%. The mean ARD values were 11.3% (SD, 10.9%), 10.6% (SD, 10.3%), and 9.1% (SD, 8%) on Days 1–3, respectively. The range of blood glucose levels was stratified, and a blood glucose level between 40 and 80 mg/dL was designated as the mean absolute difference (MAD). Blood glucose levels greater than 80 mg/dL were described as ARD. The results showed that the deviation between the sensor readings and YSI glucose values decreased with the increment of blood glucose level, and the lowest deviation occurred in the 120–240 mg/dL range.
Correlation analysis
The correlation analysis of sensor readings and YSI values revealed that there was a significant positive correlation between them (r=0.900, P<0.001).
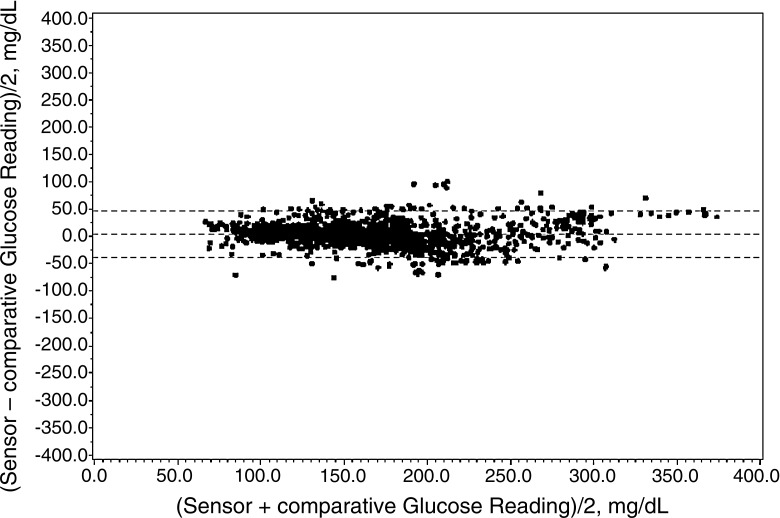
Bland–Altman analysis
The Bland–Altman scatter plots were created based on the mean paired YSI–sensor values (horizontal coordinate) and the difference between the YSI values and sensor readings (vertical coordinate). The plots revealed that the mean blood glucose level was 3.84 mg/dL (95% confidence interval −38.83 to 46.51 mg/dL). There was no change in the difference of the paired values at different blood glucose level ranges (Fig. 3).
FIG. 3.
Bland–Altman scatter plots of paired YSI–sensor glucose values.
Trend analysis
The initial blood glucose levels were stratified into hypoglycemia (<70 mg/dL), normal blood glucose range (70–180 mg/dL), and hyperglycemia (>180 mg/dL). The trend of change in the blood glucose level was observed. The trend analysis of the change of YSI values showed that there were minor ROCs in the blood glucose level under conditions of hypoglycemia, hyperglycemia, or normal blood glucose levels. The range of change was concentrated within −0.5 and 0.5 mg/dL/min. The ROC of sensor readings appeared similar to that of YSI glucose values. The difference analysis of the ROC of sensor readings and YSI values showed that 86.1% of the difference of ROC was concentrated within −1 to 1 mg/dL/min, and only 1.3% of the absolute difference of the paired values was more than 3 mg/dL/min (Table 3).
Table 3.
Difference Analysis of YSI Glucose Values and Rate of Change of Sensor Glucose Readings
|
Relative deviation |
Absolute value of relative deviation |
||||
|---|---|---|---|---|---|
| Group (mg/dL/min) | Number | % | Group (mg/dL/min) | Number | % |
| <−3 | 10 | 0.8 | — | — | — |
| −3 to <−2 | 16 | 1.3 | — | — | — |
| −2 to <−1 | 57 | 4.5 | — | — | — |
| −1 to ≤1 | 1,093 | 86.1 | (0, 1) | 1,093 | 86.1 |
| 1 to ≤2 | 67 | 5.3 | (1, 2) | 124 | 9.8 |
| 2 to ≤3 | 19 | 1.5 | (2, 3) | 35 | 2.8 |
| >3 | 7 | 0.6 | >3 | 17 | 1.3 |
Survival of sensor
In total, 48 sensors completed the consecutive 3-day test. Only three sensors failed to complete the test because of failure in adjustment, connection, did not initialize, or other causes. The survival rate of the sensor was 94.1%. In addition, the functional data obtained from the sensor revealed that 81.6% of the sensor functioned for 70 successive hours.
Safety
There were no adverse events related to instruments or operations. However, nine patients experienced skin-associated adverse events. Three subjects had erythema on three occasions due to sensor implantation. Moreover, erythema was present on the tape-adhesive areas in a subject once. One subject had a bruise, two subjects had bleeding on two occasions, and two patients suffered from itching. However, no phyma, exudation, or scleroma was found.
Discussion
CGM measures glucose concentration in the subcutaneous interstitial fluid but not in the plasma; thus the assessment of its accuracy has been given high priority. Currently, common procedures of systematic assessment are based on using plasma glucose levels as reference by drawing venous blood frequently (once every 15 min). However, there is little information regarding the assessment of the accuracy of RT-CGM using frequent venous blood collection in the Chinese population. Hence, we conducted the current multicenter study to address the issue. The accuracy of CGM includes both point accuracy and trend accuracy. Both comprise two aspects: numerical accuracy and clinical accuracy. It is known that system error usually emerges if linear regression and correlation analysis are used to assess the accuracy of CGM. When the aforementioned two methods are used for measuring the same sample, the possibility of major measurement error between them cannot be excluded even if a high correlation is achieved, so the high correlation is inadequate to assure the alternative application of the two measurement methods in clinical practice. Therefore, linear regression and correlation analysis are not suitable for assessing the agreement between two methods for measuring blood glucose levels.
In the past decade, there were a few studies illustrating the accuracy of the CGM system by various statistical methods. Kerssen et al.13 used the Pearson correlation analysis, MAD, and Clarke error grid analysis and found that the CGM system was accurate in pregnant women with type 1 diabetes mellitus. Morrow et al.10 tested an electrochemical CGM system in patients undergoing automated glucose clamp procedure. Djakoure-Platonoff et al.14 used non-calibration capillary glucose data as reference to demonstrate the accuracy of the CGM system through methods of correlation coefficient, error grid analysis, and MAD. Weinstein et al.15 compared the FreeStyle® Navigator (Abbott Diabetes Care, Alameda, CA) CGM system measurements with venous blood glucose values by mean and median ARDs and Clarke error grid analysis and proved that the CGM system was consistent and accurate. Zisser et al.16 compared CGM measurements against YSI values taken once every 20 min and showed the accuracy of the DexCom™ (San Diego, CA) SEVEN™ system. Rabiee et al.17 evaluated the accuracy of the RT-CGM system in an intensive care unit using a study using hyperinsulinemic-euglycemic and hyperglycemic clamps and found that the underestimation of hypoglycemia by RT-CGM made it unreliable in the intensive care unit setting.
In the present study, the accuracy of CGM was comprehensively evaluated using multiple statistical methods, including the agreement between the sensor readings and the YSI values at deviations of 20% and 30%, error grid analysis, ARD, linear model analysis, Bland–Altman analysis, and trend analysis.18 The agreement analysis between CGM values and YSI values, ARD, correlation analysis, and Bland–Altman analysis reflected point numerical accuracy, Clarke and consensus error grid analyses reflected point clinical accuracy, trend analysis reflected trend numerical accuracy, and continuous error grid analysis reflected trend clinical accuracy. Agreement analysis was the major method that was used. Our findings showed that 88.3% of the sensor readings were within±20% of the YSI values, and 95.7% were within±30% of the YSI values. This suggested that a high rate of agreement was achieved between the sensor readings and reference blood glucose values. Mastrototaro et al.19 investigated the accuracy and efficacy of an RT-CGM sensor in 72 subjects with type 1 diabetes and found that the overall percentages of sensor readings were 75.6% and 86.8%, respectively, within±20% and±30% agreement of the reference glucose readings. In addition, the rate of agreement between sensor readings and YSI values increased with time. Similar results were observed in ARD, which was thought to be due to the increased adjustment times, leading to the enhanced accuracy of sensor readings.20
Clinical accuracy is defined as clinical decision-making based on the obtained results. If a high rate of agreement is achieved between two detecting techniques, an accurate clinical decision can be made. Otherwise, a wrong medical decision may result. The Clarke error grid, an analysis proposed by Clarke et al.12 in 1987, is usually used to assess clinical precision. This analysis is an effective approach that combines the precision of glucose measurement with clinical results. It breaks down a scatterplot of a reference glucose meter and an evaluated glucose meter into five zones. Zone A values are within 20% of the reference sensor; Zone B contains points that are outside of 20% but would not lead to inappropriate treatment. Our findings showed that 99.1% of the paired YSI–sensor values fell within Zones A and B of the Clarke error grid, exceeding the critical value of accuracy that met the clinical requirements. Similar results were achieved using consensus error grid analysis.21
In the current study, we also used continuous error grid analysis, which is an improved error grid analysis used to assess the accuracy of the sensor readings. Continuous error grid analysis is a method that combines the blood glucose levels at different time points with the ROC of the blood glucose level.22 Results from continuous error grid analysis showed that 99% of the sensor readings reached the clinical critical value of accuracy, and the trend of change of 96.9% of the sensor readings reached the clinical critical value of accuracy. The ratios of the YSI–sensor values in regions of accurate reading, benign errors, and erroneous readings were 96.4%, 1.8%, and 1.8%, respectively. The mean ARD was 10.4%, and the median ARD was 7.8%. These values are in accordance with the previous study.9 In addition, consensus error grid analysis was used to assess the clinical precision of the paired YSI–sensor values during hypoglycemia (40–80 mg/dL). The results showed that of the 26 paired data, 96.2% fell within Zones A and B of the error grid, which indicated high clinical precision. However, it is noted that the agreement between the YSI values and sensor readings indicated that only 50% of the sensor readings were within±20% agreement of the YSI values during hypoglycemia. It is thought that there are more than 90% of patients with type 2 diabetes, in whom a lower incidence of hypoglycemia occurs compared with those with type 1 diabetes, thus leading to possible deviation when minor sample size is observed. It is suggested that the fingertip blood glucose test, in combination with error grid analysis, should be conducted for the diagnosis of hypoglycemia.
The present study also assessed the agreement of the trend of change between the sensor readings and the reference values during hypoglycemia, normal glucose levels, and hyperglycemia. The results revealed a high rate of agreement between the sensor readings and reference values. Trend analysis indicated that 86.1% of the difference of the ROCs occurred in the range of 1 mg/dL/min, which suggested that the RT-CGM system would reflect the changes of blood glucose levels very well.
The multiple statistical methods used in this study showed that the Paradigm insulin pump was both numerically and clinically accurate. However, the performance in detecting hypoglycemia remains to be further explored. Therefore, we suggest that the Paradigm insulin pump is a useful tool in the management of diabetes. For example, it can provide much more glycemic information, including magnitude, duration, and frequency of blood glucose levels, so as to provide evidence for optimal treatment decisions. It has alert and forecasting functions that help to instantly regulate blood glucose levels.
A few limitations of this study should be recognized. First, the number of type 1 diabetes patients included in the study was relatively small. Second, at the beginning of the study, we did not set specific limitation on patients' diabetes duration, therapeutic regimen, and level of hemoglobin A1c as enrollment requirements. It will be very interesting to confirm our result in patients with different diabetes durations, therapeutic regimens, and initial hemoglobin A1c in the future.
In conclusion, the Paradigm insulin pump displays high accuracy in both monitoring RT continuous changes and predicting the trend of changes in blood glucose level.
Acknowledgments
We would like to thank all the involved clinicians, nurses, and technicians at all the participating centers for their dedication to the study. This work was supported by the National Natural Science Foundation of China (grant 81100590), the Shanghai United Developing Technology Project of Municipal Hospitals (grant SHDC12010115), the Shanghai Medical Program for Outstanding Young Talent (grant XYQ2011013), and the Shanghai Municipal Health Bureau Youth Research Projects (grant 2009Y038).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battelino T. Bode BW. Continuous glucose monitoring in 2010. Int J Clin Pract Suppl. 2011;170:11–15. doi: 10.1111/j.1742-1241.2010.02573.x. [DOI] [PubMed] [Google Scholar]
- 3.Deiss D. Bolinder J. Riveline JP. Battelino T. Bosi E. Tubiana-Rufi N. Kerr D. Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 4.Raccah D. Sulmont V. Reznik Y. Guerci B. Renard E. Hanaire H. Jeandidier N. Nicolino M. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes. The RealTrend study. Diabetes Care. 2009;32:2245–2250. doi: 10.2337/dc09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV. Beck RW. Bode BW. Buckingham B. Chase HP. Clemons R. Fiallo-Scharer R. Fox LA. Gilliam LK. Hirsch IB. Huang ES. Kollman C. Kowalski AJ. Laffel L. Lawrence JM. Lee J. Mauras N. O'Grady M. Ruedy KJ. Tansey M. Tsalikian E. Weinzimer S. Wilson DM. Wolpert H. Wysocki T. Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 6.Bergenstal RM. Tamborlane WV. Ahmann A. Buse JB. Dailey G. Davis SN. Joyce C. Peoples T. Perkins BA. Welsh JB. Willi SM. Wood MA STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 7.Ehrhardt NM. Chellappa M. Walker MS. Fonda SJ. Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5:668–675. doi: 10.1177/193229681100500320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breton M. Kovatchev B. Analysis, modeling, and simulation of the accuracy of continuous glucose sensors. J Diabetes Sci Technol. 2008;2:853–862. doi: 10.1901/jaba.2008.2-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keenan DB. Cartaya R. Mastrototaro JJ. Accuracy of a new real-time continuous glucose monitoring algorithm. J Diabetes Sci Technol. 2010;4:111–118. doi: 10.1177/193229681000400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrow L. Hompesch M. Tideman AM. Matson J. Dunne N. Pardo S. Parkes JL. Schachner HC. Simmons DA. Evaluation of a novel continuous glucose measurement device in patients with diabetes mellitus across the glycemic range. J Diabetes Sci Technol. 2011;5:853–859. doi: 10.1177/193229681100500406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lü LF. Wang C. Yang YZ. He LP. Liu GJ. Chen DW. Zhong L. Chen LH. Tian HM. Zhou J. Jia WP. Ran XW. [Accuracy and safety of continuous glucose monitoring system in diabetic and non-diabetic subjects] Zhonghua Yi Xue Za Zhi. 2010;90:2967–2970. [PubMed] [Google Scholar]
- 12.Clarke WL. Cox D. Gonder-Frederick LA. Carter W. Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10:622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 13.Kerssen A. de Valk HW. Visser GH. The Continuous Glucose Monitoring System during pregnancy of women with type 1diabetes mellitus: accuracy assessment. Diabetes Technol Ther. 2004;6:645–651. doi: 10.1089/dia.2004.6.645. [DOI] [PubMed] [Google Scholar]
- 14.Djakoure-Platonoff C. Radermercker R. Reach G. Slama G. Selam JI. Accuracy of the continuous glucose monitoring system in inpatient and outpatient conditions. Diabetes Metab. 2003;29:159–162. doi: 10.1016/S1262-3636(07)70023-X. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein RL. Schwartz SL. Brazg RL. Bugler JR. Peyser TA. McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator continuous glucose monitoring system: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30:1125–1130. doi: 10.2337/dc06-1602. [DOI] [PubMed] [Google Scholar]
- 16.Zisser HC. Bailey TS. Schwartz S. Ratner RE. Wise J. Accuracy of the SEVEN continuous glucose monitoring system: comparison with frequently sampled venous glucose measurements. J Diabetes Sci Technol. 2009;3:1146–1154. doi: 10.1177/193229680900300519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabiee A. Andreasik RN. Abu-Hamdah R. Galiatsatos BS. Khouri Z. Gibson BR. Andersen DK. Elahi D. Numerical and clinical accuracy of a continuous glucose monitoring system during intravenous insulin therapy in the surgical and burn intensive care units. J Diabetes Sci Technol. 2009;3:951–959. doi: 10.1177/193229680900300443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wentholt IM. Hart AA. Hoekstra JB. Devries JH. How to assess and compare the accuracy of continuous glucose monitors? Diabetes Technol Ther. 2008;10:57–68. doi: 10.1089/dia.2007.0216. [DOI] [PubMed] [Google Scholar]
- 19.Mastrototaro J. Shin J. Marcus A. Sulur G STAR 1 Clinical Trial Investigators. The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with type 1 diabetes. Diabetes Technol Ther. 2008;10:385–390. doi: 10.1089/dia.2007.0291. [DOI] [PubMed] [Google Scholar]
- 20.Diabetes Research in Children Network (DirecNet) Study Group. Buckingham BA. Kollman C. Beck R. Kalajian A. Fiallo-Scharer R. Tansey MJ. Fox LA. Wilson DM. Weinzimer SA. Ruedy KJ. Tamborlane WV. Evaluation of factors affecting CGMS calibration. Diabetes Technol Ther. 2006;8:318–325. doi: 10.1089/dia.2006.8.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkes JL. Slatin SL. Pardo S. Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23:1143–1148. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 22.Wentholt IM. Hoekstra JB. Devries JH. A critical appraisal of the continuous glucose-error grid analysis. Diabetes Care. 2006;29:1805–1811. doi: 10.2337/dc06-0079. [DOI] [PubMed] [Google Scholar]