Abstract
We investigated changes in the incidence of amyotrophic lateral sclerosis (ALS) in the Koza/Kozagawa/Kushimoto area (K. area) in the Kii Peninsula, Japan in 1960–2009. Probable and definite ALS cases diagnosed using El Escorial criteria were collected during a five-decade period: period I-V, 1960–2009. Forty-three ALS patients matched the selection criteria in the overall K. area, including three patients on Oshima, a small island opposite the mainland K. area. The age- and gender-adjusted incidence of ALS in the overall K. area (standardized for the 2005 Japanese population) decreased from 5.47/100,000 (95% CI 1.86–9.08) in period I to 0.61/100,000 (95% CI-0.28–1.50) in period III, and then increased to 4.39/100,000 (95% CI 1.70–7.07) in periodV. On Oshima, the age- and gender-adjusted incidence of ALS was 9.45/100,000 (95% CI—7.39–26.29) in period V. The present research indicates an increase of ALS incidence in the K. area, especially on Oshima. A limitation of this study was the small population.
Keywords: Focus area, Kii-ALS, incidence, new cluster
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating adult-onset degenerating disease of unknown etiology of the motor neuron systems. The Koza, Kozagawa and Kushimoto (K.) area in the Kii Peninsula of Japan was reported to have a higher incidence of ALS in the 1950s than other areas of the world (1–5). Epidemio-logic research showed that drinking water sourced from Kozagawa River in the K. area contained severely low levels of Ca and Mg, and Ca/Mg deficiency was speculated to have a role in the development of ALS in these areas (5,6). On Oshima, a small island municipally included in the K. area, the source of drinking water was changed from regional water to the Kozagawa River in 1975. To clarify whether ALS epidemiology on Oshima changed after altering the water source, we investigated changes in ALS incidence on Oshima and in the K. area in 1960–2009.
Methods
Area of investigation
The K. area (population: 23,357 in 2005 census, 430.3 km2) is located in the southern part of Wakayama Prefecture of the Kii peninsula, Japan (Figure 1). Oshima (population: 1279 in 2005 census, 9.93 km2) is an island opposite the K. area. The K. area has a local public healthcare and welfare center, two local public hospitals, two private hospitals and 19 medical clinics.
Figure 1.
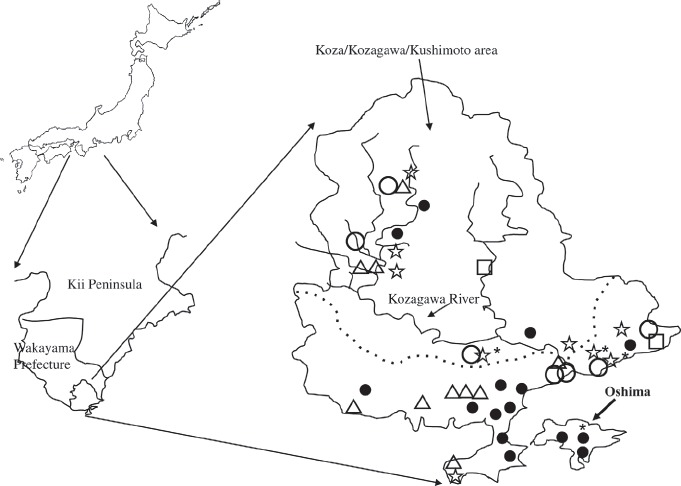
Geography of Koza/Kozagawa/Kushimoto area and Oshima, and distribution of patients with ALS. Patients with ALS between 1960 and 1969 (period I: º), between 1970 and 1979 (period II: Δ): between 1980 and 1989 (period III: □), between 1990 and 1999 (period IV: ☆), and between 2000 and 2009 (period V: •) were plotted. *: ALS/PDC
Incidence of ALS in 1960–2009
ALS patients on Oshima and overall in the K. area were enrolled from multiple sources, including hospital medical records and reports from local medical staff over a five-decade period: period I, 1960–1969; period II, 1970–1979; period III, 1980–1989; period IV, 1990–1999; period V, 2000–2009. Regional physicians including neurologists in hospitals and clinics and the staff of the public healthcare and welfare center in the K. area were requested by our team to report all patients with possible motor neuron disease every year (7). To ensure complete case identification, Wakayama Prefecture's List of Patients with Intractable Disease certified by the Ministry of Health and Welfare of Japan was used. The selection criteria were as follows: 1) patients who our neurologists examined and diagnosed using the El Esco-rial criteria (8); 2) patients with probable or definite ALS who had been living in the K. area, including Oshima, for at least one year before diagnosis. Patients who showed Parkinsonism or dementia during the disease course (ALS/Parkinsonism dementia complex: ALS/PDC) were also included.
The present research was approved by the ethics committee of Wakayama Medical University and Kansai University of Health Sciences.
Statistics
A direct method was used to standardize the annual incidence rates by age and gender, using populations in the 2005 census in Japan: 127,537,189. Unpaired t-test was performed and two-sided p<0.05 was considered significant.
Results
Patient population and clinical characteristics
We enrolled 50 patients with definite or probable ALS in the K. area, including Oshima, in 1960–2009, and 43 patients matched the selection criteria (Table I) (seven were excluded because they were living outside the K. area when diagnosed). Patient distribution was restricted to the mainland side of the K. area during periods I-IV. Three ALS patients were found on Oshima (two males and one female) in period V (Figure 1).
Table I.
Patients with ALS in the K. area, including Oshima in each period.
| Population& | ALS patients matched for the selection criteria | |||||||
|---|---|---|---|---|---|---|---|---|
| Period | Overall K area | Oshima | Total ALS patients enrolled (») | Overall K area | Oshima | Age at onset Mean (S.D.) | M/F ratio | FH n (%) |
| I | 18,251# | 2,823 | 7 | 7 | 0 | 56.1 (10.8) | 2.5 | 0(0) |
| II | 32,128 | 2,095 | 12 | 10 | 0 | 55.3 (10.6) | 1.5 | 1 (10) |
| III | 29,732 | 1,756 | 4 | 2 | 0 | 59.0 (7.1) | 2.0 | 0(0) |
| IV | 26,405 | 1,508 | 11 | 9 | 0 | 57.7 (11.5) | 2.0 | 3 (33) |
| V | 23,357 | 1,279 | 16 | 15 | 3 | 67.6 (12.3)* | 0.88 | 2(13) |
| Total | 50 | 43 | 3 | 1.53 | 6 (14.0) | |||
K.: Koza/Kozagawa/Kushimoto area; M/F: male/female ratio; FH: familial history.
: populations in 1965, 1975, 1985, 1995 and 2005 census.
: Koza/Kozagawa area.
: £<0.01 when compared with the mean age at onset in period I.
The mean age at onset in period V was the highest among the periods (Table I). The male: female ratio was low in period V compared to periods I-IV. The frequency of cases with a positive familial history in a detailed interview was 14.0%. Cu/Zn super-oxide dismutase (SOD1) genes were analyzed in three of six familial cases; none had a SOD1 gene mutation. Three ALS/PDC patients were found in the K. area in period IV and one patient on Oshima in period V.
Incidence of ALS in 1960–2009
The mean annual crude ALS incidence in the K. area in period V was 6.42/100,000 and that on Oshima was 23.46/100,000. The age- and gender-adjusted ALS incidence in the K. area decreased from 5.47/100,000 (95% CI 1.86–9.08) in period I to 0.61/100,000 (95% CI-0.28–1.50) in period III and then increased to 4.39/100,000 (95% CI 1.70–7.07) in period V (Figure 2). On Oshima, the age-and gender-adjusted incidence of ALS was 9.45/100,000 (95% CI-7.39–26.29) in period V.
Figure 2.
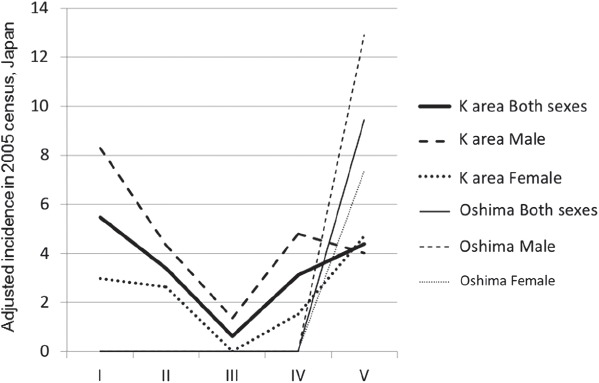
Changes in incidence of ALS in the overall Koza/Kozagawa/Kushimoto (K.) area and Oshima between 1960 and 2009. Comparing the incidence by the periods, the age- and gender-adjusted incidence of ALS in the overall K. area for the 2005 Japanese population was 5.47/100,000 (95% CI 1.86–9.08), male: 8.29/100,000 (95% CI 1.84–14.73), female: 2.98/100,000 (95% CI-0.69–6.66) in period I, markedly decreased to 0.61/100,000 (95% CI-0.28–1.50), male: 1.36/100,000 (95% CI-0.58–3.30), female: 0 in period III, but recently increased again to 4.39/100,000 (95% CI 1.70–7.07), male: 4.01/100,000 (95% CI 0.22–7.81), female: 4.71/100,000 (95% CI 0.93–8.49) in period V.
Discussion
The age- and gender-adjusted ALS incidence in the K. area decreased from 5.47 in 1960–1969 to 0.61 in 1980–1989, and then increased to 4.39 in 2000–2009. The declining trend of the male: female ratio in the past 10 years was comparable with other reports (9,10), and the recent increase of ALS incidence in females could be related to some type of environmental or cultural factor pertaining in females in this area and the increased confirmation of older female patients (11). The reason for the decline in 1980-1989 is not clear, but might be partially due to missing cases from the data set, emigration, and environmental, lifestyle and cultural changes. Recent reports of annual age-adjusted ALS incidences ranged from 0.42/100,000 (12) to 2.96/100,000 (13) in other areas in the world (14–17). Taken together, the age-and gender-adjusted incidence in the K. area and Oshima in 2000-2009 was higher than in other areas. On Oshima, no patient with ALS was found in previous research in 1946–1965 (18). It is noteworthy that a high ALS incidence was first found on Oshima in 2000–2009 after the drinking water source was changed to the Kozagawa River in 1975. The present research indicated an increase of ALS incidence in the K. area, especially on Oshima. A limitation of this study was the small population. Continuous study over a longer period is needed in this area.
Acknowledgments
The authors especially thankYoshiroYase and Fumio Yoshimasu of Kansai University of Health Sciences, Mikio Arita and Miyoko Utsumi of Wakayama Medical University and Ralph M. Garruto of the State University of New York at Binghamton for their helpful advice and encouragement.
Declaration of interest
This work was supported by grants-in-aid from the Research Committee of Muro disease (Chairman, Yasumasa Kokubo), the Ministry of Health, Labour and Welfare of Japan, and a grant-in-aid for Scientific Research of Japan.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Kusui K. Epidemiological study on amyotrophic lateral scle rosis (ALS) and other neighboring motor neuron diseases in Kii Peninsula. Psychiat Neurol Jpn. 1962;65:86–99. [PubMed] [Google Scholar]
- 2.Kimura K. Studies of amyotrophic lateral sclerosis in the Kozagawa district in the Kii Peninsula. Jpn. Wakayama Med J. 1965;9:177–92. [PubMed] [Google Scholar]
- 3.Yase Y, Matsumoto N, Yoshimasu F, Handa Y, Kumamoto T. Motor neuron disease in the Kii Peninsula, Japan. Proc Aust Assoc Neurol. 1968;5:335–9. [PubMed] [Google Scholar]
- 4.Kurland LT, Mulder DW. Epidemiologic investigations of amyotrophic lateral sclerosis. 1. Preliminary report on geo graphic distribution, with special reference to the Mariana Islands, including clinical and pathological observations. Neurology. 1954;4:355–78. doi: 10.1212/wnl.4.5.355. 438–48. [DOI] [PubMed] [Google Scholar]
- 5.Yase Y. The pathogenesis of amyotrophic lateral sclerosis. Lancet. 1972;2:292–6. doi: 10.1016/s0140-6736(72)92903-0. [DOI] [PubMed] [Google Scholar]
- 6.Garruto RM, Yanagihara R, Gajdusek DC, Arion DM. Con centrations of heavy metals and essential minerals in garden soil and drinking water in the Western Pacific. In: Chen KM, Yase Y, editors. Amyotrophic lateral sclerosis in Asia and Oceania. Shyan-Fu Chou: National Taiwan University; 1984. pp. 265–330. [Google Scholar]
- 7.Kihira T, Yoshida S, Hironishi M, Miwa H, Okamoto K, Kondo T. Changes in the incidence of amyotrophic lateral sclerosis in Wakayama, Japan. Amyotroph Lateral Scler. 2005;6:155–63. doi: 10.1080/14660820510030031. [DOI] [PubMed] [Google Scholar]
- 8.World Federation of Neurology Subcommittee on Motor Neuron Disease: El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci. 1994;124(Suppl):98–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 9.Cima V, Logroscino G, D'Ascenzo C, Palmieri A, Volpe M, Briani C, et al. Epidemiology of AT S in Padova district, Italy, from 1992 to 2005. Eur J Neurol. 2009;16:920–4. doi: 10.1111/j.1468-1331.2009.02623.x. [DOI] [PubMed] [Google Scholar]
- 10.Manjaly ZR, Scott KM, Abhinav K, Wijesekera L, Ganesalingam J, Goldstein LH, et al. Sex ratio in amyotrophic lateral sclerosis: a population based study. Amyotroph Lateral Scler. 2010;11:439–2. doi: 10.3109/17482961003610853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abhinav K, Stanton B, Johnston C, Hardstaff J, Orrell RW, Howard R, et al. Amyotrophic lateral sclerosis in south-east England: a population based study. The South-East England Register for Amyotrophic Lateral Sclerosis (SEALS Registry) Neuroepidemiology. 2007;29:44–8. doi: 10.1159/000108917. [DOI] [PubMed] [Google Scholar]
- 12.Sajjadi M, Etemadifar M, Nemati A, Ghazavi H, Basiri H, Khoundabi B, et al. Epidemiology of amyotrophic lateral sclerosis in Isfahan, Iran. Eur J Neurol. 2010;17:984–9. doi: 10.1111/j.1468-1331.2010.02972.x. [DOI] [PubMed] [Google Scholar]
- 13.Chio A, Mora G, Calvo A, Mazzini L, Bottacchi E, Mutani R, Parals, et al. Epidemiology of ALS in Italy: a 10-year prospective population based study. Neurology. 2009;24:725–31. doi: 10.1212/01.wnl.0000343008.26874.d1. [DOI] [PubMed] [Google Scholar]
- 14.Piemonte and Valle d'Aosta Register for Amyotrophic Lat eral Sclerosis (PARALS) Incidence of ALS in Italy: evidence for a uniform frequency in Western countries. Neurology. 2001;6:239–4. doi: 10.1212/wnl.56.2.239. [DOI] [PubMed] [Google Scholar]
- 15.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Incidence and prevalence of ALS in Ireland, 1955–1997: a population based study. Neurology. 1999;52:504–9. doi: 10.1212/wnl.52.3.504. [DOI] [PubMed] [Google Scholar]
- 16.Wolfson C, Kilborn S, Oskoui M, Genge A. Incidence and prevalence of amyotrophic lateral sclerosis in Canada: a sys tematic review of the literature. Neuroepidemiology. 2009;33:79–88. doi: 10.1159/000222089. [DOI] [PubMed] [Google Scholar]
- 17.Logroscino G, Traynor BJ, Hardiman O, Chio A, Mitchell D, Swingler RJ, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81:385–90. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yase Y. Epidemiological investigation on amyotrophic lat eral sclerosis in the Kii peninsula. Shinkeishinpo. 1973;17:30–3. [Google Scholar]


