TLR1/TLR2 complexes are required for induction of IL-6 and IL-23 to generate protective TH17-mediated immunity and IgA production after oral but not systemic Yersinia enterocolitica infection.
Abstract
The balance between regulatory and inflammatory immune responses is critical to maintain intestinal homeostasis. Furthermore, the nature of the inflammatory response needs to be tailored to the tissue to provide proper protective immunity while preserving host integrity. TLR2 (Toll-like receptor 2) is a unique TLR in that it has been shown to promote regulatory and inflammatory T cell responses. Using Yersinia enterocolitica, we show that oral infection promotes TH17 immunity, whereas systemic infection promotes TH1 immunity. Furthermore, induction of TH17 immunity during oral infection is dependent on TLR1 and results from the combinatorial effect of TLR2/TLR1-induced IL-6 and IL-23 and the presence of TGF-β in the intestinal environment. Interestingly, TLR2/TLR1 was not involved in TH1 immune responses during systemic infection, whereas the TLR2/TLR6 receptor complex induced IL-10+ regulatory T cell responses during both systemic and oral infections. Our results reveal that the route of infection is central in determining which pathways provide protective immunity. Furthermore, they also demonstrate that TLR2 has dual immune functions in the gut and identify TLR1 as a critical innate receptor for protective intestinal TH17 immunity.
Classically, we view the mammalian host as mounting inflammatory immune responses to clear or contain pathogenic microbial organisms while also inducing regulatory immune responses to allow colonization of the host by commensals. This view has been replaced by the more complex concept that there is no clear delineation between the two (Sansonetti and Di Santo, 2007; Weaver and Hatton, 2009) and that microorganisms use both inflammatory and regulatory immune pathways to their advantage (Sansonetti and Di Santo, 2007). Furthermore, it is becoming increasingly accepted that the type of TH response induced is determined not only by the nature of the pathogen but also by environmental cues, including the characteristics of the tissue (Coombes et al., 2007; Matzinger and Kamala, 2011), its nutritional status (Sun et al., 2007), and the presence or absence of inflammatory signals (DePaolo et al., 2011).
Yersinia enterocolitica is a gram-negative extracellular bacteria, which is pathogenic for humans and rodents. A food-borne pathogen that causes gastrointestinal symptoms (Cornelis, 2002), Y. enterocolitica has a tropism for lymphoid tissue, though its main mucosal colonization site is the terminal ileum of the small intestine (Trülzsch et al., 2007). Despite studies suggesting an association of Y. enterocolitica infection and the development of inflammatory bowel disease (Saebo et al., 2005), uveitis (Tanaka et al., 1996), and arthritis (Lahesmaa-Rantala et al., 1989), no host–pathogen interactions have been identified. TLR2 (Toll-like receptor 2) is a unique TLR because of its ability to bind with multiple coreceptors such as TLR1, TLR6, TLR10, Dectin-1, CD36, and CD14 and to promote both inflammatory (Cleveland et al., 1996) and regulatory IL-10 T cell responses in vivo (DePaolo et al., 2008; Round et al., 2011). Using Y. enterocolitica as a model pathogen, we studied how host–pathogen interactions dictated by the route of infection determine the type of T cell responses induced and required for protective immunity. Y. enterocolitica also provides an example of a pathogen using TLR2 to promote both pro- and antiinflammatory immunity and identifies TLR6 and TLR1 as innate receptors that promote tolerogenic IL-10 and proinflammatory IL-17 T cell responses in the intestinal environment, respectively.
RESULTS AND DISCUSSION
TLR1 is critical for controlling mucosal but not systemic Y. enterocolitica infection
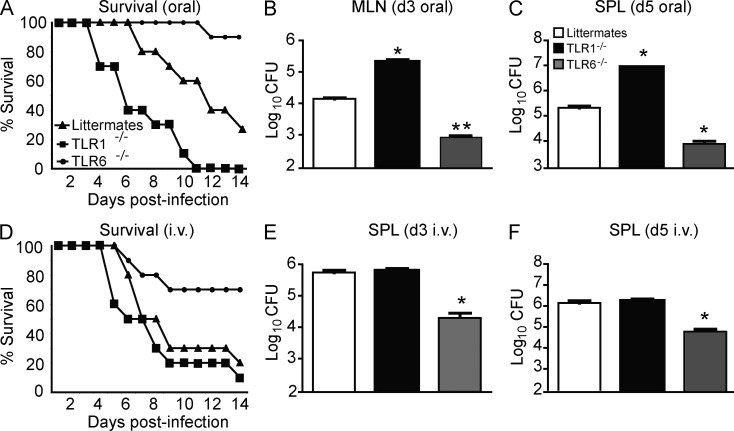
We hypothesize that the nature of the immune response induced by TLR2 depends on the partner with which it associates. This hypothesis was based on our finding that TLR2/TLR6 ligands promoted tolerogenic DCs and regulatory IL-10 T cell responses in vitro and in vivo, whereas TLR1 primarily induced IL-12p40 production in DCs in vitro (DePaolo et al., 2008). However, we were unable to demonstrate a role for TLR1 in vivo, so we sought to reassess the role using Y. enterocolitica. Because this species normally infects orally, we administered bacteria via gavage to TLR1−/−, TLR6−/−, and littermate control (TLR1+/− and TLR6+/−) mice (Fig. 1, A–C). In accordance with a previous study demonstrating that TLR2 is involved during oral Y. enterocolitica infection (Sing et al., 2002), we confirmed that TLR2−/− mice had a defect in IL-10 production and a reduced bacterial burden in the mesenteric LNs (MLNs); however, overall mortality was not affected (not depicted). Furthermore, TLR6−/− and TLR1−/− had opposing effects; absence of TLR6 dramatically increased survival (Fig. 1 A) and decreased bacterial burden (Fig. 1 B), whereas the absence of TLR1 led to decreased survival (Fig. 1 A), higher bacterial burden in MLNs (Fig. 1 B), and faster systemic dissemination (Fig. 1 C).
Figure 1.
Dependence on TLR1 is determined by the route of infection. (A) TLR1−/−, TLR6−/−, or heterozygous littermate controls (littermates) were infected orally with 105 CFU Y. enterocolitica strain 8081 and followed for survival. Data are pooled from three individual experiments (n = 5 mice per group for each experiment). Statistical significance was determined by Wilcoxon log-rank test (TLR6−/−, P < 0.001; TLR1−/−, P < 0.01). (B) Bacterial burden in MLNs at 3 d after infection. (C) Bacterial burden in the SPLs of orally infected mice 7 d after infection. (D) Survival curve of TLR1−/−, TLR6−/−, or littermates infected i.v. with 105 CFU Y. enterocolitica. Data are pooled from three individual experiments (n = 5 mice per group for each experiment). Statistical significance was determined by Wilcoxon log-rank test (TLR6−/−, P < 0.01). (E) Bacterial burden in the SPL at 3 d after infection. (B, C, and E) Data shown are the mean ± SEM (n = 7). (F) Bacterial burden in SPL 5 d after i.v. infection. Data shown are the mean ± SEM (n = 6). *, P < 0.01; **, P < 0.001 (unpaired Student’s t test).
Our previous work has demonstrated that TLR1 had no impact on the survival of mice during systemic infection with Yersinia pestis, the causative agent of bubonic plague (DePaolo et al., 2008). We sought to test whether this difference was caused by the genetic makeup of the bacteria or by the route of infection. We decided to test the latter possibility first, as the intestinal environment is unique in that it expresses high levels of TGF-β and the vitamin A metabolite retinoic acid (RA). In support of this hypothesis, we found that TLR1−/− mice infected systemically with Y. enterocolitica displayed no phenotype as compared with infected littermate controls (Fig. 1, D–F), even at doses of infection as low as 103 CFU (not depicted). These data demonstrate that TLR1 and TLR6 play opposing roles during oral Y. enterocolitica infection. Furthermore, they indicate that the host uses different innate receptors to mount protective immunity against a pathogen depending on the route of infection.
TLR1 is critical for the induction of mucosal TH17 and IgA responses but is not involved in the induction of systemic TH1 responses during Y. enterocolitica infection
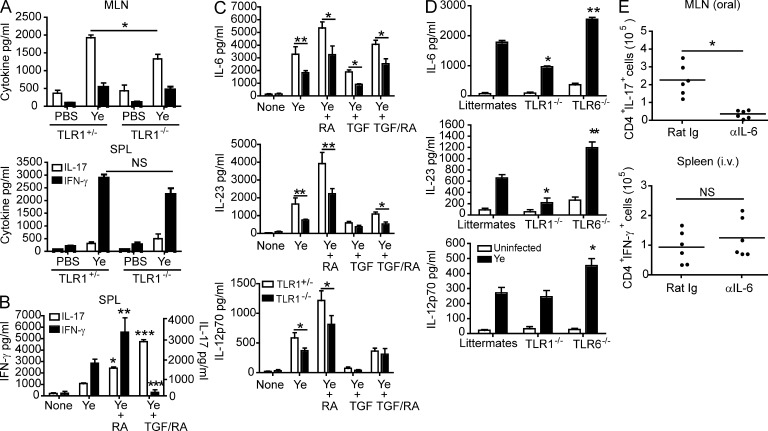
We next wanted to determine whether the differences we observed in TLR1 involvement were linked to differences in the TH responses induced during oral and systemic Y. enterocolitica infection. Cytokine analysis of TLR1−/− mice and littermate controls revealed that TH17 polarizing innate cytokines (Fig. S1 and see Fig. 3 D) and TH17 cell responses (Fig. 2 A) were dependent on the presence of TLR1 and were selectively induced during mucosal Y. enterocolitica infection. In contrast, IL-12p70 (Fig. S1) and TH1 responses (Fig. 2 B) were observed during systemic Y. enterocolitica infection and conserved in the absence of TLR1. We have previously shown that IL-10 T cell responses were dependent on TLR6 expression during peripheral infection (DePaolo et al., 2008), and in this study we demonstrate that TLR6 is also required during oral infection with Y. enterocolitica (Fig. 2, A and B), indicating that TLR6 is a critical innate receptor with tolerogenic properties in and outside of the intestinal environment,
Figure 3.
TLR1-dependent IL-6 is critical for inducing TH17 cells during oral infection. (A) Levels of IFN-γ and IL-17 from co-cultures of naive SPL CD4+ T cells and MLN or SPL DCs from TLR1+/− or TLR1−/− mice stimulated with 10 µg/ml Y. enterocolitica lysate (Ye). Data shown are the mean ± SEM (n = 5) from two individual experiments. *, P < 0.05 (unpaired Student’s t test). (B) Levels of IFN-γ and IL-17 from co-cultures of SPL DCs and CD4+ T cells as described in A but with the addition of 10 µM RA or 2 ng/ml TGF-β and RA. *, P < 0.05; **, P < 0.01; ***, P < 0.05 (unpaired Student’s t test) compared with Y. enterocolitica treated. (C) Levels of IL-6, IL-23, and IL-12p70 from TLR1+/− (open bars) and TLR1−/− (closed bars) SPL DCs treated with Y. enterocolitica lysate plus RA, TGF-β, or RA/TGF-β. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test) comparing TLR1+/− and TLR1−/− for each treatment. (D) Levels of IL-6, IL-23, and IL-12p70 in the MLNs from TLR1+/− or TLR6−/− mice 3 d after infection. *, P < 0.05; **, P < 0.01 (Student’s t test comparing infected knockouts with infected littermates). (B–D) Data are the pooled mean ± SEM (n = 6) from two individual experiments. (E) Total CD4+IL-17+ cells in MLNs of orally infected mice and total CD4+IFN-γ+ cells in the SPLs of i.v. infected mice as determined by flow cytometry. Data are pooled from two individual experiments (n = 6). Horizontal bars represent the mean. *, P < 0.05 (paired Student’s t test).
Figure 2.
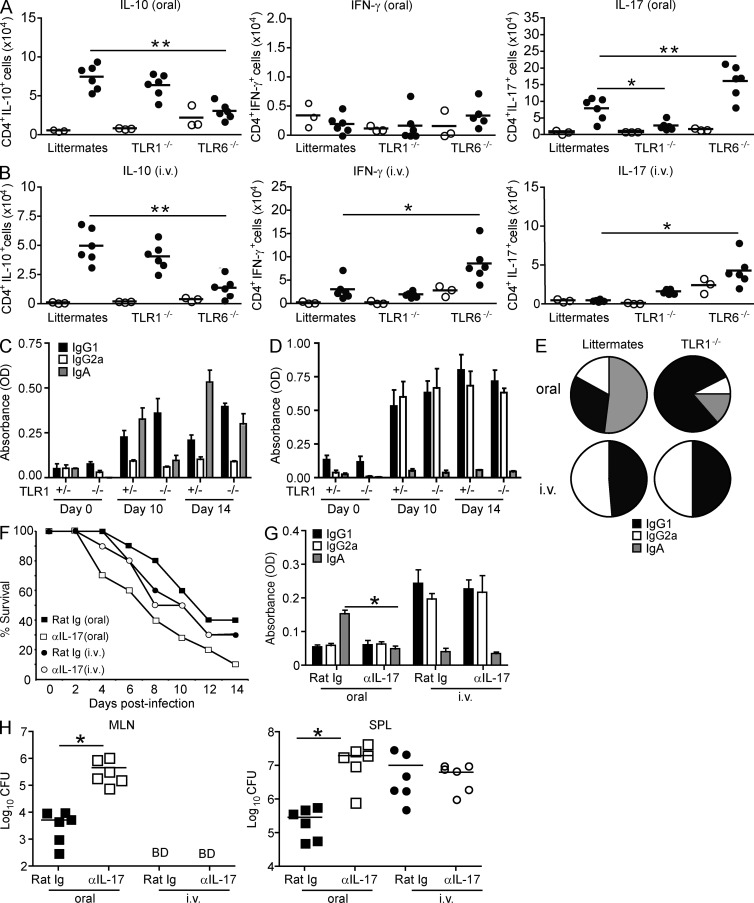
TLR1 is important for inducing IL-17–mediated immunity during oral infection. (A and B) TLR1−/−, TLR6−/−, and littermates were infected orally (A) or i.v. (B) with 105 CFU Y. enterocolitica. 6 d after infection, MLNs (oral) or SPLs (i.v.) were harvested. Intracellular cytokine production was analyzed by flow cytometry; plots are representative of one out of six infected mice. Total cell counts were determined for naive (open circles) or infected (closed circles) mice. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test). (C and D) Levels of anti-Yersinia IgA, IgG1, and IgG2a from TLR1−/− or TLR1+/− mice infected orally (C) or i.v. (D) with 105 CFU Y. enterocolitica. Fresh fecal pellets or serum were collected on days 0, 10, and 14, and antibody titers were detected by ELISA. Data are represented as the mean ± SEM and are pooled from two experiments (n = 6). (E) The ratio of anti-Yersinia IgA, IgG1, and IgG2a from infected TLR1+/− and TLR1−/− mice at day 14. (F) Survival curve of C57BL/6 mice infected orally (squares) or i.v. (circles) with 105 CFU Y. enterocolitica and injected i.p. with 100 µg monoclonal anti–IL-17 (open symbols) or isotype control (rat Ig; closed symbols) every other day for 8 d. Data are pooled from two individual experiments (n = 10). Statistical significance was determined by Wilcoxon Log-rank test (P = 0.0125, oral; P = 0.8972, i.v.). (G) Fecal antibody levels of IgG1, IgG2a, and IgA in anti–IL-17– or isotype control–treated mice. Data are the mean ± SEM (n = 6) from two independent experiments. *, P < 0.01 (paired Student’s t test). (H) Bacterial burden of orally (squares) or i.v. (circles) infected mice treated with isotype control (closed symbols) or anti–IL-17 (open symbols). MLNs and SPLs were harvested at days 3 and 7, respectively, for oral infections, and both organs were harvested at 7 d for i.v. infection. Data are from two pooled experiments (n = 6). *, P < 0.01 (paired Student’s t test). BD, below detection. (A, B, and H) Horizontal bars represent the mean.
The discovery that TH17 cells are important for protection against oral but not systemic infection is in agreement with observations that IL-6−/− mice have a more severe disease after oral (Dube et al., 2004) but not i.v. (Matteoli et al., 2008) infection with Y. enterocolitica. Furthermore, our findings also explain seemingly contradictory studies showing that infection in IL-12p40−/− mice results in increased bacterial burden and dissemination (Hein et al., 2001), whereas administration of bioactive IL-12p70 has no effect in mice orally infected with Y. enterocolitica (Bohn and Autenrieth, 1996). Our data also demonstrate that TLR1 is critical for the induction of TH17 in response to oral Y. enterocolitica infections. IL-17 was previously shown to be induced homeostatically by segmented filamentous bacteria (Ivanov et al., 2009) in addition to having a critical role in the protection against intestinal bacterial pathogens such as Citrobacter rodentium (Mangan et al., 2006; Ishigame et al., 2009). However, the molecular mechanism behind the generation of TH17 responses at mucosal surfaces has remained poorly understood to this point. The importance of TLR1 in promoting TH17 immunity in response to pathogens other than Y. enterocolitica or commensal bacteria remains to be determined.
The type of antibody response to an antigen is linked to the nature of the TH cells induced. In particular, TH17 and TH1 cells have been shown to promote IgA (Jaffar et al., 2009) and IgG2a antibody responses, respectively (Stevens et al., 1988). In accordance with the TH cells induced (Fig. 2, A and B), Yersinia-specific IgA antibodies were found in the feces of orally infected mice, whereas IgG2a antibodies were prominently found in the serum of systemically infected mice (Fig. 2, C–E). IgG1 was induced in response to both types of infection. Supporting a role for TLR1 during oral infection in IgA-mediated immunity against Y. enterocolitica, TLR1−/− mice did not generate Yersinia-specific IgA antibodies (Fig. 2, C–E). The absence of IgA antibodies was associated with an increase in IgG1 antibodies, whereas IgG2a antibodies overall remained unchanged (Fig. 2, C–E). As anticipated, absence of TLR1 had no significant effect on the antibody response during systemic infection (Fig. 2, C–E).
To directly assess the link between TH17 responses and protective immunity against Y. enterocolitica, we treated mice with a neutralizing antibody against IL-17 or an IgG2a isotype control. Mice receiving neutralizing IL-17 antibody began losing weight earlier (day 2 vs. day 5; not depicted) and had increased mortality (P < 0.05; Fig. 2 F). In line with a role for IL-17 in mucosal IgA responses (Jaffar et al., 2009), mice treated with neutralizing anti–IL-17 antibody had a significant decrease in fecal levels of anti-Yersinia IgA antibodies (Fig. 2 G). Furthermore, anti–IL-17–treated mice also had higher bacterial burden and a more disseminated infection with 2-log more bacteria in the spleen (SPL) 7 d after infection as compared to mice receiving isotype control antibody (Fig. 2 H). As anticipated, blocking IL-17 had no effect during systemic Y. enterocolitica infection (Fig. 2, F–H). The discovery that Y. enterocolitica selectively induces TH17-dependent IgA responses during oral infection fits from a teleological perspective given that TH17 responses preferentially induce secretory IgA antibodies (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009; Jaffar et al., 2009), which are highly resistant to the proteolytic enzymes present in the intestinal environment because of their secretory component and thus are uniquely capable of neutralizing luminal antigens (Endt et al., 2010; Hapfelmeier et al., 2010). This strongly emphasizes the importance of the tissue microenvironment in determining which type of TH response is induced.
TLR1-induced IL-6 is critical for mucosal TH17 responses
Because TLR1 is expressed and functions similarly between mucosal and systemic DCs (unpublished data; Edwards et al., 2003) and the conditioning of DCs determines the type of T cell response induced, we hypothesized that intestinal but not SPL DCs would induce TH17 cell differentiation when stimulated with Y. enterocolitica lysates. A distinctive feature of small intestinal DCs is that they operate in an environment rich in TGF-β and vitamin A and express ALDHA2 (aldehyde dehydrogenase-1 member A2), a retinal dehydrogenase involved in the conversion of retinal into RA (Coombes et al., 2007; Sun et al., 2007). Expression of TLR2 on CD4 T cells has been shown to promote TH17 cells (Reynolds et al., 2010). To directly examine the effect of TLR1 deficiency in DCs, we used a co-culture system with wild-type naive CD4 T cells and DCs from TLR1-deficient mice. As anticipated, when stimulated with Y. enterocolitica lysates, MLN DCs from TLR1+/− mice induced TH17 cells in vitro, whereas SPL DCs induced TH1 cell differentiation (Fig. 3 A). In accordance with a selective role for TLR1 in TH17 cell differentiation in vivo (Fig. 2, A and B), TLR1−/− MLN DCs induced significantly fewer TH17 cells than littermate control MLN DCs, whereas no differences were observed for TH1 polarization (Fig. 3 A). We next determined whether RA and/or TGF-β would be sufficient to confer the ability to induce TH17 responses to SPL DCs. As anticipated by previous studies showing that under inflammatory conditions RA acquires adjuvant properties and has the ability to promote TH1 and TH17 responses (DePaolo et al., 2011; Hall et al., 2011), incubation of SPL DCs with RA significantly promoted TH1 and, to a lesser degree, TH17 cell differentiation (Fig. 3 B). When TGF-β was added (reproducing the conditions found in the intestinal environment), TH1 polarization was lost and TH17 polarization was observed (Fig. 3 B); however, neutralization of TGF-β in the cultures was able to restore TH1 polarization (not depicted). These results emphasize the critical role played by the tissue in determining which type of immune response will be mounted against a given pathogen. It also reaffirms that RA loses its tolerogenic properties under inflammatory conditions and suggests that depending on the level of TGF-β, it may preferentially promote TH1 or TH17 immunity.
We next assessed the in vitro contribution of TLR1 in the induction of innate cytokines involved in TH1 and TH17 polarization in the presence of Y. enterocolitica lysates (Fig. 3 C). We found that TLR1 signaling in DCs was involved in the production of IL-6 and IL-23 (Fig. 3 C) but not TGF-β (not depicted). These results are consistent with observations that TLR1-deficient mice displayed decreased levels of IL-6 and IL-23 (Fig. 3 D) but normal levels of TGF-β (not depicted) in MLNs 3 d after oral infection. In accordance with the data showing normal TH1 responses in TLR1-deficient mice during systemic Y. enterocolitica infection (Fig. 2 B), we found no role for TLR1 in IL-12p70 induction in vivo (Fig. 3 D). However, the in vitro experiments indicated a possible role for TLR1 in IL-12p70 induction under conditions in which TGF-β was absent (Fig. 3 C).
Together, these data demonstrate that although TLR1 is important for the induction of IL-6 by DCs, the combinatorial effect of tissue-derived TGF-β and IL-6 is necessary for driving anti-Yersinia TH17 responses. Finally, having demonstrated that TLR1 was required for induction of IL-6 and for TH17 polarization, we also found that IL-6 was required for mucosal TH17 but not for peripheral TH1 responses during Y. enterocolitica infection (Fig. 3 E).
TLR1 signaling is important for recruitment of DCs during oral infection
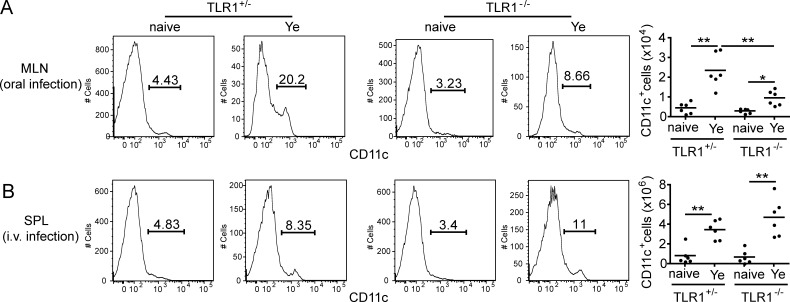
Given that we observed a more drastic and specific role for TLR1 in the induction of TH17 responses in vivo than in vitro, we tested whether TLR1 had an additional role in vivo during oral Y. enterocolitica infection. Taking into account previous findings demonstrating that TLR2/TLR1 ligands imprint a gut-tropic phenotype on T cells (Wang et al., 2011) and low levels of IL-6 and IL-23 in MLNs of TLR1–deficient mice, we hypothesized that TLR1 may also have an effect on DC migration. In accordance with this hypothesis, mice deficient for TLR1 had a 50% reduction in percentage and total DC number in MLNs compared with littermate control mice (Fig. 4 A). In contrast, the absence of TLR1 did not affect the number of DCs in the SPLs of i.v. infected mice (Fig. 4 B). These data suggest that TLR1 controls induction of TH17 responses during oral Y. enterocolitica infection by inducing IL-6 and Il-23 in DCs and allowing their migration to MLNs. We have preliminary evidence that TLR1 may be capable of regulating the production of chemokines necessary for DC recruitment (not depicted).
Figure 4.
TLR1 also contributes to trafficking of CD11c+ cells during oral but not i.v. infection. TLR1+/− and TLR1−/− mice were infected with 105 Y. enterocolitica. (A) MLNs were harvested 3 d after oral infection, and flow cytometry was performed to examine the percentage (left panels) and total cell number (right panel) of CD11c+ cells. Cells were gated on FSC and SSC and CD45. (B) SPLs were harvested 3 d after i.v. infection, and flow cytometry was performed as described in A. Data are pooled from two individual experiments (n = 6). Horizontal bars represent the mean. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
Altogether our study emphasizes that TLR2 is unique in that it possesses dual and opposing immunological functions in the intestinal mucosa when associated with TLR1 or TLR6. Given that TLR2/TRL1 and TLR2/TLR6 ligands are triacylated and diacylated lipopeptides, respectively (Omueti et al., 2005; Jin et al., 2007; Kang et al., 2009), there is the intriguing possibility that enzymes involved in their acylation may dictate whether they will act as pro- or antiinflammatory mediators. Alterations in the acylation status of LPS with similar consequences have been reported (D’Hauteville et al., 2002; Montminy et al., 2006). These observations suggest that by fine-tuning the activity of enzymes mediating acylation, bacteria may have the means to positively or negatively modulate the host immune response, thus allowing for controlled colonization of the host. This is in line with the more general concept that bacteria have evolved ways to promote both pro- and antiinflammatory responses in a concerted manner for their own benefit (Sansonetti and Di Santo, 2007).
Our findings also have implications in the field of vaccine development, as these data highlight the importance of accounting for both the type of pathogen and the site of action when selecting an immune response to maximize protection while avoiding collateral tissue damage. In particular, they stress that the route of vaccination is critical in determining the nature of the TH immune response induced. Finally, in line with other studies (DePaolo et al., 2011; Hall et al., 2011), our observation that RA promotes TH1 and TH17 cell polarization in vitro in the presence of Y. enterocolitica lysates suggests that RA may be a useful adjuvant for vaccines that comprise proinflammatory innate ligands.
MATERIALS AND METHODS
Mice.
TLR1−/− and TLR6−/− mice were generously provided by S. Akira (Osaka University, Suita, Osaka, Japan) and then bred to C57BL/6 mice. TLR1+/− and TLR6+/− were used as controls, unless otherwise noted in figure legends. All mice were maintained at the University of Chicago, and all animal experiments were performed in accordance with institutional guidelines following experimental protocol review and approval by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee.
Bacterial strains and infection.
Y. enterocolitica strain 8081 was grown overnight at 26°C in tryptic soy broth (BD), and the culture was refreshed the next morning for 2 h. Bacterial density was measured by OD at 600 nM and diluted to 106 CFU/ml in sterile PBS. Mice were administered 100 µl by intragastric gavage or i.v. In some experiments, mice were treated i.p. with 100 µg anti–IL-17 or anti-IgG2a (R&D Systems) or 100 µg anti–IL-6 or anti-IgG1 (eBioscience) beginning at the time of infection and then every other day for 5 d as previously described (Meng et al., 2009). Bacterial burden was determined by performing serial dilutions of MLNs or SPLs on tryptic soy agar plates (BD). Plates were grown for 48 h at 26°C.
Yersinia-specific antibody ELISA.
96-well plates were coated overnight at 4°C with 10 µg/ml Y. enterocolitica lysate prepared as previously described (DePaolo et al., 2008). In brief, strain 8081 was grown as described in the previous section. The bacteria was pelleted and reconstituted in 0.5 ml sterile PBS. Silicon beads were added to the bacteria, and the solution was lysed using a BeadBeater (BioSpec) for 5 min. Protein determination was performed on the lysate, and 100 µl lysate was plated to make sure all the bacteria were killed. Fecal samples were prepared by collecting fresh fecal pellets and dissolving in 1 ml lysis buffer containing soybean trypsin. Serum was prepared via terminal bleed of the retroorbital plexus. Undiluted fecal supernatants and serum (diluted 1:2) were added to bacteria-coated plates for 2 h at room temperature. Plates were washed and then incubated with HRP-conjugated anti–mouse IgA (Santa Cruz Biotechnology, Inc.) or rabbit anti–mouse IgG1, rabbit anti–mouse IgG2a, or rabbit anti–mouse IgM followed by anti–rabbit HRP (BD). TMB substrate (Dako) was used for detection, and absorbance was read at 495 nm OD. Antibody titers were detected by ELISA and calculated according to the formula: (OD450nm sample − OD450nm of blank) × serum dilution.
Flow cytometry.
MLN and SPL cells (0.5–106) were incubated overnight with 10 µg/ml heat-killed Y. enterocolitica (HKY) and the next day were stimulated for 2 h with PMA and ionomycin in the presence of Golgi plug (BD). The cells were washed and incubated with anti–mouse FcRII/III (CD16/32 clone 2.4G2; BD) for 15 min at 4°C to prevent nonspecific Fc binding. Cells were washed in PBS with 10% BSA (Sigma-Aldrich) and labeled directly with monoclonal antibodies specific for lymphocyte surface markers CD4 PeCy5 (L3T4; BD), IFN-γ PE (XMG1.2; eBioscience), IL-17 PE (17B7; eBioscience), and IL-10 APC (JES5-16E3; eBioscience) at 4°C for 15 min. For CD11c experiments, MLNs and SPLs were digested as described in In vitro DC and T cell co-cultures. Cells were stained with CD45 APC-Cy7 (3011; BD), CD11c FITC (HL3; BD), CD3e PE (145-2C11; BD), and CD19 PE (ID3; BD). Expression was determined on a FACSCanto flow cytometer (BD) using FlowJo software (Tree Star).
Detection of cytokines.
MLNs or SPLs were homogenized in 1 ml PBS, and ELISA was performed according to product instructions from BD OptEIA kits (IL-10, IL-12p40, and IL-12p70) and R&D Systems kits (IL-23 and TGF-β).
In vitro DC and T cell co-cultures.
MLN DCs and SPL DCs were isolated as previously described (DePaolo et al., 2011). In brief, MLNs or SPLs were dissociated using collagenase IV (Sigma-Aldrich), and monocytes were purified using a density gradient. CD11c+ cells were purified using CD11c-microbeads (Miltenyi Biotec). 5 × 104 DCs were stimulated with 10 µg/ml Y. enterocolitica lysate generated through sonication, and 105 CD4 T cells were harvested from SPLs of wild-type mice via CD4-positive selection (autoMACS; Miltenyi Biotec). Cultures were then stimulated with 10 nM RA or 10 nM RA plus 2 ng/ml TGF-β and in some experiments 1 µg/ml anti–TGF-β (clone 9016; R&D Systems), and supernatants were analyzed after 48 h for cytokines IFN-γ and IL-17 using ELISA.
Statistics.
Paired and unpaired Student’s t tests were used when noted in figure legends. Tests that had an interaction of P < 0.05 were considered significant. Wilcoxon log-rank test was used for analysis of survival.
Online supplemental material.
Fig. S1 shows the levels of innate cytokines from TLR1−/−, TLR6−/−, or littermate control mice infected orally or i.v. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20112339/DC1.
Supplementary Material
Acknowledgments
We thank Benjamin Sally for critical reading of the manuscript.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases K-award DK8275 (to R.W. DePaolo) and Crohn’s and Colitis Foundation of America Senior research award 2831 (to B. Jabri).
The authors declare no competing financial interests.
Author contributions: R.W. DePaolo conceived the idea, supervised all investigations, designed and performed experiments, analyzed data, and wrote the manuscript; S. Khakpour, Y. Sugiura, and W. Wang provided technical assistance; B. Jabri provided input into the conceptual development of the experiments and wrote the manuscript; K. Kamdar performed experiments and helped write the manuscript.
Footnotes
Abbreviations used:
- MLN
- mesenteric LN
- RA
- retinoic acid
- SPL
- spleen
References
- Bohn E., Autenrieth I.B. 1996. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J. Immunol. 156:1458–1468 [PubMed] [Google Scholar]
- Cleveland M.G., Gorham J.D., Murphy T.L., Tuomanen E., Murphy K.M. 1996. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect. Immun. 64:1906–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G.R. 2002. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat. Rev. Mol. Cell Biol. 3:742–752 10.1038/nrm932 [DOI] [PubMed] [Google Scholar]
- D’Hauteville H., Khan S., Maskell D.J., Kussak A., Weintraub A., Mathison J., Ulevitch R.J., Wuscher N., Parsot C., Sansonetti P.J. 2002. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J. Immunol. 168:5240–5251 [DOI] [PubMed] [Google Scholar]
- DePaolo R.W., Tang F., Kim I., Han M., Levin N., Ciletti N., Lin A., Anderson D., Schneewind O., Jabri B. 2008. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 4:350–361 10.1016/j.chom.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaolo R.W., Abadie V., Tang F., Fehlner-Peach H., Hall J.A., Wang W., Marietta E.V., Kasarda D.D., Waldmann T.A., Murray J.A., et al. 2011. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 471:220–224 10.1038/nature09849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube P.H., Handley S.A., Lewis J., Miller V.L. 2004. Protective role of interleukin-6 during Yersinia enterocolitica infection is mediated through the modulation of inflammatory cytokines. Infect. Immun. 72:3561–3570 10.1128/IAI.72.6.3561-3570.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A.D., Diebold S.S., Slack E.M., Tomizawa H., Hemmi H., Kaisho T., Akira S., Reis e Sousa C. 2003. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 α+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33:827–833 10.1002/eji.200323797 [DOI] [PubMed] [Google Scholar]
- Endt K., Stecher B., Chaffron S., Slack E., Tchitchek N., Benecke A., Van Maele L., Sirard J.C., Mueller A.J., Heikenwalder M., et al. 2010. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 6:e1001097 10.1371/journal.ppat.1001097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V., Rakotobe S., Lécuyer E., Mulder I., Lan A., Bridonneau C., Rochet V., Pisi A., De Paepe M., Brandi G., et al. 2009. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 31:677–689 10.1016/j.immuni.2009.08.020 [DOI] [PubMed] [Google Scholar]
- Hall J.A., Cannons J.L., Grainger J.R., Dos Santos L.M., Hand T.W., Naik S., Wohlfert E.A., Chou D.B., Oldenhove G., Robinson M., et al. 2011. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 34:435–447 10.1016/j.immuni.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S., Lawson M.A., Slack E., Kirundi J.K., Stoel M., Heikenwalder M., Cahenzli J., Velykoredko Y., Balmer M.L., Endt K., et al. 2010. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 328:1705–1709 10.1126/science.1188454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein J., Sing A., Di Genaro M.S., Autenrieth I.B. 2001. Interleukin-12 and interleukin-18 are indispensable for protective immunity against enteropathogenic Yersinia. Microb. Pathog. 31:195–199 10.1006/mpat.2001.0458 [DOI] [PubMed] [Google Scholar]
- Ishigame H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H., et al. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 30:108–119 10.1016/j.immuni.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., et al. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 139:485–498 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar Z., Ferrini M.E., Herritt L.A., Roberts K. 2009. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J. Immunol. 182:4507–4511 10.4049/jimmunol.0900237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M.S., Kim S.E., Heo J.Y., Lee M.E., Kim H.M., Paik S.G., Lee H., Lee J.O. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 130:1071–1082 10.1016/j.cell.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Kang J.Y., Nan X., Jin M.S., Youn S.J., Ryu Y.H., Mah S., Han S.H., Lee H., Paik S.G., Lee J.O. 2009. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity. 31:873–884 10.1016/j.immuni.2009.09.018 [DOI] [PubMed] [Google Scholar]
- Lahesmaa-Rantala R., Toivanen A., Granfors K., Yurdakul S., Hamuryudan V., Yazici H. 1989. Anti-Yersinia antibodies in patients with Behçet’s disease. Arthritis Rheum. 32:1494–1495 10.1002/anr.1780321132 [DOI] [PubMed] [Google Scholar]
- Mangan P.R., Harrington L.E., O’Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234 10.1038/nature04754 [DOI] [PubMed] [Google Scholar]
- Matteoli G., Fahl E., Warnke P., Müller S., Bonin M., Autenrieth I.B., Bohn E. 2008. Role of IFN-gamma and IL-6 in a protective immune response to Yersinia enterocolitica in mice. BMC Microbiol. 8:153 10.1186/1471-2180-8-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P., Kamala T. 2011. Tissue-based class control: the other side of tolerance. Nat. Rev. Immunol. 11:221–230 10.1038/nri2940 [DOI] [PubMed] [Google Scholar]
- Meng G., Zhang F., Fuss I., Kitani A., Strober W. 2009. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 30:860–874 10.1016/j.immuni.2009.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy S.W., Khan N., McGrath S., Walkowicz M.J., Sharp F., Conlon J.E., Fukase K., Kusumoto S., Sweet C., Miyake K., et al. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7:1066–1073 10.1038/ni1386 [DOI] [PubMed] [Google Scholar]
- Omueti K.O., Beyer J.M., Johnson C.M., Lyle E.A., Tapping R.I. 2005. Domain exchange between human toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J. Biol. Chem. 280:36616–36625 10.1074/jbc.M504320200 [DOI] [PubMed] [Google Scholar]
- Reynolds J.M., Pappu B.P., Peng J., Martinez G.J., Zhang Y., Chung Y., Ma L., Yang X.O., Nurieva R.I., Tian Q., Dong C. 2010. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 32:692–702 10.1016/j.immuni.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J.L., Lee S.M., Li J., Tran G., Jabri B., Chatila T.A., Mazmanian S.K. 2011. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 332:974–977 10.1126/science.1206095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saebo A., Vik E., Lange O.J., Matuszkiewicz L. 2005. Inflammatory bowel disease associated with Yersinia enterocolitica O:3 infection. Eur. J. Intern. Med. 16:176–182 10.1016/j.ejim.2004.11.008 [DOI] [PubMed] [Google Scholar]
- Sansonetti P.J., Di Santo J.P. 2007. Debugging how bacteria manipulate the immune response. Immunity. 26:149–161 10.1016/j.immuni.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Sing A., Rost D., Tvardovskaia N., Roggenkamp A., Wiedemann A., Kirschning C.J., Aepfelbacher M., Heesemann J. 2002. Yersinia V–antigen exploits toll-like receptor 2 and CD14 for interleukin 10–mediated immunosuppression. J. Exp. Med. 196:1017–1024 10.1084/jem.20020908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T.L., Bossie A., Sanders V.M., Fernandez-Botran R., Coffman R.L., Mosmann T.R., Vitetta E.S. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 334:255–258 10.1038/334255a0 [DOI] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775–1785 10.1084/jem.20070602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Yamakawa N., Yamaguchi H., Okada A.A., Konoeda Y., Ogawa T., Kamiya S., Usui M. 1996. Common antigenicity between Yersinia enterocolitica-derived heat-shock protein and the retina, and its role in uveitis. Ophthalmic Res. 28:284–288 10.1159/000267916 [DOI] [PubMed] [Google Scholar]
- Trülzsch K., Oellerich M.F., Heesemann J. 2007. Invasion and dissemination of Yersinia enterocolitica in the mouse infection model. Adv. Exp. Med. Biol. 603:279–285 10.1007/978-0-387-72124-8_25 [DOI] [PubMed] [Google Scholar]
- Wang S., Villablanca E.J., De Calisto J., Gomes D.C., Nguyen D.D., Mizoguchi E., Kagan J.C., Reinecker H.C., Hacohen N., Nagler C., et al. 2011. MyD88-dependent TLR1/2 signals educate dendritic cells with gut-specific imprinting properties. J. Immunol. 187:141–150 10.4049/jimmunol.1003740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C.T., Hatton R.D. 2009. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 9:883–889 10.1038/nri2660 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.