IL-1α promotes a cascade of cytokine production from epithelial cells culminating in Th2 immunity to house dust mite allergens.
Abstract
House dust mite (HDM) is one of the most common allergens worldwide. In this study, we have addressed the involvement of IL-1 in the interaction between HDM and the innate immune response driven by lung epithelial cells (ECs) and dendritic cells (DCs) that leads to asthma. Mice lacking IL-1R on radioresistant cells, but not hematopoietic cells, failed to mount a Th2 immune response and did not develop asthma to HDM. Experiments performed in vivo and in isolated air–liquid interface cultures of bronchial ECs showed that TLR4 signals induced the release of IL-1α, which then acted in an autocrine manner to trigger the release of DC-attracting chemokines, GM-CSF, and IL-33. Consequently, allergic sensitization to HDM was abolished in vivo when IL-1α, GM-CSF, or IL-33 was neutralized. Thymic stromal lymphopoietin (TSLP) became important only when high doses of allergen were administered. These findings put IL-1α upstream in the cytokine cascade leading to epithelial and DC activation in response to inhaled HDM allergen.
Allergic asthma is characterized by a Th2-dominated immune response to inhaled allergens like house dust mite (HDM), cockroach, and animal dander, which leads to eosinophilic inflammation of the airways, goblet cell metaplasia (GCM), and bronchial hyperreactivity (Barnes, 2008). The incidence of allergic sensitization to inhaled allergens has steadily risen over the last 60 yr, and in some countries up to one third of children have a positive skin prick test to environmental allergen (Umetsu et al., 2002). The various molecularly defined allergens found within the HDM Dermatophagoides pteronyssinus are the most common triggers of allergic asthma worldwide, which has led to the development of animal models that use inhaled HDM extracts to study the cellular and molecular mechanisms of allergic sensitization. Recent studies have shown that inhaled HDM extract stimulates Th2 immunity by acting on barrier epithelial cells (ECs), antigen-presenting DCs, and innate immune cells like basophils and mast cells (Hammad et al., 2010; Wills-Karp, 2010; Lambrecht and Hammad, 2012). One predominant way by which HDM induces Th2 immunity is by triggering the TLR4 receptor expressed on bronchial ECs, through its major allergen Der p 2 together with endotoxin contained in fecal pellets of the mite (Trompette et al., 2009). There is also evidence that components of HDM trigger C-type lectin or protease-activated receptors and thus contribute to the recruitment and activation of innate and adaptive immune cells (Barrett et al., 2009; Nathan et al., 2009; Lewkowich et al., 2011).
Research from various laboratories has shown that triggering of pattern recognition receptors on ECs leads to release of innate pro-Th2 cytokines like thymic stromal lymphopoietin (TSLP), GM-CSF, IL-25, and the IL-1 family member IL-33 that share the capacity of activating DCs (Hammad et al., 2009; Phipps et al., 2009; Kool et al., 2011). In this regard, TSLP has received much attention as it promotes Th2 development by DCs, activation of innate lymphoid cells, and basophil hematopoiesis (Kitajima et al., 2011; Siracusa et al., 2011). Transgenic (Tg) overexpression of TSLP in lung ECs led to enhanced Th2 responses to inhaled environmental antigens (Headley et al., 2009; Lei et al., 2011). Studies in human severe asthmatics demonstrated increased levels of TSLP messenger RNA and protein in bronchial biopsy specimens and identified associations between TSLP promoter region polymorphisms and risk of developing asthma (Harada et al., 2011; Shikotra et al., 2012). Although TSLP has become a prime target for intervention in allergic disease, other cytokines like IL-33, GM-CSF, or IL-25 could also control Th2 immunity to inhaled allergens (Fort et al., 2001; Hurst et al., 2002; Cates et al., 2004; Besnard et al., 2011).
The signals that control innate cytokine and chemokine release from bronchial ECs are poorly understood, and it is particularly unclear whether released cytokines could act as a cascade, with one cytokine stimulating the release of another, and thus acting as a controller of allergic sensitization. This possibility was raised when in vitro studies on cultured lung ECs found that IL-1 is a potent upstream inducer of TSLP production (Allakhverdi et al., 2007; Lee and Ziegler, 2007; Lee et al., 2008).
In this study, we have addressed the involvement of IL-1 in the interaction between HDM allergen, lung ECs, and DCs that leads to asthma. We found that mice lacking IL-1R on radioresistant cells, but not hematopoietic cells, were totally protected from mounting Th2 immunity and asthma to low doses of HDM. Experiments performed in vivo and in isolated air–liquid interface (ALI) cultures of bronchial ECs showed that this was caused by the TLR4-mediated release of IL-1α, which acted in an autocrine manner on bronchial ECs to release DC-attractive chemokines, GM-CSF, and IL-33 but not TSLP. Consequently, allergic sensitization to low-dose HDM was only abolished in vivo when IL-1α, GM-CSF, or IL-33 was neutralized. However, TSLP and its receptor became important when mice were exposed to high doses of HDM. These findings put IL-1α upstream in the cytokine cascade leading to epithelial and DC activation in response to inhaled HDM allergen.
RESULTS
IL-1RI signaling is crucial for development of asthma to HDM
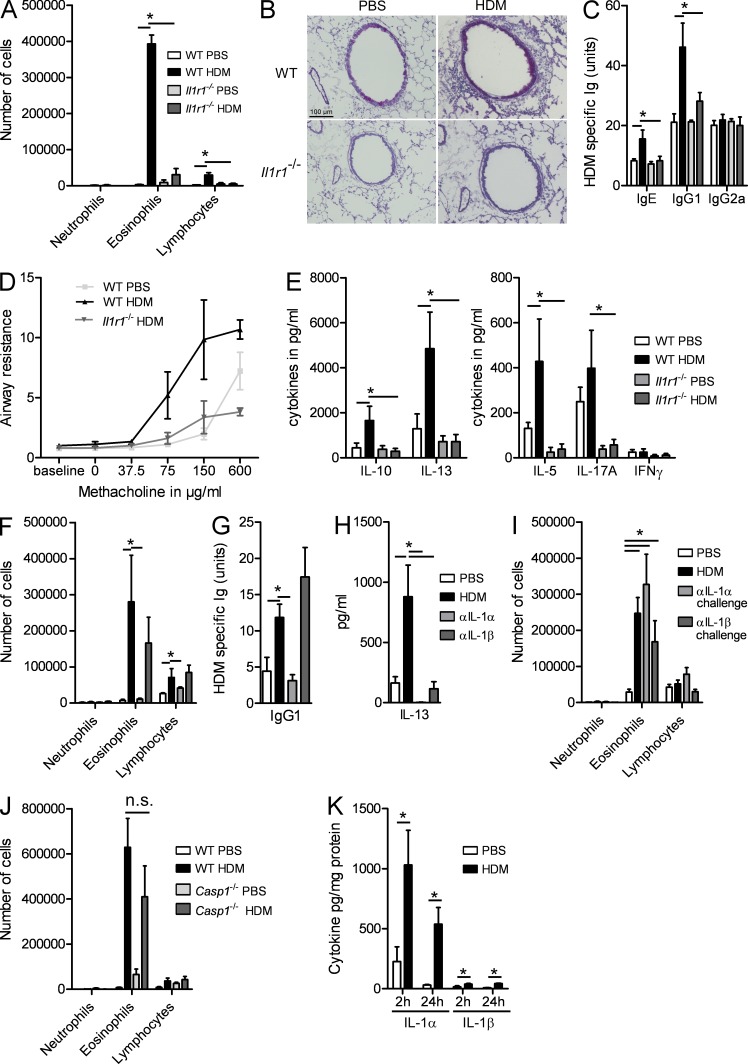
IL-1α and IL-1β are closely related cytokines that share biological activity by acting exclusively on the IL-1R1 on various cell types. To address how these two cytokines affect development of asthma, we sensitized WT and Il1r1−/− mice using 1 µg HDM or PBS as a control and challenged all mice intranasally 1 wk later on five consecutive days with 10 µg HDM. This protocol differs from previously published work on HDM-driven asthma, where we (Hammad et al., 2009) and others (Cates et al., 2004; Lewkowich et al., 2005; Phipps et al., 2009) administered higher doses of HDM. Using this protocol, we observed that HDM-sensitized WT mice developed signs of allergic asthma, such as influx of eosinophils and lymphocytes in the bronchoalveolar lavage (BAL) fluid (Fig. 1 A), peribronchial and perivascular infiltration of mononuclear cells, lymphocytes, and eosinophils, and GCM (Fig. 1 B), whereas sham PBS-sensitized mice exposed to HDM did not. In their serum, HDM-sensitized mice produced HDM-specific IgG1 and IgE, whereas Th1 cytokine–dependent IgG2a antibodies were not induced (Fig. 1 C). HDM-sensitized and challenged mice developed bronchial hyperreactivity to methacholine, whereas sham-sensitized mice did not (Fig. 1 D). These features of asthma were accompanied by enhanced production of Th2 cytokines IL-13, IL-5, and IL-10 in the mediastinal LNs (MLNs; Fig. 1 E). The levels of IL-17 and IFN-γ were not induced significantly by active HDM sensitization. Importantly, when these experiments were performed in Il1r1−/− mice, there was a strong reduction in lung eosinophilia and lymphocytosis, GCM, HDM-specific IgG1 and IgE production, and bronchial hyperreactivity to methacholine. In addition to severely impaired Th2 cytokine production, we also noticed that production of IL-17 was impaired in Il1r1−/− mice, consistent with a known role for this cytokine in Th17 development (Sutton et al., 2006).
Figure 1.
IL-1RI signaling is crucial for the development of HDM-induced asthma. (A–E) WT and Il1r1−/− mice were sensitized on day 0 with HDM or PBS and were challenged with HDM on days 7–11. (A) Differential cell counts were determined by flow cytometry 72 h later. (B) PAS staining of lung sections. (C) Levels of serum HDM–specific Igs. (D) Airway resistance in response to increasing concentrations of methacholine. (E) Cytokine levels in MLN cells restimulated for 3 d with 15 µg/ml HDM. (F–H) C57BL/6 mice were sensitized with HDM in the presence or absence of blocking antibodies to IL-1α or IL-1β and were challenged with HDM. (F) Differential cell counts were determined by flow cytometry 72 h later. (G) IgG1 levels in sera. (H) IL-13 levels in MLN cells restimulated for 3 d with HDM. (I and J) C57BL/6 mice were sensitized with HDM or PBS and were administered blocking antibodies to IL-1α or IL-1β on the last 3 d of HDM challenge. (I) Differential cell counts were determined by flow cytometry 72 h later. (J) WT and Casp1−/− mice were sensitized with HDM or PBS as a control and were rechallenged with HDM. Differential cell counts were determined by flow cytometry 72 h later. (K) WT mice were administered with PBS or HDM. IL-1α and IL-1β contents were determined in lung homogenates 2 and 24 h later. *, P < 0.05. Results show one representative experiment out of three. Five to six mice/group were used. Results are shown as mean ± SEM.
HDM sensitization depends mainly on IL-1α, not IL-1β
These experiments demonstrated that IL-1RI signaling is crucial for development of asthma but did not address whether IL-1 is required during sensitization (i.e., the first administration of HDM) or challenge phase (days 7–12) of the response. Also, as IL-1α and IL-β both trigger the IL-1RI equally well, we addressed their relative contribution during sensitization or challenge. Neutralizing IL-1α with a blocking antibody during sensitization led to strongly reduced airway eosinophilia and lymphocytosis (Fig. 1 F), serum HDM–specific Ig levels (Fig. 1 G), Th2 cytokines (Fig. 1 H; only IL-13 shown as representative Th2 cytokine), and periodic acid-Schiff (PAS)–positive GCM (not depicted). Neutralizing IL-1β during sensitization had no statistically significant effect on eosinophilic influx in the lung and on serum HDM–specific Igs (Fig. 1, F and G). IL-1β neutralization did reduce levels of Th2 cytokine production in the MLNs, however to a lesser extent than IL-1α blockade (Fig. 1 H). When either IL-1α or IL-1β was blocked during the challenge phase, there was no effect on BAL fluid cellular composition (Fig. 1 I) or any of the other parameters (not depicted).
Caspase-1 (a.k.a. IL-1 converting enzyme 1) is necessary to process pro–IL-1β into biologically active secreted IL-1β, whereas it is not necessary for IL-1α biological activity (Dinarello, 2009). We therefore sensitized WT and casp1−/− mice to HDM and challenged them with HDM. No differences on lung inflammation (Fig. 1 J) or any of the other parameters (not depicted) were observed in casp1−/− mice, further supporting the idea that mainly IL-1α is necessary for HDM-induced Th2 immunity.
One possible explanation for the differential effect of IL-1α versus IL-1β neutralization on development of HDM asthma could be different levels of production of either cytokine. We therefore measured the levels of IL-1α and IL-1β in lungs, 2 and 24 h after HDM administration of naive mice and found that IL-1α levels were strongly induced at 2 and 24 h, whereas IL-1β was barely detected (Fig. 1 K).
IL-1RI is necessary on radioresistant cells of the lungs
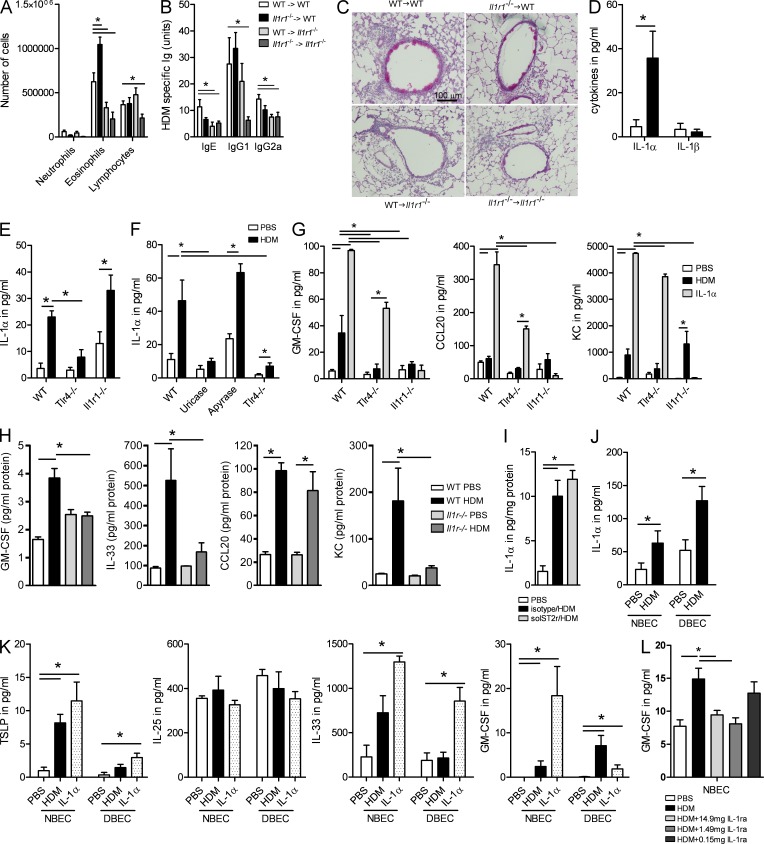
Because we observed that IL-1α was necessary for promoting Th2 immunity to natural allergens in the lung, we next questioned how it promoted Th2 immunity. The IL-1R1 is not only expressed on various immune cells like macrophages, DCs, T lymphocytes, mast cells, and eosinophils, but also on structural cells like fibroblasts and ECs. To address on which cell type IL-1R was necessary, we made a series of bone marrow chimeric mice. WT and Il1r1−/− mice were sublethally irradiated and received donor WT or Il1r1−/− bone marrow to allow for hematopoietic reconstitution. 10 wk later, we sensitized and challenged them with HDM. As in previous experiments (Fig. 1), WT → WT chimeric mice mounted higher degrees of airway eosinophilia and lymphocytosis (Fig. 2 A), serum HDM–specific IgG1 (Fig. 2 B), and GCM (Fig. 2 C) compared with Il1r1−/− → Il1r1−/− mice, essentially illustrating that the irradiation and chimerism procedure did not affect the fundamental outcome of the experiment previously performed in unmanipulated WT and Il1r1−/− mice. We observed a similar reduction in BAL fluid eosinophilia and lymphocytosis, HDM-specific Igs, and GCM in mice that lacked the IL-1RI on radioresistant structural cells (WT → Il1r1−/−), whereas no reduction was seen in mice lacking IL-1RI on hematopoietic cells (Il1r1−/− → WT).
Figure 2.
IL-1RI is necessary on radioresistant structural cells of the lungs. (A–C) Various chimeric mice (coded as bone marrow donor → recipient) were sensitized and challenged with HDM as described in Fig. 1. (A) Differential cell counts were determined 72 h later. (B) Levels of serum HDM–specific Igs. (C) PAS staining of lung sections. Five to six mice/group were used. (D) ALI cultures of primary tracheal ECs from WT mice were exposed to HDM or PBS as a control. Levels of IL-1α and IL-1β were measured in supernatants. (E) ALI cultures of primary tracheal ECs from WT, Tlr4−/−, and Il1r1−/− mice were exposed to HDM or PBS. Levels of IL-1α were measured in supernatants. (F) C57BL/6 WT mice were injected i.t. with PBS, uricase, or apyrase 30 min before exposure to PBS or HDM. Tlr4−/− mice were exposed i.t. to PBS or HDM. BAL was collected after 2 h and was analyzed for the presence of IL-1α. (G) Levels of GM-CSF, CCL20, and KC were measured in BAL fluids 24 h after exposure to HDM, IL-1α, or PBS. (H) GM-CSF, IL-33, CCL20, and KC levels in supernatants of ALI cultures of WT and Il1r−/− mice exposed to PBS or HDM. (I) WT mice were injected i.p. with solST2r and with HDM i.t. IL-1α levels were measured in lung homogenates 24 h later. (J) Primary ECs from healthy donors (NBECs) and from asthmatic patients (DBECs) were exposed to HDM or PBS. Levels of IL-1α were measured 24 h later. (K) TSLP, IL-25, GM-CSF, and IL-33 levels in supernatants of NBEC and DBEC cultures exposed to HDM, IL-1α, or PBS. (L) Primary ALI cultures of human healthy donors (NBECs) were exposed to PBS or HDM in the presence or not of different doses of IL-1ra. GM-CSF was analyzed in the supernatant of these cultures after 24 h. *, P < 0.05. Results show one representative experiment out of three. Results are shown as mean ± SEM.
Autocrine IL-1α acts on ECs to promote production of chemokines and innate pro-Th2 cytokines
Because of their exposed position as barrier cells of the lungs, bronchial ECs are prime candidates among radioresistant cells for producing IL-1, as well as responding to it. To address this possibility, we set up ALI cultures of primary ECs obtained from digested tracheas of various WT and gene-deficient mice. When these cultures were stimulated with HDM extract, they produced mainly IL-1α but not IL-1β (Fig. 2 D), reflecting the situation seen upon in vivo exposure of the lung to HDM (Fig. 1 K). We and others have previously reported that TLR4 stimulation of ECs is a crucial event in the development of Th2 immunity to HDM (Hammad et al., 2009; Trompette et al., 2009). When ALI cultures were set up using tracheal ECs of Tlr4−/− mice, HDM was less able to induce IL-1α production (Fig. 2 E). When ALI cultures were set up derived from Il1r1−/− mice, however, HDM was however fully able to induce IL-1α production (Fig. 2 E). Moreover, in vivo, the levels of IL-1α were also increased in the BAL fluids of WT mice administered with HDM (Fig. 2 F). The HDM-induced production of IL-1α seen in the BAL of WT animals was absent in Tlr4−/− mice (Fig. 2 F). In addition, we also found no increase in IL-1α levels in the BAL of mice treated with uricase at the time of HDM administration (Fig. 2 F), indicating that uric acid (UA) produced in response to HDM exposure could induce IL-1α production. However, apyrase pretreatment to degrade any HDM-induced ATP production did not show an effect on IL-1α release (Fig. 2 F).
Various pro-Th2 innate cytokines like TSLP, GM-CSF, IL-33, and IL-25, as well as chemokines like KC and CCL20 that can be produced by lung ECs, have been found to be induced in the lungs of HDM-exposed mice in vivo (Hammad and Lambrecht, 2008). We next questioned whether epithelial IL-1α and IL-1RI signaling was involved in an autocrine loop influencing the production of these factors. When tracheal epithelial ALI cultures of WT mice were stimulated with HDM extracts, GM-CSF and KC were produced, in a process requiring TLR4 (Fig. 2 G). The levels of TSLP, IL-25, and IL-33 in ALI cultures were around the detection limit of the cytokine ELISA and were inconsistent between repeat experiments, so we focused our attention on the production of GM-CSF and chemokines. ALI cultures stimulated with rIL-1α produced GM-CSF, CCL20, and KC, and these effects were not seen in Il1r1−/− mice, showing that these effects were not caused by some contaminant in the IL-1 preparations (Fig. 2 G). Strikingly, HDM was unable to induce GM-CSF and CCL20 production in ALI cultures generated from Il1r1−/− mice, essentially demonstrating that autocrine release of IL-1α was inducing these factors in a process requiring TLR4 triggering by HDM. We next studied the in vivo production of early innate pro-Th2 cytokines and chemokines in WT and Il1r1−/− mice by measuring their presence in lung homogenates 12 h after administration of HDM intratracheally (i.t.). As shown in Fig. 2 H, HDM administration in WT mice led to the production of GM-CSF, IL-33, CCL20, and KC, whereas TSLP and IL-25 were around the limit of detection in this assay (not depicted). The production of GM-CSF, IL-33, and KC but not that of CCL20 was severely reduced in Il1r1−/− mice (Fig. 2 H), suggesting that these cytokines are downstream of IL-1. Moreover, the blockade of IL-33 at the time of HDM administration did not affect IL-1α production in the lung, showing that IL-1 is upstream of IL-33 (Fig. 2 I).
Human ALI cultures produce IL-1 and GM-CSF in response to HDM allergen
To investigate the translational aspect of these findings, we stimulated ALI cultures of commercially available primary human bronchial ECs (healthy: normal bronchial ECs [NBECs]; asthmatic: diseased bronchial ECs [DBECs]) with HDM allergen after three passages of culture. Exposure of these cultures led to increased IL-1α secretion on the apical side, particularly in ALI cultures set up from DBECs (Fig. 2 J). Exposure of the human ECs to HDM also led to GM-CSF and TSLP secretion on the basolateral side but not of IL-25 (Fig. 2 K). Exposure of human ALI cultures to IL-1α also led to GM-CSF and IL-33 induction in NBEC cultures, but the GM-CSF response was significantly blunted in DBECs (Fig. 2 K). We next addressed whether human IL-1RI signaling was also involved in an autocrine loop influencing the production GM-CSF, as shown previously in mice (Fig. 2 G). NBEC cultures were exposed to PBS or HDM in the presence of different doses of a blocking IL-1R antagonist. IL-1Ra did not induce any cell death in the cultures as assessed by the absence of tight junction disruption (not depicted). As shown in Fig. 2 L, the addition of IL-1Ra to the cultures decreased the production of GM-CSF induced by HDM, demonstrating that the autocrine release of IL-1α was inducing GM-CSF production in human ECs.
Development of Th2 immunity to HDM depends on GM-CSF and IL-33
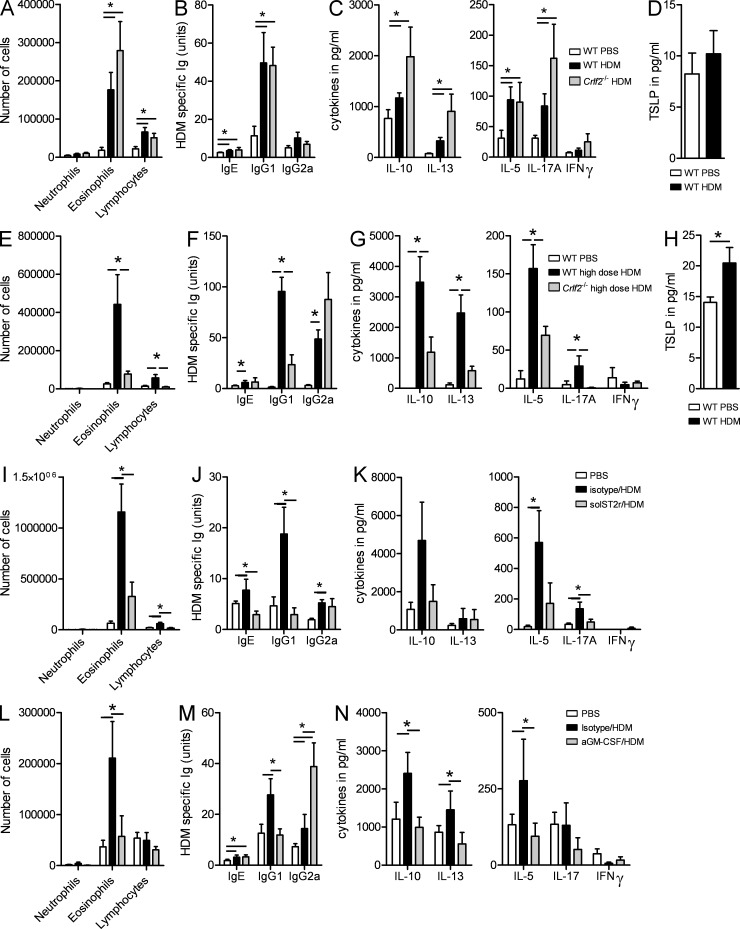
GM-CSF, IL-33, and TSLP are potentially important cytokines that were produced in the epithelial ALI cultures and/or in vivo upon HDM exposure in an IL-1R1–dependent manner. To fully understand the importance of these cytokines in the process of sensitization to HDM, we first sensitized and challenged TSLP receptor–deficient (Crlf2−/−) and WT C57BL/6 control mice to HDM. Using a low dose of HDM to sensitize and challenge mice (1 µg for sensitization followed by 10 µg for challenge for 5 d, as in all experiments), we observed that TSLP receptor signaling was not necessary to induce sensitization to HDM because these knockout mice developed identical eosinophilic and lymphocytic influx in the lungs (Fig. 3 A) and identical HDM-specific IgG1 levels (Fig. 3 B) after HDM challenge and even increased cytokine levels after restimulation of MLN cells (Fig. 3 C). The levels of IL-17 were increased in Crlf2−/− mice. In addition, the administration of a low dose of HDM did not induce TSLP production in the BAL fluids compared with mice exposed to PBS (Fig. 3 D). Similar results were obtained when Tslp−/− C57BL/6 mice or BALB/c Crlf2−/− were used for experiments, suggesting that these results hold true across two strains of mice and when either receptor or its ligand is inhibited (not depicted). However, when we used a higher dose of HDM (100 µg) to sensitize and challenge mice, Crlf2−/− mice developed less airway eosinophilia (Fig. 3 E) accompanied by a reduced Th2 cytokine production by MLN cells (Fig. 3 G) and showed increased IgG2a levels (Fig. 3 F), indicative of a switch to a Th1 type of immunity. Moreover, exposure to a high dose of HDM was accompanied by an increase in BAL fluid levels of TSLP (Fig. 3 H). These findings suggest that the role of TSLP in asthma is related to severity of the disease, as it is in humans. Interestingly, when IL-1R−/− mice were administered the high dose of HDM, they still failed to develop asthma features (not depicted).
Figure 3.
Development of Th2 immunity to HDM depends on IL-33 and GM-CSF and not TSLP. (A–D) WT and Crfl2−/− were sensitized and challenged with HDM or PBS as described in Fig. 1. (A) Differential cell counts were determined 72 h later. (B) Levels of serum HDM–specific Igs. (C) Cytokine levels in MLN cells restimulated for 3 d with HDM. (D) TSLP levels measured in BAL fluids. (E–H) WT and Crfl2−/− were sensitized and challenged with 100 µg HDM or PBS. (E) Differential cell counts were determined 72 h later. (F) Levels of serum HDM–specific Igs. (G) Cytokine levels in MLN cells restimulated for 3 d with HDM. (H) TSLP levels measured in BAL fluids. (I–K) C57BL/6 mice were injected i.p. with blocking soluble ST2 receptor or isotype control at the time of HDM sensitization. (I) Differential cell counts were determined 72 h after the last HDM challenge. (J) Levels of serum HDM–specific Igs. (K) Cytokine levels in MLN cells restimulated for 3 d with HDM. (L–N) C57BL/6 mice were injected i.p. with blocking anti–GM-CSF or isotype control antibodies at the time of HDM sensitization. (L) Differential cell counts were determined 72 h after the last HDM challenge. (M) Levels of serum HDM–specific Igs. (N) Cytokine levels in MLN cells restimulated for 3 d with HDM. *, P < 0.05. Results show one representative experiment out of at least three. Five mice/group were used. Results are shown as mean ± SEM.
To investigate the role of IL-33 in sensitization to low-dose HDM, we injected mice with recombinant soluble ST2 receptor (solST2r) to block IL-33 signaling. We found that the administration of solST2r at the time of sensitization led to a decrease in the number of eosinophils and lymphocytes in the BAL (Fig. 3 I) and in HDM-specific IgE and IgG1 in the serum (Fig. 3 J). rST2 did not significantly affect the levels of cytokines produced by MLNs (Fig. 3 K).
Because GM-CSF–deficient mice spontaneously develop alveolar proteinosis, we were not able to analyze the contribution of genetic deficiency of GM-CSF on HDM-driven asthma (Stanley et al., 1994). To address the functional importance of GM-CSF, we administered a neutralizing GM-CSF antibody at the time of low-dose sensitization to HDM. This lead to reduced eosinophilic influx in the lungs (Fig. 3 L), reduced HDM-specific IgG1 (Fig. 3 M), and Th2 cytokines in the MLNs (Fig. 3 N).
Administration of IL-1α is sufficient to promote sensitization to inhaled protein antigens
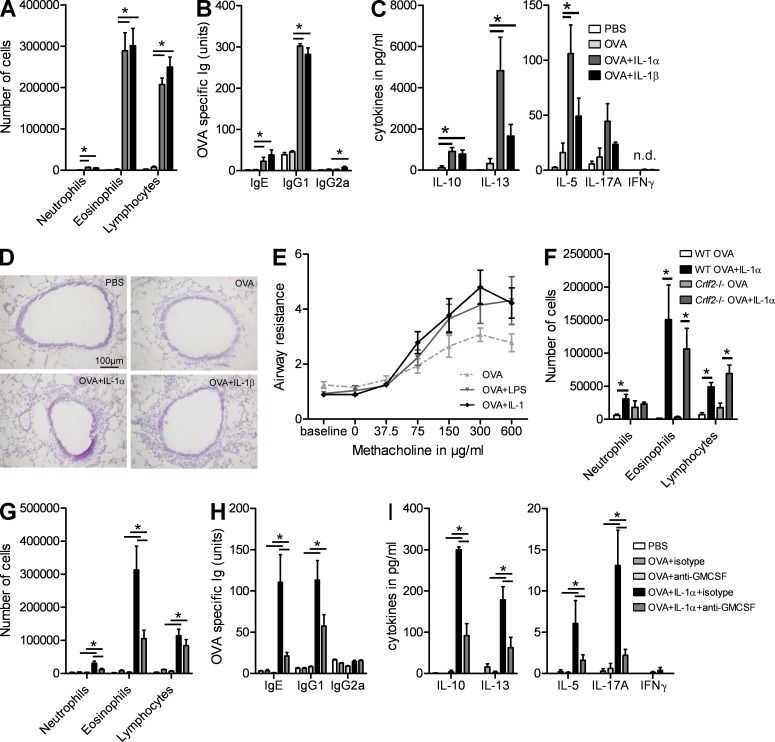
The aforementioned experiments demonstrated that IL-1α was necessary for inducing Th2 immunity to HDM via activation of an epithelial cytokine cascade. As many parallel pathways might exist to induce Th2 immunity to natural allergens in the lungs, we next wanted to study whether IL-1α was also sufficient to induce Th2 immunity. To address this, we administered an otherwise tolerizing dose of harmless OVA antigen to the lungs of naive C57BL/6, in the presence or absence of rIL-1α. 10 d later, all mice were challenged with OVA aerosols. Addition of IL-1α resulted in a significant influx of eosinophils and lymphocytes in the BAL fluid compared with mice sensitized with only OVA or with PBS as a control (Fig. 4 A). Co-administration of OVA and IL-1α also induced increased levels of Th2-dependent OVA-specific IgE and IgG1 in the serum (Fig. 4 B), a Th2 cytokine profile in MLNs (Fig. 4 C), GCM in lung ECs (Fig. 4 D), and bronchial hyperreactivity (Fig. 4 E). As in HDM-induced Th2 immunity, IL-1 had a mild stimulatory effect on IL-17 production, whereas IFN-γ was not induced. These stimulatory effects of IL-1α on Th2 immunity to OVA failed to develop in Il1r1−/− mice, suggesting that they were not caused by some off-target contaminating moiety in the cytokine (not depicted). Similar results were obtained when we administered OVA together with recombinant IL-1β, consistent with the idea that IL-1RI is stimulated equally well by IL-1α and IL-1β (Fig. 3, A–D). Interestingly LPS, an innate adjuvant known to promote Th2 sensitization when administered at low dose, was able to increase bronchial hyperreactivity to the same extent as IL-1 (Fig. 3 E).
Figure 4.
IL-1α is sufficient to promote sensitization to inhaled protein antigens. (A–E) C57BL/6 mice were sensitized on day 0 with OVA in the presence of IL-1α, IL-1β, or PBS. Mice were challenged with OVA aerosols on days 10–12. (A) Differential cell counts were determined 24 h after the last challenge. (B) Levels of serum OVA–specific Igs. (C) Cytokine levels in MLN cells restimulated for 4 d with OVA. (D) PAS staining of lung sections. (E) Bronchial hyperreactivity was analyzed in mice sensitized with OVA + IL-1 or OVA + LPS. (F) C57BL/6 and Crlf2−/− mice were sensitized with OVA or with OVA + IL-1α and were exposed to OVA aerosols. Differential cell counts were determined 24 h after the last challenge. (G–I) C57BL/6 mice sensitized with OVA in the presence or absence of IL-1α and injected with anti–GM-CSF or isotype control antibodies were challenged with OVA aerosols. (G) Differential cell counts were determined 24 h after the last challenge. (H) Levels of serum OVA-specific Igs. (I) Cytokine levels in MLN cells restimulated for 3 d with HDM. *, P < 0.05. Results show one representative experiment out of three. Five mice/group were used. Results are shown as mean ± SEM.
We then addressed the relative contribution of TSLP and GM-CSF in mediating the Th2-promoting effects of IL-1α. The induction of Th2 immunity to OVA + IL-1α (as shown in Fig. 4 F for BAL fluid cellular composition, representative of all other parameters) was unaffected in Crlf2−/− mice lacking TSLPR. Moreover, the levels of TSLP measured in BAL fluids of the mice were very low and were not increased by IL-1α (not depicted). We also neutralized GM-CSF at the time of sensitization to OVA and IL-1α and subsequently challenged mice with OVA aerosol. Compared with mice receiving isotype antibody, GM-CSF neutralization reduced the influx of eosinophils and lymphocytes in the BAL fluid (Fig. 4 G), production of OVA-specific IgE and IgG1 (Fig. 4 H), and Th2 cytokines (Fig. 4 I).
IL-1 induces Th2 sensitization via indirect effects on lung DCs
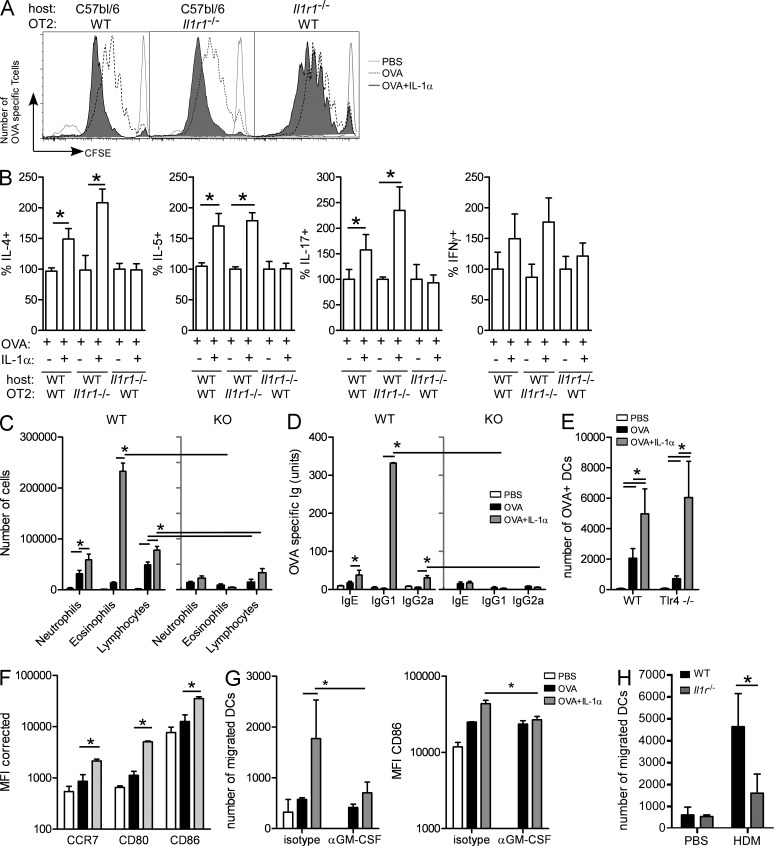
As eosinophilic airway inflammation, GCM, and allergen-specific Ig production are all controlled by CD4 T cell responses, we next addressed how mucosal IL-1 and IL-1R signaling could affect CD4 T cell activation and polarization. For that reason, we injected the model antigen OVA and measured T cell responses in the MLNs after first transferring a cohort of CFSE-labeled OVA-specific TCR Tg OTII cells. As the IL-1RI is also expressed on CD4 T cells and IL-1 was shown to directly affect T cell activation when given systemically or subcutaneously, some mice received IL-1RI–deficient OTII T cells (obtained from Il1r1−/− × OTII mice). To eliminate the contribution of IL-1 on non-T cells and study whether mucosal IL-1 directly signals to T cells, WT OTII cells were also injected in Il1r1−/− recipient mice. 1 d later, we instilled i.t. OVA ± IL-1α or PBS as a control and studied T cell activation 3 d later. In WT recipient mice, OVA-specific T cells had divided more vigorously when IL-1α was injected together with OVA, regardless of whether the OTII cells were responsive to IL-1 or not (Fig. 5 A). However, this effect was not observed in Il1r1−/− recipient mice. Fig. 5 B shows increased IL-4, IL-5, and IL-17A expression in WT mice injected with WT or Il1r1−/− OTII cells. However, if the recipient was deficient of IL-1RI, these cytokines were not increased above the level seen in mice receiving only OVA. These data show that mucosal IL-1α promotes Th2 immunity not by directly affecting antigen-specific CD4 T cells.
Figure 5.
IL-1 induces Th2 sensitization through indirect effects on lung DCs. (A and B) C57BL/6 and Il1r1−/− mice were injected with 10 × 106 CFSE-labeled OVA-specific WT OT2 cells or Il1r1−/− cells and were administered i.t. with PBS, OVA, or OVA + IL-1α. (A) Proliferation of CFSE-labeled T cells was determined by flow cytometry 4 d later. (B) Percentage of CFSE+ T cells positive for IL-4, IL-5, IL-17A, and IFN-γ was determined by flow cytometry. (C and D) MHCIIfl/fl × CD11c-Cre and WT mice were sensitized with OVA or OVA + IL-1α and were challenged with OVA aerosols. (C) Differential cell counts were determined 24 h after the last challenge. (D) Levels of serum OVA–specific Igs. (E) WT and Tlr4−/− mice were administered fluorescent OVA i.t. The number of migrating OVA+ MHCIIhiCD11c+ DCs was determined in the MLNs 24 h later. (F) WT mice were administered i.t. with OVA, OVA + IL-1α, or PBS as a control. The levels of expression of CCR7, CD80, and CD86 were determined using flow cytometry. (G) WT mice were administered OVA i.t., OVA + IL-1α, or PBS. Mice were also injected with blocking antibodies to GM-CSF at the time of sensitization. The number of migrating MHCIIhiCD11c+ DCs and their levels of CD86 expression were determined in the MLNs 24 h later. (H) WT and Il1r1−/− mice were administered HDM or PBS. The number of MHCIIhiCD11c+ DCs was determined in the MLNs 24 h later. *, P < 0.05. Results show one representative experiment out of at least two experiments. Four to five mice/group were used. Results are shown as mean ± SEM.
As inhaled OVA is presented to CD4 T cells mainly by lung DCs and given the fact that CCL20 is a prototypical chemokine attracting immature DCs to the lungs and GM-CSF is an important development and maturation cytokine for DCs, we next studied whether IL-1 and the downstream cytokine cascade promoted development of Th2 immunity by promoting the antigen-presenting function of lung DCs. To address this, we administered OVA + IL-1 to mice in which CD11c+ DCs do not express MHCII (MHCIIfl/fl × Cd11cCre+). MHCIIfl/fl × Cd11cCre− littermates served as controls. In control mice, OVA + IL-1 induced eosinophilia and lymphocytosis (Fig. 5 C), as well as serum Th2–dependent OVA-specific IgG1 (Fig. 5 D), Th2 cytokines in MLN cultures (not depicted), and GCM (not depicted). These features of asthma were strongly reduced in mice lacking MHCII on CD11chi cells.
We finally wanted to address whether and how IL-1α affected DC function and activation in vivo. To trace DC migration, we injected mice with fluorescently labeled OVA with or without IL-1α. 1 d later, the MLNs were analyzed for the number of OVA-carrying DCs and their degree of maturation. Compared with OVA alone, the addition of IL-1α to OVA resulted in enhanced migration of DCs to the MLNs and increased expression of maturation markers CD80 and CD86 on the migrated DCs (Fig. 5, E and F). The effect was also observed in Tlr4−/− mice, demonstrating that the effect of IL-1 was not caused by endotoxin contamination in the recombinant IL-1α. Enhanced DC migration and increased expression of maturation markers were inhibited if GM-CSF was neutralized at the time of OVA + IL-1α injection (Fig. 5 G). To study whether DC functions were also affected by endogenous IL-1R, we studied the migration of lung DCs to the MLNs in WT and Il1r1−/− mice exposed to HDM, a trigger for IL-1α release. As shown in Fig. 5 H, HDM administration led to enhanced DC migration to the MLNs, an effect strongly reduced in Il1r1−/− mice.
DISCUSSION
In this study, we have uncovered a crucial role for IL-1R and IL-1α in causing Th2 sensitization to inhaled HDM. The precise role of IL-1α and IL-1β in development of allergy has been unclear and studied mainly using the model antigen OVA. In some experiments in which OVA alum was injected i.p., there was no role for either cytokine as Il1r1−/− mice developed all features of asthma (Schmitz et al., 2003). However, the administration of recombinant IL-1α at the time of OVA/alum sensitization was shown to reduce asthma features (Caucig et al., 2010). In that study, IL-1α administration at later time points exacerbated the disease. In a milder model in which sensitization was induced in the absence of alum in Il1r1−/− mice, asthma was reduced (Schmitz et al., 2003). The most logical conclusion from these conflicting data is that the strength of model, the route of administration, and the cell population targeted might determine the requirement for IL-1 in disease development. The i.p. injection of IL-1α primarily targets peritoneal macrophages and induces Th1 responses that can suppress Th2 immunity (Caucig et al., 2010). In our study, the i.t. administration of the cytokine preferentially triggers lung ECs, thus favoring Th2 immunity. Using radiation bone marrow chimeric mice and exploiting the natural route of pulmonary exposure to HDM, we provide evidence that IL-1R triggering on radioresistant lung ECs promotes the innate immune response to natural allergens, a feature which was also observed in cigarette smoke–exposed mice (Botelho et al., 2011). Development of Th2 immunity to inhaled HDM or model antigens requires triggering of the TLR4 receptor on radioresistant cells (Hammad et al., 2009; Tan et al., 2010; Kool et al., 2011). This induces the release of epithelial cytokines (TSLP, GM-CSF, IL-33, and IL-25) and chemokines (KC and CCL20) that promote Th2 immunity by activating basophils, eosinophils, and DCs (Fort et al., 2001; Hurst et al., 2002; Cates et al., 2004; Saenz et al., 2008; Besnard et al., 2011). We have set up ALI cultures of primary ECs from the conducting airways of mice and confirmed that GM-CSF, IL-1α, and KC were produced by HDM-stimulated ECs in a process requiring TLR4. We did not find consistent induction of TSLP, IL-33, and IL-25 in the ALI cultures, which does not exclude a role for them in vivo. Strikingly, the production of GM-CSF was impaired in ALI cultures set up from Il1r1−/− mice, uncovering the presence of an endogenous autocrine loop of IL-1α acting on IL-1RI on ECs to promote cytokine production. We also found evidence for this innate pro-Th2 amplification loop in vivo, as exposure of Il1r1−/− mice to HDM led to significantly reduced production of GM-CSF, IL-33, and KC in lung homogenates, suggesting an important role for endogenous IL-1 in promoting Th2 immunity to allergens. Conversely, when we administered IL-1α to naive mouse lungs in vivo, we could induce production of GM-CSF and CCL20 (unpublished data). We have recently reported that endogenous danger signals like UA or ATP played an important role in the process of Th2 sensitization (Kool et al., 2011). In this study, we show that HDM-induced UA production was required for IL-1α production. However the neutralization of ATP with apyrase did not affect IL-1α secretion in vivo, indicating that ATP might be downstream of IL-1α production or might be involved in a different pathway.
There are some subtle differences between the induction of cytokines and chemokines in the ALI cultures in vitro and the response seen in vivo. IL-33 was not induced by HDM in vitro but was consistently induced in vivo in a process requiring IL-1RI. This could be a potentially important observation, as IL-33 has been shown to promote Th2 immunity to allergens by stimulating the function of DCs and innate lymphoid cells (Besnard et al., 2011; Eiwegger and Akdis, 2011). We have previously reported that Th2 immunity induced by inflammatory DCs is blocked when IL-33 signaling is blocked by administration of the soluble T1/ST2 receptor (Lambrecht et al., 2000). In support, we also observed a decrease in the number of BAL eosinophils and HDM-specific Igs when IL-33 signaling was blocked at the time of sensitization in this HDM model.
A central role has been attributed to TSLP in the process of allergic sensitization (Zhou et al., 2005; Liu et al., 2007; Headley et al., 2009). Several groups have reported that IL-1 can induce TSLP (Allakhverdi et al., 2007; Lee and Ziegler, 2007), and a recent study demonstrated that lung DCs produce TSLP upon exposure to HDM (Kashyap et al., 2011). We found that the development of Th2 immunity driven by IL-1 or by a low dose of HDM did not require TSLP or its receptor. When a high dose of HDM (100 µg) was used to sensitize and challenge mice, airway eosinophilia was reduced in mice lacking TSLPR. Exactly why there is this allergen dose–dependent effect of TSLP is unclear at present. In the high-dose model, IgG2a levels were increased, indicative of a mixed Th1 and Th2 response. As Th1 responses can counteract Th2 immunity, we speculate that the absence of TSLP or of its receptor further shifts the immune response to Th1 response. In the low-dose model, the threshold for Th1 induction might be too high for TSLP blockade to have this enhancing effect.
GM-CSF is a hematopoietic cytokine associated with Th2 immunity in the lung (Stämpfli et al., 1998; Ohta et al., 1999; Cates et al., 2004; Bleck et al., 2006). This cytokine was reliably induced in vitro and in vivo by both HDM and IL-1α. Not surprisingly, neutralization of GM-CSF at the time of sensitization to HDM or OVA + IL-1α in vivo strongly reduced the salient feature of asthma. Human bronchial ECs have been shown to make IL-1α and IL-1β in an asthmatic setting and also express the IL-1RI (Mattoli et al., 1990; Marini et al., 1991). Our findings on cultures of human ECs demonstrated that IL-1α was increased after HDM exposure and that IL-1α could induce GM-CSF. It is therefore likely that the pathway that we discovered is also operative in humans exposed to allergens and could also help explain the adjuvant effects of air pollutants (Bleck et al., 2006).
In the current study, we identify IL-1α and not IL-1β as the predominant IL-1 cytokine driving the innate cytokine cascade, but we have not studied how IL-1α is produced. IL-1α can be released as a cytokine by inflammatory cells or as an alarmin by dying cells (Chen et al., 2007; Dinarello, 2009). We have found it to be produced by bronchial ECs in ALI cultures and by alveolar macrophages early in the response to HDM (unpublished data). However, we have never detected dying cells after HDM administration (unpublished data), but more studies are required to rule out the possibility that dying cells contribute to IL-1α release. Experiments with casp1−/− mice did not support a role for IL-1β in Th2 immunity, in line with a previous publication on the lack of involvement of the NLRP3 inflammasome in HDM-driven asthma (Kool et al., 2011). However, various papers show that IL-1β could be cleaved to its active form outside the cell by enzymes (proteinase3 and elastase) secreted by neutrophils (Joosten et al., 2009). We found no significant reduction in HDM-induced Th2 immunity when neutrophils were depleted using antibodies at the time of sensitization (unpublished data).
DCs are necessary and sufficient to cause Th2 immunity to HDM (Hammad et al., 2010). We found that the type of Th2 immunity induced by IL-1α relied completely on antigen presentation by DCs and that IL-1α induced the migration and activation of DCs in a GM-CSF–dependent way. Clearly, the process of Th2 development induced by mucosal administration of IL-1 or the enhanced proliferation of antigen-specific T cells seen did not require direct signaling of IL-1RI on T cells, as Th2 immunity was still induced when OVA and IL-1 were administered to the lungs of mice harboring OVA-specific TCR Tg T cells lacking IL-1RI. Strikingly, the same observation was made for induction of Th17 responses in the lung-draining nodes, which did not require IL-1RI on T cells. This is in contrast to studies in which IL-1 was given systemically or subcutaneously and promoted proliferation and Th2/Th17 immunity by directly acting on T cells (Sutton et al., 2006; Ben-Sasson et al., 2009). One explanation could be that administration to the lung does not allow sufficient IL-1α to reach the T cells. In our opinion, the promotion of enhanced T cell proliferation and Th2 immunity is best explained by effects of IL-1α and GM-CSF on DCs, which subsequently promote T cell expansion and differentiation. In parallel, other cytokines like IL-33 that are simultaneously induced could affect T cells directly.
In conclusion, our experiments in mice and human bronchial ECs have unraveled a new mechanism that helps explain sensitization to HDM allergen. HDM triggers ECs to produce IL-1α in a TLR4-dependent manner. The IL-1α subsequently acts in an autocrine manner on the lung ECs, leading to secretion of proinflammatory chemokines, GM-CSF, and IL-33. Together, these recruit and activate inflammatory DCs that induce adaptive Th2 immunity to the allergen. It will be interesting to study whether this cascade of innate cytokines programming adaptive immunity is induced by other natural allergens, environmental pollutants, and respiratory viruses that cause allergy.
MATERIALS AND METHODS
Mice.
TSLP receptor−/− (Crlf2−/−) mice on C57BL/6 background (backcrossed for at least 10 generations to C57BL/6) were provided M. Comeau (Amgen, Thousand Oaks, CA); Il1r1−/− mice (backcrossed for 8 generations to C57BL/6) were provided by B. Ryffel (University of Orléans, Orléans, France); Casp1−/− mice were provided by T. Vandenberghe (Flanders Institute for Biotechnology, Zwijnaarde, Belgium); and Mhc2fl/fl CD11cCre+ and Mhc2fl/fl CD11cCre− mice were provided by A. Liston (University of Leuven, Leuven, Belgium; backcrossed for at least 10 generations to C57BL/6). Female C57BL/6 WT mice were obtained from Harlan. Tlr4−/− (backcrossed for eight generations to C57BL/6) mice and MHCII-restricted OVA-TCR Tg OTII mice were obtained from the Jackson Laboratory. Mice were housed under specific pathogen–free conditions in individually ventilated cages in a controlled day–night cycle and given food and water ad libitum. All experiments were approved by the animal ethics committee of Ghent University.
Reagents.
HDM extracts were obtained from Greer Laboratories. Recombinant soluble ST2 was provided by H. Braun (Flanders Institute for Biotechnology). Rasburicase (uricase) was purchased from Fasturtec, and human IL-1ra (anakinra) was obtained from Amgen. For sensitization of mice, endotoxin low OVA was obtained from Hyglos and Worthington Biochemicals, whereas for use during antigen challenge, Grade III OVA and apyrase were obtained from Sigma-Aldrich. We obtained purified anti–IL-1α, recombinant mouse and human IL-1α, IL-1β, and IL-33, and ELISA Duoset for mIL-13, mKC, mCCL20, mIL-25, mIL-33, hIL-1α, hIL-1b, hIL-33, hTSLP, and hGM-CSF from R&D Systems. ELISA to detect hIL-25 was obtained from Wuhan EIAab Science. FITC-labeled antibody to MHCII, PE-Cy5–labeled antibodies to CD3 and CD19, PE-Cy7–labeled antibody against CD49b, and APC-labeled antibody to CD11c, MHCII, and F4/80, and ELISA sets for mMCP1, mTSLP, and mIL-17A were acquired from eBioscience. FITC-labeled antibody to Ly6C, PE-labeled antibody against Siglec-F and Ly6G, Horizon V450–labeled antibody against CD11b, as well as mIL-1α, mIL-1β, mIL-4, mIL-5, mIL-10, mIFN-γ, and mGM-CSF ELISA sets and antibody pairs to mouse IgE, IgG1, and IgG2a to measure Igs in serum were obtained from BD. Aqua, CFSE, PE–Texas red–labeled antibody against CD11c and OVA Alexa Fluor 488 were purchased from Molecular Probes/Invitrogen. PerCp-Cy5.5–labeled antibody against MHCII and LEAF-purified anti–IL-1α, anti–IL-1β, anti–GM-CSF, and isotype control antibodies for neutralization experiments were obtained from BioLegend.
Generation of bone marrow chimeras.
8–10-wk-old Il1r1−/− and WT mice were sublethally irradiated (8 Gy) and received 2 × 106 bone marrow cells i.v. from either Il1r1−/− or WT C57BL/6 donors 4 h after irradiation. Mice were used in experiments at least 10 wk after bone marrow reconstitution.
Model of HDM-induced asthma.
Mice were anesthetized with isoflurane and received 1 µg HDM intranasally 7 d later, mice were challenged with 10 µg HDM on five consecutive days under anesthesia. 3 d after the last challenge, mice were sacrificed and organs were dissected for analysis. BAL was performed using 3× of 1 ml EDTA-containing PBS and analyzed, and lungs were inflated with PBS/OCT (1:1) solution and snap frozen in liquid nitrogen and kept at −80°C until further processing. Blood was taken to collect serum and analyzed for HDM-specific Igs. MLNs were dissected, and single cell suspensions were prepared by pressing through a 100-µm cell sieve and restimulated in vitro with 15 µg/ml HDM for 3 d. Supernatant was collected from these cultures, and cytokine profiles were assayed by ELISA. Lung function was performed using Flexivent invasive measurement of dynamic resistance as described previously (Hammad et al., 2007). In neutralization experiments, IL-1α and IL-1β were blocked at the time of sensitization to HDM by use of 70 µg blocking antibody injected i.t. In other experiments, 150 µg anti–GM-CSF antibody or 200 µg recombinant soluble ST2 was injected i.p. at the time of sensitization.
Early innate immune response to HDM.
Mice were i.t. instilled with PBS or 100 µg HDM. Apyrase- and uricase-treated mice were injected i.t. with 4 U/ml apyrase or 100 µg uricase 30 min before exposure to PBS or HDM. Mice were injected i.p. with 200 µg recombinant soluble ST2 (rST2). Mice were sacrificed 2 or 24 h after the injection. BAL was collected, and the left lung was snap frozen in liquid nitrogen and stored at −80°C for preparation of lung homogenates. It was then homogenized with a tissue homogenizer in 500 µl of cold lysis buffer (20 mM Tris-HCl, pH 8.0, 0.14 M NaCl, 10% glycerol [vol/vol], 1 mM PMSF, 1 mM sodium orthovanadate [Na3VO4], 1 µM NaF, 40 mg/ml aprotinin, and 20 mg/ml leupeptin) using a tissue homogenizer (IKA) with the addition of 1% Igepal after homogenization. Samples where then kept on ice for 30 min with agitation each 10 min, followed by a centrifugation to pellet debris. Cleared lysate was quantified for protein concentration with NanoOrange reagent (Invitrogen) according to the manufacturer’s protocol.
Cell suspensions were made of the right lung and used for FACS analysis. Innate cytokines were measured by ELISA on lung homogenates, and concentrations were corrected for the protein content.
OVA experiments.
DC migration was investigated by injecting 100 µg OVA Alexa Fluor 488 i.t. and dissecting MLNs after 24 h. Cells were stained for flow cytometry, and DCs positive for Alexa Fluor 488 and expressing high levels of MHCII and CD11c were considered to be migratory DCs. For i.t. administration of PBS, 100 µg OVA-w ± 80 ng IL-1α, 80 ng IL-1β, 80 ng LPS, and 8 or 80 ng IL-33 mice were anesthetized with isoflurane at day 0 and 1. At day 10, mice were challenged with OVA aerosols (1% OVA grade III solution) for 30 min on three consecutive days. Mice were sacrificed 24 h after the last aerosol. BAL was performed and cells were analyzed as described previously (van Rijt et al., 2004). MLNs were dissected and restimulated for 4 d with 10 µg/ml OVA-w. Supernatant was harvested at day 4 and analyzed for cytokines by ELISA. For T cell division experiments, lymphocytes from LNs and spleen from OTII TCR Tg mice were isolated and stained with CFSE and injected i.v. in recipient mice. 1 d later, mice received an i.t. injection of PBS, 100 µg OVA-Endograde ± 80 ng IL-1α or 80 ng IL-1β. After 4 d, the MLNs were analyzed for T cell divisions and cultured for 3 d for cytokine response, without restimulation. Flow cytometry was performed on LSRII (BD).
ALI cultures of mouse and human tracheal ECs.
The isolation and culture of tracheal ECs were performed with small adaptations as previously described (Mayer et al., 2008). 8-wk-old mice were sacrificed with CO2, and trachea were dissected free and digested with pronase E and DNase I overnight at 4°C. Cell suspensions were allowed to adhere for 2 h in a Petri dish at 37°C. Nonadherent cells were grown for 4–7 d until confluence was reached (>5 kOhm, measured by transepithelial resistance) in a transwell system on collagen (Sigma-Aldrich)-coated membranes (Corning). These cells were cultured for 3 wk as ALI cultures and subsequently exposed for 24 h to 100 µg HDM, 10 ng IL-1α, or 10 ng IL-1β or PBS as a control. Cytokines secreted in medium were measured by ELISA.
Human NBECs were purchased from Lonza. Cells were cultured in T80 culture flask (Thermo Fisher Scientific) to expand the cell numbers in BEGM growth medium as proposed by the manufacturer, until ∼85–90% confluence of cells. Cells were harvested and plated on collagen (Sigma-Aldrich)-precoated transwells (Corning) until cells reached confluence. Medium on the apical side was removed, and medium at the basolateral side was replaced by B-ALI growth medium (Lonza). Cells were cultured for 4 wk in B-ALI growth medium and pulsed overnight with PBS, 100 µg HDM ± IL-1ra, or 10 ng recombinant hIL-1α. Cytokine levels were measured in supernatant by ELISA.
Statistical analyses.
For all experiments, we calculated the difference between groups with the Mann-Whitney U test for unpaired data (SPSS version 15.0). Differences were considered significant when the p-value was <0.05.
Acknowledgments
We thank Tom Boterberg for his help with the irradiation of mice and Lotte Schmidt for her help setting up the mouse ALI cultures in our laboratory.
B.N. Lambrecht is a recipient of an Odysseus Grant of the Flemish Organization for Scientific Research (FWO) and a recipient of a European Research Council Consolidator grant and a Ghent University Multidisciplinary Research Partnership grant (Group-ID). H. Hammad and B.N. Lambrecht are supported by National Institutes of Health grant number 5R21AI083690-02. H. Hammad is a recipient of an FWO program grant. B.N. Lambrecht is a recipient of a GOA Concerted Research Initiative from Ghent University.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- ALI
- air–liquid interface
- BAL
- bronchoalveolar lavage
- DBEC
- diseased bronchial EC
- EC
- epithelial cell
- GCM
- goblet cell metaplasia
- HDM
- house dust mite
- i.t.
- intratracheal(ly)
- MLN
- mediastinal LN
- NBEC
- normal bronchial EC
- PAS
- periodic acid-Schiff
- Tg
- transgenic
- TSLP
- thymic stromal lymphopoietin
- UA
- uric acid
References
- Allakhverdi Z., Comeau M.R., Jessup H.K., Yoon B.R., Brewer A., Chartier S., Paquette N., Ziegler S.F., Sarfati M., Delespesse G. 2007a. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 204:253–258 10.1084/jem.20062211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P.J. 2008. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 8:183–192 10.1038/nri2254 [DOI] [PubMed] [Google Scholar]
- Barrett N.A., Maekawa A., Rahman O.M., Austen K.F., Kanaoka Y. 2009. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J. Immunol. 182:1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson S.Z., Hu-Li J., Quiel J., Cauchetaux S., Ratner M., Shapira I., Dinarello C.A., Paul W.E. 2009. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. USA. 106:7119–7124 10.1073/pnas.0902745106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard A.G., Togbe D., Guillou N., Erard F., Quesniaux V., Ryffel B. 2011. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur. J. Immunol. 41:1675–1686 10.1002/eji.201041033 [DOI] [PubMed] [Google Scholar]
- Bleck B., Tse D.B., Jaspers I., Curotto de Lafaille M.A., Reibman J. 2006. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation. J. Immunol. 176:7431–7437 [DOI] [PubMed] [Google Scholar]
- Botelho F.M., Bauer C.M., Finch D., Nikota J.K., Zavitz C.C., Kelly A., Lambert K.N., Piper S., Foster M.L., Goldring J.J., et al. 2011. IL-1α/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLoS ONE. 6:e28457 10.1371/journal.pone.0028457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates E.C., Fattouh R., Wattie J., Inman M.D., Goncharova S., Coyle A.J., Gutierrez-Ramos J.C., Jordana M. 2004. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J. Immunol. 173:6384–6392 [DOI] [PubMed] [Google Scholar]
- Caucig P., Teschner D., Dinges S., Maxeiner J.H., Reuter S., Finotto S., Taube C., von Stebut E. 2010. Dual role of interleukin-1alpha in delayed-type hypersensitivity and airway hyperresponsiveness. Int. Arch. Allergy Immunol. 152:303–312 10.1159/000288283 [DOI] [PubMed] [Google Scholar]
- Chen C.J., Kono H., Golenbock D., Reed G., Akira S., Rock K.L. 2007. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 13:851–856 10.1038/nm1603 [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519–550 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- Eiwegger T., Akdis C.A. 2011. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur. J. Immunol. 41:1535–1538 10.1002/eji.201141668 [DOI] [PubMed] [Google Scholar]
- Fort M.M., Cheung J., Yen D., Li J., Zurawski S.M., Lo S., Menon S., Clifford T., Hunte B., Lesley R., et al. 2001. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 15:985–995 10.1016/S1074-7613(01)00243-6 [DOI] [PubMed] [Google Scholar]
- Hammad H., Lambrecht B.N. 2008. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 8:193–204 10.1038/nri2275 [DOI] [PubMed] [Google Scholar]
- Hammad H., Kool M., Soullié T., Narumiya S., Trottein F., Hoogsteden H.C., Lambrecht B.N. 2007. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J. Exp. Med. 204:357–367 10.1084/jem.20061196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H., Chieppa M., Perros F., Willart M.A., Germain R.N., Lambrecht B.N. 2009. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15:410–416 10.1038/nm.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H., Plantinga M., Deswarte K., Pouliot P., Willart M.A., Kool M., Muskens F., Lambrecht B.N. 2010. Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 207:2097–2111 10.1084/jem.20101563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M., Hirota T., Jodo A.I., Hitomi Y., Sakashita M., Tsunoda T., Miyagawa T., Doi S., Kameda M., Fujita K., et al. 2011. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am. J. Respir. Cell Mol. Biol. 44:787–793 10.1165/rcmb.2009-0418OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley M.B., Zhou B., Shih W.X., Aye T., Comeau M.R., Ziegler S.F. 2009. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J. Immunol. 182:1641–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst S.D., Muchamuel T., Gorman D.M., Gilbert J.M., Clifford T., Kwan S., Menon S., Seymour B., Jackson C., Kung T.T., et al. 2002. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169:443–453 [DOI] [PubMed] [Google Scholar]
- Joosten L.A., Netea M.G., Fantuzzi G., Koenders M.I., Helsen M.M., Sparrer H., Pham C.T., van der Meer J.W., Dinarello C.A., van den Berg W.B. 2009. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 60:3651–3662 10.1002/art.25006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap M., Rochman Y., Spolski R., Samsel L., Leonard W.J. 2011. Thymic stromal lymphopoietin is produced by dendritic cells. J. Immunol. 187:1207–1211 10.4049/jimmunol.1100355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Lee H.C., Nakayama T., Ziegler S.F. 2011. TSLP enhances the function of helper type 2 cells. Eur. J. Immunol. 41:1862–1871 10.1002/eji.201041195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M., Willart M.A., van Nimwegen M., Bergen I., Pouliot P., Virchow J.C., Rogers N., Osorio F., Reis e Sousa C., Hammad H., Lambrecht B.N. 2011. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 34:527–540 (published erratum appears in Immunity. 2011. 34:627) 10.1016/j.immuni.2011.03.015 [DOI] [PubMed] [Google Scholar]
- Lambrecht B.N., Hammad H. 2012. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu. Rev. Immunol. 30:243–270 10.1146/annurev-immunol-020711-075021 [DOI] [PubMed] [Google Scholar]
- Lambrecht B.N., De Veerman M., Coyle A.J., Gutierrez-Ramos J.C., Thielemans K., Pauwels R.A. 2000. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Invest. 106:551–559 10.1172/JCI8107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.C., Ziegler S.F. 2007. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc. Natl. Acad. Sci. USA. 104:914–919 10.1073/pnas.0607305104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.C., Headley M.B., Iseki M., Ikuta K., Ziegler S.F. 2008. Cutting edge: Inhibition of NF-kappaB-mediated TSLP expression by retinoid X receptor. J. Immunol. 181:5189–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L., Zhang Y., Yao W., Kaplan M.H., Zhou B. 2011. Thymic stromal lymphopoietin interferes with airway tolerance by suppressing the generation of antigen-specific regulatory T cells. J. Immunol. 186:2254–2261 10.4049/jimmunol.1002503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich I.P., Herman N.S., Schleifer K.W., Dance M.P., Chen B.L., Dienger K.M., Sproles A.A., Shah J.S., Köhl J., Belkaid Y., Wills-Karp M. 2005. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 202:1549–1561 10.1084/jem.20051506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich I.P., Day S.B., Ledford J.R., Zhou P., Dienger K., Wills-Karp M., Page K. 2011. Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation. Respir. Res. 12:122 10.1186/1465-9921-12-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Soumelis V., Watanabe N., Ito T., Wang Y.H., Malefyt Rde.W., Omori M., Zhou B., Ziegler S.F. 2007. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 25:193–219 10.1146/annurev.immunol.25.022106.141718 [DOI] [PubMed] [Google Scholar]
- Marini M., Soloperto M., Mezzetti M., Fasoli A., Mattoli S. 1991. Interleukin-1 binds to specific receptors on human bronchial epithelial cells and upregulates granulocyte/macrophage colony-stimulating factor synthesis and release. Am. J. Respir. Cell Mol. Biol. 4:519–524 [DOI] [PubMed] [Google Scholar]
- Mattoli S., Miante S., Calabrò F., Mezzetti M., Fasoli A., Allegra L. 1990. Bronchial epithelial cells exposed to isocyanates potentiate activation and proliferation of T-cells. Am. J. Physiol. 259:L320–L327 [DOI] [PubMed] [Google Scholar]
- Mayer A.K., Bartz H., Fey F., Schmidt L.M., Dalpke A.H. 2008. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment. Eur. J. Immunol. 38:1689–1699 10.1002/eji.200737936 [DOI] [PubMed] [Google Scholar]
- Nathan A.T., Peterson E.A., Chakir J., Wills-Karp M. 2009. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J. Allergy Clin. Immunol. 123:612–618 10.1016/j.jaci.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., Yamashita N., Tajima M., Miyasaka T., Nakano J., Nakajima M., Ishii A., Horiuchi T., Mano K., Miyamoto T. 1999. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: essential role of GM-CSF. J. Allergy Clin. Immunol. 104:1024–1030 10.1016/S0091-6749(99)70084-9 [DOI] [PubMed] [Google Scholar]
- Phipps S., Lam C.E., Kaiko G.E., Foo S.Y., Collison A., Mattes J., Barry J., Davidson S., Oreo K., Smith L., et al. 2009. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 [corrected] responses. Am. J. Respir. Crit. Care Med. 179:883–893 (published erratum appears in Am. J. Respir. Crit. Care Med. 2009. 180:1032) 10.1164/rccm.200806-974OC [DOI] [PubMed] [Google Scholar]
- Saenz S.A., Taylor B.C., Artis D. 2008. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226:172–190 10.1111/j.1600-065X.2008.00713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N., Kurrer M., Kopf M. 2003. The IL-1 receptor 1 is critical for Th2 cell type airway immune responses in a mild but not in a more severe asthma model. Eur. J. Immunol. 33:991–1000 10.1002/eji.200323801 [DOI] [PubMed] [Google Scholar]
- Shikotra A., Choy D.F., Ohri C.M., Doran E., Butler C., Hargadon B., Shelley M., Abbas A.R., Austin C.D., Jackman J., et al. 2012. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J. Allergy Clin. Immunol. 129:104–111: e1–e9 10.1016/j.jaci.2011.08.031 [DOI] [PubMed] [Google Scholar]
- Siracusa M.C., Saenz S.A., Hill D.A., Kim B.S., Headley M.B., Doering T.A., Wherry E.J., Jessup H.K., Siegel L.A., Kambayashi T., et al. 2011. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 477:229–233 10.1038/nature10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stämpfli M.R., Wiley R.E., Neigh G.S., Gajewska B.U., Lei X.F., Snider D.P., Xing Z., Jordana M. 1998. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J. Clin. Invest. 102:1704–1714 10.1172/JCI4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E., Lieschke G.J., Grail D., Metcalf D., Hodgson G., Gall J.A., Maher D.W., Cebon J., Sinickas V., Dunn A.R. 1994. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl. Acad. Sci. USA. 91:5592–5596 10.1073/pnas.91.12.5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H., Lavelle E.C. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203:1685–1691 10.1084/jem.20060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A.M., Chen H.C., Pochard P., Eisenbarth S.C., Herrick C.A., Bottomly H.K. 2010. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J. Immunol. 184:3535–3544 10.4049/jimmunol.0900340 [DOI] [PubMed] [Google Scholar]
- Trompette A., Divanovic S., Visintin A., Blanchard C., Hegde R.S., Madan R., Thorne P.S., Wills-Karp M., Gioannini T.L., Weiss J.P., Karp C.L. 2009. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 457:585–588 10.1038/nature07548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu D.T., McIntire J.J., Akbari O., Macaubas C., DeKruyff R.H. 2002. Asthma: an epidemic of dysregulated immunity. Nat. Immunol. 3:715–720 10.1038/ni0802-715 [DOI] [PubMed] [Google Scholar]
- van Rijt L.S., Kuipers H., Vos N., Hijdra D., Hoogsteden H.C., Lambrecht B.N. 2004. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J. Immunol. Methods. 288:111–121 10.1016/j.jim.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Wills-Karp M. 2010. Allergen-specific pattern recognition receptor pathways. Curr. Opin. Immunol. 22:777–782 10.1016/j.coi.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Comeau M.R., De Smedt T., Liggitt H.D., Dahl M.E., Lewis D.B., Gyarmati D., Aye T., Campbell D.J., Ziegler S.F. 2005. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6:1047–1053 10.1038/ni1247 [DOI] [PubMed] [Google Scholar]