Abstract
Leptin signaling deficient rodents have emerged as models of obesity/insulin resistance syndrome. Altered leptin signaling, however, can affect axial and appendicular bone geometrical properties differently, and, thus, we hypothesized that leptin-deficiency would differentially influence mechanical properties of vertebrae and tibiae compared to lean rats. Mature (9 mo) leptin receptor deficient obese (cp/cp; n = 8) and lean (+/?; n = 7) male JCR:LA-corpulent rats were used to test that hypothesis. Tibiae and the sixth lumbar vertebrae (L6) were scanned with micro-CT and were broken in three point-bending (tibiae) or axial loading (L6). Supporting the hypothesis, vertebrae and tibiae were differentially affected by leptin signaling deficiency. Tibiae, but not vertebrae, were significantly shorter in obese rats and achieved a significantly greater load (>18%), displacement (>15%), and stress (>18%) at the proportional limit, relative to the lean rats. Conversely, L6 in obese rats had significantly reduced displacement (>25%) and strain (>32%) at proportional limit, relative to the lean rats. Those combined results suggest that the etiology and duration of obesity may be important determinants of bone mechanical properties, and axial and appendicular bones may be affected differently.
1. Introduction
Obesity and its attendant complications threaten the sustainability of many health care systems worldwide [1]. While an increased risk of developing type 2 diabetes, cardiovascular disease, and certain cancers is a well-recognized corollary of obesity, recent evidence points to the risk of perturbations in bone health as well [2–4]. Several mechanisms have been proposed to explain the interrelation between obesity and bone metabolism, including increased proinflammatory cytokines and excessive leptin secretion [2].
The discovery of leptin as an adipose-derived hormone involved in energy balance provided key insights into the molecular pathogenesis of obesity [5, 6]. Leptin is a 14 kD protein secreted by white adipose cells [7] and is strongly correlated with body fat mass [8]. While leptin plays an important role in appetite regulation and energy metabolism, it is also a powerful inhibitor of bone mass accrual [9].
Animals lacking functional leptin signaling manifest severe obesity and its related diseases. The JCR:LA-corpulent rat is such a model. JCR:LA-corpulent rats have a mutation in the leptin receptor (cp) gene, leading to a complete absence of functional leptin receptors [10]. Rats homozygous for that mutation (cp/cp) are highly obese and are used as an animal model for obesity/insulin resistance syndrome (see [11] for review). Rats heterozygous for the cp mutation (+/cp) and homozygous normal rats (+/+) are phenotypically indistinguishable and can collectively be used as lean controls (+/?) [12].
Leptin has a plethora of influences on hormonal and trophic factors, all of which can influence bone metabolism. Exogenous administration of leptin can act via the central nervous system to inhibit bone formation [13, 14] or peripherally to enhance bone formation [15], although more recent studies with lower doses of intracerebroventricular leptin administration show enhanced bone formation with increased osteoprotegerin (OPG), osteocalcin, and receptor activator of nuclear factor κβ ligand (RANKL) levels [16]. Impaired leptin signaling influenced bones in a site-specific manner. Leptin-deficient mice had suppressed femoral bone mineral density (BMD) and enhanced lumbar BMD, relative to lean mice [17]. Those changes were reported for bone geometry and not specific to the bone material irrespective of geometry. Because JCR:LA-corpulent rats manifest an obesity/insulin resistant phenotype, and diabetes mellitus can cause a degeneration in bone material properties [18, 19], potentially, the mechanical properties of obese/insulin resistant rat bones would differ from those of controls. Thus, we hypothesized that leptin receptor deficiency would differentially influence mechanical properties of vertebrae and tibiae of JCR:LA-corpulent rats compared to lean rats.
2. Materials and Methods
2.1. Animals
Mature (9 months) male obese (cp/cp; n = 8) and lean (+/?; n = 7) JCR:LA-corpulent rats were obtained from Dr. J. Russell's colony (University of Alberta). The JCR:LA-corpulent rats are patently obese at 3 weeks (wk) of age, and moderate hyperinsulinaemia can be observed already at 4 wk [20]. Rats were housed in a temperature- and humidity-controlled room with a reverse 12 h light/dark cycle (19:00 to 07:00). Rats were housed 2 per cage at the University of Calgary 1 wk prior to sacrifice. Standard rat chow (5P14 ModRod EQ, Richmond, IN, USA) and water were provided ad libitum for both lean and obese rats. All procedures were ethically approved by the University of Calgary Animal Welfare Committee.
2.2. Tibial and L6 Preparation
Immediately after euthanization (halothane anaesthesia followed by cervical dislocation) tibiae and the sixth lumbar vertebrae (L6) were dissected and cleaned of adherent muscular and connective tissues. To isolate the vertebral centrum, the neural spine, transverse processes, and zygapophyses were removed from each vertebra with a diamond wafer saw (Buehler Isomet, Lake Bluff, IL, USA) [21]. To ensure parallel caudal and rostral vertebral surfaces (for biomechanical testing), those surfaces were cleaned of intervertebral discs and a wafer was cut from the caudal surface parallel to the rostral surface bringing the total length of the tested centrum to 80% of the centrum length in its entirety [21]. Bones were individually wrapped in saline-soaked gauze (50 mM potassium phosphate buffer solution, pH = 7.4) and sealed hermetically in plastic bags that were frozen (–30°C) for less than 1 month until biomechanical testing. Freezing and thawing has been shown to not adversely affect bone mechanical properties [22].
2.3. Tibial Bone Geometry
Tibiae were thawed in 22°C buffer (50 mM potassium phosphate buffer solution, pH = 7.4) for 1 hour and subjected to micro-CT scanning (Skyscan 1073, Aartselaar, Belgium) at a magnification of 14x (resolution of 20 μm). Bitmap images generated from scanning that represented the tibial longitudinal midpoints (one image per tibia) were used as input to custom software (Matlab, Natick, MA, USA) that thresholded images and calculated geometric parameters including total cross-sectional area, distances from centroid to the lateral surface of the cross-section, and cross-sectional moment of inertia. After scanning, tibiae were rewrapped in saline soaked gauze, hermetically sealed, and frozen (–30°C) until biomechanical testing.
2.4. Tibial Biomechanical Testing
On the day of testing, tibiae were thawed in 22°C buffer (50 mM potassium phosphate buffer solution, pH = 7.4) for 1 hr. When thawed, tibial length was measured using callipers (Model 599-578-1, Brown and Sharpe, Irvine, CA, USA) and tibiae were placed on a round surfaced 13.3 mm loading span. The cross-sectional shape of tibiae (triangular) permitted stable and repeatable placement of the diaphyseal shaft on the loading span. The round-surfaced cross-head probe of a servocontrolled electromechanical testing system (Model 1122, Instron Corp., Canton, MA, USA) contacted the medial tibial surface at its longitudinal midpoint and applied a preload of 1 N; the medial surface was in compression and the lateral surface was in tension. Load was applied at 25.4 mm/min until failure, and testing order was stratified based on group to eliminate potential testing-order effects. Load deformation curves were acquired (RC Computerscope A/D Board, RC Electronics, Santa Barbara, CA, USA) at 200 Hz. From the load-deformation curves, load and displacement and energy at proportional limit, maximal load, and flexural rigidity (structural properties) were determined. Structural properties were used in conjunction with geometrical properties (μCT scanning) to calculate material properties, including stress and strain at proportional limit, maximal load, and modulus of elasticity.
2.5. L6 Bone Geometry
Vertebrae were thawed in 22°C buffer (50 mM potassium phosphate buffer solution, pH = 7.4) for 1 hr and subjected to micro-CT scanning (Skyscan 1073, Aartselaar, Belgium) at a magnification of 30x (resolution of 13 μm). In the sagittal plane, centra had an hourglass appearance. In axial biomechanical testing, the narrowest region of the centrum (i.e., centre of the hourglass) was subjected to the greatest stress, and, thus, centrum transverse sections corresponding to the narrowest region of the vertebral centra were used for geometrical determination. Bitmap images generated from scanning of that region (one image per centrum) were used as input to customized software (Matlab, MathWorks, Natick, MA, USA) that determined total cross sectional area, trabecular area, and cortical bone area (Scion Image, Frederick, MD, USA). Vertebral height was measured with callipers (Model 599-578-1, Brown and Sharpe, Irvine, CA, USA). After scanning, L6 were rewrapped in saline soaked gauze hermetically sealed, and frozen (–30°C) until biomechanical testing.
2.6. L6 Biomechanical Testing
On the day of testing, L6 vertebrae were thawed in 22°C buffer (50 mM potassium phosphate buffer solution, pH = 7.4) for at least 1 hr. The caudal surface of the centrum was placed on a stainless steel plate thinly coated with mineral oil to approximate unconstrained compression. The flat-surfaced cross head of a servocontrolled electromechanical testing system (Model 1122, Instron Corp., Canton, MA, USA) was lubricated and contacted the rostral surface of the centrum with a pre-load of 5 N. The centrum was cycled from 5–10 N 20 times at 0.001%/s to eliminate viscoelastic creep of trabecular bone [23]. Cycling was stopped at a preload of 10 N, and samples were subsequently compressed at 127 mm/min. Testing was stratified based on group to prevent a testing-order effect. Load deformation curves were acquired (RC Computerscope A/D Board) at 200 Hz and were used to determine load and displacement and energy to proportional limit, maximal load, and stiffness (structural properties). Geometrical data (micro-CT scanning) were used with the structural properties to determine stress and strain at the proportional limit and at maximal load and the apparent elastic modulus [24].
2.7. Ash Analyses
Crushed L6 centra and a 2 mm section of the tibial diaphysis immediately distal to the mid-diaphyseal fracture site were dehydrated in 100% ethanol for 5 d, defatted in acetone for 5 d, and dried at 100°C (Thermolyne F62700, Dubuque, IA, USA) for 48 hr in a ceramic crucible. Dried bone samples were weighed (Mettler AE 163, Anaheim, CA, USA; ±10 μg) to determine dry bone mass. After weighing, samples were incinerated at 600°C for 5 d. The ash was weighed, and mineral ash fraction was calculated as (ash mass)/(dry mass) × 100%.
2.8. Plasma Leptin
Following an overnight fast, blood was collected from each rat into a chilled tube containing 1 mg/mL of EDTA, aprotinin (500 kallikrein inhibitor units/mL; Sigma Chemical Co.), and diprotin-A (0.1 mM). The blood was centrifuged at 1600 ×g for 15 min at 4°C and plasma aliquots stored at –80°C. A Rat Endocrine LINCOplex kit was used to quantify leptin (Linco Research Inc., Millipore, Billerica, MA, USA) with a sensitivity of 6.2 pM.
2.9. Statistics
Mann-Whitney comparisons determined where intergroup significant differences were present (SPSS, Chicago, IL, USA). Where no significant differences were present, the sample size required per group for the values to reach significance was calculated based on a significance level of 0.05 and a power of 0.8. A significance level of P ≤ 0.05 was used for all statistical tests. Reported values are means ± SD.
3. Results
3.1. Tibiae
Obese rats had significantly greater body mass (>107%), and their tibiae were significantly shorter relative to lean rats (Table 1). Tibial cross-sectional structural properties, however, were not adversely affected in the obese rats (Figure 1, Table 2). There were minimal significant differences between obese and lean rats, and those differences were confined to load and displacement at proportional limit. Load and displacement at proportional limit were significantly greater in obese rats. Similar to structural properties, there were minimal significant differences between obese and lean tibial material properties (Table 3). Obese tibiae had a significantly greater stress at proportional limit, relative to lean tibiae. There were no significant differences in structural or material properties at maximal load.
Table 1.
Tibial geometrical properties.
| Lean | Obese | n | |
|---|---|---|---|
| Tibial length (mm) | 42.01 ± 1.02∗ | 40.10 ± 0.67 | |
| Tibial mass (g) | 1.37 ± 0.19 | 1.27 ± 0.09 | 31 |
| Cross-sectional area (mm2) | 5.42 ± 0.46 | 5.53 ± 0.28 | 178 |
| Cross-sectional moment of inertia Ixx (mm4) | 3.40 ± 0.56 | 3.44 ± 0.44 | 2453 |
| Body mass (g) | 389.5 ± 32.7∗ | 807.5 ± 53.0 |
Values are means ± SD. ∗denotes statistically significant (P ≤ 0.05). n denotes the sample size per group needed for the recorded differences to become statistically significant based on a power of 0.8.
Figure 1.
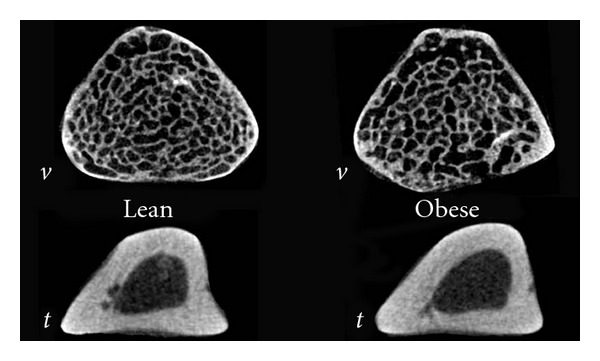
Exemplar L6 (v) and tibial (t) transverse cross-sectional micro-CT images for lean and genetically obese rats.
Table 2.
Tibial structural properties.
| Lean | Obese | n | |
|---|---|---|---|
| Flexural rigidity (N·mm2·103) | 5.37 ± 0.81 | 5.40 ± 0.34 | 5868 |
| Load at proportional limit (N) | 25.49 ± 2.94∗ | 30.22 ± 3.41 | |
| Displacement at proportional limit (mm) | 0.26 ± 0.04∗ | 0.30 ± 0.02 | |
| Maximal load (N) | 36.13 ± 4.48 | 39.34 ± 3.67 | 26 |
| Displacement at maximal load (mm) | 0.61 ± 0.10 | 0.60 ± 0.08 | 1272 |
| Energy to proportional limit (N·mm·103) | 4.12 ± 0.76 | 5.06 ± 0.89 | 49 |
| Energy to maximal load (N·mm·104) | 1.78 ± 0.45 | 1.56 ± 0.78 | 123 |
Values are means ± SD. ∗denotes statistically significant (P ≤ 0.05). n denotes the sample size per group needed for the recorded differences to become statistically significant based on a power of 0.8.
Table 3.
Tibial material properties.
| Lean | Obese | n | |
|---|---|---|---|
| Modulus of elasticity (GPa) | 1.60 ± 0.24 | 1.59 ± 0.25 | 9423 |
| Stress at proportional limit (MPa) | 27.90 ± 2.81∗ | 33.12 ± 6.14 | |
| Strain at proportional limit (%) | 1.97 ± 0.36 | 2.29 ± 0.09 | 8 |
| Stress at maximal load (MPa) | 39.53 ± 4.45 | 42.96 ± 6.16 | 38 |
| Strain at maximal load (%) | 4.60 ± 0.98 | 4.58 ± 0.76 | 29705 |
| Mineral ash fraction (%) | 71.94 ± 0.28 | 72.53 ± 0.43 | 9 |
Values are means ± SD. ∗denotes statistically significant (P ≤ 0.05). n denotes the sample size per group needed for the recorded differences to become statistically significant based on a power of 0.8.
3.2. Vertebrae
There were no significant differences in vertebral geometrical properties between obese and lean L6 (Table 4). Similarly, there were minimal significant differences in vertebral structural properties (Table 5). Conversely to the tibiae, displacement at proportional limit was significantly less in the obese L6, relative to lean L6. As with the structural properties, there were minimal significant differences in the majority of L6 material properties (Table 6). The only difference that was significant was that strain at proportional limit was greater in lean rats. There were no significant differences in structural or material properties at maximal load.
Table 4.
L 6 geometrical properties.
| Lean | Obese | n | |
|---|---|---|---|
| Vertebral height (mm) | 7.46 ± 0.32 | 7.57 ± 0.51 | 224 |
| Total area (mm2) | 12.55 ± 2.10 | 14.06 ± 1.43 | 22 |
| Trabecular area (mm2) | 7.59 ± 2.08 | 8.81 ± 1.17 | 28 |
| Cortical area (mm2) | 4.96 ± 0.83 | 5.24 ± 1.00 | 168 |
Values are means ± SD. n denotes the sample size per group needed for the recorded differences to become statistically significant.
Table 5.
L 6 structural properties.
| Lean | Obese | n | |
|---|---|---|---|
| Stiffness (N·mm−1) | 244.01 ± 19.80 | 248.52 ± 34.23 | 564 |
| Load at proportional limit (N) | 120.84 ± 19.56 | 101.34 ± 20.34 | 17 |
| Displacement at proportional limit (mm) | 0.75 ± 0.15∗ | 0.56 ± 0.09 | |
| Maximal load (N) | 137.88 ± 17.90 | 132.58 ± 20.16 | 203 |
| Displacement at maximal load (mm) | 1.07 ± 0.32 | 1.30 ± 0.46 | 46 |
| Energy to proportional limit (N·mm·103) | 8.69 ± 2.82 | 5.91 ± 1.95 | 12 |
| Energy to maximal load (N·mm·104) | 1.87 ± 0.71 | 2.64 ± 1.31 | 27 |
Values are means ± SD. ∗denotes statistically significant (P ≤ 0.05). n denotes the sample size per group needed for the recorded differences to become statistically significant based on a power of 0.8.
Table 6.
L 6 material properties.
| Lean | Obese | n | |
|---|---|---|---|
| Apparent elastic modulus (MPa) | 115.83 ± 26.17 | 107.63 ± 8.65 | 71 |
| Stress at proportional limit (MPa) | 9.42 ± 2.37 | 7.25 ± 1.53 | 13 |
| Strain at proportional limit (%) | 13.47 ± 2.54∗ | 9.07 ± 2.09 | |
| Stress at maximal load (MPa) | 10.68 ± 2.11 | 9.47 ± 1.40 | 34 |
| Strain at maximal load (%) | 19.01 ± 5.04 | 21.00 ± 7.94 | 167 |
| Mineral ash fraction (%) | 63.43 ± 0.85 | 63.44 ± 1.14 | 155412 |
Values are means ± SD. ∗denotes statistically significant (P ≤ 0.05). n denotes the sample size per group needed for the recorded differences to become statistically significant based on a power of 0.8.
3.3. Plasma Leptin
Plasma leptin was 193.7 ± 18.8 pM in lean rats and 728.6 ± 17.1 pM in obese rats which was significantly different (P = 0.001).
4. Discussion
Obese cp/cp JCR:LA-corpulent rats are leptin receptor deficient and have been used as a model of genetic-related obesity with accompanying hyperleptinemia and hyperinsulinemia [25]. We show that plasma leptin was approximately 4-fold higher in the obese versus lean rats, a pattern that is similar to the 12-fold higher plasma insulin we have previously shown in these rats [26]. The current study assessed the mechanical properties of the tibiae and vertebrae of those rats and found that tibiae and vertebrae were differentially affected by leptin receptor deficiency. Tibiae were significantly shorter in obese rats and had significantly greater loads, displacements, and stresses at the proportional limit, relative to the lean rats. Conversely, vertebrae in obese rats had significantly smaller displacements and strains at proportional limit, relative to the lean rats. Changes in tibial and vertebral mechanical properties were minimal in that there were no significant differences when mechanical properties were assessed at maximal load.
Detecting minimal changes between obese and lean rats was unanticipated given that leptin deficiency is associated with a myriad of factors favoring bone loss including hypogonadism, hypercortisolism, reduced trophic factors, and reduced activity levels [13, 17, 27]. One potential explanation for the minimal change in bone structural and material properties is that leptin deficiency causes significant obesity, and that an increase in body mass could subject bones to greater mechanical forces, which may be protective against bone loss [28]. It could also be suggested that if the obese rats attain maximal body weight earlier, the continued growth trajectory of the lean rats could minimize differences between the groups. Our previous work with this model in fact shows the opposite and it is the obese rats that continue to accrue mass at a greater magnitude than the lean rats [29]. Additionally, a lack of cerebral leptin signaling may enhance osteoblast activities thereby augmenting bone formation [13]. The minimal changes observed in mechanical properties could suggest that the overall influence of leptin deficiency on bone may be a dynamic balance between the bone-formation favoring and the bone loss-promoting actions of leptin.
The current study was consistent with previous studies in that the leptin-deficient rodents were significantly heavier [11, 25] and their appendicular bones were significantly shorter with a lower bone mineral density [30, 31] than lean rodents. Those shortened bones may have resulted from elevated 1,25-(OH)2D3 levels [30], suppressed trophic factors (such as IGF-1, GH, or TGFβ-1) [17, 32], or decreased muscle masses [17]. Prisby and colleagues [31] showed that longitudinal growth was compromised in tibiae and femora in the prediabetes state (7 wk of age) in Zucker diabetic fatty (ZDF) rats, which similar to the JCR:LA cp rats, are leptin receptor deficient. The impaired growth was evident prior to the development of overt hyperglycemia, thereby suggesting that factors other than those related to excess glucose were involved. Hamann and coworkers [33] showed that suppressed osteoblastogenesis could explain the low bone mass in ZDF rats with well-established insulin-resistant type 2 diabetes.
In contrast to shortened appendicular bones, vertebral height and bone mineral density were greater in leptin-deficient (ob/ob) mice, relative to the lean mice [17]. The current results also revealed different trends in vertebral and tibial properties. Tibiae in the obese rats underwent greater displacement at the proportional limit and carried a greater load at the proportional limit. The corresponding material property, stress at proportional limit, was greater in the obese rats suggesting that changes in material properties contributed to the observed changes in structural properties. Vertebrae in the obese rats underwent significantly less displacement to the proportional limit, relative to lean controls. The corresponding material property, strain at proportional limit, was also significantly less in the obese vertebrae suggesting again that material properties contributed to changes in structural properties.
It remains unclear why vertebrae and tibiae were differentially affected. This site difference, however, has been suggested by some [31] to perhaps account for controversial reports of protective or detrimental effects of type 2 diabetes on skeletal properties in the human literature. One factor could have related to tibial and vertebral growth plates responding differently to leptin deficiency [17]; in the current study tibiae were significantly shorter, and the vertebrae were unaltered, relative to the controls. Alternatively, different responses may have arisen from perturbations secondary to leptin-deficiency, such as alterations in muscle mass [17], different mechanical environments of the tibia and spine, or alterations in the marrow environment [17].
The current study, in conjunction with diet-induced obesity studies [34–36], suggested that the mechanism of obesity was an important factor in how obesity affected bone properties. While the current study showed that decrements in bone mechanical properties in the leptin receptor deficient obese rats (genetic-related obesity) were minimal and limited to the proportional limit, Zernicke and colleagues [35] showed that decrements in bone mechanical properties for high-fat sucrose (HFS) fed obese rats (diet-related obesity) were substantial at proportional limit and maximal load (30% reduction in L6 maximal stress). Moreover, Lorincz and coworkers [36] showed that structural and morphological properties of tibiae were adversely affected in mice fed an HFS diet. Lower cortical thickness, cross-sectional area, and load at maximum were seen in HFS versus control mice [36]. It was suggested that upregulation of RANKL and cyclo-oxygenase-2 mRNA levels might reflect elevated osteoclast activity in response to the inflammatory state of the diet-induced obesity. Collectively, those dissimilar findings suggest that disparate effects exist between dietary factors that promote obesity and the array of adipokines (e.g., leptin) and other factors released from adipose tissue. Taken together, the data suggest that the etiology of obesity (i.e., genetic versus diet-related) is an important consideration when assessing the influence of obesity on bone mechanical properties.
5. Conclusions
Mechanical properties of obese and lean JCR:LA-corpulent rat tibiae and vertebrae were assessed. Changes in mechanical properties were minimal and limited to the proportional limit. Supporting the premise that leptin-deficiency affects tibiae and vertebrae differently, the mechanical properties of obese tibiae were enhanced while those of vertebrae were diminished, relative to the lean controls. The reported changes specific to leptin-deficiency-induced obesity were different than previously reported changes that occur with dietary induced obesity. Collectively, those data suggest the etiology of obesity may influence how obesity affects bone mechanical properties.
Acknowledgments
This work was funded in part by the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada. The authors declare no conflict of interests.
References
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.Cao JJ. Effects of obesity on bone metabolism. Journal of Orthopaedic Surgery and Research. 2011;6, article 30 doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Migliaccio S, Greco EA, Fornari R, Donini LM, Lenzi A. Is obesity in women protective against osteoporosis? Diabetes, Metabolic Syndrome and Obesity. 2011;4:273–282. doi: 10.2147/DMSO.S11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holeckiand M, Wiecek A. Relationship between body fat mass and bone metabolism. Polskie Archiwum Medycyny Wewnetrznej. 2010;120(9):361–367. [PubMed] [Google Scholar]
- 5.Chehab FF, Qiu J, Ogus S. The use of animal models to dissect the biology of leptin. Recent Progress in Hormone Research. 2004;59:245–266. doi: 10.1210/rp.59.1.245. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 7.Cinti S, Frederich RC, Zingaretti MC, De Matteis R, Flier JS, Lowell BB. Immunohistochemical localization of leptin and uncoupling protein in white and brown adipose tissue. Endocrinology. 1997;138(2):797–804. doi: 10.1210/endo.138.2.4908. [DOI] [PubMed] [Google Scholar]
- 8.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Medicine. 1995;1(11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 9.Elefteriou F, Takeda S, Ebihara K, et al. Serum leptin level is a regulator of bone mass. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koletsky S. Pathologic findings and laboratory data in a new strain of obese hypertensive rats. American Journal of Pathology. 1975;80(1):129–142. [PMC free article] [PubMed] [Google Scholar]
- 11.Russell JC, Graham S, Hameed M. Abnormal insulin and glucose metabolism in the JCR:LA-corpulent rat. Metabolism. 1994;43(5):538–543. doi: 10.1016/0026-0495(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 12.Richardson M, Schmidt AM, Graham SE, Achen B, DeReske M, Russell JC. Vasculopathy and insulin resistance in the JCR:LA-cp rat. Atherosclerosis. 1998;138(1):135–146. doi: 10.1016/s0021-9150(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 13.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 14.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 15.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regulatory Peptides. 2000;92(1–3):73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 16.Bartell SM, Rayalam S, Ambati S, et al. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. Journal of Bone and Mineral Research. 2011;26(8):1710–1720. doi: 10.1002/jbmr.406. [DOI] [PubMed] [Google Scholar]
- 17.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34(3):376–383. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Hou JCH, Zernicke RF, Barnard RJ. Experimental diabetes, insulin treatment, and femoral neck morphology and biomechanics in rats. Clinical Orthopaedics and Related Research. 1991;264:278–285. [PubMed] [Google Scholar]
- 19.Reddy GK, Stehno-Bittel L, Hamade S, Enwemeka CS. The biomechanical integrity of bone in experimental diabetes. Diabetes Research and Clinical Practice. 2001;54(1):1–8. doi: 10.1016/s0168-8227(01)00273-x. [DOI] [PubMed] [Google Scholar]
- 20.Brindley DN, Russell JC. Animal models of insulin resistance and cardiovascular disease: some therapeutic approaches using the JCR:LA-cp rat. Diabetes, Obesity and Metabolism. 2002;4(1):1–10. doi: 10.1046/j.1463-1326.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- 21.Salem GJ, Zernicke RF, Martinez DA, Vailas AC. Adaptations of immature trabecular bone to moderate exercise: geometrical, biochemical, and biomechanical correlates. Bone. 1993;14(4):647–654. doi: 10.1016/8756-3282(93)90087-q. [DOI] [PubMed] [Google Scholar]
- 22.Pelker RR, Friedlaender GE, Markham TC, Panjabi MM, Moen CJ. Effects of freezing and freeze-drying on the biomechanical properties of rat bone. Journal of Orthopaedic Research. 1984;1(4):405–411. doi: 10.1002/jor.1100010409. [DOI] [PubMed] [Google Scholar]
- 23.Linde F, Hvid I. The effect of constraint on the mechanical behaviour of trabecular bone specimens. Journal of Biomechanics. 1989;22(5):485–490. doi: 10.1016/0021-9290(89)90209-1. [DOI] [PubMed] [Google Scholar]
- 24.Salem GJ, Zernicke RF, Martinez DA, Vailas AC. Biomechanical and biochemical changes in lumbar vertebrae of rapidly growing rats. American Journal of Physiology. 1989;2561(1, part 2):R259–R263. doi: 10.1152/ajpregu.1989.256.1.R259. [DOI] [PubMed] [Google Scholar]
- 25.Reimer RA, Russell JC. Glucose tolerance, lipids, and GLP-1 secretion in JCR:LA-cp rats fed a high protein fiber diet. Obesity. 2008;16(1):40–46. doi: 10.1038/oby.2007.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parnell JA, Reimer RA. Differential secretion of satiety hormones with progression of obesity in JCR:LA-corpulent rats. Obesity. 2008;16(4):736–742. doi: 10.1038/oby.2007.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruegsegger P, Medici TC, Anliker M. Corticosteroid-induced bone loss. A longitudinal study of alternate day therapy in patients with bronchial asthma using quantitative computed tomography. European Journal of Clinical Pharmacology. 1983;25(5):615–620. doi: 10.1007/BF00542348. [DOI] [PubMed] [Google Scholar]
- 28.Thomas T, Burguera B. Is leptin the link between fat and bone mass? Journal of Bone and Mineral Research. 2002;17(9):1563–1569. doi: 10.1359/jbmr.2002.17.9.1563. [DOI] [PubMed] [Google Scholar]
- 29.Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA cp rats. British Journal of Nutrition. 2012;107(4):601–613. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsunuma A, Kawane T, Maeda T, Hamada S, Horiuchi N. Leptin corrects increased gene expression of renal 25-hydroxyvitamin D 3-1α-hydroxylase and -24-hydroxylase in leptin-deficient, ob/ob mice. Endocrinology. 2004;145(3):1367–1375. doi: 10.1210/en.2003-1010. [DOI] [PubMed] [Google Scholar]
- 31.Prisby RD, Swift JM, Bloomfield SA, Hogan HA, Delp MD. Altered bone mass, geometry and mechanical properties during the development and progression of type 2 diabetes in the Zucker diabetic fatty rat. Journal of Endocrinology. 2008;199(3):379–388. doi: 10.1677/JOE-08-0046. [DOI] [PubMed] [Google Scholar]
- 32.Thomas T, Gori F, Spelsberg TC, Khosla S, Riggs BL, Conover CA. Response of bipotential human marrow stromal cells to insulin-like growth factors: effect on binding protein production, proliferation, and commitment to osteoblasts and adipocytes. Endocrinology. 1999;140(11):5036–5044. doi: 10.1210/endo.140.11.7128. [DOI] [PubMed] [Google Scholar]
- 33.Hamann C, Goettsch C, Mettelsiefen J, et al. Delayed bone regeneration and low bone mass in a rat model of insulin-resistant type 2 diabetes mellitus is due to impaired osteoblast function. American Journal of Physiology. 2011;301(6):E1120–E1128. doi: 10.1152/ajpendo.00378.2011. [DOI] [PubMed] [Google Scholar]
- 34.Li KC, Zernicke RF, Barnard RJ, Li AFY. Effects of a high fat-sucrose diet on cortical bone morphology and biomechanics. Calcified Tissue International. 1990;47(5):308–313. doi: 10.1007/BF02555914. [DOI] [PubMed] [Google Scholar]
- 35.Zernicke RF, Salem GJ, Barnard RJ, Schramm E. Long-term, high-fat-sucrose diet alters rat femoral neck and vertebral morphology, bone mineral content, and mechanical properties. Bone. 1995;16(1):25–31. doi: 10.1016/s8756-3282(00)80007-1. [DOI] [PubMed] [Google Scholar]
- 36.Lorincz C, Reimer RA, Boyd SK, Zernicke RF. High-fat, sucrose diet impairs geometrical and mechanical properties of cortical bone in mice. British Journal of Nutrition. 2010;103(9):1302–1308. doi: 10.1017/S0007114509993084. [DOI] [PubMed] [Google Scholar]


