Abstract
Doripenem dosing regimens for patients receiving continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodiafiltration (CVVHDF) were devised based on an established efficacy criterion (free plasma doripenem concentrations above the minimum inhibitory concentration [fT > MIC] of 1 mg/L for ≥35% of the dosing interval) while maintaining exposure below that with the highest studied dose of 1000 mg infused over 1 hour every 8 hours in healthy subjects. Simulations were utilized to assure ≥90% probability of achieving the efficacy criterion with the recommended doripenem regimens. Inflated intersubject variability of 40% (coefficient of variation) was used for pharmacokinetic parameters (representative of clinical variation) and nonrenal clearance was doubled to account for potential changes with acute renal insufficiency. Results indicate that a reduction in doripenem dose will be needed for critically ill patients receiving CVVH or CVVHDF. This work was conducted to fulfill a health authority request and resulted in the addition of dosing recommendations to the Doribax Summary of Product Characteristics.
1. Introduction
Doripenem is a parenteral carbapenem with broad-spectrum microbiological activity, inclusive of multidrug-resistant gram-negative pathogens [1, 2]. It is approved at a dose of 500 mg administered as a 1-hour infusion every 8 hours (q8h) in the United States of America (USA) and the European Union for the treatment of complicated intra-abdominal infections and complicated urinary tract infections, including pyelonephritis. Doripenem is also approved in the European Union as a 500 mg 1- or 4-hour infusion q8h for the treatment of nosocomial pneumonia, including ventilator-associated pneumonia, in adults [3, 4]. Renal excretion is the major route of doripenem elimination [5] and a strong direct relationship between doripenem clearance (CL) and creatinine clearance (CrCL) has been noted [CL = 13.6·(CrCL/98)0.659] [6]. Systemic exposure (mean area under the curve [AUC] from time 0 extrapolated to infinite time) of doripenem in subjects with mild (CrCL 51–79 mL/min), moderate (CrCL 31–50 mL/min), and severe (CrCL ≤ 30 mL/min) renal impairment was 1.6-, 2.8-, and 5.1-fold higher, respectively, than that of age-matched healthy subjects with normal renal function (CrCL ≥ 80 mL/min) [3, 7]. Consequently, a dosage regimen of 250 mg, infused over 1 hour, every 12 hours (q12h), is recommended for subjects with a CrCL >10 and <30 mL/min) [3].
Continuous venovenous hemofiltration (CVVH) and venovenous hemodiafiltration (CVVHDF) are often used to manage hemodynamically unstable patients who are volume overloaded and patients with acute kidney injury [8–10]. CVVHDF employs diffusion as well as convection for solute and extracellular fluid removal, while CVVH is dependent on ultrafiltration alone. Both of these methodologies enhance extracellular fluid and drug clearance in patients with impaired renal function [11–13]. The clinical impact of altered drug clearance also depends on the CVVH or CVVHDF modality and flow rate [11, 13]. In clinical practice, these therapies are tailored to the individual patient's needs by modifying the blood flow, ultrafiltration, dialysate, and replacement fluid flow rates.
Cirillo et al. characterized the pharmacokinetics of doripenem and its inactive metabolite, doripenem-M-1, during continuous renal replacement therapy (CRRT) in hemodialysis-dependent subjects with Stage 5 chronic kidney disease and have summarized their findings using noncompartmental analysis [14]. Despite significant removal of drug by CVVH (38% of administered dose) and CVVHDF (29% of dose), systemic exposure (AUC) of doripenem and doripenem-M-1 was approximately 3- and 5-fold greater, respectively, in subjects who received CRRT compared to healthy subjects. CRRT significantly augmented the clearance of doripenem in subjects without renal function, but not as effectively as a functioning kidney, and doripenem dosing regimens for those who are on CRRT would therefore still need to be adjusted.
This investigation was designed to devise doripenem dosage recommendations for critically ill patients receiving CVVH or CVVHDF therapy using model-based pharmacokinetic parameters derived from the data of Cirillo et al. [14] as well as efficacy and safety considerations. The analysis described herein was conducted to address a postapproval commitment with the European Medicines Agency in which the sponsor was required to (a) perform an analysis of pharmacokinetic data from the study in subjects with CRRT and, if possible, propose a dosing regimen for doripenem in patients on CRRT; (b) the analysis had to include a comprehensive assessment of the inactive M-1 metabolite pharmacokinetics given the potential for accumulation; (c) the dosing regimen would have to be supported by a pharmacokinetic/pharmacodynamic argument based on a target attainment analysis involving Monte Carlo simulations with higher variability estimates that are representative of the variation seen in the clinical situation; (d) the simulations would require a sensitivity analysis using prolonged infusion time to assess pharmacokinetic/pharmacodynamic coverage for less susceptible pathogens; (e) the CRRT study was performed in chronic kidney disease subjects for ethical reasons. However, the pharmacokinetic/pharmacodynamic assessments had to include a sensitivity analysis that took into consideration acute kidney impairment where CRRT is more commonly used and is often associated with increased non-renal clearance (CLNR) [15].
The doripenem dosage recommendations for subjects needing CVVH or CVVHDF therapy were designed to (1) maintain free plasma doripenem concentrations above the minimum inhibitory concentration (fT > MIC) for at least 35% of the dosing interval, a recognized efficacy target for carbapenems [16–18]; (2) maintain steady-state peak concentration (Cmax,ss) and total daily area under the curve (AUC24,ss), respectively, under 44.0 mg/L and 208.8 mg·h/L for doripenem and 9.81 mg/L and 63.9 mg·h/L for doripenem-M-1. These safety metrics are based on the exposure observed with the highest safely studied doripenem dose of 1000 mg, infused over 1 hour q8h in healthy subjects.
2. Methods
2.1. Pharmacokinetic Study
The pharmacokinetic data used in this analysis were from the previous study of Cirillo et al. that characterized the pharmacokinetics of doripenem and its inactive metabolite, doripenem-M-1, in hemodialysis-dependent subjects with Stage 5 chronic kidney disease while they were undergoing a 12-hour CVVH or CVVHDF session, which commenced approximately 1 hour before the subjects received a single intravenous 1-hour infusion of doripenem 500 mg [14]. The CRRT procedure was conducted on a nondialysis day.
2.2. Safety and Efficacy Criteria for Simulations
2.2.1. Safety Criteria
The safety metric for this analysis was to maintain doripenem and doripenem-M-1 exposures lower than the observed Cmax,ss and AUC24,ss with doripenem 1000 mg administered q8h in healthy subjects. These safety metrics were obtained from a randomized, double-blind, placebo- and positive-controlled crossover study that was conducted to evaluate electrocardiogram QTc intervals in healthy adults [3]. The safety threshold was defined by Cmax,ss and AUC24,ss of, respectively, 44.0 mg/L and 208.8 mg·h/L for doripenem and 9.81 mg/L and 63.9 mg·h/L for doripenem-M-1.
2.2.2. Efficacy Criteria
For doripenem, like other carbapenem antibiotics, the fT > MIC for infecting pathogens is the target that best correlates with subject outcomes [16–18]. It has been reported that the recommended 500 mg dose of doripenem, for individuals with normal renal function, infused over 1 hour q8h is expected to be effective against bacilli with doripenem MICs of ≤1 mg/L based on a fT >MIC of 35% in critically ill subjects [19]. Target attainment up to an MIC of 1 mg/L is desirable since the majority of the pathogens (clinical isolates from studies of subjects with intra-abdominal and complicated urinary tract infections and nosocomial pneumonia, including subjects in the intensive care unit) have an MIC90 of ≤1 mg/L [19]. Pharmacokinetic/pharmacodynamic target attainment probabilities for fT >MIC of 35% were therefore evaluated for various dosing regimens across a range of MICs, which were chosen based on the variety of pathogens and their doripenem susceptibility in large Phase 3 clinical studies and surveillance studies [19]. Protein binding of 8.5% was applied to correct for plasma protein binding in the simulated data [19].
2.3. Steady-State Simulations for Safety Assessment Using Nonparametric Superposition
Steady-state simulations of doripenem and doripenem-M-1 plasma concentration time data with various dosing regimens were performed using nonparametric superposition. The pharmacokinetic profile for nonparametric superposition was derived from chronic kidney disease subjects who underwent a 12-hour CVVH or CVVHDF procedure [14]. Steady-state simulated concentration-time data were analyzed using standard noncompartmental methods to generate Cmax,ss, AUC24,ss, and AUC during a dosing interval at steady-state (AUCτ). No formal statistical analyses were performed on these data, which were summarized descriptively by doripenem dose and CRRT modality. Nonparametric superpositioning and noncompartmental analyses were performed using validated WinNonlin (Version 5.2, Pharsight Corporation) software. For CVVH, 500 mg q8h, 250 mg q8h, and 250 mg q12h 1-hour infusions were simulated to steady-state. Similarly, for CVVHDF, 500 mg q8h, 375 mg q12h, 250 mg q8h, and 250 mg q12h 1-hour infusions were simulated to steady-state. The nonparametric superposition simulations assumed pharmacokinetic linearity at lower doses and stationary (or time-independent) pharmacokinetics upon multiple dosing.
2.4. Pharmacokinetic Parameter Estimation for Utilization in Target Attainment Simulations
The actual doripenem plasma concentrations observed by Cirillo et al. [14] in hemodialysis-dependent chronic kidney disease subjects undergoing CRRT were fitted using a 2-compartment model with zero-order input. An additive error model with log-transformed data was used to describe the residual variability. The model was parameterized in terms of clearance (CL), central volume (V1), peripheral volume (V2), and distributional clearance (Q). The subjects in the study were classified as “anephric” since they all had a CrCL of less than 5 mL/min. CL under this CRRT situation had 2 components, CLNR and machine clearance (CLCRRT), with CL = CLNR + CLCRRT where CLNR is an estimated parameter like Q, V1, and V2, while CLCRRT is fixed. CLCRRT was fixed in these analyses for each individual subject based on previously reported findings [14].
2.5. Monte-Carlo-Based Efficacy Target Attainment Simulations
A 5,000-subject Monte Carlo simulation was implemented in the S-Plus software (Insightful Corporation, Seattle, WA, USA), using mean pharmacokinetic parameter estimates (see Supplementary Table 1, Supplementary Materials available online at doi:10.5402/2012/782656) and an inflated between-subject variability of 40% coefficient of variation (CV) for all parameters. Simulations were conducted to generate concentration-time profiles for several dosing regimens of doripenem. Residual variability was not introduced into the calculations of simulated concentrations since it was previously found to be insignificant [6, 19].
The following dose regimens, each infused q12h over 1 hour, were simulated: 250 mg administered for CVVH subjects with CrCL of 30 mL/min; 250 mg administered for CVVHDF subjects with CrCL of 0; 500 mg administered for CVVHDF subjects with CrCL of 30 mL/min. The 250 mg q12h regimen represents the highest possible regimen based on the safety assessment. For simulations with residual kidney function, CrCL was set at 30 mL/min because it represents the worst case scenario with CrCL fixed at the upper end of the severe renal impairment category. In the presence of residual kidney function, CL was computed as the sum of CLNR, renal clearance, and CLCRRT. The doripenem renal clearance for a CrCL of 30 mL/min is 2.5 L/hr based on the population pharmacokinetic model reported by Nandy et al. [6] and the CLNR reported by Cirillo et al. [5]. Based on the doripenem prescribing information [3, 4], the dose in severe renal impairment (CrCL < 30 mL/min) should be a q12h infusion of 250 mg. If this population also undergoes CVVHDF, which is the most efficient CRRT modality, then it would be anticipated that the dose should be increased. We therefore investigated the 500 mg dose of doripenem for CVVHDF in subjects with residual kidney function (CrCL = 30 mL/min). The higher dose is not warranted for CVVH since it is not as efficient as CVVHDF for eliminating doripenem from the systemic circulation [14]. Likewise, since CVVH removes drug from the systemic circulation using a single ultrafiltration process (as compared to CVVHDF, which also uses convection), only the higher clearance scenario with CrCL of 30 mL/min was simulated for CVVH. To assess the probability of achieving the efficacy target for higher MICs, these simulations were repeated with a 4-hour infusion. The percentage of subjects with fT > MIC ≥ 35% was computed for each dosing regimen with MICs from 1 to 8 mg/L.
To account for the potential impact of pharmacokinetic variability, an inflated between-subject variability of 40% CV was used for all simulations. This inflated %CV was selected based on two sources of recently published information: (1) New Clinical and Laboratory Standards Institute (CLSI) interpretive criteria (breakpoints) for carbapenems against Enterobacteriaceae, which were based on Monte Carlo simulations, utilized inflated between-subject variability of 40% CV [20] and (2) population pharmacokinetic parameters of doripenem based on data from Phase 1/2/3 studies of critically ill subjects, which showed that the %CV estimates range from 34% to 55% for all model-estimated pharmacokinetic parameters [6]. All pharmacokinetic parameters had variability close to 40% or less, except peripheral volume, which had a variability estimate of 55%.
To illustrate the influence of inflated variability, simulations were conducted for the single-dose scenario studied by Cirillo et al. [14]. These simulations used mean pharmacokinetic parameter estimates (see Supplementary Table 1) with a between-subject variability of 40% CV for all parameters. The results of the simulations were compared with the observed data from Cirillo et al. [14] in Figure 1. The results clearly illustrate that the simulated concentrations exhibit greater variability than the biological variation seen in the otherwise healthy chronic kidney disease volunteers. Thus, the inflated variability is a reasonable approximation of the clinical scenario that can be anticipated in critically ill patients on CRRT and requiring doripenem therapy.
Figure 1.
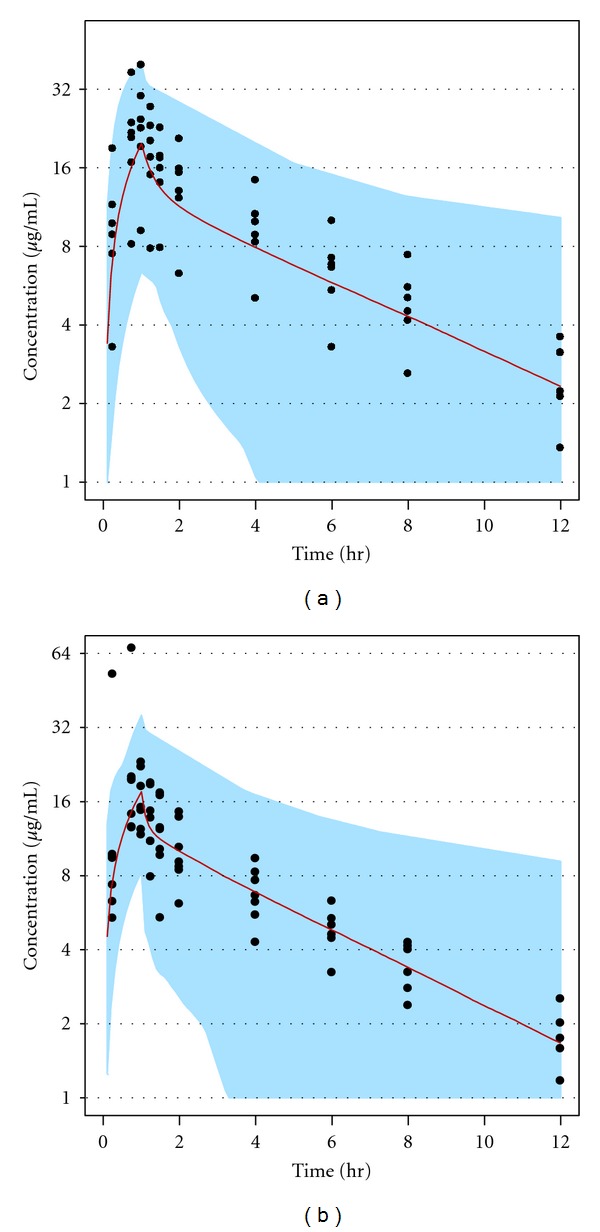
A representation of the inflated variance (40% CV) for between-subject variability in pharmacokinetic parameters. The (a) and (b) panels represent CVVH and CVVHDF data, respectively, from a previously published study [14]. The filled circles are observed study data and the lines and shaded areas represent the median and range, respectively, of minimum and maximum simulated concentrations.
The majority of patients who are treated with CRRT have acute kidney impairment [11]. Patients with acute kidney impairment often have multifactorial clinical complications, including inflammation, sepsis, and trauma. These factors may have additional complicating influences on pharmacokinetics such as induction of CLNR. Since doripenem CLNR may be 2-fold higher in patients with acute kidney impairment, as seen with other carbapenems [15], the whole efficacy target attainment portion of this study was also conducted with a CLNR for doripenem that was twice the value observed in chronic kidney disease patients (see Supplementary Table 1). Target attainment was calculated for the proposed CVVH and CVVHDF dosing regimens with both a 1-hour and a 4-hour infusion in acute kidney impairment.
2.6. Assumptions for the Simulations
The target attainment simulation strategy described above was based on the following assumptions: (1) a series of simulations were conducted to determine the probability of efficacy target attainment with the proposed doripenem dosing regimens for subjects with acute kidney impairment being treated with CRRT; the CLNR for doripenem was doubled and set at 7.4 L/hr for CVVH and 9.4 L/hr for CVVHDF; (2) pharmacokinetic linearity was assumed; the CRRT study was performed at the 500 mg doripenem dose and profiles for lower doses were simulated assuming that the pharmacokinetics will stay linear at the lower doses in CRRT patients; (3) stationary (or time-independent) pharmacokinetics was assumed, which implies that target attainment determined from single-dose simulations apply to steady-state multiple-dosing scenarios. This assumption for doripenem is permissible because the parent drug does not accumulate to a significant extent, since it has a 1- to 4-hour plasma elimination half-life for various subject populations including subjects with severe chronic kidney disease [5, 6, 14, 19]; (4) stationary (or time-independent) pharmacodynamics was assumed wherein time-dependent phenomena such as postantibiotic effect and resistance development were not incorporated in the modeling process; (5) no formal model was developed to describe dropouts and complete compliance was assumed during simulations. This is a fair assumption because doripenem is administered intravenously under the supervision of a healthcare professional in a clinical setting; (6) interindividual variability for several parameters (e.g., protein binding and residual kidney function) was not incorporated in the simulations because such detailed information is not available; (7) the reported protein binding of 8.5% for doripenem [19] was also assumed to apply to CVVH and CVVHDF subjects; (8) residual variability was not introduced into the calculations of simulated concentrations since it was found to be insignificant; (9) the pharmacokinetic/pharmacodynamic target is based on plasma drug exposure associated with bacteriostatic effect (fT > MIC 35% target). If clinical outcome is correlated with other more challenging endpoints such as 2- to 3-log kill or bactericidal effect, then the calculated target attainment may represent an overprediction; (10) the pathogen distributions obtained from large Phase 3 doripenem studies [19] and surveillance data [19] were considered to be representative of the general widespread microbial population occurring in the clinical situation for CRRT subjects; (11) the uncertainty and analytical error in MIC determinations was not added as a source of variation in the simulations.
2.7. Software for Pharmacokinetic Parameter Estimation and TAR Simulations
Each subject's pharmacokinetic parameters for CVVH and CVVHDF were estimated separately using the first-order estimation method to characterize the time course of plasma concentrations with the NONMEM V level 1.1 software package (GloboMax, Hanover, MD, USA) including NM-TRAN (version III level 1.0) and PREDPP (version IV level 1.0). Compilations were achieved using Digital Visual Fortran version 6.0.a (Digital Equipment Corporation, Maynard, MA, USA). Graphical data visualization, evaluation of NONMEM outputs, construction of goodness of fit plots, and simulation based target attainment analysis were performed using the S-Plus version 6.0 package for Windows (Insightful Inc., Data Analysis Products Division, Seattle, WA, USA).
3. Results
3.1. Safety Criteria
The simulated Cmax,ss and AUC24,ss of doripenem and doripenem-M-1 determined by nonparametric superposition and noncompartmental analyses of the single-dose mean data from subjects receiving CVVH and CVVHDF [14] are shown in Table 1. The Cmax,ss and AUC24,ss for a 250 mg q12h 1-hour infusion regimen in CVVH subjects were, respectively, 13.2 mg/L and 114.2 mg·h/L for doripenem, and 3.27 mg/L and 58.6 mg·h/L for doripenem-M-1. The values of Cmax,ss and AUC24,ss in CVVHDF subjects were 12 mg/L and 86.6 mg·h/L for doripenem and 3.49 mg/L and 54.2 mg·h/L for doripenem-M-1. The simulated AUC24,ss and Cmax,ss values obtained for a 250 mg q12h 1-hour infusion regimen were lower than the prespecified safety thresholds for both doripenem and doripenem-M-1. All the higher evaluated doses (Table 1) yielded doripenem M-1 values in excess of prespecified safety threshold and therefore the 250 mg q12h regimen was considered the maximum dose for patients with minimal, to no, residual renal function. The next goal was to test whether the dose chosen based on the safety metric would also be satisfactory from an efficacy standpoint based on a target attainment analysis (see below).
Table 1.
Results of nonparametric superposition and noncompartmental analysis performed on mean data for doripenem and doripenem-M-1.
| Continuous venovenous hemofiltration | Continuous venovenous hemodiafiltration | Healthy subjectsa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1a | Simulated steady-state (day 10) | Day 1a | Simulated steady-state (day 10) | |||||||
| 500 mg q12h |
500 mg q8h |
250 mg q8h |
250 mg q12h |
500 mg q12h |
500 mg q8h |
375 mg q12h |
250 mg q8h |
250 mg q12h |
1000 mg q8h |
|
| Doripenem | ||||||||||
| Cmax,ss (mg/L) | 24.1 | 29.6 | 14.9 | 13.2 | 22.5 | 26.5 | 17.9 | 13.2 | 12.0 | 44.0 |
| AUCτ (mg·h/L) | 97.6 | 114 | 57.1 | 57.1 | 88.4 | 86.5 | 64.9 | 43.3 | 43.3 | 69.6 |
| AUC24,ss (mg·h/L) | N/A | 342.0 | 171.3 | 114.2 | N/A | 259.5 | 129.8 | 129.9 | 86.6 | 208.8 |
| Doripenem-M-1 | ||||||||||
| Cmax,ss (mg/L) | 3.02 | 8.86 | 4.44 | 3.27 | 3.66 | 9.15 | 5.23 | 4.57 | 3.49 | 9.81 |
| AUCτ (mg·h/L) | 24.4 | 58.6 | 29.3 | 29.3 | 21.8 | 54.2 | 40.7 | 27.1 | 27.1 | 21.3 |
| AUC24,ss (mg·h/L) | N/A | 175.8 | 87.9 | 58.6 | N/A | 162.6 | 81.4 | 81.3 | 54.2 | 63.9 |
AUC24,ss: total daily area under the curve at steady state; AUCτ: area under the curve at steady state during the dosing interval τ; Cmax,ss: maximum plasma concentration at steady state; q8h: every 8 hours; q12h: every 12 hours.
aThe day 1 500 mg q12h CRRT data and the healthy subject data are observed results from single- and multiple dose studies. All doripenem dosing regimens were a 1-hour infusion duration.
3.2. Efficacy Criteria
3.2.1. Pharmacokinetic Parameter Estimation for Monte Carlo Simulations
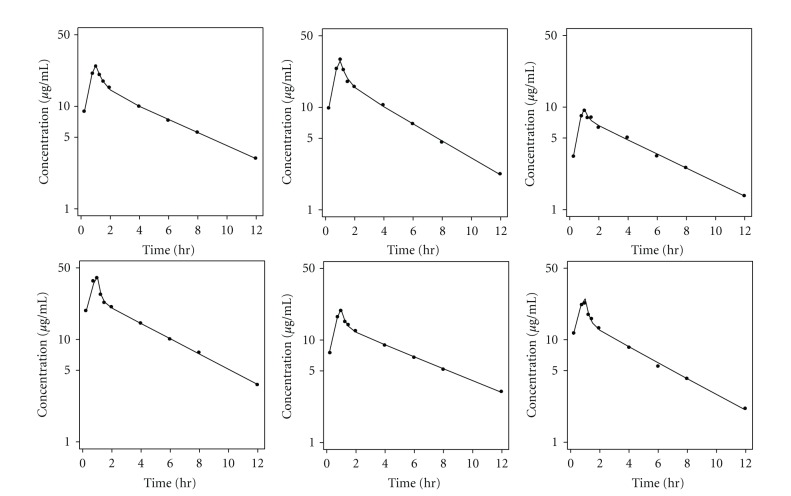
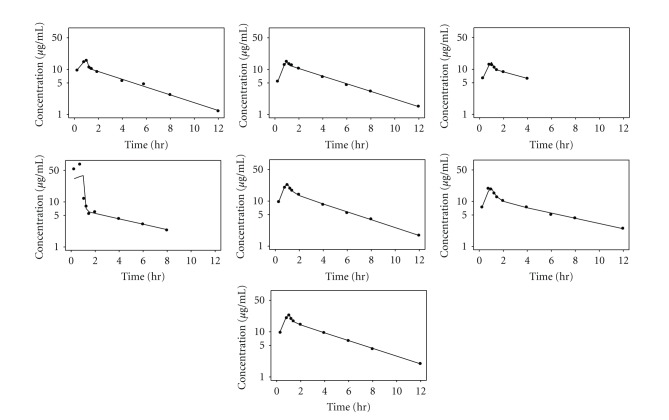
Individual fitting of the plasma pharmacokinetic data for CVVH subjects (n = 6) is depicted in Figure 2 and the parameter estimates are reported in Supplementary Table 1. Figure 3 and Supplementary Table 1 provide the corresponding model fit and pharmacokinetic parameters for CVVHDF (n = 7). The model-derived value of CLNR for CVVH (3.7 L/h) and CVVHDF (4.7 L/h) subjects are quite consistent with the estimate of CLNR of 3.7 L/h in healthy subjects reported by Cirillo et al. [5]. This suggests that, unlike other carbapenems, there is no significant decline in the CLNR of doripenem in chronic kidney disease.
Figure 2.
Individual fitting of the plasma pharmacokinetic data for CVVH subjects using a 2-compartment model.
Figure 3.
Individual fitting of the plasma pharmacokinetic data for CVVHDF subjects using a 2-compartment model.
3.2.2. Time above MIC Analysis in Chronic Kidney Disease
The results of the Monte Carlo simulations, based on a 1-hour doripenem infusion, are presented in Supplementary Tables 2 to 4. The recommended dosing regimen for CVVH and CVVHDF achieved the efficacy metric (fT > MIC ≥ 35%) with greater than 90% probability for MICs ≤1 mg/L at all possible ultrafiltration flow rates for both CRRT modalities, even in patients with CrCL up to 30 mL/min (see Tables 2 and 3). The results of Monte Carlo simulations, based on a 4-hour doripenem infusion, are presented in Supplementary Tables 5 to 7. The probability of target attainment did improve at the lower flow rates for higher MICs but complete coverage for MICs ≥2 mg/L at the higher CRRT flow rates was not achieved.
Table 2.
Probability of pharmacokinetic/pharmacodynamic target (fT > MIC ≥ 35%) attainment by CVVH flow rate in acute kidney impairment subjects with residual kidney function (250 mg q12h, 4-hour infusion).
| MIC (mg/L) | CVVH flow rate (L/hr) | |||
|---|---|---|---|---|
| 0.5 | 1 | 2 | 3 | |
| 1 | 0.984 | 0.981 | 0.973 | 0.963 |
| 2 | 0.603 | 0.573 | 0.514 | 0.455 |
| 4 | 0.021 | 0.017 | 0.012 | 0.008 |
| 8 | 0.000 | 0.000 | 0.000 | 0.000 |
Pharmacokinetic-pharmacodynamic target attainment probabilities for CVVH are based on a simulation of 5000 subjects using mean pharmacokinetic parameter estimates [14], with inflated between-subject variability (40% CV) and assuming a protein binding of 8.5% along with the presence of residual kidney function. Total drug CL represented a sum of 2 × CLNR, renal CL, and CVVH CL. The renal component of the clearance allowed residual kidney function with a CrCL of 30 mL/min.
Table 3.
Probability of pharmacokinetic/pharmacodynamic target (fT > MIC ≥ 35%) attainment by CVVHDF flow rate in acute kidney impairment subjects.
| MIC (mg/L) | CVVHDF flow rate (L/hr) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.25 | 1.75 | 2.25 | 2.5 | 2.75 | 3.25 | 3.5 | 3.75 | 4.5 | |
| Anephric subjectsa (250 mg q12h, 4-hour infusion) | |||||||||
|
| |||||||||
| 1 | 0.985 | 0.981 | 0.978 | 0.975 | 0.973 | 0.968 | 0.966 | 0.963 | 0.954 |
| 2 | 0.578 | 0.547 | 0.514 | 0.500 | 0.482 | 0.452 | 0.439 | 0.424 | 0.379 |
| 4 | 0.017 | 0.014 | 0.011 | 0.010 | 0.009 | 0.008 | 0.006 | 0.006 | 0.005 |
| 8 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
|
| |||||||||
| Subjects with residual kidney functionb (500 mg q12h, 4-hour infusion) | |||||||||
|
| |||||||||
| 1 | 0.999 | 0.999 | 0.999 | 0.998 | 0.998 | 0.998 | 0.998 | 0.998 | 0.998 |
| 2 | 0.952 | 0.944 | 0.937 | 0.933 | 0.929 | 0.919 | 0.914 | 0.911 | 0.896 |
| 4 | 0.367 | 0.345 | 0.323 | 0.311 | 0.3 | 0.279 | 0.269 | 0.259 | 0.231 |
| 8 | 0.005 | 0.003 | 0.003 | 0.002 | 0.002 | 0.002 | 0.002 | 0.001 | 0.001 |
aPharmacokinetic/pharmacodynamic target attainment probabilities for CVVHDF are based on a simulation of 5000 anephric subjects using mean pharmacokinetic parameter estimates from Cirillo et al. [14] with inflated between-subject variability (40% CV) and assuming a protein binding of 8.5%. Total CL represented the sum of 2 × CLNR and CVVHDF clearance.
bThe renal component of the clearance allowed residual kidney function with a CrCL of 30 mL/min.
3.2.3. Time above MIC Analysis in Acute Kidney Injury
Monte Carlo simulations for subjects with acute kidney impairment, and therefore potential elevated CLNR, treated with CVVH or CVVHDF indicated that the efficacy criterion (fT > MIC ≥ 35%) is achievable with ≥90% probability only up to an MIC of 0.5 mg/L with a 1-hour infusion (Supplementary Tables 8 to 10), whereas this criterion was achieved with ≥90% probability for MICs up to 1 mg/L when the infusion duration was prolonged to 4 hours (Tables 2 and 3).
4. Discussion
The goals of renal replacement therapy in critically ill patients, especially those with acute kidney impairment, are to avert the life-threatening consequences of acidosis, electrolyte imbalances, uremia, and fluid overload, thereby preserving life and allowing organ function to recover [21]. CRRT has become the main form of renal replacement therapy for critically ill patients with acute kidney impairment and its use is preferable in hemodynamically unstable patients [21]. The choice between CVVH and CVVHDF is largely dependent on institutional preferences and the experience of the support staff while the dose of therapy can be tailored to the patient's needs. The pharmacokinetics of many drugs are altered in the presence of acute kidney impairment and critical illness [15]. Appropriate dosing in these critically ill patients is complicated by a number of factors, including evolving illness, severity of illness, and organ dysfunction, as well as the initiation of CRRT. Thus, drug dosing for these critically ill patients is dependent on (a) the patient's residual kidney function; (b) altered drug disposition parameters due to accompanying illness; (c) the efficiency of the CRRT procedure. Several empiric methods for estimating the appropriate drug dosing for patients receiving CRRT have been proposed, mostly focused on antimicrobials [11].
Since CRRT influences the disposition of most carbapenems [22–24], which may necessitate dosage adjustment, a study was conducted to evaluate the influence of CVVH and CVVHDF on the pharmacokinetics of doripenem [14]. With the goal of developing a dosing regimen, we utilized data from Cirillo et al. [14] and conducted Monte Carlo simulations to propose doripenem dosage recommendations for subjects undergoing CVVH and CVVHDF. The simulations were performed using a pharmacokinetic model that was built using doripenem concentration time data from hemodialysis-dependent Stage 5 chronic kidney disease subjects who received CVVH or CVVHDF. These new dosing recommendations (in the Doribax Summary of Product Characteristics) suggest that 250 mg infused over 4 hours q12h is suitable for patients receiving CVVH (Table 4) [25]. This dose was also deemed appropriate for patients receiving CVVHDF who have a CrCL <5 mL/min. A higher dose of 500 mg infused over 4 hours q12h is recommended for patients with residual kidney function (CrCL of 5–30 mL/min) who are receiving CVVHDF. Patients being treated with CRRT who have chronic kidney disease can be adequately treated with either a 1- or 4-hour doripenem infusion time but a 4-hour infusion time may be more suitable for the treatment of infections due to less susceptible pathogens. Patients with new onset acute kidney impairment being treated with CRRT may have improved outcomes if doripenem is infused over 4 hours.
Table 4.
Dosing recommendations for patients on CRRT [25].
| CRRT procedure | Estimated CrCL | Dose | Frequency | Infusion timea,b | Target attainment (MIC) |
|---|---|---|---|---|---|
| CVVH | ≤30 mL/min | 250 mg | q12h | 4 hours | ≤1 mg/L |
| CVVHDF | <5 mL/min | 250 mg | q12h | 4 hours | ≤1 mg/L |
| CVVHDF | 5–30 mL/min | 500 mg | q12h | 4 hours | ≤1 mg/L |
aFor patients with acute kidney impairment on CRRT, an infusion time of 4 hours is required, taking into consideration the possible increases in nonrenal clearance of carbapenems in patients with acute renal insufficiency.
bPatients with chronic kidney disease on CRRT can be treated with either a 1- or 4-hour infusion time. Based mainly on pharmacokinetic/pharmacodynamic considerations, a 4-hour infusion time may be more suitable to maximize the percentage time during the dosing interval that the free plasma concentration of doripenem exceeds the minimum inhibitory concentration (% fT > MIC).
From a safety standpoint, a substantial dose reduction would be anticipated for patients with severe chronic kidney disease and those who may be experiencing acute kidney impairment to prevent the concentrations of doripenem-M-1 from accumulating extensively when multiple doses of doripenem are administered. Exposure to the metabolite doripenem-M-1 in patients on CRRT may be increased to levels where no in vivo safety data are presently available. While the metabolite lacks target pharmacological activity, other possible pharmacological effects are unknown. To address this safety concern, nonparametric super-position analysis was performed, using the single-dose mean pharmacokinetic data reported by Cirillo et al. for both doripenem and doripenem-M-1 [14]. The simulated exposure metrics (Cmax,ss and AUC24,ss) with doripenem 250 mg infused over 1 hour q12h were lower than the prespecified thresholds (see Table 1), thereby ensuring that there is sufficient safety margin for both parent and metabolite in these patient populations.
The results of Monte Carlo simulations indicate that the recommended dosing regimens for CVVH and CVVHDF achieved the efficacy coverage criterion (fT > MIC ≥ 35%) for organisms with an MIC ≤1 mg/L for greater than 90% of the subjects at all possible ultrafiltration flow rates, even in patients with CrCL up to 30 mL/min. This level of efficacy coverage is also achievable with a 1-hour 500 mg q8h doripenem infusion at MICs of ≤1 mg/L in ≥90% of the general patient population [19].
It should be noted that subjects who had Stage 5 chronic kidney disease and were on CRRT had a 50% higher volume of distribution as compared to normal volunteers [14]. Therefore, increasing the infusion duration for chronic kidney disease patients did not extend the coverage beyond 1 mg/L because of the dilution effect from the higher volume of distribution (Supplementary Tables 5 to 7). However, a prolonged, 4-hour infusion regimen may be desirable for infections due to less susceptible pathogens in the chronic kidney disease population at lower CRRT flow rates. Model-derived values of CLNR for CVVH (3.7 L/h) and CVVHDF (4.7 L/h) subjects were consistent with values observed in healthy subjects (CLNR = 3.7 L/h) [5], and it appears that doripenem CLNR is minimally affected by the presence of chronic kidney disease. However, some have reported higher CLNR of carbapenems in patients with acute kidney impairment versus those with chronic kidney disease who require CRRT [15]. Thus, in acute kidney impairment a 4-hour doripenem infusion is recommended to maintain fT > MIC for ≥35% of the dosing interval in the majority of this population for MICs ≤1 mg/L.
In conclusion, dosing regimens were developed (Table 4) for patients receiving CRRT such as CVVH with a CrCL ≤30 mL/min and CVVHDF with CrCL <5 mL/min and 5–30 mL/min based on simulations using data from a conventional pharmacokinetic study [25]. The simulations indicate that the patients will reach a ≥90% probability of target attainment (>35% fT > MIC) for pathogens with MICs ≤1 mg/L. Pathogens with a higher MIC are not sufficiently covered and increasing the dose would lead to increased exposure of the doripenem-M-1 metabolite, which should be avoided. The results of this analysis have resulted in the first labeled dosing recommendation for a carbapenem in subjects being treated with CRRT.
Supplementary Material
Supplementary Tables: Mean (± SD) pharmacokinetic parameter estimates for subjects undergoing continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodiafiltration (CVVHDF) are provided in Supplementary Table 1. The results of the Monte Carlo simulations for time above MIC analyses in chronic kidney disease are presented in Supplementary Tables 2 to 4 (based on a 1-hour doripenem infusion) and Supplementary Tables 5 to 7 (based on a 4-hour doripenem infusion). The results of the Monte Carlo simulations for time above MIC analyses in acute renal insufficiency based on a 1-hour doripenem infusion are presented in Supplementary Tables 8 to 10.
Conflict of Interests
M. N. Samtani, P. Nandy, I. Cirillo, and N. Vaccaro are employees of Janssen Pharmaceutical Companies of Johnson & Johnson, Raritan and Titusville, N. J., USA. G. R. Matzke has worked as an investigator with a number of pharmaceutical companies, including Janssen Research and Development.
Authors' Contribution
All authors had access to the study data, provided direction and comments on the paper, and made the final decision about where to publish these data and approved the final draft submission to the journal. G. R. Matzke, I. Cirillo, and N. Vaccaro contributed to study design and data interpretation. M. N. Samtani performed the pharmacokinetic analyses and provided interpretation of that data with help from P. Nandy. M. N. Samtani and P. Nandy provided an initial concept and draft report that was further developed by Dr. Sandra Norris, with direction provided by all authors.
Disclosure
All authors met International Council of Medical Journal Editors criteria and all those who fulfilled those criteria are listed as authors. The pharmacokinetic data reported in this paper have been summarized and previously published using noncompartmental analysis.
Acknowledgments
Janssen Pharmaceutical Companies of Johnson & Johnson funded this study and was responsible for study design and data collection, analysis, and its interpretation. The sponsor also was responsible for deciding to publish the data. Sandra Norris, Pharm. D., Norris Communications Group LLC provided writing assistance.
References
- 1.Jones RN, Huynh HK, Biedenbach DJ, Fritsche TR, Sader HS. Doripenem (S-4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluations. Journal of Antimicrobial Chemotherapy. 2004;54(1):144–154. doi: 10.1093/jac/dkh298. [DOI] [PubMed] [Google Scholar]
- 2.Pillar CM, Torres MK, Brown NP, Shah D, Sahm DF. In vitro activity of doripenem, a carbapenem for the treatment of challenging infections caused by gram-negative bacteria, against recent clinical isolates from the United States. Antimicrobial Agents and Chemotherapy. 2008;52(12):4388–4399. doi: 10.1128/AAC.00381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortho-McNeil-Janssen Pharmaceuticals, Inc. Doribax Prescribing Information 2007. 2010, http://doribax.com/shared/pi/doribax.pdf.
- 4.European Medicines Authority. Summary of product characteristics: Doribax. 2011, http://www.emea.europa.eu/humandocs/Humans/EPAR/doribax/doribax.htm.
- 5.Cirillo I, Vaccaro N, Turner K, Solanki B, Natarajan J, Redman R. Pharmacokinetics, safety, and tolerability of doripenem after 0.5-, 1-, and 4-hour infusions in healthy volunteers. Journal of Clinical Pharmacology. 2009;49(7):798–806. doi: 10.1177/0091270009337012. [DOI] [PubMed] [Google Scholar]
- 6.Nandy P, Samtani MN, Lin R. Population pharmacokinetics of doripenem based on data from phase 1 studies with healthy volunteers and phase 2 and 3 studies with critically ill patients. Antimicrobial Agents and Chemotherapy. 2010;54(6):2354–2359. doi: 10.1128/AAC.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirillo I, Vaccaro N, Castaneda-Ruiz B, Turner K, Redman R. Pharmacokinetics of doripenem in subjects with varying degrees of renal impairment (abstract A-1886). Proceedings of the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and the 46th Annual Meeting of the Infectious Diseases Society of America; October 2008; Washington, DC, USA. [Google Scholar]
- 8.Cerdá J, Ronco C. Modalities of continuous renal replacement therapy: technical and clinical considerations. Seminars in Dialysis. 2009;22(2):114–122. doi: 10.1111/j.1525-139X.2008.00549.x. [DOI] [PubMed] [Google Scholar]
- 9.Joy MS, Matzke GR, Armstrong DK, Marx MA, Zarowitz BJ. A primer on continuous renal replacement therapy for critically ill patients. Annals of Pharmacotherapy. 1998;32(3):362–375. doi: 10.1345/aph.17105. [DOI] [PubMed] [Google Scholar]
- 10.Palevsky PM. Dialysis modality and dosing strategy in acute renal failure. Seminars in Dialysis. 2006;19(2):165–170. doi: 10.1111/j.1525-139X.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 11.Heintz BH, Matzke GR, Dager WE. Antimicrobial dosing concepts and recommendations for critically Ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacotherapy. 2009;29(5):562–577. doi: 10.1592/phco.29.5.562. [DOI] [PubMed] [Google Scholar]
- 12.Kuang D, Verbine A, Ronco C. Pharmacokinetics and antimicrobial dosing adjustment in critically ill patient during continous renal replacement theraphy. Clinical Nephrology. 2007;67(5):267–284. doi: 10.5414/cnp67267. [DOI] [PubMed] [Google Scholar]
- 13.Pea F, Viale P, Pavan F, Furlanut M. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clinical Pharmacokinetics. 2007;46(12):997–1038. doi: 10.2165/00003088-200746120-00003. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo I, Vaccaro N, Balis D, Redman R, Matzke GR. Influence of continuous venovenous hemofiltration and continuous venovenous hemodiafiltration on the disposition of doripenem. Antimicrobial Agents and Chemotherapy. 2011;55(3):1187–1193. doi: 10.1128/AAC.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Critical Care. 2008;12(6):p. 235. doi: 10.1186/cc7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clinical Infectious Diseases. 1998;26(1):1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 17.Drusano GL. Antimicrobial pharmacodynamics: critical interactions of “bug and drug”. Nature Reviews Microbiology. 2004;2(4):289–300. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 18.Turnidge JD. The pharmacodynamics of β-lactams. Clinical Infectious Diseases. 1998;27(1):10–22. doi: 10.1086/514622. [DOI] [PubMed] [Google Scholar]
- 19.Samtani MN, Flamm R, Kaniga K, Nandy P. Pharmacokinetic-pharmacodynamic-model-guided doripenem dosing in critically ill patients. Antimicrobial Agents and Chemotherapy. 2010;54(6):2360–2364. doi: 10.1128/AAC.01843-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, Supplement to M-100, June 2010 Update. AST Meeting Minutes, January 2010.
- 21.Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advances and future research. Nature Reviews Nephrology. 2010;6(9):521–529. doi: 10.1038/nrneph.2010.100. [DOI] [PubMed] [Google Scholar]
- 22.Burkhardt O, Hafer C, Langhoff A, et al. Pharmacokinetics of ertapenem in critically ill patients with acute renal failure undergoing extended daily dialysis. Nephrology Dialysis Transplantation. 2009;24(1):267–271. doi: 10.1093/ndt/gfn472. [DOI] [PubMed] [Google Scholar]
- 23.Fish DN, Teitelbaum I, Abraham E. Pharmacokinetics and pharmacodynamics of imipenem during continuous renal replacement therapy in critically ill patients. Antimicrobial Agents and Chemotherapy. 2005;49(6):2421–2428. doi: 10.1128/AAC.49.6.2421-2428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langgartner J, Vasold A, Glück T, Reng M, Kees F. Pharmacokinetics of meropenem during intermittent and continuous intravenous application in patients treated by continuous renal replacement therapy. Intensive Care Medicine. 2008;34(6):1091–1096. doi: 10.1007/s00134-008-1034-7. [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency. European Public Assessment Report (EPAR)—Doribax. 2012, http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000891/human_med_000744.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124#.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables: Mean (± SD) pharmacokinetic parameter estimates for subjects undergoing continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodiafiltration (CVVHDF) are provided in Supplementary Table 1. The results of the Monte Carlo simulations for time above MIC analyses in chronic kidney disease are presented in Supplementary Tables 2 to 4 (based on a 1-hour doripenem infusion) and Supplementary Tables 5 to 7 (based on a 4-hour doripenem infusion). The results of the Monte Carlo simulations for time above MIC analyses in acute renal insufficiency based on a 1-hour doripenem infusion are presented in Supplementary Tables 8 to 10.