Abstract
Objective. This prospective study evaluated laparoscopic sleeve gastrectomy for its safety and efficiency in excess weight loss (%EWL) in super superobese patients (BMI >60 Kg/m2). Results. Thirty patients (33 women and 7 men) were included, with mean age of 35 years (range 18 to 59). Mean preoperative BMI was 66 Kg/m2 (range 60 to 85). The study included one patient with complete situs inversus and 4 (14%) with previous restrictive gastric banding. The mean operative time was 120 minutes (range 80 to 220 min) and the mean hospital stay was 7.5 days (4 to 28 days). There was no postoperative mortality or need for a laparotomy conversion. Two subphrenic hematomas, one gastric fistula, and one pulmonary embolism, were the major complications. After 18 months 17 (77%) had sufficient weight loss and six had insufficient results, leading to either re-sleeve gastrectomy (3), or gastric bypass (2). Three years after the initial laparoscopic sleeve gastrectomy, the mean EWL was 51% (range 21 to 82). Conclusion. The laparoscopic sleeve gastrectomy is a safe and efficient operating procedure for treating super superobesity. In the case of insufficient weight loss, a second-stage operation like resleeve gastrectomy or gastric bypass can be proposed.
1. Introduction
Surgery is the only effective treatment of morbid obesity. It is accepted that surgical treatment of super superobese patients (BMI > 60 kg/m²) and high-risk patients with comorbidities, is responsible for an increased risk of postoperative morbidity and mortality after bariatric surgery [1, 2].
Sleeve gastrectomy is a recently used surgical technique, with an acceptable rate of postoperative complications [3]. It has been described as a first step before a gastric bypass or biliopancreatic diversion with duodenal switch [3]. The restrictive technique of sleeve gastrectomy reduces the gastric capacity by approximately 80%. In his study, we report our experience with sleeve gastrectomy in super superobese patients.
2. Methods
This is a prospective study that included 30 consecutive super superobese patients and was designed to study the efficacy and safety of the sleeve gastrectomy in this group of these patients. The patients were operated between May 2004 and November 2009. For the 30 super superobese patients, we evaluated: the duration of intervention, the early (less than 30 days) and late postoperative complications, hospital stay, loss of excess weight (% EWL), and the need for a second operation in the case of insufficient weight loss.
All patients were operated by laparoscopy, and they were placed in the operating table according to the French position (the surgeon was positioned between the legs of the patient). Each intervention involved six trocars. The gastrocolic ligament was opened 4 cm from the pylorus. The greater curvature of the stomach was freed with the Ligasure (Covidien, Norwalk, CT, United States) until the angle of Hiss. The left crura of the diaphragm was systematically visualised and posterior attachments of the stomach released. A 34 French boogie was introduced into the stomach and positioned along the lesser curvature. The stomach was cut with an incision parallel to the boogie using a linear stapler (Endo GIA 45 Covidien, 4.8 mm, Norwalk, CT, United States). In each case, the resected stomach was placed in a plastic bag and was extracted via the umbilical trocar. Staple line was consistently reinforced by a nonabsorbable suture (Endostich Surgidac-2/0, Covidien, Norwalk, CT, United Kingdom). The fascia of each port site greater than 12 mm in diameter was always closed. A methylene blue test was performed to eliminate a leak. A drain was placed along the staple line. An upper gastrointestinal contrast study was performed the third postoperative day. Cautious refeeding was permitted in the absence of fistula, and patients were discharged after removal of the drain. Iron, vitamin, and essential mineral supplements were systematically given to the patient at their discharge.
3. Results
Thirty patients underwent a laparoscopic sleeve gastrectomy, 23 women and 7 men. The average age was 35 years. All patients were super superobese with a mean BMI of 66 kg/m² (range from 60 to 85 kg/m²). The average preoperative weight was 168 kg (range 140–258 kg). Comorbidities are summarized in Table 1. One patient had a complete situs inversus and 4 others (14%) had previous gastric banding.
Table 1.
Co-morbidities of the 30 super superobese patients.
| Nb patients | % | |
|---|---|---|
| Sleep apnea syndrome | 17 | 58% |
| Hypertension | 15 | 50% |
| Arthritis | 15 | 50% |
| Diabetes | 13 | 42% |
| Dyslipidemia | 13 | 42% |
| Asthma | 6 | 21% |
| Stress incontinence | 1 | 3% |
Mean operative time was 120 minutes (range 80 to 220 min). There was no conversion to laparotomy and no postoperative mortality. The average length of hospital stay was 7.5 days (range 4–28 days). Immediate postoperative complications occurred in 4 patients (14%): two presented subphrenic hematomas, one developed a gastric fistula, and one had pulmonary embolism. All three surgical complications required the creation of a laparoscopic drainage with no need for further surgical treatment.
The mean followup was 24 months. Of the 23 patients that had a followup greater than 18 months, weight loss was satisfactory in 17 patients (77%). Six patients had insufficient weight loss defined by a BMI between 35 to 40 kg/m², progressive weight regain or persistence of comorbidities supposed to improve with further weight loss. If the patient gave his consent for a second operation, then a gastro-intestinal contrast study was performed, in the presence of a dilated gastric pouch due either to incomplete gastric resection or to the persistence of hyperphagia responsible for a mechanical dilation a resleeve was proposed, otherwise, the choice of a gastric bypass was made (Figure 1). The choice of resleeve as a revisional surgery was made for 2 reasons firstly it is reasonable to reoperate a stomach when there is still a secondary gastric pouch a known cause of weight loss failure and secondly, resleeve according to our previous experience, although we do not have a long term followup, has been shown to have better results associated to less morbidity and mortality rates for superobese patients [4]. Three out of six patients were reoperated with a resleeve gastrectomy, and two with a gastric bypass surgery 18 to 23 months after the initial procedure. The last patient refused a second operation. Three years after the original sleeve gastrectomy, the average loss of BMI was 20 kg/m² (range 10 to 39 kg/m², Figure 2). The average percentage of excess weight loss at 3 years was 51% (range 21–82%, Figure 3), while the average weight loss was 56 kg (range 28–144 kg) (Figure 4). During the followup, a port site hernia requiring surgical treatment was observed in 2 patients (7%). There were no other complications or iron and vitamin nutritional deficiencies during the followup.
Figure 1.
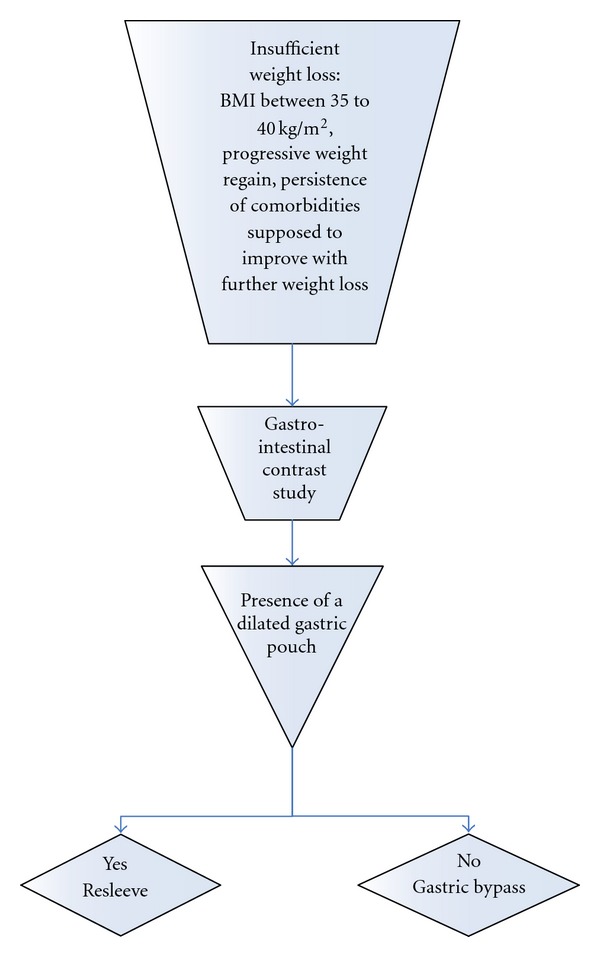
Decisional algorithm for the choice of revisional operation in the case of insufficient weight loss.
Figure 2.
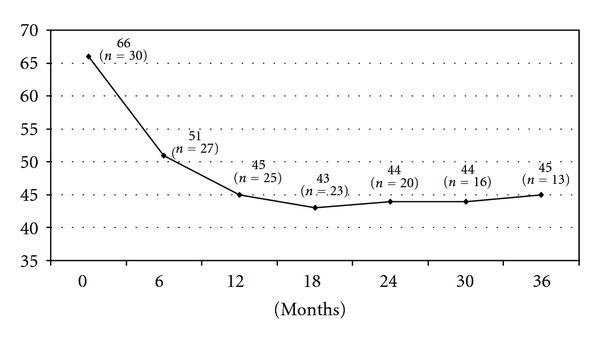
BMI evolution (Kg/m2).
Figure 3.
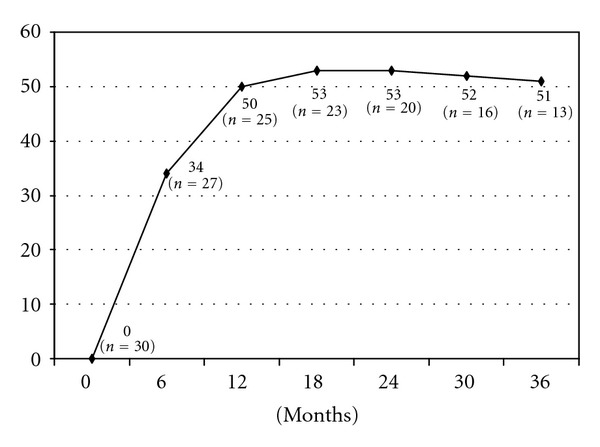
Variation in percentage of excess weight lost (% EWL).
Figure 4.
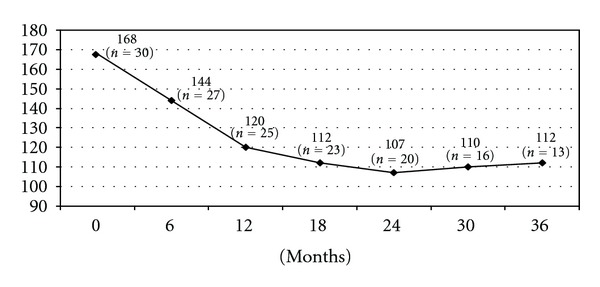
Weight evolution (Kg).
4. Discussion
Perioperative risks (morbidity and mortality) are known to be high for patients with super superobesity. The multidisciplinary team preoperatively should carefully evaluate the benefit/risk ratio of bariatric surgery in any given patient. Gastric banding presents less risk but is also less effective in super superobese patients [5]. Gastric bypass and biliopancreatic diversion although associated to a higher morbidity ratio demonstrate excellent efficiency in regard to weight loss [6]. The Magenstrasse technique uses a longitudinal section of the stomach without gastric resection has been proposed in super superobesity because of its lower morbidity and mortality [7]. This procedure that divides the stomach into two compartments should be distinguished from sleeve gastrectomy, the technique reported in this paper. Preliminary results showed that the sleeve gastrectomy, after a 24-month period postroperatively was equally effective as gastric bypass in terms of weight loss in the super superobese patients while presenting lower risk for complications (3,6% to 9% resp.). The frequency of complications after gastric bypass in the super superobese can reach up to 23% [8] and up to 38% for duodenal switch [6]. In the super superobese patients, the death rate was assessed up to 2.7% after gastric bypass [8] and 6.25% after duodenal switch [6]. Among our 30 patients, we had 3 immediate postoperative complications like hematoma or fistula that were successfully treated with laparoscopic drainage. Current studies on the sleeve gastrectomy have published an average followup of less than 3 years [7, 9, 10] while two separate studies [6, 9] using a stapler line reinforcement, that is, the same technique with our team, reported also no postoperative morbidity and mortality.
The initial rapid weight loss, reported by previous studies [4, 9, 11], reached a plateau at 18 months after surgery [11]. We found the presence of insufficient weight loss in only 23% of patients at 18 months. In our group of 30 patients, 23 had a followup more than 18 months, 5 had a second operation 3 of them had a resleeve gastrectomy and 2 a gastric bypass. It is known that the sleeve gastrectomy in the super superobese can be a definitive treatment for an average loss of excess weight by 50% at one year [3, 10]. We have shown that the sleeve gastrectomy for the super superobese can allow an average loss of excess weight by 53% at 18 months. If weight loss was insufficient, the sleeve gastrectomy could then be followed by a second operating procedure, like gastric bypass [3] or biliopancreatic diversion [9]. The second operation presented less technical difficulties thanks to the weight loss already achieved since the first sleeve gastrectomy. Five patients were operated for a second time, three of them had a resleeve gastrectomy, and two a gastric bypass as a second-step procedure. Although we present a small group in this study, there were no postoperative complications associated to the excellent weight loss and these results demonstrate the efficacy as well as the safety of the second intervention.
It is known that bariatric surgery provokes weight loss through dietary restriction or through the malabsorption it imposes. The mechanisms responsible for decreased appetite are poorly understood. To make things more complicated some of the excellent early results like the decrease in blood sugar and insulin resistance seem to be independent from the weight loss [12]. A great interest has been reported for the role of ghrelin [13], a hormone that is secreted by the gastric fundus a part of the stomach that is largely resected in a sleeve gastrectomy. Ghrelin is secreted under the influence of cholinergic stimulation and has probably a major role in controlling appetite since its receptors are present in the pituitary and hypothalamus. In humans, the serum concentration of ghrelin is increased in anorexia nervosa, Prader Willi syndrome, and in patients with a reduced caloric diet [13]. Ghrelin's concentrations are decreased by food intake and after gastrectomy or gastric bypass [13, 14]. The absence of contact between food and the lining of the stomach producing ghrelin inhibits its secretion [14]. In the case of sleeve gastrectomy, the removal of the ghrelin-producing area could eventually explain the weight loss. This effect was reversed experimentally by the exogenous administration of ghrelin [15]. Two separate studies reached the same conclusions with our team and have provided evidence for both an early and late ghrelin decrease after the sleeve gastrectomy [16, 17]. These specific hormonal mechanisms leading to weight loss need to be further elucidated [18].
Acknowledgments
The authors would like to thank ViVi Fysekidou for her comments and help in text editing.
References
- 1.Dresel A, Kuhn JA, McCarty TM. Laparoscopic Roux-en-Y gastric bypass in morbidly obese and super morbidly obese patients. American Journal of Surgery. 2004;187(2):230–232. doi: 10.1016/j.amjsurg.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez AZ, Jr., De Maria EJ, Tichansky DS, et al. Multivariate analysis of risk factors for death following gastric bypass for treatement of morbid obesity. Annals of Surgery. 2004;239(5):698–703. doi: 10.1097/01.sla.0000124295.41578.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regan JP, Inabnet WB, Gagner M, Pomp A. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obesity Surgery. 2003;13(6):861–864. doi: 10.1381/096089203322618669. [DOI] [PubMed] [Google Scholar]
- 4.Catheline JM, Fysekidis M, Bihan H, Boschetto A, Dbouk R, et al. Better results in weight loss after the second gastrectomy in re-sleeve gastrectomy. Journal of Obesity and Weight Loss Therapy. 2011;1, article 107 [Google Scholar]
- 5.Zinzindohoué F, Chevallier JM, Douard R, et al. Laparoscopic gastric banding: a minimally invasive surgical treatment for morbid obesity: prospective study of 500 consecutive patients. Annals of Surgery. 2003;237(1):1–9. doi: 10.1097/00000658-200301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obesity Surgery. 2000;10(6):514–523. doi: 10.1381/096089200321593715. [DOI] [PubMed] [Google Scholar]
- 7.Johnston D, Dachtler J, Sue-Ling HM, King RFGJ, Martin IG. The magenstrasse and mill operation for morbid obesity. Obesity Surgery. 2003;13(1):10–16. doi: 10.1381/096089203321136520. [DOI] [PubMed] [Google Scholar]
- 8.Msika S. La chirurgie de l’obésité morbide de l’adulte. Journal de Chirurgie. 2003;140:4–21. [PubMed] [Google Scholar]
- 9.Consten ECJ, Gagner M, Pomp A, Inabnet WB. Decreased bleeding after laparoscopic sleeve gastrectomy with or without duodenal switch for morbid obesity using a stapled buttressed absorbable polymer membrane. Obesity Surgery. 2004;14(10):1360–1366. doi: 10.1381/0960892042583905. [DOI] [PubMed] [Google Scholar]
- 10.Krawczykowski D. La sleeve gastrectomie laparoscopique de premiere intention après cerclage gastrique: resultats préliminaires. 2004;50:29–33. [Google Scholar]
- 11.Almogy G, Crookes PF, Anthone GJ. Longitudinal gastrectomy as a treatment for the high-risk super-obese patient. Obesity Surgery. 2004;14(4):492–497. doi: 10.1381/096089204323013479. [DOI] [PubMed] [Google Scholar]
- 12.Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Annals of Surgery. 2002;236(5):554–559. doi: 10.1097/00000658-200211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghigo E, Broglio F, Arvat E, Maccario M, Papotti M, Muccioli G. Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clinical Endocrinology. 2005;62(1):1–17. doi: 10.1111/j.1365-2265.2004.02160.x. [DOI] [PubMed] [Google Scholar]
- 14.Cummings DE, Weigle DS, Scott Frayo R, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. The New England Journal of Medicine. 2002;346(21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 15.Frühbeck G, Diez Caballero A, Gil MJ. Fundus functionality and ghrelin concentrations after bariatric surgery. The New England Journal of Medicine. 2004;350(3):308–309. doi: 10.1056/NEJM200401153500323. [DOI] [PubMed] [Google Scholar]
- 16.Langer FB, Hoda MAR, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obesity Surgery. 2005;15(7):1024–1029. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 17.Cohen R, Uzzan B, Bihan H, Khochtali I, Reach G, Catheline JM. Ghrelin levels and sleeve gastrectomy in super-super-obesity. Obesity Surgery. 2005;15(10):1501–1502. doi: 10.1381/096089205774859335. [DOI] [PubMed] [Google Scholar]
- 18.Aarts EO, Janssen IMC, Berends FJ. The gastric sleeve: losing weight as fast as micronutrients? Obesity Surgery. 2011;21(2):207–211. doi: 10.1007/s11695-010-0316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


