Abstract
Background
The Clinical Dementia Rating Scale Sum of Boxes (CDR-SOB) score is commonly used, although the utility regarding this score in staging dementia severity is not well established.
Obiective
To investigate the effectiveness of CDRSOB scores in staging dementia severity compared with the global CDR score.
Design
Retrospective study.
Setting
Texas Alzheimer's Research Consortium minimum data set cohort.
Participants
A total of 1577 participants (110 controls, 202 patients with mild cognitive impairment, and 1265 patients with probable Alzheimer disease) were available for analysis.
Main Outcome Measures
Receiver operating characteristic curves were generated from a derivation sample to determine optimal cutoff scores and ranges, which were then applied to the validation sample.
Results
Optimal ranges of CDR-SOB scores corresponding to the global CDR scores were 0.5 to 4.0 for a global score of 0.5, 4.5 to 9.0 for a global score of 1.O, 9.5 to 15.5 for a global score of 2.0, and 16.0 to 18.0 for a global score of 3.0. When applied to the validation sample, κ scores ranged from 0.86 to 0.94 (P <.001 for all), with 93.0% of the participants falling within the new staging categories.
Conclusions
The CDR-SOB score compares well with the global CDR score for dementia staging. Owing to the increased range of values, the CDR-SOB score offers several advantages over the global score, including increased utility in tracking changes within and between stages of dementia severity. Interpretive guidelines for CDR-SOB scores are provided.
Staging of Alzheimer Disease (AD) severity via global assessment measures is commonplace in clinical and research settings and is useful for many reasons. Clinically, knowledge of dementia severity is helpful for rapid communication about the disease, for making management decisions, and for selection of pharmacologic options that have been approved for different levels of diseas severity.1–3 In research settings, staging of dementia severity is valuable for operationally defining homogeneous patient populations for comparison purposes and end points for research studies monitoring progression.2 In addition, staging of dementia severity is critical for clinical trials, as outlined by the US Food and Drug Administration's Guidelines for the Clinical Evaluation of Antidementia Drugs,4(p15) which states that the stage and severity of the dementia affecting each subject participating in a clinical trial must be assessed and recorded systematically in a manner that will be readily understood by other workers in the field.
The Washington University Clinical Dementia Rating Scale (CDR) is a global assessment instrument that yields global and Sum of Boxes (SOB) scores, with the global score regularly used in clinical and research settings to stage dementia severity.5 Although the CDR-SOB score has been considered a more detailed quantitative general index than the global score6 and provides more information than the global CDR score in patients with mild dementia,7 its utility in formally staging dementia severity remains untested.
The utilization of CDR-SOB scores for staging dementia severity offers several advantages over the global score because the optimal characteristics of both scores can be combined into a single score. First, CDR-SOB scores are much simpler to calculate than the global score and they do not require an algorithm for computation, which will ultimately result in fewer calculation errors for those not using the online system. Second, CDR-SOB scores can be treated as interval data in statistical analyses, whereas global CDR scores are ordinal by the nature of the algorithm approach to condensing the data. Finally, the most significant advantage to using CDR-SOB scores for staging of dementia severity is the increased precision afforded for tracking changes across time.
This study was designed to evaluate the utility of CDRSOB scores in staging AD severity compared with the global CDR score and to provide interpretive guidelines for CDRSOB scores. It was hypothesized that CDR-SOB scores would correctly stage many participants in the present study. Next, we evaluated the utility of CDR-SOB scores in distinguishing between patients diagnosed as having mild cognitive impairment (MCI) vs those diagnosed as having very early AD. It was hypothesized that CDR-SOB scores would accurately predict diagnosis (MCI or AD) in most patients with global CDR scores of 0.5.
METHODS
PARTICIPANTS
The Texas Alzheimer's Research Consortium (TARC) is a statefunded collaborative group of investigators from Baylor College of Medicine, Texas Tech University Health Sciences Center, University of North Texas Health Science Center, and University of Texas Southwestern Medical Center. The TARC database includes a retrospective minimum data set containing clinical and demographic data on individuals enrolled since 2001 in AD research programs at TARC member sites and was patterned after the National Alzheimer's Coordinating Center minimum data set. The present study conlprises 1577 participants (110 controls, 202 patients with MCI, and 1265 patients with probable AD) from the TARC minimum data set who had available demographic data and CDR global and SOB scores at the initial visit. The breakdown of global CDR scores was as follows: CDR 0, 112 individuals (110 controls and 2 patients with MCI); CDR O.5, 457 individuals (196 with MCI and 261 with AD); CDR 1, 582 individuals (4 with MCI and 578 with AD); CDR 2, 304 individuals (all with AD); and CDR 3. 122 individuals (all with AD). All the participants met consensusbased diagnoses based on the following criteria: patients with AD met criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Alzheimers Disease and Related Disorders Association Work Group classification of probable AD,8 patients with MCI met the Mayo Clinic research criteria,9 and controls performed within normal limits on psychometric assessment and were assigned a global CDR score of 0. The CDR scores were assigned independent of consensus diagnosis.
MEASURES
The CDR is obtained through semistructured interviews of patients and informants, and cognitive functioning is rated in 6 domains of functioning: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. Each domain is rated on a 5-point scale of functioning as follows: 0, no impairment; 0.5, questionable impairment; 1, mild impairment; 2, moderate impairment; and 3, severe impairment (personal care is scored on a 4-point scale without a 0.5 rating available). The global CDR score is computed via an algorithm.10 Herein, each global score was calculated using the Washington University online algorithm (http://www.biostat.wustl.edu/~adrc/cdrpgm/index.html). Domain scores were entered inlo the online algorithm independently by 2 different research assistants, and discrepancies in computed global CDR scores (n=8) were double checked for entry errors and resolved. The CDR-SOB score is obtained by summing each of the domain box scores, with scores ranging from 0 to 18. The CDR demonstrates good reliability11,12 and has been validated against neuropathologic finding13–15
STATISTICAL ANALYSES
Analyses were conducted using staistical software packages (Stata 9 [StataCorp LP, College Station, Texas] and SAS version 9.1.3 [SAS Institute Inc, Cary, North Carolina]). Analyses to evaluate the utility of CDR-SOB scores in staging dementia severity relative to global scores took place in 2 uhases. First. the sample was randomly divided by diagnostic category into 2groups (derivation sample and validation sample). The derivation sample consisted of 788 participants (55 controls, 101 patients wiih MCI, and 632 patients with AD). Receiver operating characteristic (ROC) curves were generated for every 2 adjacent global CDR score categories (0 and O.5,0.5 and 1, 1 and 2, and 2 and 3) to determine optimal CDR-SOB cutoff scores. Sum of Boxes score ranges were established between each pair of bordering cutoff scores. The validation sample consisted of 789 participants (55 controls, 101 patients with MCI. and 633 patientswith AD). The cutoff scores derived from the derivation sample were then applied to the validation sample. The Cohen κ was used to evaluate agreement. Next, analyses were restricted to patients in the validation sample whose global CDR score was 0.5 (147 with MCI and 85 with AD), and a ROC curve was calculated to determine the diagnostic accuracy of CDR-SOB scores in classifying patients with MCI and AD. To evaluate the utility of CDRSOB scores in distinguishing between patients with MCI and those with very early AD, logistic regression was used, with diagnostic group as the dependent variable and CDR-SOB scores as the independent variable, adjusting for the simultaneous effects of sex along with age and years of education as continuous variables.
RESULTS
The demographic characteristics of the study population are provided in Table 1. Patients with AD were significantly older and controls were significantly younger than patients with MCI. The AD group had significantly fewer years of education than the control and MCI groups, which were not different from one another. As expected, there were more women than men across all diagnostic groups, with the percentage of women significantly higher in the AD group compared with the MCI group. As expected, the control group performed significantly better on the Mini-Mental State Examination than did patients with MCl, who scored significantly better than the AD sample. Mean CDR-SOB scores were highest in the AD group, followed by the MCI group and then the control group.
Table 1.
Baseline Study Population Characteristicsa
| Characteristics | AD Group (n=1265) | MCI Group (n=202) | Controls (n=110) | Total (N=1577) |
|---|---|---|---|---|
| Sex, No. (%) | ||||
| Male | 417 (33.0) | 88 (43.6) | 42 (38.2) | 547 (34.7) |
| Female | 848 (67.0)b | 114 (56.4) | 68 (61.8) | 1030 (65.3) |
| Age, y | ||||
| Mean (SD) | 75.5 (8.1)c | 73.1 (9.4) | 69.1 (10.2)c | 74.8 (8.6) |
| Range | 44–95 | 43–97 | 47–90 | 43–97 |
| Educational level, y | ||||
| Mean (SD) | 12.5 (3.6)c | 14.1 (3.1) | 14.8 (2.8) | 12.9 (3.6) |
| Range | 0–20 | 3–20 | 5–20 | 0–20 |
| MMSE score | ||||
| Mean (SD) | 17.9 (6.8)c | 27.5 (2.0) | 29.0 (1.2)d | 19.9 (7.4) |
| Range | 0–30 | 17–30 | 24–30 | 0–30 |
| CDR Sum of Boxes score | ||||
| Mean (SD) | 7.8 (4.4)c | 1.5 (1.1) | 0.04 (0.1)b | 6.4 (4.8) |
| Range | 0.5–18.0 | 0.5–7.0 | 0–0.5 | 0–18.0 |
Abbreviations: AD, Alzheimer disease; CDR, Clinical Dementia Rating; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
All statistical comparisons are based on MCI as the reference group and the χ2 statistic (categorical data) or analysis of variance (continuous data).
P<,01.
P<.001.
P<.05.
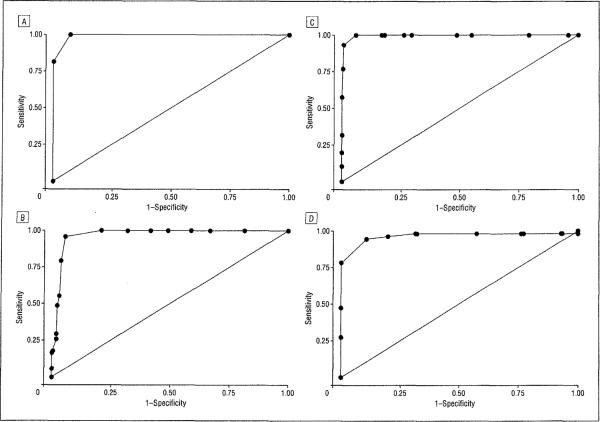
Optimal ranges of CDR-SOB scores that mapped onto global CDR scores and area under the ROC curve values for each pair of adjacent global score categories obtained from the derivation sample are given in Table 2; κ values obtained from the validation sample are also provided. Finally, the percentage of participants with a score within the newly derived CDR-SOB ranges that were correctly staged into the appropriate global CDR score stages in the validation sample is given. The ROC curves for CDR-SOB scores are presented in Figure 1.
Table 2.
Ranges and κ Values for CDR Sum of Boxes Scores by Global CDR Score Stage
| Global CDR Score |
|||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 2.0 | 3.0 | |
| CDR Sum of Boxes | |||||
| Range | 0 | 0.5–4.0 | 4.5–9.0 | 9.5–15.5 | 16.0–18.0 |
| AUC | NA | 0.99 | 0.97 | 0.99 | 0.97 |
| κ Valuea | 0.94 | 0.94 | 0.90 | 0.86 | 0.88 |
| Correctly classified, %b | 100.0 | 95.1 | 89.6 | 91.5 | 100.0 |
Abbreviations: AUC, area under the receiver operating characteristic curve (all were significant at P<.001); CDR, Clinical Dementia Rating; NA, not applicable.
Overall κ=0.90.
Correctly classified reflects the percentage of participants in the derived Sum of Boxes ranges who were in the appropriate global score range.
Figure 1.
Receiver operating characteristic curves for Clinical Dementia Rating (CDR) Sum of Boxes scores mapping onto global CDR scores of 0 and 0.5 (area under the receiver operating characteristic curve [AUC]=0.99) (A), 0.5 and 1.0 (AUC=0.97) (B), 1.0 and 2.0 (AUC>0.99) (C), and 2.0 and 3.0 (AUC=0.97) (D).
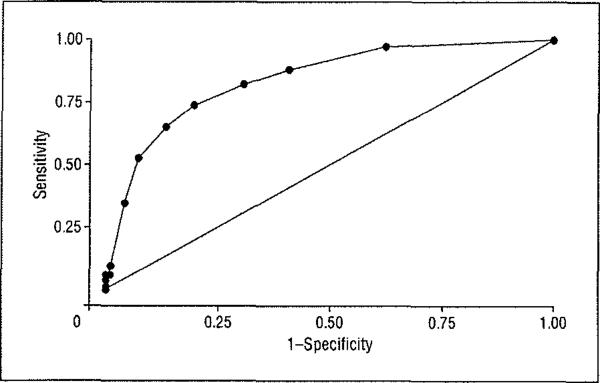
Next, using the ROC curve restricted to validation sample patients with MCI and AD and a global CDR score of 0.5, a CDR-SOB cutoff score of 2.5 or greater was obtained (sensitivity = 0.74 and specificity = 0.81), whereby 76.9% of patients with AD and MCI were correctly classified. The obtained ROC curve is given in Figure 2 (area under the ROC curve of 0.85). Finally, logistic regression analyses were conducted to evaluate the utility of CDR-SOB scores in discriminating between these patients with MCI and AD. After adjustment for age, sex, educational level, and Mini-Mental State Examination scores, an odds ratio of 2.87 (95% confidence interval, 2.22–3.70) was obtained for CDR-SOB scores, indicating that for each 1-point increment in CDR-SOB score, there is a 2.9-fold increased liltelihood of being diagnosed as having AD.
Figure 2.
Receiver operating characteristic curve for Clinical Dementia Rating (CDR) Sum of Boxes scores for identifying patients with Alzheimer disease and mild cognitive impairment and a global CDR score of 0.5 (area under the receiver operating characteristic curve = 0.85).
COMMENT
The staging of dementia severity is important for clinical and research purposes, with the CDR being one of the most commonly used instruments. To our knowledge, however, there are no published guidelines available for staging dementia severity based on CDR-SOB scores. In the present sample, 93.0% of participants (AD, MCI, and control groups) were staged correctly using the proposed CDR-SOB score guidelines (Table 3).
Table 3.
Sum of Boxes Staging Category
| CDR Sum of Boxes Range | Staging Category |
|---|---|
| 0 | Normal |
| 0.5–4.0 | Questionable cognitive impairment |
| 0.5–2.5 | Questionable impairment |
| 3.0–4.0 | Very mild dementia |
| 4.5–9.0 | Mild dementia |
| 9.5–15.5 | Moderate dementia |
| 16.0–18.0 | Severe dementia |
Abbreviation: See Table 1.
Previous research suggests that CDR-SOB scores may have potential for discriminating between patients with MCI and very early AD who are assigned a global CDR score of 0.5. Gmndman et al,16 analyzing data from the Memory lmpairment Study,17 found that patients with MCI (global CDR score of 0.5) were assigned significantly lower CDR-SOB scores (mean [SD] score, 1.8 [0.8]) than were patients with very mild AD who also were assigned a global score of 0.5 (mean [SD] score, 3.0 [0.8]). When restricting analyses to patients with MCI and AD who were assigned a global CDR score of 0.5, the present study found a nearly 3-fold increased risk of being diagnosed as having AD for every 1-point increment in CDR-SOB scores, which is slightly higher than hut consistent with a previous investigation7 that found an odds ratio of 2.3 for the diagnosis of dementia in patients with a CDR score of 0.5.
As mentioned previously, CDR-SOB scores for staging dementia severity offer several advantages over the global score. The present results can be used to further expand on the potential for increased precision in tracking change across time. Tracking progression in AD studies has traditionally been conducted using the global CDR score, with primary end points being transitioned from one category (eg, mild AD, global CDR of 1) to another (eg, moderate AD, global CDR of 2). The proposed guidelines afford a more precise interpretation of progression. For example, the present results suggest that mild AD spans CDR-SOB scores from 4.5 to 9.0, whereas moderate AD spans CDR-SOB scores from 9.5 to 15.5. Therefore, using the global CDR score, there would be no way to distinguish between a patlent who progresses from a score of 4.5 to 9.5 and one who progresses from a score of 9.0 to 9.5, although both have progressed from mild to moderate AD. The present guidelines also afford researchers the ability to monitor progression within stages (eg, mild AD) in addition to between stages of dementla.
The present results demonstrate that CDR-SOB scores can be used to accurately stage patients with AD, and interpretive guidelines are presented. These data also demonstrate that CDR-SOB scores can, with reasonable accuracy, discriminate between patients with very early AD and those with MCI, which is impossible using global CDR scores owing to the nature of the scale. However, given that this study did not analyze other forms of mild dementia syndromes (eg, frontotemporal dementia or Parkinson disease), the generalizability of the guidelines should be tested with additional patient populations. These results offer clinicians and researchers alike an alternative interpretive strategy for the CDR that affords greater precision in patient care and analysis.
Acknowledgments
Funding/Support: This study was made possible by the Texas Alzheimer's Research Consortium (TARC), funded by the state of Texas through the Texas Council on Alzheimer's Disease and Related Disorders; the Alzheimer's Association Zenith award and the RSMIS Foundation (Dr Doody); and grant P30AG12300 from the National Institute on Aging, National Institutes of Health (University of Texas Southwestern Medical Center Alzheimer's Disease Center).
Footnotes
Author Contributions: Drs O'Bryant and Waring had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: O'Bryant, Waring, and Cullum. Acquisition of data: O'Bryant, Waring, Cullum, Hall, Lacritz, Reisch, and Doody. Analysis and interpretation of data: O'Bryant, Waring, Cullum, Massman, Lupo, Reisch, and Doody. Drafting of the manusctipt: O'Bryant, Waring, and Cullum. Critical revision of the manuscript for important intellectual content: O'Bryant, Waring, Cullum, Hall, Lacritz, Massman, Lupo, Reisch, and Doody. Statistical analysis: O'Bryant, Waring, Massman, Lupo, and Reisch.
Texas Alzheimer's Research Consortium Investigators: Violeta Capriles, MD, MPH, Eveleen Darby, MA, MS, Kinga Szigeti, MD, PhD, Baylor College of Medicine, Houston, Texas; Randolph Schiffer, MD, Merena Tindall, RN, Patricia Sutker, PhD, Yan Zhang, PhD, Texas Tech University Health Sciences Center, Lubbock; Jessica Alexander, BA, Thomas Fairchild, PhD, Janice Knebl, DO, Douglas Mains, PhD, University of North Texas Health Science Center, Fort Worth; Ramon Diaz-Arrastia, MD, PhD, Joey Naylor, BA, Roger Rosenberg, MD, Doris Svetlik, RN, Keverly Williams, BA, University of Texas Southwestern Medical Center, Dallas.
Financial Disclosure: None reported.
REFERENCES
- 1.Horowitz F. Developmental theory, prediction, and the developmental equation in follow-up research. In: Friedman S, Haywood HC, editors. Developmental Follow-up: Concepts, Domains, and Methods. Academic Press; San Diego. CA: 1994. pp. 27–44. [Google Scholar]
- 2.Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: Mini-Mental State Examination and Clinical Dementia Rating. Am J Geriar Psychiatry. 2006;14(2):139–144. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 3.Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 4.Leber P. Guidelines for the Clinical Evaluahon of Antidementia Drugs. US Food and Drug Administration; Rockville. MD: 1990. [Google Scholar]
- 5.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 6.Berg L, Miller JP, Storandt M, et al. Mild senile dementia of the Alzheimer type, 2: longitudinal assessment. Ann Neurol. 1988;23(5):477–484. doi: 10.1002/ana.410230509. [DOI] [PubMed] [Google Scholar]
- 7.Lynch CA, Walsh C, Blanco A, et al. The clinical dementia rating sum of box score in mild dementia. Dement Geriatr Cogn Disord. 2006;21(1):40–43. doi: 10.1159/000089218. [DOI] [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, editor. Mild Cognitive Impairment: Aging to Alzheimer's Disease. Oxford University Press; New York, NY: 2003. [Google Scholar]
- 10.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 11.Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer's Disease Cooperative Study experience. Neurology. 1997;48(6):1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 12.Burke WJ, Miller JP, Rubin EH, et al. Reliability of the Washington University Clinical Dementia Rating. Arch Neurol. 1988;45(1):31–32. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- 13.Berg L, McKeel DW, Jr, Miller JP, Baty J, Morris JC. Neuropathological indexes of Alzheimer's disease in demented and nondemented persons aged 80 years and older. Arch Neurol. 1993;50(4):349–358. doi: 10.1001/archneur.1993.00540040011008. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC, McKeel DW, Jr, Storandt M, et al. Very mild Alzheimer's disease: informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991;41(4):469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC, Storandt M, McKeel DW, Jr, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46(3):707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 16.Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61(1):59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Andersen K, Lolk A, Kragh-Sorensen P, Petersen NE, Green A. Depression and the risk of Alzheimer disease. Epidemiology. 2005;16(2):233–238. doi: 10.1097/01.ede.0000152116.32580.24. [DOI] [PubMed] [Google Scholar]