Abstract
Purpose
Patients question whether multiple biopsy sessions cause worse prostate cancer outcomes. Therefore, we investigated whether there is an association between the number of prior biopsy sessions and biochemical recurrence after radical prostatectomy.
Materials and Methods:
Men in the SEARCH (Shared Equal Access Regional Cancer Hospital) database who underwent radical prostatectomy between 1988 and 2010 after a known number of prior biopsies were included in the analysis. Number of biopsy sessions (range 1 to 8) was examined as a continuous and categorical (1, 2 and 3 to 8) variable. Biochemical recurrence was defined as a prostate specific antigen greater than 0.2 ng/ml, 2 values at 0.2 ng/ml or secondary treatment for an increased prostate specific antigen. The association between number of prior biopsy sessions and biochemical recurrence was analyzed using the Cox proportional hazards model. Kaplan-Meier estimates of freedom from biochemical recurrence were compared among the groups.
Results
Of the 2,739 men in the SEARCH database who met the inclusion criteria 2,251 (82%) had only 1 biopsy, 365(13%) had 2 biopsies and 123 (5%) had 3 or more biopsies. More biopsy sessions were associated with higher prostate specific antigen (p <0.001), greater prostate weight (p <0.001), lower biopsy Gleason sum (p = 0.01) and more organ confined (pT2) disease (p = 0.017). The Cox proportional hazards model demonstrated no association between number of biopsy sessions as a continuous or categorical variable and biochemical recurrence. Kaplan-Meier estimates of freedom from biochemical recurrence were similar across biopsy groups (log rank p = 0.211).
Conclusions
Multiple biopsy sessions are not associated with an increased risk of biochemical recurrence in men undergoing radical prostatectomy. Multiple biopsy sessions appear to select for a low risk cohort.
Keywords: biopsy, prostatic neoplasms, prostatectomy, recurrence, diagnosis
Since the introduction of PSA testing there has been a significant stage migration in prostate cancer.1 Whereas before the PSA era biopsies were usually performed because of abnormal findings on digital rectal examination, in the current era biopsy is most commonly performed because of an abnormal serum PSA.2 In addition, many men with an abnormal serum PSA and a negative biopsy result are followed carefully, and undergo repeat biopsy because of an increasing PSA or subtle changes detected by digital rectal examination. In addition, diagnoses that may require another biopsy are common, such as high grade prostatic intraepithelial neoplasia and atypia or atypical small acinar proliferation.3-5 These factors have contributed to an increasing number of men undergoing single as well as multiple prostate biopsies.6
Prostate biopsy is the current standard for the diagnosis of prostate cancer and assignment of Gleason grade. In the absence of other reliable diagnostic methods such as accurate and precise prostate imaging, a number of men will undergo multiple biopsy sessions. Patients are often concerned about the potential risks of multiple biopsies. There are theoretical risks of cancer spreading via needle biopsy and of the initiation of inflammatory processes. These might increase the technical difficulty of a subsequent operation and possibly result in higher positive margin rates or directly influence tumor pathology.7 It has not been fully elucidated if there is an association between multiple prostate biopsies and localized prostate cancer recurrence or adverse outcomes after RP. Therefore, we addressed whether the number of prostate biopsies affects the risk of biochemical recurrence after RP.
METHODS
Study Population
This is an institutional review board approved analysis of the SEARCH database of men who underwent RP between 1988 and 2010, and who were treated at Veterans Affairs medical centers in West Los Angeles and Palo Alto, California; Augusta, Georgia; and Asheville and Durham, North Carolina.8 The analysis included men who had a known number of prior biopsy sessions. We excluded patients who underwent primary treatment with androgen deprivation or radiation therapy. We also excluded men if time between biopsy and surgery was more than 365 days (152), suggesting an initial active surveillance treatment strategy. The cohort was analyzed in groups based on the number of prior biopsy sessions required to diagnose cancer (1, 2 and 3 to 8). The primary outcome was BCR, which was defined as PSA greater than 0.2 ng/ml, 2 values at 0.2 ng/ml or secondary treatment for an increased PSA.
Statistical Analysis
We evaluated the association between the number of prior biopsy sessions (range 1 to 8) and clinical and pathological characteristics using Kruskal-Wallis and chi-square analyses. Number of biopsy sessions was examined as a categorical variable (1, 2 and 3 to 8), as were Gleason sum, clinical stage, center and pathological stage. PSA, age, year of surgery and prostate weight were examined as continuous variables. We analyzed the association between number of prior biopsy sessions and BCR using Cox proportional hazards models, which adjusted for demographic, clinical and pathological characteristics from the surgical specimen. This analysis included biopsy sessions as a categorical (1, 2 and 3 to 8) and continuous (range 1 to 8) variable. Information on the number of cores on the diagnostic biopsy was missing for 415 men (15%). We were concerned that including this variable in our multivariate models would lead to loss of power. Therefore, we explored whether including the number of cores obtained in our multivariate models would influence the results. We noted that the number of cores obtained was not related to BCR, and inclusion (or exclusion) of this variable in our models did not alter the hazard ratio or p values for the association between number of biopsy sessions and BCR. Therefore, the number of cores was not included in our final multivariate models. Freedom from BCR was plotted using Kaplan-Meier analysis. We evaluated a possible association between the number of biopsies and freedom from BCR using the log rank test. All statistical analyses were performed using STATA® 9.1 and R version 2.11.1.
RESULTS
Patient Characteristics
Of the 2,739 men who met the inclusion criteria 2,251 (82%) had only 1 biopsy, 365 (13%) had 2 biopsies and 123 (5%) 3 or more biopsies to diagnose cancer. Preoperative cohort characteristics are shown in table 1. A larger number of biopsy sessions was associated with older age (p = 0.017), higher median PSA (1 biopsy—6.3 ng/ml, 2 biopsies—7.7 ng/ml, 3 or more biopsies—8.1 ng/ml, p <0.001) and greater median prostate weight (1 biopsy—38 gm, 2 biopsies—43 gm, 3 or more biopsies—50 gm, p <0.001). Men who underwent more biopsies had a lower clinical stage biopsy Gleason sum (p = 0.010) and were more likely to have organ confined (pT2) disease (p = 0.017).
Table 1.
Patient characteristics
| 1 Biopsy | 2 Biopsies | 3–8 Biopsies | p Value | ||||
|---|---|---|---|---|---|---|---|
| Median age, (IQR) | 62 | (58–67) | 63 | (59–67) | 63 | (60–68) | 0.017* |
| No. Caucasian (%) | 1,354 | (60) | 207 | (57) | 60 | (49) | 0.027† |
| Median kg/m2 body mass index (IQR) | 28 | (25–31) | 28 | (25–31) | 28 | (26–30) | 0.979* |
| Median ng/ml baseline PSA (IQR) | 6.3 | (4.6–10.0) | 7.7 | (5.2–11.2) | 8.1 | (6.2–12.8) | <0.001* |
| Median yr of surgery (IQR) | 2002 | (1996–2006) | 2002 | (1997–2005) | 2002 | (1998–2006) | 0.316* |
| Median biopsy cores obtained (IQR) | 8 | (6–11) | 8 | (6–12) | 10 | (8–12) | 0.001* |
| No. clinical stage (%): | 0.002† | ||||||
| T1 | 1,107 | (60) | 218 | (66) | 81 | (75) | |
| T2 or Greater | 729 | (40) | 111 | (34) | 27 | (25) | |
| No. biopsy Gleason sum (%): | 0.010† | ||||||
| 2–6 | 1,333 | (61) | 247 | (68) | 85 | (70) | |
| 7 | 653 | (30) | 80 | (22) | 31 | (25) | |
| 8–10 | 201 | (9) | 34 | (9) | 6 | (5) | |
| No. pathological stage (%): | 0.017† | ||||||
| T0 | 1 | (less than 1) | 0 | 1 | (1) | ||
| T2 | 1,548 | (72) | 260 | (76) | 98 | (82) | |
| T3–4 | 596 | (28) | 83 | (24) | 21 | (18) | |
| No. postop Gleason sum (%): | 0.095† | ||||||
| 2–6 | 908 | (41) | 160 | (44) | 58 | (48) | |
| 7 | 1,045 | (47) | 164 | (46) | 54 | (45) | |
| 8–10 | 262 | (12) | 36 | (10) | 9 | (7) | |
| No. pos surgical margin (%) | 934 | (42) | 152 | (43) | 41 | (34) | 0.148† |
| Median gm prostate wt (IQR) | 38 | (30–50) | 43 | (32–56) | 50 | (39–69) | <0.001* |
Kruskal-Wallis test.
Chi-square test.
Biochemical Recurrence
After adjusting for clinically and statistically significant variables including pathological features, we found no association between BCR and number of biopsy sessions when analyzed as a continuous (HR 1.04, 95% CI 0.92-1.17, p = 0.516) or categorical (3 or more biopsies HR 1.07, 95% CI 0.72-1.61, p = 0.727) variable (table 2). Kaplan-Meier estimates of freedom from BCR were similar among the groups with a median followup of 37 months (log rank p = 0.211, see figure). Median time to recurrence was 150 months for 1 biopsy, and was not reached for 2 or for 3 or more biopsies.
Table 2.
Multivariate Cox proportional hazards model examining the association of number of biopsy sessions with BCR
| HR (95% CI)* | p Value | HR (95% CI)† | p Value | |
|---|---|---|---|---|
| No. biopsy sessions as continuous variable |
0.91 (0.81–1.03) | 0.132 | 1.04(0.92–1.17) | 0.516 |
| No. biopsy sessions as categorical variable: |
||||
| 1 | Reference | Reference | ||
| 2 | 1.05(0.85–1.19) | 0.673 | 1.21 (0.97–1.51) | 0.098 |
| 3 or more | 0.73 (0.50–1.09) | 0.128 | 1.07 (0.72–1.61) | 0.727 |
Adjusted for age, race, body mass index, PSA, clinical stage, biopsy Gleason sum, center and year of surgery.
Adjusted for age, race, body mass index, PSA, pathological stage, postoperative Gleason sum, prostate weight, center and year of surgery.
Figure.
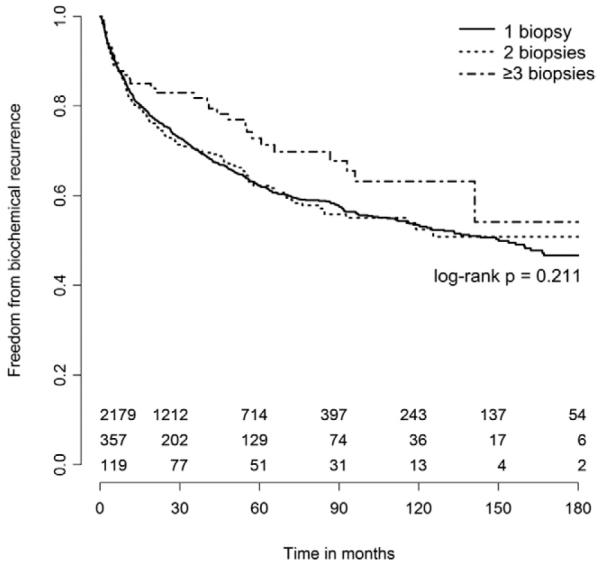
Kaplan-Meier estimates of freedom from biochemical recurrence stratified by number of biopsy sessions.
DISCUSSION
In the current study we found no independent association between the number of biopsy sessions and BCR in patients who undergo RP. This finding is applicable to a large number of men who will undergo more than 1 prostate biopsy in their lifetime, whether due to persistently increased PSA, abnormal examination findings or other risk factors. In our cohort of patients who eventually underwent RP 18%, or nearly 1 in 5, underwent more than 1 biopsy before being diagnosed with cancer. Other studies have demonstrated that 20% to 40% of patients with prostate cancer require more than 1 biopsy for diagnosis.6,9,10 With nearly 220,000 men in the United States diagnosed with prostate cancer in 2010, this equates to tens of thousands of men undergoing multiple prostate biopsies in their lifetime.11 Many will undoubtedly be concerned about whether each additional biopsy increases the risk of cancer spread and a poor cancer outcome. Although previous studies have investigated the relationship between multiple prostate biopsies and pathological findings,3-6,9,10 to our knowledge this is the largest cohort analysis investigating the impact of multiple biopsies on long-term cancer outcomes, specifically BCR.
We found no independent association between the number of biopsy sessions and BCR when biopsies were evaluated as a continuous or categorical variable in the Cox proportional hazards model. Moreover biochemical-free survival estimates were similar among the groups on Kaplan-Meier analysis at a median followup of 37 months. If validated in future studies these findings suggest that patients may be reassured that undergoing multiple biopsies does not increase cancer risk. This conclusion is supported by analogous results from a study from Memorial Sloan-Kettering Cancer Center of nearly 1,400 men with a mean followup of 32 months.6 Lopez-Corona et al demonstrated similar 5-year progression-free probabilities for patients with 1 biopsy compared to those with 2 or more biopsies.6
Although biochemical-free survival was similar among the groups, patients undergoing more biopsies were more likely to have organ confined disease (83% for 3 or more biopsies, p = 0.017). Epstein et al reported that organ confined disease was present in 73% of RP specimens from patients with benign initial biopsies.12 Our findings are consistent with those of others, including Djavan et al, who noted that organ confined disease in prostatectomy specimens was more likely in patients undergoing a third vs first biopsy (p = 0.001), and in those undergoing a fourth vs first biopsy (p = 0.001).10 Similarly Lopez-Corona et al reported organ confined cancer in 61% of men diagnosed on initial biopsy vs 75% of men diagnosed after 2 or more biopsies (p <0.0001).6 Furthermore, the authors demonstrated higher Gleason scores in the prostatectomy specimens of patients with 1 biopsy (p = 0.038) but similar rates of positive margins. We also identified similar margin status among the groups. However, differences in postoperative Gleason sum did not reach significance in our cohort (p = 0.095). The similar rates of positive margins suggest that gross inflammatory changes after repeat biopsies do not lead to technical challenges that would increase the risk of positive margins. It is also noteworthy that we found no evidence of needle biopsies causing local tumor seeding. Despite these pathological findings, men undergoing more biopsies in the Memorial Sloan-Kettering cohort6 and in the current study did not have improved biochemical-free survival compared to those with only 1 biopsy. This finding could be explained by a followup length that was insufficient to demonstrate a significant difference in survival.
Patients undergoing multiple biopsies are more likely to have a larger prostate and unilateral and lower volume cancer on biopsy.6,9,10 Surgical prostate weight was substantially greater (p <0.001) in men with at least 3 biopsies (50 gm) compared to those with only 1 biopsy (38 gm), which likely contributed to higher baseline PSA (p <0.001). We identified a lower Gleason sum at biopsy for men undergoing more than 1 biopsy (p = 0.010), and a similar result has been shown by others.6,9,10 However, Tan et al revealed that a clinically substantial proportion of men with multiple biopsies may have Gleason sum 8-10 disease,9 a finding that has not been reproduced in other studies. They attributed this finding to their biopsy schema, which predicted the number of positive cores and percent of positive cores on multivariate analysis, and may have improved cancer sampling in this setting.9,13 Moreover, others have shown that many of these high grade cancers will be downgraded after surgery.14 In our cohort men with multiple biopsies were more likely to have surgery closer to the present date. Thus, it is possible that the contemporary shift in Gleason scoring may have a more substantial impact on those men who underwent more than 1 biopsy compared to those who only had 1 biopsy,15,16 although the magnitude between dates of surgery was small in our study.
We demonstrated that multiple prostate biopsies are not associated with worse cancer outcomes, yet the procedure still carries risks. In comparing patients undergoing a fourth vs a first biopsy, Djavan et al noted that the former experienced significantly more discomfort, rectal bleeding, recurrent mild hematuria and hematospermia.10 These patients also experienced more dysuria and had a trend toward more urinary tract infections. More recent data have shown increasing rates of infection complications after prostate biopsy, in part due to antibiotic resistant bacteria.17,18 The overall rates of infection in these series were low (2% to 3%), but the risks of resultant sepsis and death remain. These outcomes were outside the scope of our current study.
This study is limited by the retrospective nature of the analysis, variations in prostate biopsy practices among centers and lack of central pathology review. Although the number of biopsy sessions was not independently associated with biochemical recurrence, we must be cognizant that this is a surgical cohort and our findings may not be applicable to patients on active surveillance or to those undergoing other primary therapies. However, the association between an increased number of biopsy sessions and low risk features (eg low grade disease and organ confined disease) suggests that cancer outcomes for these patients might follow less aggressive pathological disease characteristics. Median followup was only 37 months in our Kaplan-Meier estimates. We might expect that men undergoing even more biopsies during a longer followup period would have lower risk features and, therefore, a better rate of biochemical-free survival, although studies with longer followup would be needed for confirmation. Strengths of this study are its multicenter design and relatively large sample of patients with well-defined risk factors and complete followup.
CONCLUSIONS
The number of prostate biopsy sessions is not associated with BCR after prostatectomy. Patients undergoing multiple prostate biopsies are more likely to have a larger prostate, higher PSA and lower risk pathological features. As validated in other cohorts these findings suggest patients may be reassured that repeat prostate biopsy does not lead to a worse cancer outcome compared to cancer diagnosed on first biopsy. Additional studies with longer followup are necessary to further elucidate the effects of multiple biopsies on disease-free and overall survival, and to evaluate the impact of repeat biopsies on men known to have prostate cancer.
Acknowledgments
Supported by the Department of Veterans Affairs, the Department of Defense, Prostate Cancer Research Program (SJF), NIH R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), Georgia Cancer Coalition (MKT) and the American Urological Association Foundation/Astellas Rising Star in Urology Award (SJF). Views and opinions of and endorsements by the author(s) do not reflect those of the U.S. Army, U.S. Navy or the Department of Defense.
Abbreviations and Acronyms
- BCR
biochemical recurrence
- PSA
prostate specific antigen
- RP
radical prostatectomy
REFERENCES
- 1.Cooperberg MR, Lubeck DP, Mehta SS, et al. Time trends in clinical risk stratification for prostate cancer: implications for outcomes (data from CaPSURE) J Urol, suppl. 2003;170:S21. doi: 10.1097/01.ju.0000095025.03331.c6. [DOI] [PubMed] [Google Scholar]
- 2.Potter SR, Horniger W, Tinzl M, et al. Age, prostate-specific antigen, and digital rectal examination as determinants of the probability of having prostate cancer. Urology. 2001;57:1100. doi: 10.1016/s0090-4295(01)00980-3. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006;175:820. doi: 10.1016/S0022-5347(05)00337-X. [DOI] [PubMed] [Google Scholar]
- 4.Kopp RP, Parsons JK, Shiau J, et al. Prostate atypia: clinical and pathological variables associated with cancer diagnosis on repeat biopsy. Prostate Cancer Prostatic Dis. 2011;14:149. doi: 10.1038/pcan.2010.53. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Shinohara K, Grossfeld GD, et al. Prostate cancer detection in men with prior high grade prostatic intraepithelial neoplasia or atypical prostate biopsy. J Urol. 2001;165:1409. [PubMed] [Google Scholar]
- 6.Lopez-Corona E, Ohori M, Wheeler TM, et al. Prostate cancer diagnosed after repeat biopsies have a favorable pathological outcome but similar recurrence rate. J Urol. 2006;175:923. doi: 10.1016/S0022-5347(05)00350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein EA, Silverman R. Inflammation, infection, and prostate cancer. Curr Opin Urol. 2008;18:315. doi: 10.1097/MOU.0b013e3282f9b3b7. [DOI] [PubMed] [Google Scholar]
- 8.Banez LL, Loftis RM, Freedland SJ, et al. The influence of hepatic function on prostate cancer outcomes after radical prostatectomy. Prostate Cancer Prostatic Dis. 2010;13:173. doi: 10.1038/pcan.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan N, Lane BR, Li J, et al. Prostate cancers diagnosed at repeat biopsy are smaller and less likely to be high grade. J Urol. 2008;180:1325. doi: 10.1016/j.juro.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Djavan B, Ravery V, Zlotta A, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001;166:1679. [PubMed] [Google Scholar]
- 11.Altekruse SF, Krapcho M, Neyman N, et al. SEER Cancer Statistics Review, 1975-2007. National Cancer Institute; Bethesda, Maryland: 2010. [Google Scholar]
- 12.Epstein JI, Walsh PC, Akingba G, et al. The significance of prior benign needle biopsies in men subsequently diagnosed with prostate cancer. J Urol. 1999;162:1649. [PubMed] [Google Scholar]
- 13.Jones JS. Saturation biopsy for detecting and characterizing prostate cancer. BJU Int. 2007;99:1340. doi: 10.1111/j.1464-410X.2007.06868.x. [DOI] [PubMed] [Google Scholar]
- 14.Kane CJ, Presti JC, Jr, Amling CL, et al. Changing nature of high risk patients undergoing radical prostatectomy. J Urol. 2007;177:113. doi: 10.1016/j.juro.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 15.Ghani KR, Grigor K, Tulloch DN, et al. Trends in reporting Gleason score 1991 to 2001: changes in the pathologist’s practice. Eur Urol. 2005;47:196. doi: 10.1016/j.eururo.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Thompson IM, Canby-Hagino E, Lucia MS. Stage migration and grade inflation in prostate cancer: Will Rogers meets Garrison Keillor. J Natl Cancer Inst. 2005;97:1236. doi: 10.1093/jnci/dji286. [DOI] [PubMed] [Google Scholar]
- 17.Zaytoun OM, Vargo EH, Rajan R, et al. Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology. 2011;77:1035. doi: 10.1016/j.urology.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 18.Feliciano J, Teper E, Ferrandino M, et al. The incidence of fluoroquinolone resistant infections after prostate biopsy-are fluoroquinolones still effective prophylaxis? J Urol. 2008;179:952. doi: 10.1016/j.juro.2007.10.071. [DOI] [PubMed] [Google Scholar]


