Abstract
Alzheimer’s disease (AD) is generally associated with lower omega-3 fatty acid intake from fish but despite numerous studies, it is still unclear whether there are differences in omega-3 fatty acids in plasma or brain. In matched plasma and brain samples provided by the Memory and Aging Project, fatty acid profiles were quantified in several plasma lipid classes and in three brain cortical regions. Fatty acid data were expressed as % composition and as concentrations (mg/dL for plasma or mg/g for brain). Differences in plasma fatty acid profiles between AD, mild cognitive impairment (MCI), and those with no cognitive impairment (NCI) were most apparent in the plasma free fatty acids (lower oleic acid isomers and omega-6 fatty acids in AD) and phospholipids (lower omega-3 fatty acids in AD). In brain, % DHA was lower only in phosphatidylserine of mid-frontal cortex and superior temporal cortex in AD compared to NCI (−14% and −12%, respectively; both p < 0.05). The only significant correlation between plasma and brain fatty acids was between % DHA in plasma total lipids and % DHA in phosphatidylethanolamine of the angular gyrus, but only in the NCI group (+0.77, p < 0.05). We conclude that AD is associated with altered plasma status of both DHA and other fatty acids unrelated to DHA, and that the lipid class-dependent nature of these differences reflects a combination of differences in intake and metabolism.
Keywords: Alzheimer’s disease, brain lipids, docosahexaenoic acid, free fatty acids, mild cognitive impairment, memory and aging project, oleic acid, omega-3 fatty acids, polyunsaturates, phospholipids
INTRODUCTION
Several studies show that fish consumption decreases the risk of dementing illnesses such as Alzheimer’s disease (AD) [1–6]. The nutrients in fish most clearly associated with protecting brain function in the elderly are the omega-3 fatty acids, particularly docosahexaenoic acid (DHA). Plasma levels of DHA usually correlate positively with fish and/or DHA intake [7–9], so if low fish intake is associated with a higher risk of AD and aging-associated cognitive decline, one would expect to see lower plasma DHA in persons affected by AD. Indeed, some prospective and cohort studies report lower plasma and red blood cell DHA in AD [7, 10–12], but this relationship is by no means always observed. A review of ten independent studies reporting blood fatty acid profiles showed that plasma or red cell DHA in persons with cognitive impairment varied widely and, overall, was not different compared to those with no cognitive impairment (NCI) [13]. In cross-sectional studies of all-cause dementia or dementias other than AD, plasma DHA actually varies from 50% lower to 5 fold higher in AD than in NCI depending on the plasma lipid class analyzed [13–16]. In a nested case-control study, plasma DHA was 35% higher in those who developed dementia than in NCI [17]. Similarly, DHA in brain cortex in AD ranges from 50 to 130% of that in age-matched NCI, with some studies showing little or no difference [13, 18].
Hence, despite relatively good agreement that low fish and DHA intake are associated with a higher risk of AD and other forms of aging-related cognitive decline, plasma DHA, which is the biomarker of lower DHA intake, does not seem to reliably reflect lower DHA intake in AD. Furthermore, we are aware of no previous publication reporting blood and brain DHA from the same individuals. In the present study, we therefore had two objectives: (i) To assess plasma and brain fatty acid profiles in the same individuals stratified according to cognitive status (NCI, mild cognitive impairment [MCI], or AD). (ii) To conduct more detailed fatty acid analysis of brain and plasma lipid classes than is commonly done, which might also help to explain some of the inconsistencies in the literature pertaining to plasma or brain DHA in AD.
The brain and plasma lipid classes we chose to analyze reflect those most commonly studied. For instance, in the brain, fatty acids are almost exclusively present in four major phospholipid classes, of which phosphatidylethanolamine is the most enriched in DHA [19]. In plasma, phospholipids have the highest concentration of omega-3 fatty acids and most closely reflect long term trends in dietary fatty acid intake. In contrast, plasma triglycerides are a short term reflection of dietary fatty acid intake, while free fatty acids may be a key pool for fatty acid transfer to the brain [20]. Cholesteryl esters are the other main plasma fatty acid pool, the turnover of which relatively little is known.
METHODS
Subjects and sample collection
Cases were selected from deceased participants of the ongoing Memory and Aging Project (MAP), a clinical neuropathologic prospective cohort study of 1,306 residents of retirement communities in the Chicago area that began in 1997 (Table 1). Study participants were free of clinically diagnosed dementia at enrolment and all agreed to annual clinical evaluations and organ donation at death. All protocols were approved by the Institutional Review Board of Rush University Medical Center. Background about MAP pertinent to the present study has been previously described in detail [21]. Briefly, MAP participants undergo a uniform, structured clinical evaluation each year that includes a neurological examination and neuropsychological testing. In MAP, clinical diagnoses of dementia and AD are made based on criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association. The diagnosis of MCI is made using criteria previously described and validated in this cohort [21].
Table 1.
Subject characteristics
| Age (y) | 85 ± 6* |
| Male/female | 19/17 |
| Education (y) | 14.8 |
| Mini mental state exam | |
| No cognitive impairment (n = 12) | 29.2 ± 1.0a |
| Mild cognitive impairment (n = 12) | 27.1 ± 1.9b |
| Alzheimer’s disease (n = 12) | 13.3 ± 8.4c |
mean ± SD (n = 36);
Different superscripts denote a statistically significant between groups (p ≤ 0.05; Kruskal-Wallis, followed by Mann-Whitney test).
Thirty six autopsied cases were randomly sampled within strata defined by clinical diagnosis before death (n = 12 NCI, n = 12 MCI, and n = 12 AD). The sampled cases were analyzed for fatty acid profiles in predominantly gray matter of three brain cortical regions: superior temporal gyrus, midfrontal gyrus, and angular gyrus. The mean postmortem lag time to brain autopsy was 5.9 h. Plasma was available for 26 of the 36 cases for which brain samples had been obtained. Mean lag time between the blood samples obtained for these analyses and death of the participant was 1.1 y. Blood samples were collected annually at scheduled time points while the participants were still alive and were frozen at −80°C until analyzed; the last sample obtained before death was used for these analyses. Nutrient intake was not available for this sample.
Fatty acid analyses
All fatty acid analyses were done blind on samples shipped frozen on dry ice from Chicago, USA, to Sher-brooke, QC, Canada. All solvents were HPLC grade (Fisher Scientific, Fair Lawn, NJ, USA). Total plasma lipids were extracted into chloroform-methanol (2 : 1, vol : vol) containing 0.02% butylated hydroxytoluene by the classical Folch method we use routinely [22]. As internal standard, heptadecanoic acid was added in the free fatty acid form. For quantification of fatty acids extracted from plasma total lipids, the extract was saponified using KOH-methanol and the fatty acids subsequently methylated with BF3/MeOH at 90°C for 30 min. For quantification of fatty acids in individual plasma lipid classes, a 120 μl aliquot of plasma was used and internal standards of heptadecanoic acid in the free fatty acid, triglyceride, cholesteryl ester, and total phospholipid form were added to each sample (Sigma, St. Louis, MO, USA). Plasma lipid classes were then separated by silica thin layer chromatography using the solvent system—petroleum ether: diethyl ether: acetic acid: methanol 85 : 15 : 2.5 : 1 (vol : vol : vol : vol)—on 20 by 20 cm plates (Silica Gel 60A, 250 μm, Whatman, Fisher Scientific). Fatty acids in plasma phospholipids and cholesteryl esters were saponified prior to methy-lation with BF3/MeOH.
For each brain sample, total lipids from 0.4–0.8 g of tissue were extracted into chloroform-methanol (2 : 1, vol : vol) containing 0.02% butylated hydrox-ytoluene. Heptadecanoic acid was added as an internal standard for three phospholipid classes: phosphatidylethanolamine and phosphatidylserine (Avanti Polar Lipids, Alabama, USA), and phosphatidylcholine (Sigma). Phospholipid classes were then separated by silica thin layer chromatography using chloroform: methanol: water: ammonium hydroxide (65 : 35 : 4:4, vol : vol : vol : vol). To maximize fatty acid recovery, the silica bands were first saponified using KOH-methanol and the fatty acids subsequently methylated with BF3/MeOH at 90°C for 30 min. Fatty acid methyl esters were analyzed by gas chromatography as previously described [22], with sample injection in pulsed splitless mode at 250°C onto a fused silica capillary column (30 m long by 0.25 mm i.d., 0.25 μm film thickness; model DB-23, Chromatographic Specialties, Brockville, Canada) with flame ionization detection at 250°C (Agilent model 6890 GC, Palo Alto, CA). The identity of individual fatty acids was determined by comparing retention times with standard mixtures of fatty acids (NuChek 68A, and NuChek 411, NuChek 455; NuChek Prep, Inc., Elysian, MN), as well as by our own customized standards. Fatty acid data were analyzed using ChemStation (Agilent).
Data presentation, analysis and statistics
All data are shown as mean ± SD. With the internal standards, fatty acid profiles could be calculated as concentration (mg/dL for plasma or mg/g wet tissue for brain) or as % composition. Given the large amount of data generated, in general, only % composition or concentration is presented but not both, the choice being dependent on whether greater differences between groups were present as concentrations (mostly in plasma) or % composition (mostly in brain). Fatty acid data were not normally distributed, so the non-parametric Kruskal-Wallis test was used followed by a Mann-Whitney test (SPSS; Chicago, Illinois, USA) with p < 0.05 as the cut-off for statistical significance between groups.
RESULTS
Plasma fatty acids
The concentration of plasma free fatty acids as a whole was 43% lower in MCI and 52% lower in AD compared to NCI (p < 0.05). The free monounsaturates, especially free oleic acid and oleic acid isomers, were ~80% lower in AD and ~70% lower in MCI compared to NCI (p < 0.05; Table 2). Free linoleic acid and the sum of free omega-6 fatty acids were 50–80% lower in AD and MCI than in NCI (p < 0.05). Compared to the NCI group, neither free DHA nor the sum of free omega-3 fatty acids was significantly lower in AD. The concentration of free saturated fatty acids (mainly palmitate and stearate) was not statistically different between the three groups.
Table 2.
Fatty acid profile of plasma free fatty acids (mg/dL) in persons with no cognitive impairment (NCI), mild cognitive impairment (MCI) or Alzheimer’s disease (AD)1
| NCI (n = 10) | MCI (n = 7) | AD (n = 9) | |
|---|---|---|---|
| Palmitate (16 : 0) | 4.7 ± 2.5* | 3.0 ± 1.2 | 3.0 ± 1.0 |
| Stearate (18 : 0) | 4.0 ± 1.0 | 3.1 ± 1.6 | 3.4 ± 1.0 |
| Σ Saturates | 9.0 ± 3.5 | 6.3 ± 2.8 | 6.7 ± 2.0 |
| Oleate (18 : 1n-9) | 4.5 ± 3.5a | 1.0 ± 0.4b | 0.8 ± 0.9c |
| Oleate isomers | 1.2 ± 1.5a | 0.5 ± 0.5ab | 0.2 ± 0.2b |
| 22 : 1n-9 | 1.5 ± 0.8 | 0.9 ± 1.0 | 0.1 ± 0.004 |
| Σ Monounsaturates | 7.1 ± 5.0a | 2.4 ± 1.1b | 1.2 ± 1.2c |
| Linoleate | 1.7 ± 1.3a | 0.4 ± 0.1b | 0.3 ± 0.4b |
| Σ n-6 polyunsaturates | 2.4 ± 1.4a | 1.1 ± 0.2b | 1.0 ± 0.4b |
| α-linolenate | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.1 ± 0.03 |
| Docosahexaenoate | 0.1 ± 0.04 | 0.03 ± 0.02 | 0.1 ± 0.05 |
| Σn-3 polyunsaturates | 0.5 ± 0.3 | 1.1 ± 1.0 | 0.3 ± 0.1 |
| SUM | 18.9 ± 9.6a | 10.8 ± 4.2b | 9.1 ± 3.2b |
Mean ± SD;
arachidonate, eicosapentaenoate and n-3 docosapen-taenoate are not shown because they were present at <0.1 mg/dL;
Different superscripts denote a statistically significant between groups (p ≤ 0.05; Kruskal-Wallis, followed by Mann-Whitney test).
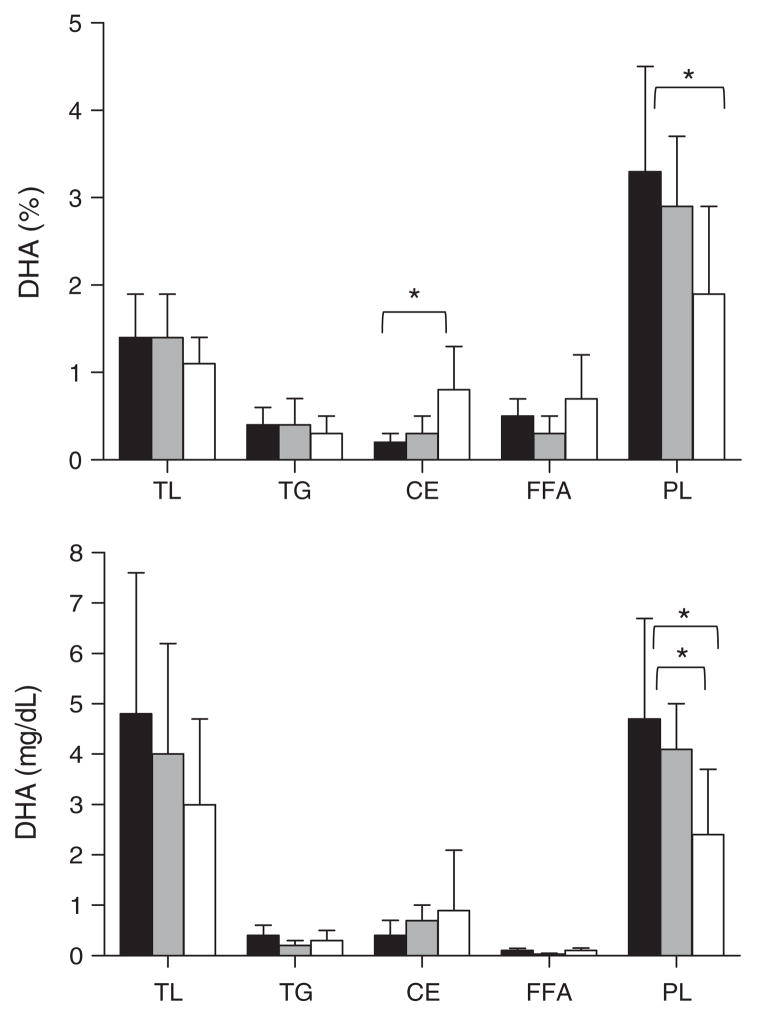
The concentration of plasma total phospholipids did not differ across groups. In plasma total phospholipids, the concentration (mg/dl) of palmitate (16 : 0), eicos-apentaenoic acid (20 : 5n-3) and DHA were 30–50% lower while α-linolenate (18 : 3n-3) was 8-fold higher in AD compared to NCI (p < 0.05; Table 3). With the exception of 3 fold higher % DHA in plasma cholesteryl esters of AD cases compared to NCI (Fig. 1, p < 0.05), there was no significant difference between groups in the fatty acid profile of the plasma triglycerides or cholesteryl esters.
Table 3.
Fatty acid profile of plasma total phospholipids (mg/dL) in persons with no cognitive impairment (NCI), mild cognitive impairment (MCI) or Alzheimer’s disease (AD)
| NCI (n = 10) | MCI (n = 6) | AD (n = 9) | |
|---|---|---|---|
| Palmitate (16 : 0) | 38.0 ± 9.8a* | 41.2 ± 8.7a | 26.3 ± 10.9b |
| Stearate (18 : 0) | 23.0 ± 5.1 | 22.9 ± 3.7 | 21.6 ± 8.7 |
| ΣSaturates | 68.5 ± 23.1 | 65.1 ± 11.8 | 48.6 ± 17.9 |
| Palmitoleate (16 : 1n-7) | 0.8 ± 0.2ab | 1.2 ± 0.4b | 0.6 ± 0.3a |
| Oleate (18 : 1n-9) | 13.7 ± 2.0 | 15.3 ± 2.9 | 12.1 ± 3.5 |
| Oleate isomers | 3.9 ± 1.8 | 5.3 ± 2.1 | 6.3 ± 2.9 |
| 22 : 1n-9 | 0.6 ± 1.1 | 1.2 ± 1.6 | 0.4 ± 0.3 |
| ΣMonounsaturates | 19.2 ± 3.0 | 23.0 ± 5.1 | 19.2 ± 4.7 |
| Linoleate | 23.1 ± 15.2 | 30.1 ± 5.3 | 25.8 ± 10.0 |
| Arachidonate | 16.0 ± 4.7 | 15.6 ± 3.8 | 10.6 ± 4.7 |
| Σn-6 polyunsaturates | 45.1 ± 16.4 | 52.0 ± 10.5 | 42.0 ± 12.2 |
| α-Linolenate | 0.1 ± 0.1a | 0.2 ± 0.1ab | 0.8 ± 1.5b |
| Eicosapentaenoate | 0.9 ± 0.5a | 1.1 ± 0.7a | 0.5 ± 0.2b |
| Docosapentaenoate | 1.1 ± 0.4 | 1.2 ± 0.3 | 0.9 ± 0.4 |
| Docosahexaenoate | 4.7 ± 2.0a | 4.1 ± 0.9a | 2.4 ± 1.3b |
| Σn-3 polyunsaturates | 7.2 ± 2.4 | 7.0 ± 1.7 | 5.2 ± 3.5 |
| SUM | 140 ± 31 | 147 ± 25 | 115 ± 27 |
Mean ± SD;
Different superscripts denote a statistically significant between groups (p ≤ 0.05; Kruskal-Wallis followed by Mann-Whitney test).
Fig. 1.
Docosahexaenoic acid (DHA) in plasma total lipids (TL), triglycerides (TG), cholesteryl esters (CE), free fatty acids (FFA) and phospholipids (PL) expressed as % composition (upper panel) or concentration (mg/dL; lower panel). Bars are for no cognitive impairment (■, n = 10), mild cognitive impairment (□, n = 7), or Alzheimer’s disease (
 , n = 9). Data for Alzheimer’s disease were significantly different from the no cognitive impairment group and/or the mild cognitive impairment group (*p < 0.05; Kruskal-Wallis followed by Mann-Whitney tests).
, n = 9). Data for Alzheimer’s disease were significantly different from the no cognitive impairment group and/or the mild cognitive impairment group (*p < 0.05; Kruskal-Wallis followed by Mann-Whitney tests).
Brain fatty acids
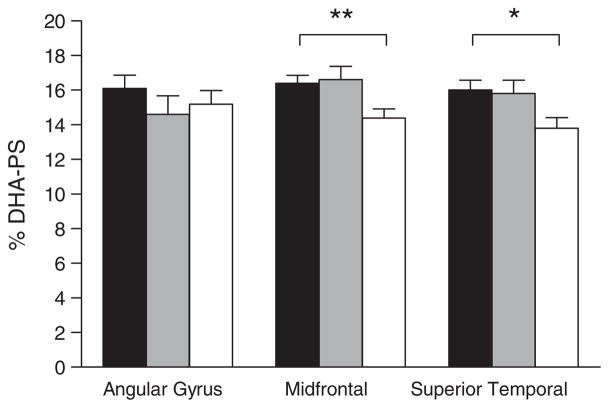
There was no significant difference in the concentration (mg/g) of phosphatidylethanolamine, phosphatidylcholine, or phosphatidylserine between the three groups in any of the three brain cortical regions studied (data not shown). Despite the separation of individual phospholipid classes, very few significant differences in the concentration or % composition of individual fatty acids were observed across the three groups in any of the three brain cortical regions studied. The main difference was a statistically significantly lower % composition of DHA in the AD group, specifically in phosphatidylserine of the mid-frontal (−14%) and superior temporal cortex (−12%) (Fig. 2). In the superior temporal and midfrontal cortex, oleic acid isomers were 10–20% higher in AD (p < 0.05) (data not shown) but further analysis will be needed to determine the exact identity of the specific oleic acid isomers affected.
Fig. 2.
Percent composition of docosahexaenoic acid in brain phos-phatidylserine (% DHA-PS) in three brain regions in persons with no cognitive impairment. Bars are for no cognitive impairment (■, n = 12), mild cognitive impairment (
 , n = 12), or Alzheimer’s disease (□, n = 12). Data for Alzheimer’s disease were significantly different from both no cognitive impairment and mild cognitive impairment groups in the midfrontal cortex (**p = 0.014; Kruskal-Wallis followed by Mann Whitney tests) and in the superior temporal cortex (*p = 0.03; Kruskal-Wallis test). No other differences in fatty acid composition for these brain regions were observed across the three groups, or across the four brain phospholipid classes studied, regardless of whether the data were expressed as concentration (mg/g) or % composition.
, n = 12), or Alzheimer’s disease (□, n = 12). Data for Alzheimer’s disease were significantly different from both no cognitive impairment and mild cognitive impairment groups in the midfrontal cortex (**p = 0.014; Kruskal-Wallis followed by Mann Whitney tests) and in the superior temporal cortex (*p = 0.03; Kruskal-Wallis test). No other differences in fatty acid composition for these brain regions were observed across the three groups, or across the four brain phospholipid classes studied, regardless of whether the data were expressed as concentration (mg/g) or % composition.
Correlation between plasma and brain fatty acid composition
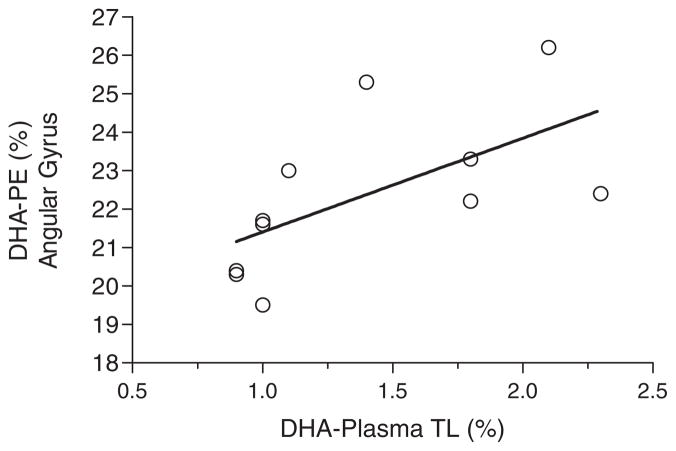
Possible correlations were examined between plasma and brain fatty acids within all the lipid classes and brain regions measured. The only statistically significant correlation was for % DHA in plasma total lipids compared to % DHA in brain phosphatidylethanolamine, specifically in the angular gyrus of the NCI group (r = +0.774; p < 0.005) (Fig. 3). No significant correlations were observed for DHA (% or mg/g) or any other fatty acids in the other brain regions or in the AD and MCI groups (data not shown).
Fig. 3.
Significant positive correlation between percent composition of docosahexaenoic acid in plasma total lipids [DHA-Plasma TL (%)] versus % DHA in phosphatidylethanolamine of the brain’s angular gyrus [DHA-PE (%)] in the no cognitive impairment group (n = 11; r = +0.77, p = 0.005). In the Alzheimer’s disease and mild cognitive impairment groups, there was no significant correlation between docosahexaenoic acid in any plasma lipid class compared to any brain lipid class or brain region.
DISCUSSION
This study presents three main observations: First, there were very few significant differences in brain cortical fatty acid profiles across the three groups, regardless of brain region or phospholipid class analyzed, or units used to present the fatty acid data (mg/g or % composition). These results are consistent with several previous reports showing that the fatty acid composition of the brain cortex may differ very little in AD or in MCI compared to NCI [13, 18]. The lower DHA in phosphatidylserine of the mid-frontal and superior temporal cortex in AD (Fig. 2) may be linked to cognitive deterioration because DHA specifically in phosphatidylserine participates in the signaling cascade leading to apoptosis [23].
Second, differences in the plasma fatty acid profiles in AD or MCI were not specific to DHA or even to omega-3 fatty acids. Indeed, in the plasma free fatty acids, oleic acid isomers and the sum of monounsaturates as well as the omega-6 fatty acids were lower in AD than were the omega-3 fatty acids. Fatty acid concentrations (mg/dL) revealed differences between AD, MCI, and NCI that were not apparent when fatty acid profiles were expressed by % composition. Hence, higher or lower plasma DHA in AD that depends on the lipid class involved will be missed if only the fatty acid profiles of plasma total lipids are analyzed rather than individual plasma lipid classes (Fig. 1). Our present data confirm that different units used to express plasma fatty acid data (%, mg/dL) may also contribute to the divergent plasma or brain fatty acid results in the AD literature [13]. These analytical nuances complicate the applicability of plasma DHA as a marker of DHA intake in disease states such as AD. For fatty acid analyses in which the pool size is known to or could be predicted to differ between the experimental groups, our results suggest that it is preferable to provide the data as both % composition and concentrations.
Third, with one exception, individual fatty acids in plasma were not correlated with those in the brain cortical regions or phospholipid classes we studied. The single exception was that % DHA in plasma total lipids correlated with % DHA in brain phosphatidylethanolamine, but only in NCI and only in the angular gyrus (Fig. 3). Hence, at least in this small cohort, plasma fatty acid profiles in MCI or AD seem to be a poor marker of those in the brain cortex, whether for omega-3 or other types of fatty acids.
As reviewed elsewhere [13], in the AD group, DHA was higher in plasma cholesteryl esters but lower in plasma phospholipids (Fig. 1). We suggest that these pool-dependent and inverse differences in plasma DHA in AD provide a preliminary indication that plasma fatty acid transport may be perturbed in AD. Such a perturbation could in turn potentially disrupt the transfer of fatty acids, particularly DHA, from the plasma to the brain. Whether such a disruption is due to aging per se or to a particular disease process associated with the development of AD is as yet unknown. However, older persons with no known cognitive impairment commonly have altered omega-3 fatty acid metabolism, including: (i) higher plasma levels of omega-3 fatty acids [24, 25]; (ii) higher plasma DHA response to short-term supplementation with a fish oil containing DHA [22]; and (iii) markedly altered transit of DHA through plasma lipid classes as measured using an oral dose of carbon-13-labeled DHA [26]. Understanding how and why omega-3 fatty acid metabolism changes during aging may therefore help uncover why aging-associated cognitive decline is not always associated with lower plasma DHA and may in fact raise DHA in plasma cholesteryl esters despite the relatively consistent observation of lower fish intake [13].
The 5.9 h delay in accessing and freezing the brain is one limitation of the present study. Biochemical studies suggest that the liberation of ‘free’ fatty acids following death or a chemical or physical insult to the brain is very rapid, i.e., within seconds if not faster [27, 28]. Clearly a postmortem delay of several hours makes assessing the true brain free fatty acid pool somewhat artificial, hence the reason we did not try. Nevertheless, the delay from death to autopsy was similar for the whole sample so the very similar DHA profiles in different phospholipid classes across the three groups and three brain cortical regions casts some doubt on the idea that the AD brain contains less DHA. The obligatory postmortem delay in obtaining the brain samples suggests brain fatty acid analysis may have limited value if the fatty acids of interest have either very slow turnover, or very fast turnover that is perturbed by death. Even if the postmortem delay were reduced to an unlikely 1–2 h, this is still orders of magnitude more than the rate of fatty acid liberation from membrane phospholipids [20, 27, 28]. Finally, grey matter atrophy in AD may alter cortical fatty acid content because cortical thickness and surface area have decreased without this necessarily being apparent when the data are expressed as concentrations. Hence, for a more complete understanding of how brain fatty acid profiles may change in AD, it would be beneficial to have measures of cortical thickness and volumes of grey and white matter throughout the brain.
We conclude that compared to NCI, AD cases from the MAP cohort have very little difference in the phospholipid content of DHA (or other fatty acids) in brain cortex but more complex differences in DHA in plasma lipids. The lipid class-dependent differences in plasma DHA content in AD may contribute to the inconsistent literature that shows on the one hand a relatively strong direct relationship between habitual fish intake and risk of AD, yet on the other, a weak and inconsistent link to plasma DHA. Since dietary DHA supplements are relatively ineffective reduced cognitive decline in AD [13, 29–31], but may be protective in those with mild aging-related cognitive decline [32], our results will hopefully stimulate further work to better understand how and why DHA metabolism changes in the elderly, especially in those at risk of MCI and AD.
Acknowledgments
This study was funded by the Rush Translational Sciences Consortium and the National Fisheries Institute. SCC obtained financial support for the analytical component of this research from the Canadian Institutes of Health Research, the Research Center on Aging, a Canada Research Chair, and laboratory infrastructure funding by the Canadian Foundation for Innovation. Mary Ann Ryan provided skilled technical assistance.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1120).
References
- 1.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 2.Maclean CH, Issa AM, Newberry SJ, Mojica WA, Morton SC, Garland RH, Hilton LG, Traina SB, Shekelle PG. Effects of omega-3 fatty acids on cognitive function with aging, dementia, and neurological diseases. Evid Rep Technol Assess. 2005 Summ;:1–3. [PMC free article] [PubMed] [Google Scholar]
- 3.Gillette Guyonnet S, Abellan Van Kan G, Andrieu S, Bar-berger Gateau P, Berr C, Bonnefoy M, Dartigues JF, de Groot L, Ferry M, Galan P, Hercberg S, Jeandel C, Morris MC, Nourhashemi F, Payette H, Poulain JP, Portet F, Roussel AM, Ritz P, Rolland Y, Vellas B. IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging. 2007;11:132–152. [PubMed] [Google Scholar]
- 4.Boudrault C, Bazinet RP, Ma DW. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer’s disease. J Nutr Biochem. 2009;20:1–10. doi: 10.1016/j.jnutbio.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: A complex association. Nat Clin Pract Neurol. 2009;5:140–152. doi: 10.1038/ncpneuro1044. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC. The role of nutrition in Alzheimer’s disease: Epidemiological evidence. Eur J Neurol. 2009;16(Suppl 1):1–7. doi: 10.1111/j.1468-1331.2009.02735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch Neurol. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 8.Sanders TA, Ellis FR, Dickerson JW. Studies of vegans: The fatty acid composition of plasma choline phosphoglycerides, erythrocytes, adipose tissue, and breast milk, and some indicators of susceptibility to ischemic heart disease in vegans and omnivore controls. Am J Clin Nutr. 1978;31:805–813. doi: 10.1093/ajcn/31.5.805. [DOI] [PubMed] [Google Scholar]
- 9.Arterburn LM, Hall EB, Oken H. Distribution, inter-conversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:S1467–S1476. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 10.Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes - The EVA Study. Am J Clin Nutr. 2003;77:803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 11.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plama n-3 fatty acids and the risk of cognitive decline in older adults; the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85:1103–1111. doi: 10.1093/ajcn/85.4.1103. [DOI] [PubMed] [Google Scholar]
- 12.Whalley LJ, Deary IJ, Starr JM, Wahle KW, Rance KA, Bourne VJ, Fox HC. n-3 Fatty acid erythrocyte membrane content, ApoE e4, and cognitive variation: An observational follow-up study in late adulthood. Am J Clin Nutr. 2008;87:449–454. doi: 10.1093/ajcn/87.2.449. [DOI] [PubMed] [Google Scholar]
- 13.Cunnane SC, Plourde M, Pifferi F, Bégin M, Féart C, Barberger-Gateau P. Fish, docosahexaenoic acid and Alzheimer’s disease. Prog Lipid Res. 2009;48:239–256. doi: 10.1016/j.plipres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Corrigan FM, Van Rhijn A, Horrobin DF. Essential fatty acids in Alzheimer’s disease. Ann N Y Acad Sci. 1991;640:250–252. doi: 10.1111/j.1749-6632.1991.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 15.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 16.Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D, Gibney MJ. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer’s disease: A case-control study. Br J Nutr. 2003;89:483–489. doi: 10.1079/BJN2002804. [DOI] [PubMed] [Google Scholar]
- 17.Laurin D, Verreault R, Lindsay J, Dewailly E, Holub BJ. Omega-3 fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis. 2003;5:315–322. doi: 10.3233/jad-2003-5407. [DOI] [PubMed] [Google Scholar]
- 18.Fraser T, Tayler H, Love S. Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer’s disease. Neurochem Res. 2009;35:503–513. doi: 10.1007/s11064-009-0087-5. [DOI] [PubMed] [Google Scholar]
- 19.Crawford MA. Docosahexaenoic acid in neural signaling systems. Nutr Health. 2006;18:263–276. doi: 10.1177/026010600601800309. [DOI] [PubMed] [Google Scholar]
- 20.Umhau J, Zhou W, Carson RE, Rapoport SI, Polozova A, Demar J, Hussein N, Bhattacharjee AK, Ma K, Esposito G, Majchrzak S, Hercovitch P, Eckelman WC, Kurdziel KA, Salem N., Jr Imaging incorporation of circulating docosahexaenoic acid into the brain using positron emission tomography. J Lipid Res. 2009;50:1259–1268. doi: 10.1194/jlr.M800530-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 22.Vandal M, Freemantle E, Tremblay-Mercier J, Plourde M, Fortier M, Bruneau J, Gagnon J, Tremblay S, Bégin M, Cunnane SC. Plasma omega-3 fatty acid response to a fish oil supplement in the healthy elderly. Lipids. 2008;43:1085–1089. doi: 10.1007/s11745-008-3232-z. [DOI] [PubMed] [Google Scholar]
- 23.Kim HY, Akbar M, Kim YS. Phosphatidylserine-dependent neuroprotective signaling promoted by docosahex-aenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2010;82:165–172. doi: 10.1016/j.plefa.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Groot RH, van Boxtel MP, Schiepers OJ, Hornstra G, Jolles J. Age dependence of plasma phospholipid fatty acid levels: Potential role of linoleic acid in the age-associated increase in docosahexaenoic acid and eicosapentaenoic acid concentrations. Br J Nutr. 2009;102:1058–1064. doi: 10.1017/S0007114509359103. [DOI] [PubMed] [Google Scholar]
- 25.Fortier M, Tremblay-Mercier J, Plourde M, Chouinard-Watkins R, Vandal M, Pifferi F, Freemantle E, Cunnane SC. Higher plasma n-3 fatty acid status in the moderately healthy elderly in southern Quebec: Higher fish intake or aging-related change in n-3 fatty acid metabolism? Prostaglandins Leukot Essent Fatty Acids. 2010;82:277–280. doi: 10.1016/j.plefa.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Plourde M, Chouinard-Watkins R, Vandal M, Zhang Y, Lawrence P, Brenna JT, Cunnane SC. Plasma incorporation, apparent retroconversion and beta-oxidation of 13 C-docosahexaenoic acid in the elderly. Nutr Metabol. 2011;8:5. doi: 10.1186/1743-7075-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CT, Liu Z, Bazinet RP. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: An intraventricular study. J Neurochem. 2011;116:363–373. doi: 10.1111/j.1471-4159.2010.07116.x. [DOI] [PubMed] [Google Scholar]
- 28.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degneration, Alzheimer’s, and other neurodegenerative diseases. Ann Rev Nutr. 2011;31:321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devore EE, Grodstein F, van Rooij FJ, Hofman A, Rosner B, Stampfer MJ, Witteman JC, Breteler MM. Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. Am J Clin Nutr. 2009;90:170–176. doi: 10.3945/ajcn.2008.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroger E, Verreault R, Carmichael PH, Lindsay J, Julien P, Dewailly E, Ayotte P, Laurin D. Omega-3 fatty acids and risk of dementia: The Canadian Study of Health and Aging. Am J Clin Nutr. 2009;90:184–192. doi: 10.3945/ajcn.2008.26987. [DOI] [PubMed] [Google Scholar]
- 31.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Jr, Weiner M, Shinto L, Aisen PS. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, Salem N, Jr, Stedman M. Benefical effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dementia. 2010;6:456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]