Abstract
One of the many advantages of multivariate pattern recognition approaches over conventional mass-univariate group analysis using voxel-wise statistical tests is their potential to provide highly sensitive and specific markers of diseases on an individual basis. However, a vast majority of imaging problems addressed by pattern recognition are viewed from the perspective of a two-class classification. In this article, we provide a summary of selected works that propose solutions to biomedical problems where the widely-accepted classification paradigm is not appropriate. These pattern recognition approaches address common challenges in many imaging studies: high heterogeneity of populations and continuous progression of diseases. We focus on diseases associated with aging and propose that clustering-based approaches may be more suitable for disentanglement of the underlying heterogeneity, while high-dimensional pattern regression methodology is appropriate for prediction of continuous and gradual clinical progression from magnetic resonance brain images.
Keywords: high-dimensional pattern analysis, clustering, pattern regression, MRI, aging, MCI, Alzheimer’s disease
I. INTRODUCTION
Morphological analysis of medical images has been used in a variety of research and clinical studies that investigate the effect of diseases and treatments on anatomical structures. To identify brain structures associated with a disease, traditional neuroimaging approaches analyze target (patient) population with respect to a control group or to another patient population. Analysis of group differences has often been performed with the help of univariate statistical methods (e.g., voxel-based morphometry [VBM]). Unfortunately, mass-univariate statistical methods may not be applicable in whole-brain magnetic resonance imaging (MRI) studies with relatively low sample size because of very high dimensionality of the data. More importantly, mass-univariate group-comparison methods have very limited diagnostic power, because in every single region detected by these methods, there is typically a significant overlap between healthy and diseased individuals.
On the other hand, high-dimensional pattern classification has gained significant attention in recent years, and represents a promising technique for capturing complex spatial patterns of pathological brain changes. Importantly, pattern classification methods have begun to provide tests of high sensitivity and specificity on an individual patient basis, in addition to characterizing group differences. As a result, these methods can potentially be used as diagnostic and prognostic tools. Pattern classification approaches were shown to work particularly well in the task of classifying patient populations from normal cohort in various clinical studies (e.g., Alzheimer’s (Duchesne 2008; Fan et al., 2008c; Kloppel et al., 2008; Misra et al., 2009), autism (Ecker et al., 2010), schizophrenia (Fan et al., 2008b), etc.). The state-of-the-art brain image classification methods work by learning a classification function from a set of labeled training examples, and then apply the learned classifier to predict labels of the test data. In most cases, these approaches assume the availability of two distinct populations (i.e., control normal and diseased), and aim at categorizing a novel image into one of the two groups. These methods belong to the family of supervised classification approaches and assume that the labels for all training data are available.
However, in many diseases, populations of patients and healthy subjects are highly heterogeneous, and clear boundaries between the clinical manifestation of various subconditions may not be always established. For example, the neurobiological heterogeneity in schizopherenia is characterized by distinct neuroanatomical (endo) phenotypes underlying different psychopathological symptom dimensions (Koutsouleris et al., 2008) and by a significant sexual dimorphism in the structural abnormalities associated with the disorder (Davatzikos, 2004). Moreover, populations of normal individuals are not necessarily homogeneous, which is particularly evident in the studies of older healthy adults (Resnick et al., 2003). Additionally, the continuous progression of diseases may result in a significant overlap between the normal and patient populations. For example, pathology associated with the Alzheimer’s disease (AD) is known to progress gradually over many years, sometimes starting decades before a final clinical stage. Therefore, it is important to estimate continuous clinical variables that might relate to disease stage, rather than categorical classification. Furthermore, the ability to predict the change in cognitive performance from baseline imaging is even more important, as it would estimate disease progression and improve patient management.
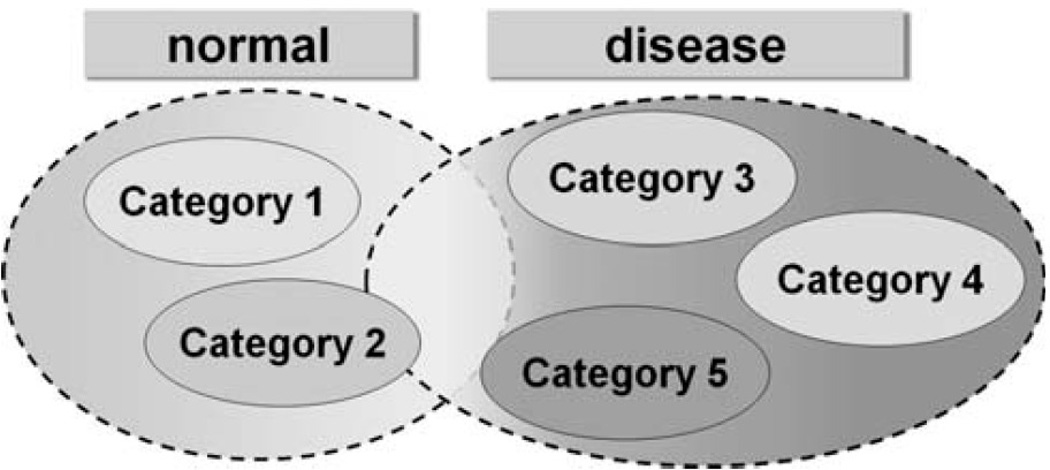
Figure 1 shows a diagram of a possible heterogeneous structure of populations in many clinical studies, as well as the significant overlap in diagnostic categories due to the progressive nature of diseases. Clearly, the high level label information (i.e., grouping into normal and patient categories) used by existing supervised approaches does not necessarily present a comprehensive picture of the disease. As a result, unknown heterogeneous structure of populations and continuous nature of many disorders are but two of many challenges that the traditional dichotomous classification is not designed to address.
Figure 1.
Possible scenario observed in a number of diseases. A number of subconditions may exist within the normal and patient groups. Additionally, the categorical boundaries between normal and patient populations may be blurred due to the continuous nature of the disease. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In this article, we present a summary of selected works on imaging problems that cannot be addressed using common two-class classification paradigm. The application focus of this article is on the studies of aging. We first discuss the potential and limitations of the univariate statistical methods in the context of AD, and review selected results of the two-class pattern classification as applied in the studies of aging. We do not attempt to be thorough in our presentation of conventional mass-univariate and two-class classification approaches, but rather use example works to highlight the range of problems the traditional methodologies are designed to address. We then describe a clustering-based approach that attempts to disentangle heterogeneity of the normal aging populations (Filipovych et al., 2011), as well as review a high-dimensional pattern regression methodology that is specifically designed to determine the stage or progressive rate of the disease development for a given individual, rather than a categorical label (Wang et al., 2010).
II. MATERIALS
The data used in the articles, discussed below as examples of the methodologies, are from the AD neuroimaging initiative (ADNI) and from the Baltimore Longitudinal Study of Aging (BLSA). The goal of ADNI (www.loni.ucla.edu/ADNI) is to recruit 800 adults, ages 55–90 years, to participate in the research: approximately 200 normal control older individuals to be followed for 3 years, 400 people with mild cognitive impairment (MCI) to be followed for 3 years, and 200 people with early AD to be followed for 2 years. For up-to-date information, see www.adni-info.org. In the ADNI, a subset of MCI subjects was diagnosed with AD during the study, i.e., MCI converters (MCI-C), whereas a vast majority of MCI subjects did not exhibit change in diagnosis during the follow-up period, i.e., MCI nonconverters (MCI-NC).
One of the current limitations of ADNI is its rather short follow-up evaluations period. On the other hand, BLSA (Resnick et al., 2003) is a prospective longitudinal study of aging, with the neuroimaging component currently in its 16th year. BLSA has followed 158 individuals (aged 55–85 years at enrollment) with annual or semiannual imaging and clinical evaluations. The neuroimaging substudy of the BLSA is described in detail by Resnick et al. (2003).
A. Image Processing
All MR images were preprocessed following mass-preserving shape transformation framework (Davatzikos et al., 2001). Gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) were segmented out from each skull-stripped MR brain image by a brain tissue segmentation method (Pham and Prince, 1999). Each tissue-segmented brain image was spatially normalized into a template space, by using a high-dimensional image warping method (Shen and Davatzikos, 2002). The total tissue mass was preserved in each region during the image warping and tissue density maps were generated in the template space. These tissue density maps give a quantitative representation of the spatial distribution of tissue in a brain, with brightness being proportional to the amount of local tissue volume before warping.
III. VBM AND TWO-CLASS CATEGORIZATION
A. Voxel-Based Morphometry
MCI has attracted a lot of attention in the recent years, in part because it offers opportunities for relatively early diagnosis of AD and a number of pharmacological interventions typically target MCI patients. A number of studies, including both region-of-interest and voxel-based analyses, have reported relatively reduced brain volumes in the hippocampus, parahippocampal gyrus, cingulate, and other brain regions in both MCI and AD patients (Jack et al., 1999; Convit et al., 2000; Chetelat et al., 2003; Fox and Schott, 2004; Karas et al., 2004).
Group comparisons were performed by Misra et al. (2009) via voxel-based statistical analysis to find brain regions that are typically affected by AD. Group comparisons involved voxel-by-voxel t-tests applied by the SPM software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) to the tissue-density maps. Several regions of relatively reduced volumes of GM in MCI-C compared with MCI-NC were evident, including the anterior hippocampus, amygdala, much of the temporal lobe GM, and the insular cortex, posterior cingulate, and orbitofrontal cortex. WM was reduced primarily in the periventricular frontal region, indicating higher periventricular leukoareosis in MCI-C when compared with MCI-NC. WM was also relatively reduced in MCI-C anterior-laterally to the right hippocampus. Finally, ventricular CSF was relatively larger in the temporal horns of the lateral ventricles bilaterally. Regions of increased periventricular abnormal WM in MCI-C, relative to MCI-NC indicated relatively more pronounced leukoareosis in the former group.
In general, the results of the group comparisons were consistent with the similar results reported in the abundant related literature. The results of the univariate statistical methods provide an important insight into the development of AD as they indicate brain structures that are typically affected by the disease. Unfortunately, VBM has very little diagnostic and prognostic value and does not allow to assess subjects on an individualized level.
B. Two-Class Categorization
In contrast to the VBM, pattern classification methods attempt to learn subtle patterns of brain pathology associated with the disease, and detect presence of the disease-related patterns in the brain of a new subject. Following the two-class categorization methodology, individual-patient analysis was performed by Misra et al. (2009) and aimed at classifying individual scans as belonging to MCI-C or MCI-NC participants based on their baseline MRI. The two-class classification approach directly relates to our ability to use quantitative MRI analysis for individual diagnosis, rather than to identify statistical differences between two potentially overlapping groups. Misra et al. (2009) applied the high-dimensional pattern classification approach (Fan et al., 2007) that considers all brain regions jointly and identifies a minimal set of brain regions whose volumes jointly maximally differentiate between the two groups under consideration on an individual scan basis. After the two-class classifier was trained, the pattern classification method provided a structural phenotypic score for every new subject, which reflects the degree to which the AD-like atrophy is present in the brain of the subject. The structural phenotypic score of a subject is essentially the value of the classification function for the brain of the subject. Positive scores (i.e., values of the classification function) indicate AD-like brain structure and negative scores indicate normal structure. More specifically, the following two classifiers were constructed.
B.1. Classifier 1. A classifier was constructed from the normal control and AD groups that were described in detail in Fan et al. (2008a), and then applied to the MCI subjects. The classifier achieved classification accuracy between AD and the control group equal to 94.3%, evaluated via leave-one-out cross-validation. The average structural phenotypic score of the MCI-C was 1.23 ± 0.7 and of the MCI-NC was 0.46 ± 1.28 (p value of two-group t-test = 0.0002).
B.2. Classifier 2. A more specific classifier that optimally differentiates between MCI-C and MCI-NC was constructed and tested using leave-one-out cross-validation. Maximum MCI-C/MCI-NC classification rate was 81.5%; however, the classification rate tended to fluctuate between 75 and 80% for various parameters of the classification algorithm. The area under the receiver operating characteristic curve that reflects the ability of the method to correctly discriminate between AD-related and non-AD brain pathology was 0.77.
In general, the two-class pattern classification approaches allowed to differentiate AD and control populations with very high accuracy. Additionally, the approach showed significant potential in predicting conversion to AD in MCI patients. A review and a benchmark comparison of the state-of-the-art two-class classification methods was done by Cuingnet et al. (accepted for publication) and suggests that most of the pattern recognition approaches perform relatively similar in the classification task. As expected, the classifiers achieve good accuracy in differentiating AD from normal groups and have significantly worse performance in predicting conversion to AD from MCI.
IV. DISENTANGLING HETEROGENEITY IN NORMAL ADULT POPULATIONS
Although the two-class (or multiclass) categorization approaches can provide highly sensitive and specific individualized biomarkers of AD, they assume a very simplistic picture of the disease. These methods attempt to categorize subjects at a very high level (i.e., AD, MCI, and normal) and are oblivious to the details of the underlying heterogeneity. At the same time, different subconditions may exist within the disease and potentially may require different treatment options.
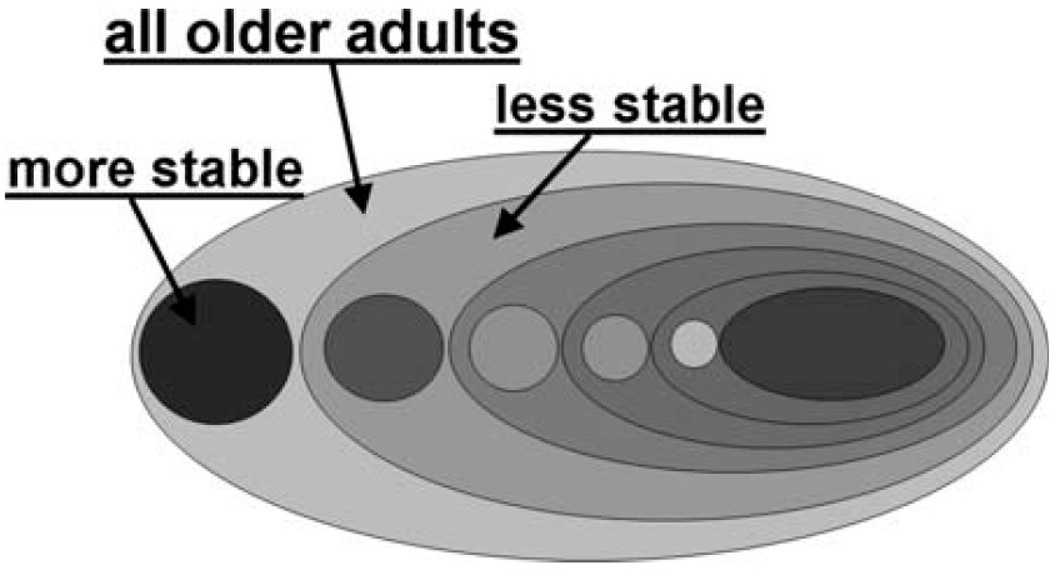
Filipovych et al. (2011) proposed a clustering-based method that is specifically designed to find coherent subpopulations within heterogeneous distributions. The method involves little supervision at the stage of dimensionality reduction, and employs a hierarchical clustering technique to automatically group MR images of healthy older adults with respect to the underlying brain pathology. After analyzing a set of 875 scans of healthy normal adults from the BLSA study, it was discovered by the approach that the most cognitively stable subjects form a small, but extremely coherent subpopulation (i.e., cluster). At the same time, all cognitively less stable individuals form a less coherent and dispersed cluster, which in turn can be viewed as a denser relatively cognitively stable subpopulation, and even more dispersed cognitively less stable subpopulation. The results suggest that the population of subjects with relatively better cognitive performance is more densely distributed than the population of less normal brain images. Overall, the population of healthy older adults can be visualized as the hierarchical structure in Figure 2.
Figure 2.
Hierarchical structure of the populations of healthy older adults. Any given heterogeneous population consists of a small homogeneous cognitively more stable subpopulation and of rather dispersed cognitively less stable and heterogeneous subpopulation. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The figure shows a population of normal adults that consists of two subpopulations, one of which is a small, yet coherent, subpopulation of cognitively more stable individuals. The other subpopulation is much more heterogeneous and on average less cognitively stable. The cognitively less stable subpopulation in turn also consists of two subgroups of relatively more stable and relatively less stable adults, and so on. These results suggest that at the very early stages, cognitive performance degrades in a continuous manner, without going through any distinct phases.
A. Cluster-Based Level of Pathology
Filipovych et al. (2011) also explored ways of using discovered subpopulations to quantify the severity of brain pathology in individual brains. Unlike the common two-class categorization paradigm that typically assumes the availability of patient and normal populations, all individuals in the BLSA study are normal, and no prior categorization of the population is available. Nonetheless, it was shown that it is possible to design an individualized marker that reflects subject’s cognitive decline. Given a cluster of images of cognitively less stable subjects, CP, and a cluster of cognitively more stable individuals, CN, a measure of the level of pathology for an image I was defined as follows:
| (1) |
where d(I,CN) and d(I,CP) are the distances between I and the centers of more stable and less stable clusters, respectively. As the result, individuals with better cognitive performance are expected to have lower level of pathology, and vice versa.
It was found that there is a very strong relationship between the proposed measure and cognitive performance. The subpopulations of healthy older adults that correspond to the extreme quartiles of the pathology measure (i.e., subjects with the level of pathology in the upper 25% and in the lower 25%, respectively) showed significantly different cognitive performance with respect to most cognitive tests. Table I shows group differences in the Mini-Mental State Exam (MMSE; Folstein et al., 1975) that assesses mental status, the immediate free recall score (sum of five immediate recall trials) on California Verbal Learning Test (CVLT; Delis et al., 1987) and the long-delay free recall score on CVLT that assess verbal learning and immediate and delayed recall, and the total number of errors from the Benton Visual Retention Test (Benton, 1974) that assesses short-term visual memory.
Table I.
Relationship between cognitive performance and level of pathology
| CVLT List A Sum |
CVLT Long-Delay Free |
BVRT Errors |
MMSE | |
|---|---|---|---|---|
| Mean scores | 0.001 | <0.001 | 0.007 | 0.048 |
| First-visit scores | 0.001 | 0.001 | 0.011 | 0.137 |
| Last visit scores | <0.001 | 0.001 | 0.042 | 0.038 |
p Values of one-sided t-test obtained for the group with level of pathology in upper 25% vs. the group with level of pathology in lower 75%.
Additionally, no significant age difference between the lower and the upper quartile groups was observed (p = 0.322), which suggested that the method proposed in Filipovych et al. (2011) captures pathology that is not solely induced by age.
Overall, the results given in Filipovych et al. (2011) suggest that clustering-based pattern recognition allows to get a better understanding of the underlying heterogeneity, and at the same time has the potential to provide quantitative markers of cognitive decline at the very early stages.
V. PREDICTING CLINICAL DEVELOPMENT OF PROGRESSIVE BRAIN CHANGES
As we mentioned earlier, many pathologies and diseases present a continuous spectrum of structural and functional change. Therefore, it is important to understand the relationship between brain changes and progressive stages of diseases. A high-dimensional pattern regression method was described by Wang et al. (2010), which is specifically designed to predict cognitive performance from MR brain images. The method works by measuring the similarity of correlation coefficients between voxel-wise measures and continuous clinical stages, and applies an adaptive regional clustering method to capture informative regional features. A subset of features with top-ranking correlation power with respect to the underlying regression problem is then selected from the original regional clusterings. Subsequently, relevance vector machines (Tipping, 2001) are built to describe the relationship between the patterns of volumetric brain regions and the corresponding clinical stages. Additionally, the approach employs a bagging framework to facilitate analysis of relatively small populations.
A. Predicting Cognitive Performance in Older Adults
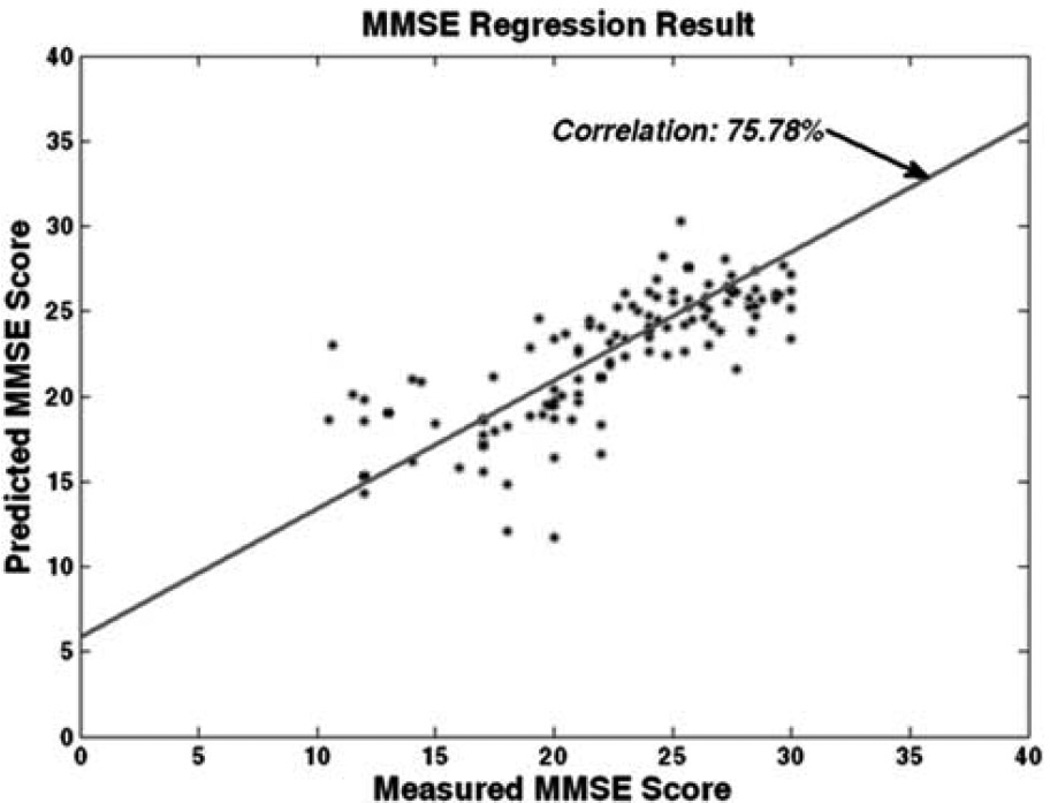
In the context of aging, the pattern regression method (Wang et al., 2010) was applied within the ADNI study to the problem of predicting the results of future cognitive evaluations (e.g., MMSE) from baseline MR brain images in the subjects ranging from healthy controls to MCI to AD. Figure 3 presents the results of predicting the future values of MMSE evaluations from the first-visit imaging evaluations. Overall, the method results in a regression rate of 75.78%, which is the correlation between the actual measured clinical scores and the predicted values. This result is very promising as it suggests that baseline images contain very rich information that is not only indicative of cognitive decline, but is also predictive of the future cognitive performance.
Figure 3.
Predictive cognitive scores, Clinical measured MMSE vs. MMSE estimated by pattern regression. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
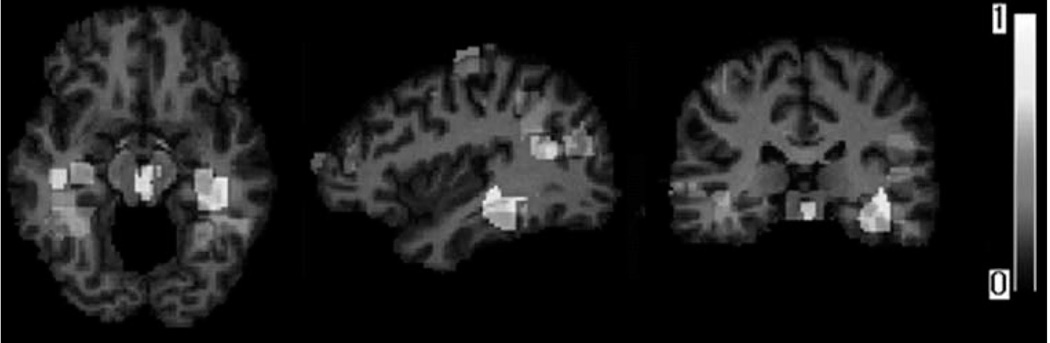
Figure 4 shows the brain regions detected by the pattern regression algorithm (Wang et al., 2010) that collectively contribute to the prediction of cognitive performance. The regions are color-coded with respect to the potential importance for prediction. Most of the locations of brain atrophy patterns highly associated with MMSE are consistent with the known clinical findings, such as the hippocampus, which is commonly taken as a biomarker of early AD.
Figure 4.
Examples of brain regions, which are most informative for prediction of cognitive evaluations. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
B. Predicting the Rate of Disease Development from Baseline Scans
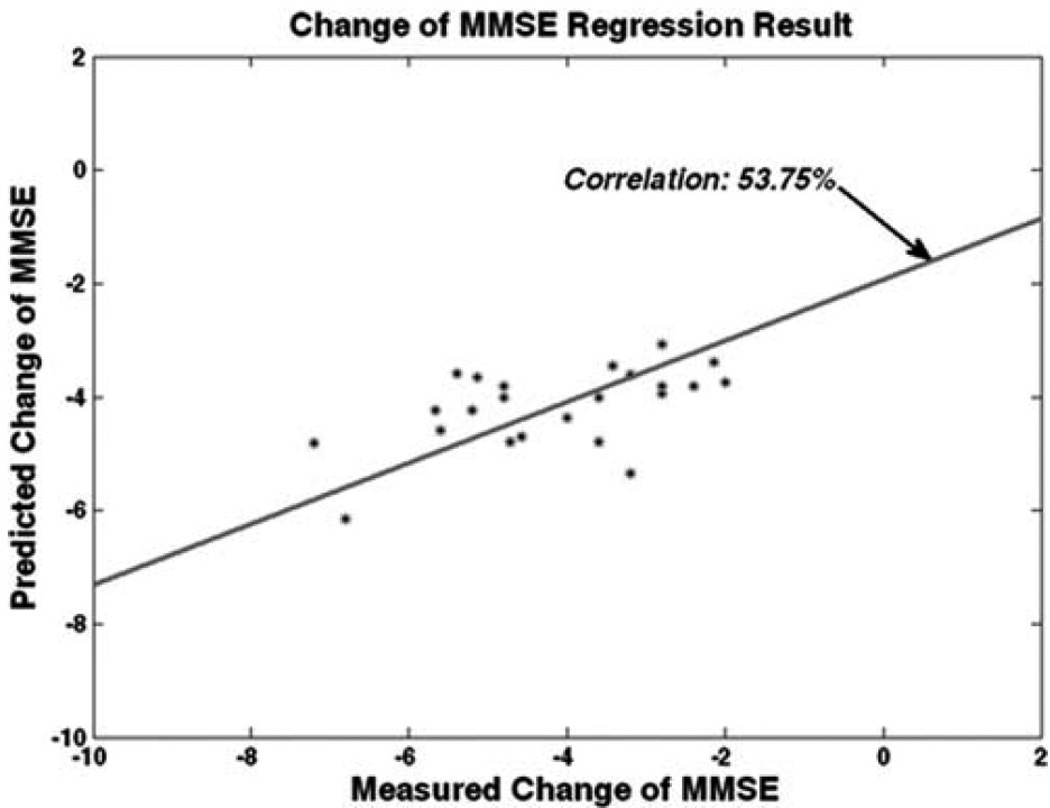
As MCI patients are at high risk of conversion to AD, it is of great clinical interest to be able to predict which of the MCI individuals are likely to progress rapidly and decline cognitively, using their baseline exams. Notice that the task of predicting the rapidity of cognitive decline is principally different from the problem of categorizing subjects with respect to the predicted conversion to AD. The pattern recognition approach geared at the prediction of the rate of decline in MMSE showed regression rate of 53.75% (i.e., Figure 5), which is worse than the prediction of the MMSE score itself. The reason might be due to the fact that the pattern recognition approach by Wang et al. (2010) was applied within the ADNI study, which is characterized by a relatively short follow-up period. The lack of longer follow-up evaluations makes estimation of the true rate of cognitive decline particularly challenging. Nonetheless, given that the rate of cognitive decline is most-likely to change nonlinearly over time, the potential of the pattern regression to capture continuous evolution of cognitive performance from base-line images is very promising.
Figure 5.
Predicting rate of change of MMSE for MCI and AD patients from ADNI study. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
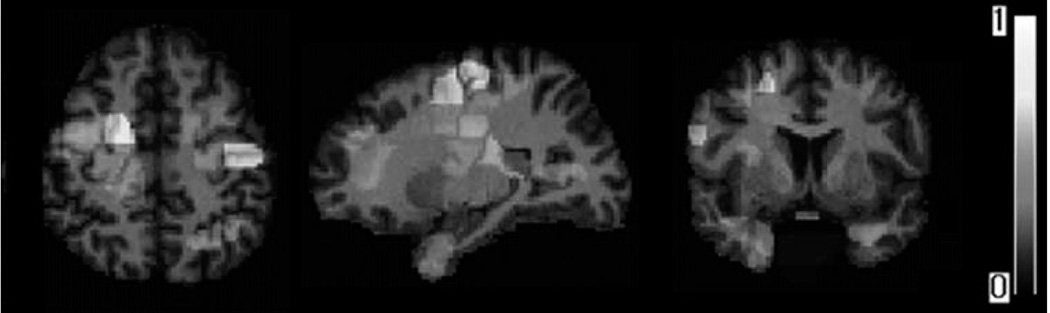
Figure 6 shows the maps of volumetric regions that were determined by the pattern regression approach (Wang et al., 2010) to be most informative for predicting the rate of cognitive decline. Some of the regional locations are similar with those used for MMSE predication. Obviously, hippocampus also contributes to cognitive decline. Tn addition, left parietal lobe appears to be the most significantly associated with cognitive declining, which is primarily involved in processing sensory information from various parts of the body, knowledge of numbers, and their relations.
Figure 6.
Examples of brain regions, which are most informative for predicting rate of change in cognitive evaluations. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
VI. SUMMARY
In this article, we discussed selected works that trace the evolution of pattern recognition approaches in neuroimaging. Our special focus was on the problems that do not fit into the common two-class categorization paradigm. The most recent findings in this area suggest that high-dimensional clustering presents a promising tool for investigating highly heterogeneous diseases with unknown nosological boundaries between subconditions. In the context of aging, the clustering-based approach indicates that in the very early stages cognitive performance declines in a continuous manner. Moreover, cognitively less stable populations seem to be extremely heterogeneous, whereas cognitively more stable individuals form rather homogeneous populations. Additionally, it is possible to derive quantitative image-based indicators of cognitive decline even if categorical labels are not available. On the other hand, high-dimensional pattern regression methodology is appropriate for prediction of continuous and gradual clinical progression from images. It was shown that pattern regression paradigm allows estimating the relationship between the spatial patterns of brain changes and the clinical evaluations. This relationship not only indicates the presence of a disease but also can describe the rapidity of its progression at any time in the future.
In summary, an emerging body of problems in neuroimaging studies require pattern recognition methodologies that go beyond two-class classification paradigm. These new methodologies need not substitute the two-class categorization methods, but rather be a complement that highlights disease properties not captured by commonly used approaches. Although pattern classification shows promising performance in the scenarios where the nosological boundaries of diseases are well established, pattern clustering may help to establish those boundaries, whereas pattern regression links imaging information with the clinical evaluations that form the basis for the diagnosis.
Acknowledgments
Grant sponsor: This work was supported in part by R01AG-14971.
REFERENCES
- Benton A. Revised visual retention test. New York: The Psychological Corporation; 1974. [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- Convit A, de Asis J, de Leon MJ, Tarshish CY, De Santi S, Rusinek H. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in nondementedelderly predict decline to Alzheimer’s disease. Neurobiol Aging. 2000;21:19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Cuingnet R, Gerardin E, Tessieras J, Auzias G, Lehéricy S, Habert MO, Chupin M, Benali H, Colliot O. The Alzheimer’s Disease neuroimaging initiative, Automatic classification of patients with Alzheimer’s disease from structural MRI: A comparison of ten methods using the ADNI database. doi: 10.1016/j.neuroimage.2010.06.013. (accepted for publication in Neuroimage) [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Gene A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–1369. doi: 10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. New York: The Psychological Corporation; 1987. California verbal learning test: Research Edition. [Google Scholar]
- Duchesne S, Caroli A, Geroldi C, Barillot C, Frisoni G, Collins D. MRI-based automated computer classification of probable ad versus normal controls. IEEE Trans Med Imaging. 2008;27:509–520. doi: 10.1109/TMI.2007.908685. [DOI] [PubMed] [Google Scholar]
- Ecker C, et al. Investigating the predictive value of whole-brain structural MR scans in autism: A pattern classification approach. Neuroimage. 2010;49:44–56. doi: 10.1016/j.neuroimage.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Fan Y, Batmanghelich N, Clark CM, Davatzikos C. Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. Neuroimage. 2008a;39:1731–1743. doi: 10.1016/j.neuroimage.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, et al. Unaffected family members and schizophrenia patients share brain structure patterns: A high-dimensional pattern classification study. Biol Psychiatry. 2008b;63:118–124. doi: 10.1016/j.biopsych.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Resnick SM, Wu X, Davatzikos C. Structural and functional biomarkers of prodromal Alzheimer’s disease: A high-dimensional pattern classification study. Neuroimage. 2008c;41:277–285. doi: 10.1016/j.neuroimage.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Shen D, Gur RC, Gur RE, Davatzikos C. COMPARE: Classification of morphological patterns using adaptive regional elements. IEEE Trans Med Imaging. 2007;26:93–105. doi: 10.1109/TMI.2006.886812. [DOI] [PubMed] [Google Scholar]
- Filipovych R, Resnick SM, Davatzikos C. Semi-supervised cluster analysis of imaging data. Neuroimage. 2011;54:2185–2197. doi: 10.1016/j.neuroimage.2010.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox N, Schott J. Imaging cerebral atrophy: normal ageing to Alzheimer’s disease. Lancet. 2004;363:392–394. doi: 10.1016/S0140-6736(04)15441-X. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos E, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas GB, Scheltens P, Rombouts SARB, Visser PJ, Schijndel RAv, Fox NC, Barkhof F. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kloppel S, et al. Automatic classification of MR scans in Alzheimer’s disease. Brain. 2008;131:681–689. doi: 10.1093/brain/awm319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Gaser C, Jäger M, Bottlender R, Frodl T, Holzinger S, Schmitt GJE, Zetzsche T, Burgermeister B. Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage. 2008;39:1600–1612. doi: 10.1016/j.neuroimage.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Misra C, Fan Y, Davatzikos C. Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: Results from ADNI. Neuroimage. 2009;44:1415–1422. doi: 10.1016/j.neuroimage.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham DL, Prince JL. Adaptive fuzzy segmentation of magnetic resonance images. IEEE Trans Med Imaging. 1999;18:737–752. doi: 10.1109/42.802752. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DG, Davatzikos CG. HAMMER: Hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–1439. doi: 10.1109/TMI.2002.803111. [DOI] [PubMed] [Google Scholar]
- Tipping ME. Sparse Bayesian learning and the relevance vector machine. J Mach Learn Res. 2001;1:211–244. [Google Scholar]
- Wang Y, Fan Y, Bhatt P, Davatzikos C. High-dimensional pattern regression using machine learning: From medical images to continuous clinical variables. Neuroimage. 2010;50:1519–1535. doi: 10.1016/j.neuroimage.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]