Abstract
The aim of the present clinical positron emission tomography study was to examine if the 5-HTT is a common target, both for tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs). Serotonin transporter (5-HTT) occupancy was estimated during treatment with TCA, SSRI and mirtazapine in 20 patients in remission from depression. The patients were recruited from out-patient units and deemed as responders to antidepressive treatment. The radioligand [11C]MADAM was used to determine the 5-HTT binding potential. The mean 5-HTT occupancy was 67% (range 28–86%). There was no significant difference in 5-HTT occupancy between TCA (n=5) and SSRI (n=14). 5-HTT affinity correlated with the recommended clinical dose. Mirtazapine did not occupy the serotonin transporter. The results support that TCAs and SSRIs have a shared mechanism of action by inhibition of 5-HTT.
Keywords: 5-HTT, depression, PET, SSRI, TCA
Introduction
The era of pharmacological treatment of depression began with the tricyclic antidepressant (TCA), imipramine, which was found to be effective in the treatment of endogenous depression (Kuhn, 1958). However, the mechanism of action was not known at the time. In 1969, Carlsson and colleagues could show that imipramine and amitriptyline inhibit neuronal uptake of 5-hydroxy tryptamine (5-HT) and norepinephrine in rodents (Carlsson et al. 1969a, b). The authors hypothesized that serotonergic activity may be of key importance for antidepressant effect. Following subsequent demonstrations of TCA binding to 5-HTT (Ross & Renyi, 1969), considerable effort was made to develop selective serotonin reuptake inhibitors (SSRI), of which zimelidine was the first such drug on the market, indeed showing significant antidepressant effect (Montgomery et al. 1980). The effect on central 5-HTT binding in patients in clinical antidepressant treatment has, however, remained largely unknown.
Molecular imaging methods such as positron emission tomography (PET) allow for measurement of drug binding to target proteins in the human brain. Recent advancements include the development of the radioligand [11C]DASB, for selective imaging of 5-HTT (Ginovart et al. 2001). In a clinical study of five different SSRIs, high serotonin transporter occupancy has been demonstrated at low therapeutic dosing (Meyer et al. 2004). Suhara et al. (2003) have used [11C](+)McN5652, a radioligand not ideal for quantitative studies, and demonstrated high serotonin transporter occupancy at low clinical doses of the TCA clomipramine. This finding represents support for the view that TCAs bind to 5-HTT, although the degree of occupancy was difficult to determine.
Our research group has developed the radioligand [11C]MADAM, a close analogue to [11C]DASB. [11C]MADAM binds selectively to 5-HTT (Halldin et al. 2005; Lundberg et al. 2005), has good reliability in measurements of 5-HTT binding in vivo in the human brain (Lundberg et al. 2006) and has been shown to be suitable for occupancy studies (Lundberg et al. 2007a).
The aim of the present PET study in 20 patients responding to antidepressant treatment was to confirm 5-HTT occupancy as a common mechanism of action for widely used antidepressants. Four SSRIs (citalopram, fluoxetine, sertraline and venlafaxine), and two TCAs (amitriptyline and clomipramine) were studied. Mirtazapine was included as a 5-HTT ‘dummy’. SSRI and TCA 5-HTT occupancy was compared and the apparent inhibitory constant expressed by dose (Ki,dose) was calculated.
Method
Subjects
The study was approved by the Ethics Committee of Karolinska Institutet and by the Radiation Safety Committee of the Karolinska University Hospital. Twenty patients (nos. 1–20, six males and 14 females, aged 22–59 yr, mean age 40 yr) on treatment with antidepressants within the recommended clinical dose range were recruited. The patients had been treated from 2 months to >1 yr (13 of the patients had been treated >1 yr). The patients were included after giving informed consent. All patients were responders to their respective antidepressant medication. Each patient had received the clinical diagnosis of major depression. At time of PET all patients but two (no. 5 and no. 12) were in full remission. The partial responders were additionally assessed with the Montgomery–Åsberg Depression Rating Scale score, patient no. 5 having 23 points out of 54 (self-rating) and patient no. 12 scoring 22 out of 60 [as rated by one of the authors (M. T.)], at time of PET measurement. Except for psychiatric history, the patients were essentially healthy, based on medical history, physical examination, blood and urine analysis and magnetic resonance imaging (MRI, Signa, 1.5 T; GE Healthcare, UK) of the brain. The patients were on monotherapy and thus not treated with any concomitant potentially serotonergic drugs.
Since the patients were recruited among responders to antidepressant treatment, baseline 5-HTT binding data could not be obtained. Binding potential (BP) values were instead obtained from a reference group consisting of 26 comparison subjects, aged 21–55 yr (all males, mean age 27 yr), assembled from earlier studies (Lundberg et al. 2005, 2006, 2007a, b). The average BP of [11C]MADAM in putamen in the reference group was 1.40 (range 0.86–2.40, s.d.=0.36).
Radiochemistry
[11C]MADAM was synthesized as described previously (Tarkiainen et al. 2001). The radioactivity injected i.v. ranged between 283 and 335 MBq. The specific radioactivity of the radioligand injected varied between 576 and 90 452 Ci/mmol, corresponding to an injected mass between 0.02 and 3.83 μg (n=15). In five of the PET measurements, analysis of specific radioactivity failed for technical reasons (nos. 1, 3, 5, 9, 10).
PET experimental procedure
Each patient was examined once with PET and the radioligand [11C]MADAM. The time between the latest dose of medication and the PET examination was at least 3 h.
The PET system used was ECAT EXACT HR 47 (Siemens, Germany), which was run in 3D mode. The inplane and axial resolution was ~3.8 and 4.0 mm, respectively, full-width at half maximum (FWHM).
In each PET measurement the subject was placed recumbent with their head in the PET system. A head fixation system with an individual plaster helmet was used (Bergström et al. 1981). A sterile physiological phosphate buffer (pH 7.4) solution containing [11C]MADAM was diluted with saline to the volume of 10 ml and then injected as a bolus for 2 s into a cannula inserted into an antecubital vein. The cannula was then immediately flushed with 10 ml saline.
Brain radioactivity was measured in a series of consecutive time-frames for 93 min. The frame sequence consisted of three 1-min frames, four 3-min frames and 13 6-min frames. After correction for attenuation, random and scatter events, images were reconstructed using a Hann filter (2 mm FWHM). The reconstructed volume was displayed as 47 horizontal sections with a centre-to-centre distance of 3.125 mm and a pixel size of 2.02×2.02 mm2.
Plasma concentrations
Blood samples to measure concentration in plasma of the respective antidepressant were obtained at 1 h before, at start, after 45 min and by the end of the PET measurement at 93 min. The plasma concentrations of each antidepressant, except mirtazapine, were determined through analysis based on chromatography with either high-performance liquid chromatography or liquid chromatography mass spectrometry, at the Karolinska University Laboratory, Department of Clinical Pharmacology. Serum mirtazapine concentration was measured at the Department of Clinical Pharmacology, Lund University Hospital, Sweden.
Image analysis
To align the PET and MRI datasets, co-registration was done according to the procedure described by Lundberg et al. (2007a) .
Regions of interest (ROIs) were defined manually by the first and second author (J.L. and M.T.), according to anatomical boundaries for the putamen and cerebellum as previously described (Lundberg et al. 2007a). All ROIs were delineated in five consecutive sections on the magnetic resonance images and transferred to the corresponding reconstructed and co-registered PET images, using an automated procedure.
The data for each ROI were pooled in order to obtain the average radioactivity for the whole volume of interest. Regional radioactivity was calculated for each frame, corrected for decay and plotted vs. time, thus providing time-activity curves for each region. For bilateral ROIs, right and left regions were averaged.
Regional BP values for [11C]MADAM binding were calculated with the simplified reference tissue model (Lammertsma & Hume, 1996), using cerebellum as the reference region, as has been described previously (Lundberg et al. 2005). PMOD software (PMOD Group, Switzerland) was used to calculate the estimated BP values.
5-HTT occupancy was calculated according to equation (1):
| (1) |
where BPtreatment refers to the BP value in the putamen for patients treated with an antidepressant and BPreference is the average BP for [11C]MADAM in the putamen of the reference group.
Assuming that there is a linear relationship between dose and drug concentration in the brain, Ki,dose, the dose at which half of the serotonin transporters are occupied, may be derived from equation (2):
| (2) |
where occmax is the assumed maximal occupancy (100%) and ‘dose’ is expressed as the daily dose (mg) of the drug. The correlation between Ki,dose and recommended clinical dose (L.D. Electronic Medicines Compendium, 2010) was tested.
Statistics
Non-parametric tests were used to compare occupancy values and Ki,dose of different treatment groups, since the samples were considered too small to be assumed to be normally distributed. All statistical analyses were conducted using PASW Statistics 18 for Windows (SPSS Inc., USA).
Results
All 20 patients in the study participated according to protocol. At time of PET, blood samples were obtained for determination of drug concentration in plasma or serum. Drug concentration values were obtained for all but one patient (no. 1), for which the amitriptyline concentration was below the lower limit of quantification.
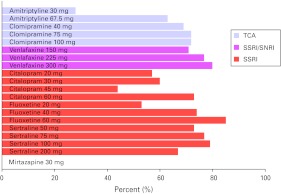
For all drugs and all doses, except for mirtazapine (subject no. 20), the BP was lower in the putamen of the treated patients than of the reference group. 5-HTT occupancy at the serotonin transporters was calculated according to equation (1) (Table 1). The mean occupancy for subjects nos. 1–19 was 67%, range 28–86% (Fig. 1, Table 1). The 5-HTT occupancy did not differ significantly between drugs (independent samples Kruskal–Wallis Test: significance=0.128). The occupancy was numerically lower for TCA than SSRI (mean 61% vs. 70%, median 69% vs. 73%), albeit not significantly different (independent samples Kruskal–Wallis test: significance=0.165).
Table 1.
Treatment characteristics
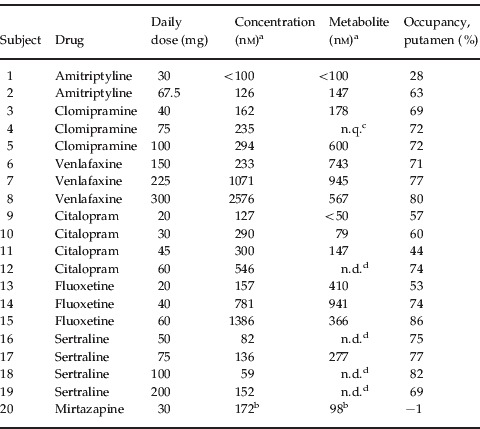
Measured in plasma at time of positron emission tomography (PET).
Measured in serum at time of PET.
Not quantifiable since 2/4 samples contained <100 nm.
Not determined.
Figure 1.
Histogram illustrating the relationship between drug, daily dose and 5-HTT occupancy. TCA, tricyclic antidepressant; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor.
The 5-HTT affinity varied five-fold between the examined antidepressants (Ki,dose; 14–66 mg in range; independent samples Kruskal–Wallis Test: significance=0.05). The affinity for each drug correlated with the corresponding recommended clinical dose (Spearman's ρ=0.93, significance <0.05, data not shown; L.D. Electronic Medicines Compendium, 2010).
Discussion
Earlier PET studies have demonstrated 5-HTT occupancy in the brain of patients treated with SSRIs (Meyer et al. 2001, 2004) and clomipramine (Suhara et al. 2003). Our results, including the two TCAs amitriptyline and clomipramine, further corroborate 5-HTT as a common target for antidepressant drug action. There was no significant difference in 5-HTT occupancy between the five patients treated with TCA and the 14 patients on SSRI treatment. In addition, the statistically significant correlation between estimated affinity in vivo and recommended clinical dose provides further support for 5-HTT occupancy as a common mechanism of action for antidepressant drugs.
On the basis of data from one of the previous PET studies on antidepressant treatment, the authors have suggested 80% 5-HTT occupancy as a required minimum for SSRI treatment of depressive episodes (Meyer et al. 2004). In our sample of patients in remission from depression, 5-HTT occupancy was significantly lower than the proposed 80% level (61–74%, 95% confidence intervals; Fig. 1, Table 1).
The lack of effect of mirtazapine on the serotonin transporter serves as a reminder of other antidepressant modes of action. The norepinephrine transporter (NET) has since been proposed as a target for TCAs, as well as for more recently developed selective NET inhibitors such as reboxetine. Inhibition of NET has been suggested as an explanation for the higher efficacy of TCAs in the treatment of major depressive disorder, reported for at least a subgroup of patients (Anderson, 1998). So far, NET occupancy of the TCA clomipramine has been examined in detail in non-human primates (Takano et al. 2011) and occupancy of nortriptyline has been confirmed in vivo in the brain of human control subjects (Sekine et al. 2010). However, quantification of NET occupancy in vivo in clinical antidepressive treatment remains to be examined in future studies.
In the present study on a small number of patients on each drug there are several methodological considerations. The putamen was chosen as the index region for 5-HTT occupancy for a number of reasons. The putamen is part of the striatum, which has been used as the index region in previous studies of 5-HTT occupancy (Meyer et al. 2001, 2004). Moreover, the test–retest reproducibility of [11C]MADAM binding has been shown to be better in the putamen than in smaller and less homogeneous structures (Lundberg et al. 2006). In addition, although serotonin transporter BP has been shown to be lower in several brain regions of patients examined during a major depressive episode, no significant difference in BP in the putamen has been reported (Meyer, 2007).
The study design with a reference group was chosen mainly to allow for recruitment of psychiatric patients in stable, long-term treatment with antidepressants. Discontinuation of treatment in order to acquire individual baseline 5-HTT BP values would, in many cases, have been unethical. Instead, an estimate for the baseline BP value was obtained from a reference group (cf. Method). Due to inter-individual variability in baseline 5-HTT BP (Lundberg et al. 2006), the approach using a reference value may correspond to a few percent error in individual occupancy values as previously discussed for D2 dopamine receptor occupancy (Farde et al. 1992).
The mean age of the patients, 40 yr, was 13 yr higher than that of the reference group. Age-related decrease in 5-HTT density has been suggested (Hesse et al. 2003). In our present data, we obtained lower 5-HTT occupancy than that previously reported by another PET centre (Meyer et al. 2004). This difference between centres cannot be explained by a potential age effect on 5-HTT binding since the age effect would yield higher occupancy estimates.
The naturalistic recruitment setting led to a selection bias, primarily including patients who tolerated the medication well. In relation to the recommended daily dose, the doses of the TCAs, and in particular amitriptyline, were relatively low when compared to the highest doses in the SSRI group. It is not known whether the low dose of amitriptyline in patient no. 1 was a result of titration with regard to clinical effect or to side-effects.
The subjects enrolled in the study had been treated for at least 2 months, or >1 yr for the majority of the patients. In rodent studies, a decrease in 5-HTT density has been reported after 3 wk of SSRI administration (Benmansour et al. 1999). It is not known if 5-HTT density in human subjects is down regulated after repeated dosing. The occupancy values reported here are not different from the occupancy values obtained in an earlier study using the same PET protocol in control subjects after a single dose of 20 mg R,S-citalopram or 10 mg S-citalopram (Lundberg et al. 2007a). It is thus not likely that a suggested treatment effect on 5-HTT density may be large enough to have a major impact on the estimated occupancy values.
For antipsychotic drugs, it is possible to relate D2 dopamine-receptor occupancy to clinical effects and extrapyramidal symptoms. An important question is if 5-HTT occupancy in a similar fashion can provide guidelines to optimal clinical treatment with antidepressants. The serotonergic side-effects of antidepressants are generally not as observable as seen in antipsychotic drug treatments. Sexual side-effects are perhaps the major threat to long-term compliance and a future possibility would be to study 5-HTT occupancy in relation to SSRI dose, clinical effect and sexual dysfunction in patients on antidepressive treatments.
In conclusion, this study provides further support for 5-HTT as a shared target for antidepressive treatments with TCAs and SSRIs.
Acknowledgements
The study was supported by the Swedish Science council (41804), AFA sjukförsäkrings jubileumsstipendier (AFA Insurance), Karolinska Institutet, the Stockholm Centre for Psychiatric Research and Education, and through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet. The subjects are gratefully acknowledged for generously volunteering in the study. The out-patient clinics involved in recruiting patients are acknowledged, in particular Serafen´s psychiatric out-patient clinic and Gustavsbergs vårdcentral. Finally we acknowledge all our colleagues at the Karolinska PET unit.
Statement of Interest
Professor Farde also holds a position as Chief Scientist, iMed CNS/Pain, AstraZeneca, Sweden. Professor Landén has served on advisory boards for AstraZeneca and Lundbeck pharmaceuticals, and received one research grant from AstraZeneca.
References
- Anderson IM. SSRIs vs. tricyclic antidepressants in depressed inpatients: a meta-analysis of efficacy and tolerability. Depression and Anxiety. 1998;7:11–17. [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA. et al. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. Journal of Neuroscience. 1999;19:10 494–10 501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström M, Boëthius J, Eriksson L, Greitz T. et al. Head fixation device for reproducible position alignment in transmission CT and positron emission tomography. Journal of Computer Assisted Tomography. 1981;5:136–141. doi: 10.1097/00004728-198102000-00027. [DOI] [PubMed] [Google Scholar]
- Carlsson A Corrodi H Fuxe K Hökfelt T 1969aEffect of antidepressant drugs on the depletion of intraneuronal brain 5-hydroxytryptamine stores caused by 4,α-dimethyl-meta-tyramine European Journal of Pharmacology 5357–366. [DOI] [PubMed] [Google Scholar]
- Carlsson A Corrodi H Fuxe K Hökfelt T 1969bEffects of some antidepressant drugs on the depletion of intraneuronal brain catecholamine stores caused by 4,α-dimethyl-meta-tyramine European Journal of Pharmacology 5367–373. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordström A-L, Wiesel F-A, Pauli S. et al. Positron emission tomographic analysis of central D1 and D2 receptor occupancy in patients treated with classical neuroleptics and clozapine. Archives of General Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Wilson AA, Meyer JH, Hussey D. et al. Positron emission tomography quantification of [11C]-DASB binding to the human serotonin transporter: modeling strategies. Journal of Cerebral Blood Flow and Metabolism. 2001;21:1342–1353. doi: 10.1097/00004647-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Halldin C, Lundberg J, Sóvágó J, Gulyás B. et al. [11C]MADAM, a new serotonin transporter radioligand characterized in the monkey brain by PET. Synapse. 2005;58:173–183. doi: 10.1002/syn.20189. [DOI] [PubMed] [Google Scholar]
- Hesse S, Bartel H, Murai T, Muller U. et al. Is correction for age necessary in neuroimaging studies of the central serotonin transporter. European Journal of Nuclear Medicine and Molecular Imaging. 2003;30:427–430. doi: 10.1007/s00259-002-1044-6. [DOI] [PubMed] [Google Scholar]
- Kuhn R. The treatment of depressive states with G 22355 (imipramine hydrochloride) American Journal of Psychiatry. 1958;115:459–464. doi: 10.1176/ajp.115.5.459. [DOI] [PubMed] [Google Scholar]
- L.D. Electronic Medicines Compendium. 2010. http://www.medicines.org.uk. http://www.medicines.org.uk ) Communications ( ). Accessed.
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lundberg J Borg J Halldin C Farde L 2007bA PET study on regional coexpression of 5-HT1A receptors and 5-HTT in the human brain Psychopharmacology 195425–433. [DOI] [PubMed] [Google Scholar]
- Lundberg J, Halldin C, Farde L. Measurement of serotonin transporter binding with PET and [11C]MADAM: a test-retest reproducibility study. Synapse. 2006;60:256–263. doi: 10.1002/syn.20297. [DOI] [PubMed] [Google Scholar]
- Lundberg J, Odano I, Olsson H, Halldin C. et al. Quantification of 11C-MADAM binding to the serotonin transporter in the human brain. Journal of Nuclear Medicine. 2005;46:1505–1515. [PubMed] [Google Scholar]
- Lundberg J Stroyer Christophersen JS Buchberg Petersen K Loft H et al. 2007aPET measurement of serotonin transporter occupancy: a comparison of escitalopram and citalopram International Journal of Neuropsychopharmacology 10777–785. [DOI] [PubMed] [Google Scholar]
- Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. Journal of Psychiatry and Neuroscience. 2007;32:86–102. [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V. et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [11C]DASB PET imaging study. American Journal of Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D. et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. American Journal of Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Rani J, McAuley R, Roy D. et al. The antidepressant efficacy of zimelidine and maprotiline. Acta Psychiatrica Scandinavica. 1980;290:219–224. doi: 10.1111/j.1600-0447.1981.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Ross SB, Renyi AL. Inhibition of the uptake of tritiated 5-hydroxytryptamine in brain tissue. European Journal of Pharmacology. 1969;7:270–277. doi: 10.1016/0014-2999(69)90091-0. [DOI] [PubMed] [Google Scholar]
- Sekine M, Arakawa R, Ito H, Okumura M. et al. Norepinephrine transporter occupancy by antidepressant in human brain using positron emission tomography with (S,S)-[18F]FMeNER-D2. Psychopharmacology. 2010;210:331–336. doi: 10.1007/s00213-010-1824-9. [DOI] [PubMed] [Google Scholar]
- Suhara T, Takano A, Sudo Y, Ichimiya T. et al. High levels of serotonin transporter occupancy with low-dose clomipramine in comparative occupancy study with fluvoxamine using positron emission tomography. Archives of General Psychiatry. 2003;60:386–391. doi: 10.1001/archpsyc.60.4.386. [DOI] [PubMed] [Google Scholar]
- Takano A, Nag S, Gulyas B, Halldin C. et al. NET occupancy by clomipramine and its active metabolite, desmethylclomipramine, in non-human primates in vivo. Psychopharmacology. 2011;216:279–286. doi: 10.1007/s00213-011-2212-9. [DOI] [PubMed] [Google Scholar]
- Tarkiainen J, Vercouillie J, Emond P, Sandell J. et al. Carbon-11 labelling of MADAM in two different positions: a highly selective PET radioligand for the serotonin transporter. Journal of Labelled Compounds and Radiopharmaceuticals. 2001;44:1013–1023. [Google Scholar]