Abstract
Bacterial flagella are naturally-occurring self-assembling protein nanofibers protruding from the bacterial surface to assist the swimming of bacteria. They are rigid and exhibit diverse morphologies depending on the ionic strength, the pH values, temperature, and subunit sequences. Here, the silica nanotubes (SNTs) with controllable morphologies were synthesized using flagella as biological templates in aqueous solution under mild conditions. The morphologies and surface features of flagella-templated SNTs can be simply tuned by adjusting the pH value or surface chemistry of flagella by peptide display. A variety of different morphologies (coiled, straight, and curly with different wavelengths) and surface features (smooth, rough, granular and pear-necklace-like) of SNTs were obtained. When pH varies from acidic to alkaline conditions, in general, SNTs varied from bundled coiled, to characteristic sinusoidal waves, helical, and straight morphology. Under genetic control, flagella displaying negatively-charged peptides exhibited thinner layer of silica condensation but rough surface. However, flagella with positively-charged peptide inserts induced the deposition of thicker silica shell with smooth surface. Incorporation of hydroxyl bearing amino acid residues such as Ser into the peptide displayed on flagella highly enhanced the biotemplated deposition of silica. This work suggests that bacterial flagella are promising biotemplates for developing an environmentally-benign and cost-efficient approach to morphology-controlled synthesis of nanotubes. Moreover, the dependency of the thickness of the silica shell on the peptides displayed on flagella helps us to further understand the mechanism of biomimetic nucleation of silica on biological templates.
Introduction
Silica nanotubes (SNTs) have gained extensive attention in the last few decades because of their intrinsic biocompatibility, hydrophilic nature and customizability via simple chemical modifications.1,2 SNTs have potential applications as chemosensors, adsorbents,3 biosensors4 and nanoscale reactors5 as well as in areas such as biospearation, biocatalysis2,6 and gene delivery.7 Among the various strategies and techniques that have been employed to synthesize SNTs, the template-directed synthesis of SNTs exhibits some advantages. For example, the dimensions and morphology of the SNTs can be finely controlled through the precise manipulation of the templates. The thickness of the silica wall can also be controlled by tuning the concentration of a precursor solution such as tetraethoxysilane (TEOS) and the reaction time. Moreover, the templates can be removed by extraction, heat treatment or chemical reactions. SNTs with distinct morphologies such as straight, helical, curly, chiral, double chiral and pearl-necklace-like have all been developed based on various templates.8–12 Recently, it was found that carbon nanotubes with curly morphology are safer than straight ones, suggesting that morphology control of nanotubes is important in biomedical applications.13
A variety of inorganic, organic, or biomolecular templates have been used for the synthesis of SNTs.2 In nature, there are great sources of biological systems with well-defined structures and complex morphologies which are potentially suitable as templates for the fabrication of inorganic materials. Tobacco mosaic virus (TMV), DNA, peptide and collagen were used as templates for the synthesis of SNTs.14–18 Hybrid organic-inorganic SNTs with multiple layers were generated using a lipid template.19 However, the morphology of SNTs is limited to the availability of the templates. Most of the above templates do not have specific controllable morphologies, resulting in SNTs with different morphologies under one synthetic condition.
The morphology controlled synthesis of SNTs induced under different pH values on flagella template has never been reported (Scheme I). Bacterial flagella are naturally-occurring protein nanofibers protruding from the bacteria surface. The flagellum has an outer diameter of 12–25 nm and up to several micrometers, which is self-assembled from more than 20,000 copies of identical major protein subunits-flagellins (FliC) and some minor proteins.20 Flagella are very rigid with a modulus of rigidity about 1 × 1011 dyn/cm2.21 At neutral pH, they exhibit helical structures. When dried on a substrate, they are flattened to a curly morphology with a sinusoidal wave. The wavelength is constant for each specific strain. Some mutant strains can give rise to different wavelength or even a straight morphology.22 Moreover, flagella can exhibit various stable conformations depending on their environment and constituent monomers such as pH value, salt concentration, temperature, and the ratio of monomers copolymerized from different strains, a property termed “flagellar polymorphism”.22,23 By tuning any of these conditions, various morphologies of flagella can be obtained.24–27
Scheme 1.
Schematic illustration of flagella structure and SNTs synthesis on flagella template. (a) Flagellum structure: Flagellum is a nanotube which is assembled from a monomer called FliC. The surface-exposed potion (D2&D3 domains) can be genetically engineered by insertion of a foreign peptide. (b) Templated nucleation of silica on flagella surface: Morphology of flagella can be controlled under different pH values and then the resultant SNTs exhibit the morphology of the flagella.
Interestingly, the N- and C- terminal regions (D0 and D1 domains) of FliC located in the center of flagellum are highly conserved. In contrast, the central region (D2 and D3 domains, Scheme I) of FliC is highly variable and surface-exposed. Some of sequences in this region can be deleted or replaced with foreign sequence without losing the self-assembly properties.28 As a consequence, the surface chemistry of flagella can be modified by displaying functional groups on the surfaces via a genetic approach.28 In this work, we take advantage of stable homogeneous conformation of flagella under specific condition and helical morphological variations by tuning any conditions that affect the flagellar morphology. For example, by turning the pH value, flagella should exhibit uniform morphology at specific pH value but distinct morphologies between different pH values. Thus, the resultant SNTs on the flagellar template would also exhibit corresponding morphology at a set pH value and changed to another kind of morphology at a different pH value (Scheme 1). Moreover, the surface features of SNTs are also modified by varying the pH values.
Experimental
Peptide display and flagella purification
The oligonucleotides which encode target peptide with complementary sequences were designed and synthesized (Invitrogen). The primers and encoded peptide sequences are summarized in Table 1. The oligonucleotides also contain Xho I and Bgl II sticky ends at the 5′-end and 3′-end, respectively, and were annealed as double-stranded DNA. The resultant double stranded oligos were then inserted into vector pLS411 which contains flagellin gene with a multi-cloning site in the central region. The recombinant vector was transformed into competent cell of salmonella SL5928, a flagellin gene knock-out stain. Individual colonies were inoculated on agar plate with ampicillin (AMP) (100 μg/mL) and the plasmids were confirmed by DNA sequencing (MCLAB). The recombinant strains were cultured in LB media with AMP (100 μg/mL) at 37 °C with shaking (250 rpm) until optical density (OD) reached 0.6–0.8. The cell culture was collected and centrifuged at 6000 g for 20 min at 15°C. The pellets were then washed twice with phosphate buffered saline (PBS) buffer (pH=7.4). Finally, the cell pellet was resuspended in deionized water and cooled in an ice-bath. The flagella were detached from the cells by vortex mixer at the highest speed for 3 min and then subjected to centrifugation at 6000 g for 20–30 min to remove bacteria. The supernatant containing detached flagella was collected. The supernatant was centrifuged again at 10,000 g for 20 min to remove debris from solution. High purity flagella were precipitated by ultra-centrifugation at 80,000 g for 2 h. Finally, the flagella were dissolved in deionized water and kept at −20 °C for further usage.
Table 1.
Primer sequences, the corresponding name and peptide sequences displayed on flagella.
| Name | Oligonucleotides | Encoded peptide sequences |
|---|---|---|
| E8+C+S8 | 5′ –GA TCT GAA GAG GAA GAG GAA GAG GAA GAA TGT AGT AGC AGT AGC AGT AGC AGT AGC C-3′ 5′ –TC GAG GCT ACT GCT ACT GCT ACT GCT ACT ACA TTC TTC CTC TTC CTC TTC CTC TTC A-3′ |
EEEEEEEECSSSSSSSS --------*OOOOOOOO |
| E8CS8 | 5′ –GA TCT GAA AGT GAG AGC GAA AGT GAG AGC TGT GAA AGT GAG AGC GAA AGT GAA AGC C-3′ 5′ –TC GAG GCT TTC ACT TTC GCT CTC ACT TTC ACA GCT CTC ACT TTC GCT CTC ACT TTC A-3′ |
ESESESESCESESESES -O-O-O-O*-O-O-O-O |
| D8CS8 | 5′ –GA TCT GAT AGT GAC AGC GAT AGT GAC AGC TGT GAT AGT GAC AGC GAT AGT GAC AGC C-3′ 5′ –TC GAG GCT GTC ACT ATC GCT GTC ACT ATC ACA GCT GTC ACT ATC GCT GTC ACT ATC A-3′ |
DSDSDSDSCDSDSDSDS -O-O-O-O*-O-O-O-O |
| GPP8 from collagen | 5′ –GA TCT GGA CCA CCT GGT CCA CCT GGT CCT CCA GGT CCA CCT GGA CCA CCT GGT CCA CCT GGT CCT CCA GGT CCA CCT C -3′ 5′ –TC GAG AGG TGG ACC TGG AGG ACC AGG TGG ACC AGG TGG TCC AGG TGG ACC TGG AGG ACC AGG TGG ACC AGG TGG TCC A-3′ |
GPPGPPGPPGPPGPPGPPGPPGPP ************************ |
| N-terminal of collagen | 5′ –GA TCT CAG CTG TCT TAT GGC TAT GAT GAG AAA TCA ACC GGA GGA ATT TCC GTG CCT C-3′ 5′ –TC GAG AGG CAC GGA AAT TCC TCC GGT TGA TTT CTC ATC ATA GCC ATA AGA CAG CTG A-3′ |
QLSYGYDEKSTGGISVP ^*OO*O--+OO***O** |
| C-terminal of collagen | 5′ –GA TCT CAG CGC TGG TTT CGA CTT CAG CTT CCT GCC CCA GCC ACC TCA AGA GAA GGC TCA CGA TGG TGG CCG CTA C-3′ 5′ –TC GAG TAG CGG CCA CCA TCG TGAGCC TTC TCT TGA GGT GGC TGG GGC AGG AAG CTG AAG TCG AAA CCA GCG CTG A-3′ |
SAGFDFSFLPQPPQEKAHDGGRYYRA O***-*O***^**^-+*^-**+OO+* |
| E8 | 5′ –GA TCT CGA GGT GAT GAA GAG GAA GAG GAA GAG GAA GAA C-3′ 5′ –TC GAG TTC TTC CTC TTC CTC TTC CTC TTC ATC ACC TCG A-3′ |
EEEEEEEE -------- |
| N-Zone of collagen | 5′ –GA TCT GGT TTG GAT GGT GCC AAG GGA GAT GCT GGT CCT GCT GGT CCT AAG GGT GAG CCT GGC AGC CCT GGT GAA AAT C-3′ 5′ –TC GAG ATT TTC ACC AGG GCT GCC AGG CTC ACC CTT AGG ACC AGC AGG ACC AGC ATC TCC CTT GGC ACC ATC CAA ACC A-3′ |
GLDGAKGDAGPAGPKGEPGSPGEN **-**+*-******+*-**O**-^ |
| C-Zone of collagen | 5′ –GA TCT GGA CCC CAA GGC CCA CGT GGT GAC AAG GGT GAG ACA GGC GAA CAG GGC GAC AGA GGC ATA AAG GGT CAC CGT C-3′ 5′ –TC GAG ACG GTG ACC CTT TAT GCC TCT GTC GCC CTG TTC GCC TGT CTC ACCCTT GTC ACC ACG TGG GCC TTG GGG TCC A-3′ |
GPQGPGDGETGEQGDRGIKGHR **^***-*-**-^*-+**+*^+ |
| KGG4 | 5′ –GA TCT AAA GGC GGT AAA GGC GGT AAA GGC GGT AAA C-3′ 5′ –TC GAG TTT ACC GCC TTT ACC GCC TTT ACC GCC TTT A-3′ |
KGGKGGKGGKGG +**+**+**+** |
| KS | 5′ –GA TCT AGC AGC AAA AAG AGC GGT AGC TAC AGC GGT AGC AAA GGT AGC AAA C -3′ 5′ –TC GAG TTT GCT ACC TTT GCT ACC GCT GTA GCT ACC GCT CTT TTT GCT GCT A-3′ |
SSKKSGSYSGSKGSK OO++O*O^O*+*O+ |
Amino acids analysis of the peptides displayed on flagella: * nonpolar, ^ polar, O amino acid with hydroxyol group, − negatively charged residue, + positively charged residue.
Coating of silica on flagella templates
Coating of silica on flagella templates used the methods we previous described12 with some modifications. Briefly, APTES (2×10−3 mmol) was mixed and agitated gently with flagella solution (500 mL) by vortex mixer. Then, the pH value of the solution was adjusted to a set value with HCl or NaOH and cooled in an ice-water bath for 3–5 min. TEOS (2.5×10−2 mmol) was added while stirring for at least 3 min. The mixed solution was left in ice-water bath for ~30 min and then aged at room temperature for 8 h. Because the condensation of TEOS is slow at the acidic conditions, longer aging time (24 h) was applied to solutions of lower pH values. The white precipitates were centrifuged at 5000 g for 10 min and washed with ethanol and water several times. The samples were mounted on TEM grids and subjected to examination by TEM (Zeiss 10).
Results and Discussion
Peptides displayed on flagella and coating of silica on flagella template
All bioengineered strains were sequenced at each step to confirm the identity of the inserted sequences. Some of engineered strains were under-expressed. That is, short and less flagella were grown on each bacterium. These strains were then inoculated on a semi-solid media to select the most motile bacteria swimming to the edge of the plate with maximum flagella expression. Finally, 11 different peptides were successfully displayed on the surface of flagella and listed in Table 1.
Coating of silica on flagellar template was carried out following our pervious description in aqueous solution under ambient conditions.12 Aminopropyltriethoxysilane (APTES) was first mixed with flagella and followed by adding TEOS. The APTES should play dual roles in directing the in situ transcription of silica nanotubes on the flagellar surface. First, according to the so-called “surface mechanism”, APTES was adsorbed on the surface of flagella through hydrogen bonding or electrostatic interactions between its amino group and the biotemplate surface.29 The APTES adsorbed on flagellar surface was then hydrolyzed, giving rise to –Si-OH groups which could serve as nuclei for silica growth upon the addition of TEOS. Then, the nuclei could mediate the hydrolysis and condensation of TEOS, resulting in the formation of silica shell. Second, as a weak base, the addition of APTES increased the pH value to 10.40, which in turn, promoted hydrolysis of TEOS. The deposition of silica on flagellar template depends on the absolute hydrolysis and condensation rates of TEOS as well as the relative rate between these two reactions.30 Hydrolysis occurs in both acidic and alkaline conditions, but the rate is lowest at pH=7.0. The condensation reaches minimum at pH value of about 2.0 and continue to increase from acidic to alkaline conditions. However, when the pH value is over 11, the rate of condensation of TEOS decreases again.31
Effects of different pH values on morphology controlled synthesis of SNTs
Because bacterial flagella are stable at pH=2–10,25 the effects of the pH value ranging from 3–11.5 on the morphology control of SNTs were evaluated. As expected, at pH≤3 or pH>11.5, no tubular structures could be observed due to the depolymerization of flagella (Fig. S1). In acidic conditions (pH=3.5–4), bundled SNTs with multi-channels appeared with a thin layer of silica nanoparticles coated on the flagellar surface and these SNTS exhibited curly or coiled morphology. At pH=2–5, hydrolysis of TEOS is favorable but condensation is the rate-determining step.31 Low rate condensation of the hydrolyzed TEOS on flagella resulted in the thin layer of silica coating. A higher proportion of SNTs with curly morphology was observed at pH=3.5. Interestingly, there was a great amount of coiled silica bundles at pH=4 (Fig. 1a, b). Because the isoelectric point of wild-type flagella is 5.3,32 the electrostatic interactions between APTES and flagella were considerably attenuated at lower pH values. In these acidic conditions, TEOS may condense directly on the flagellar surface. The flagellar filaments were bundled together by condensation reactions between hydroxyl anions on neighboring flagella (Scheme S1). Actually, we also observed similar results of SNTs using TEOS without APTES at the pH value below 5.3. At pH=5–7, only a very thin layer of silica coated on flagellar surface, indicating low condensation of TEOS (Fig. S1). These results are consistent with previous reports on silica formation.31,33
Fig. 1.
TEM images of the SNTs on wild-type flagella template at different pH values. (a) Curly and coiled bundled SNTs at pH=4. (b) Higher magnification of bundled SNTs coated with a very thin layer of silica. (c) SNTs with sinusoidal waves and coated with a thin layer of silica at pH=8. The flagella before coating silica have similar morphology indicating the in situ transcription of SNTs from original flagella (inset). (d) SNTs with shorter wavelength at pH=9. (e) Curly and coiled monodisperse SNTs at pH=10.5. (f) Close to straight SNTs at pH=11. (g) Typical high magnification images taken from the same samples shown in a-f, respectively. (Scale bar of b, c, d, e, f: 400 nm)
In alkaline conditions (pH=8–11.5), the poly-condensation of TEOS is more favorable.31 After TEOS was added to the solution, a white precipitate appeared in 15 min, indicating the formation of silica. At a pH value higher than 11.5, the precipitates could be observed in several minutes. Conversely, it took about 24 h in acidic conditions (pH=3–4) to form white precipitates, and at the pH value lower than 3, no precipitate could be observed (Fig. S2). At pH=8, silica condensation occurred simultaneously on flagella and in solution. A thin layer of silica particles was nucleated on the surface of flagella, resulting in a relatively uniform but rough appearance (Fig. 1c). There were also some free silica nanoparticles in the solution. Most of the SNTs exhibited the characteristic sinusoidal wavelength which revealed the in situ transcription on flagella template. When the pH value was increased to 9, the SNTs also showed sinusoidal wave morphology but with a shorter wavelength (Fig. 1d). Some free silica nanoparticles were also formed off the flagella. At pH=10, almost all of the SNTs exhibited curly or coiled morphology (Fig. S1). At pH=10.5, most SNTs exhibited coiled morphology (Fig. 1e). At pH=11, both slightly curly and straight morphologies of SNTs were observed (Fig. 1f). Some free spherical silica nanoparticles condensed at the intersections of SNTs. At pH=11.5 and 12, the flagella became unstable. Most flagella depolymerized and degraded into short fragments with straight morphology and coated with a layer of granular silica particles (Fig. S1). At the same time, a large number of spherical silica nanoparticles ranging from 40–300 nm with meso-structured interiors were observed because of wrapping the fragments inside. This structure is very similar to the tobacco mosaic virus templated silica nanocomposites as previously reported.34 In alkaline conditions, we believe that the APTES was attached to flagellar surface by electrostatic interactions so the presence of ions can interfere the binding of APTES on flagella. In a control experiment, different concentrations of NaCl were mixed with the flagella following silica coating process. No silica was deposited on the flagella.
Under different pH values, in addition to the morphological changes, the surface of the SNTs also showed distinct features (Fig. 1 inset). At low pH values (pH≤5), the SNTs had smooth inner and outer wall surfaces with uniform inner and outer diameters. In alkaline conditions (pH=8–10.5), thicker silica coated on flagella and the surface of SNTs became rougher with pearl-necklace-like structures. However, at pH=11–11.5, the silica shell became thinner with granular structure. The resultant SNT surface features and the thickness of silica shell can also be fine-tuned by adjusting the concentration of TEOS (original, 5×, or 10×) (Fig. 2). The thickness of silica shell increased with higher concentration of TEOS and the surface of the SNTs became flat.
Fig. 2.
TEM images of SNTs on wild-type flagella template with different surface features and thickness. (a) Original concentration of TEOS. (b) 5× TEOS. (c) 10× TEOS.
pH variation during the synthesis of SNTs
The morphology transformation of flagella at different pH values is rapid and reversible. Almost all flagella exhibited homogeneous morphology at each specific pH value.25 However, we noticed that the morphology of resultant SNTs at a given pH value was not as uniform as that of the biotemplates. In this study, the pH value was adjusted to a setting point before TEOS was added. The pH value changes after poly-condensation of silica on flagellar surface were monitored. Not only did the pH value change after each reaction but the magnitude and direction of each change also varied with different initial pH values. At pH=2 and 7, the pH value slightly increased after the reactions while the pH value changed to the opposite directions in alkaline conditions (Fig. 3). The range of pH changes seems to be related to the amount of precipitated silica (Fig. S2). The changes in the pH value during the reaction are likely caused by APTES as it is the only base in the solution. The variation in the pH value during the reaction might be the reason for the occurrence of some heterogeneous morphology of SNTs. At the early stage of the reaction, the morphologies of some flagella were “fixed” by the initial silica shell but the silica shell may be too thin to fix some other flagella, resulting in some variation of morphologies of SNTs.
Fig. 3.
The pH values before and after coating of silica on flagella template at different initial points.
Morphology and surface feature control of SNTs on bioengineered flagella displaying different functional groups
Morphology controlled synthesis of SNTS on bioengineered flagella was also investigated. Because alkaline condition is more favorable for the condensation of TEOS31,33 and we observed the bioengineered flagella became unstable in acidic condition. SNTs synthesis on flagella displaying negatively charged peptides at normal and pH=11 was studied. SNTs on E8 flagella template showed a slightly curly morphology with pearl-necklace-like surface structures and became straight with granular surfaces at pH=11 (Fig. 4a, b). E8+C+S8 flagella templated SNTs had much thicker diameter with granular structures and most SNTs were built from flagella bundles (Fig. 4c). The displayed cysteine residues on flagellar surfaces induced bundle formation between neighboring flagella by disulfide bonds. At pH=11, bundled SNTs disappeared but exhibited a similar structure as E8 flagella templated SNTs (Fig. 4d). E8CS8 flagella template-based SNTs exhibited smooth surfaces and thicker diameter than those on E8+C+S8 flagella template due to more flagella bundled inside the SNTs (Fig. 4e). Interestingly, at pH=11, many coiled SNT bundles were observed (Fig. 4f). It revealed that the arrangement of amino acids on flagellar surface can also modulate the morphology of flagella. Only a thin layer of granular silica was formed on D8CD8 flagellar surface. Most SNTs were straight and composed of flagella bundles (Fig. 4g). The bundled SNTs became monodisperse at pH=11 but their morphology did not change a lot (Fig. 4h). Because the isoelectric point of aspartic acid (3.8) is lower than that of glutamic acid (4.0), thin silica shell on D8CS8 flagellar surface indicated that negatively charged surface may be less favorable for the condensation of TEOS. The peptide-amphiphile nanofiber with negatively charged surface is also not favorable for the condensation of TEOS.35 However, the introduction of nucleophilic hydroxyl groups (in Serine) promoted the condensation because the hydroxyl group displayed on surface of flagella may mimic the hydroxyl anion to attack the silicon atom by an SN2-Si mechanism.36 On the other hand, the carboxylate groups on flagella surface interacted with amino groups of APTES. We observed that pH value was decreased from ~10.40 to ~9.20 after the flagella displaying negatively charged peptides were mixed with APTES. As a result, the degree of TEOS hydrolysis was decreased.
Fig. 4.
The morphology and surface features of SNTs on negatively-charged flagella templates. (a, b) SNTs on E8 flagella template. (c, d) SNTs on E8+C+S8 flagella template. (e, f) SNTs on E8CS8 flagella template. (g, h) SNTs on D8CS8 flagella template. (a, c, e, g) are at pH=10.4. (b, d, f, h) are at pH=11. (Scale bar: 400 nm)
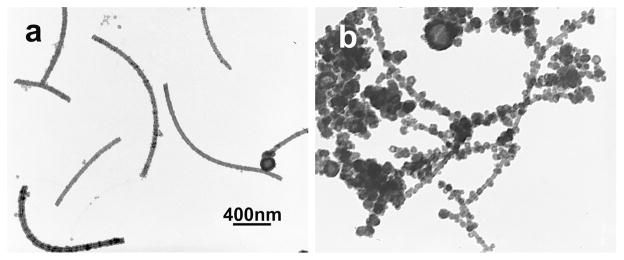
Positively charged lysine or arginine-enriched proteins promoted silica mineralization.37–39 Poly-lysine or histidine and arginine-lysine loop were found to catalyze the silica polymerization.35,40 In this study, thick uniform silica shell but with rough surfaces deposited on flagella displaying lysine residues termed KGG4 (Fig. 5a), which is similar to previous reports on positively charged templates.35,40 However, when serine residues were introduced on the surface of flagella, termed KS, a novel morphology of SNTs was obtained (Fig. 5b), where many silica nanoparticles were nucleated around the nanotubes.
Fig. 5.
The morphology and surface features of SNTs on positively-charged flagella templates (pH=10.4). (a) Morphology of SNTs on KGG4 flagella template. (b) Morphology of SNTs on KS flagella template. (Scale bar: 400 nm)
The ability to induce the deposition of silica may be different between lysine and serine. It is possible that the formation and growth of silica particles are much faster at some nucleation sites on the flagella. After the KGG4 flagella were mixed with APTES and TEOS, the APTES might not interact with lysine residues on the flagella surface directly because they both share amino groups. Deprotonated TEOS with partial hydrolysis gave rise to negatively charged species which then might preferentially interact with the positive lysine residues on flagella. However, the exact mechanism is still unknown.
The morphology of SNTs on flagella displaying nonpolar peptides (GPP8) template was similar to that on wild type flagella template (Fig. S3a). At pH=11 values, however, the deposition of silica on the flagella was severely decreased indicating the polar residues might be important for the condensation of TEOS (Fig. S3b). Flagella displaying N-, C-terminal, N-, C-zone sequences with mixed polar amino acids from type I collagen were also examined for the controlled synthesis of SNTs (Fig. 6). A very thick layer of silica was deposited on the flagella displaying N-terminal template. Relatively thinner and straight SNTs were obtained on the flagella displaying C-terminal template. Both of them exhibited a pearl-necklace-like structure. However, a much thinner layer of silica was formed on flagella displaying N- and C-zone templates. There are 6 and 4 hydroxyl bearing amino acid residues in N-terminal and C-terminal peptides, respectively, but only one and zero hydroxyl residues in N-zone and C-zone peptides, respectively. This data further confirmed that hydroxyl bearing amino acid residues such as Ser could promote nucleation of silica on the flagellar surfaces.
Fig. 6.
Morphology of flagella on flagella displaying mixed polar peptides from type I collagen. (a) SNTs on flagella displaying N-terminal of collagen. (b) SNTs on flagella displaying C-terminal of collagen. (c) SNTs on flagella displaying N-zone of collagen. (d) SNTs on flagella displaying C-zone of collagen. See Table 1 for sequences of these collagen-derived peptides. (Scale bar: 400 nm)
Synthesis of SNTs on other biological template
The in situ transcription of silica shells can also be applied to other biological templates for more different morphologies of SNTs. Here, we synthesized SNTs on a more rigid biological template, type 1 bacterial pili, which are protein nanotubes to assist the adhesion of bacteria to a solid surface.41 The pilus is a protein nanorod with 6–7 nm in diameter and 1–2 μm in length that can be detached from bacterial cells. It is assembled from protein monomers fimA with 27 subunits in eight turns.42 The pili templated SNTs are composed of bundled structures with straight morphology (Fig. S4a). Under a high pH value (pH=11), pili became unstable and broken into fragments and wrapped in spherical silica particles. Interestingly, the fragments were organized radially around a silica core (Fig. S4b inset)
Conclusion
The morphology and surface feature of SNTs on flagella template can be precisely tuned by controlling the pH value, concentration of TEOS, and peptide sequences displayed on the surface of flagella. At low pH values (pH≤4), novel bundled SNTs were obtained with curly or coiled morphology. A layer of tiny nanoparticles was mineralized on the surface of flagella. At higher pH values (pH=8–11.5), the morphology of SNTs exhibited more variations. Curly, coiled, straight, sinusoidal with different wavelength could be observed. The surface feature of SNTs also possessed smooth, rough, pearl-necklace-like and granular structures. High concentration of TEOS led to the formation of thicker and uniform silica on flagella templates. A thin layer of silica was found to form on the negatively charged surface of flagella, suggesting less favorable deposition of silica on such templates. However, when the flagellar surfaces were modified with hydroxyl groups due to the genetic display of serine-containing peptides, the thickness of silica shell was highly increased. Through comparison of the number of hydroxyl residues on flagella displaying N-, C-terminal and N-, C-zone template, we further confirmed the role of hydroxyl bearing amino acid residues in promoting silica deposition. Positively charged surface facilitates the mineralization of silica and the resultant silica shell had uniform thickness. At pH=11, SNTs exhibited distinct morphologies on E8+C+S8 and E8CS8 flagella templates. This indicated the arrangement of peptide sequence also affects the morphology of flagella. Moreover, the bundled SNTs can also be easily obtained by displaying cysteine residues on flagella. We expect that more morphologies of SNTs may be obtained by inserting other peptide sequences on flagellar surface. This flagella-based biotemplating method can also be applied to other biological templates such as pili to obtain straight SNTs. The current work opened up a new approach toward the facile fabrication of SNTs with controllable morphologies and surface features.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (DMR-0847758, CBET-0854414 and CBET-0854465). We also thank the financial support from National Institutes of Health (5R01HL092526-02, 5R21EB009909-02, 4R03AR056848-03), Oklahoma Center for the Advancement of Science and Technology (HR11-006) and Oklahoma Center for Adult Stem Cell Research.
Footnotes
Electronic Supplementary Information (ESI) available: [More morphologies of SNTs under different pH, silica precipation, schematic illustration of bundled SNTs, TEM images of SNTs on flagella displaying GPP8 and on bacterial pili]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Mitchell DT, Lee SB, Trofin LCM, Li N, Nevanen TK, Söderlund H, Martin CR. J Am Chem Soc. 2002;124:11864–11865. doi: 10.1021/ja027247b. [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Tang H, Cao K, Song H, Sheng W, Wu Q. J Mater Chem. 2011;21:6122–6135. [Google Scholar]
- 3.Jung JH, Park M, Shinkai S. Chem Soc Rev. 2010;39:4286–4302. doi: 10.1039/c002959a. [DOI] [PubMed] [Google Scholar]
- 4.He B, Son SJ, Lee SB. Langmuir. 2006;22:8263–8265. doi: 10.1021/la060187t. [DOI] [PubMed] [Google Scholar]
- 5.Ogihara H, Takenaka S, Yamanaka I, Tanabe E, Genseki A, Otsuka K. Chem Mater. 2006;18:996–1000. [Google Scholar]
- 6.Lee SB, Mitchell DT, Trofin L, Nevanen TK, Söderlund H, Martin CR. Carrier-Based Drug Delivery. 8. Vol. 879. American Chemical Society; 2004. pp. 98–118. [Google Scholar]
- 7.Chen CC, Liu YC, Wu CH, Yeh CC, Su MT, Wu YC. Adv Mater. 2005;17:404–407. [Google Scholar]
- 8.Wu X, Ruan J, Ohsuna T, Terasaki O, Che S. Chem Mater. 2007;19:1577–1583. [Google Scholar]
- 9.Zygmunt J, Krumeich F, Nesper R. Adv Mater. 2003;15:1538–1541. [Google Scholar]
- 10.Jung JH, Yoshida K, Shimizu T. Langmuir. 2002;18:8724–8727. [Google Scholar]
- 11.Wan X, et al. Nanotechnology. 2008;19:315602. doi: 10.1088/0957-4484/19/31/315602. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Li D, Mao C. Adv Funct Mater. 2008;19:4007–4013. [Google Scholar]
- 13.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, Brown S, MacNee W, Donaldson K. Nat Nano. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 14.Shenton W, Douglas T, Young M, Stubbs G, Mann S. Adv Mater. 1999;11:253–256. [Google Scholar]
- 15.Royston E, Lee SY, Culver JN, Harris MT. J Colloid Interface Sci. 2006;298:706–712. doi: 10.1016/j.jcis.2005.12.068. [DOI] [PubMed] [Google Scholar]
- 16.Numata M, Sugiyasu K, Hasegawa T, Shinkai S. Angew Chem Int Ed. 2004;43:3279–3283. doi: 10.1002/anie.200454009. [DOI] [PubMed] [Google Scholar]
- 17.Meegan JE, Aggeli A, Boden N, Brydson R, Brown AP, Carrick L, Brough AR, Hussain A, Ansell RJ. Adv Funct Mater. 2004;14:31–37. [Google Scholar]
- 18.Ono Y, Kanekiyo Y, Inoue K, Hojo J, Nango M, Shinkai S. Chem Lett. 1999;28:475–476. [Google Scholar]
- 19.Ji Q, Kamiya S, Jung JH, Shimizu T. J Mater Chem. 2005;15:743–748. [Google Scholar]
- 20.Samatey FA, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, Yamamoto M, Namba K. Nature. 2001;410:331–337. doi: 10.1038/35066504. [DOI] [PubMed] [Google Scholar]
- 21.Hoshikawa H, Kamiya R. Biophys Chem. 1985;22:159–166. doi: 10.1016/0301-4622(85)80038-7. [DOI] [PubMed] [Google Scholar]
- 22.Asakura S. Adv Biophys. 1970;1:99–155. [PubMed] [Google Scholar]
- 23.Asakura S, Iino T. J Mol Biol. 1972;64:251–256. doi: 10.1016/0022-2836(72)90334-8. [DOI] [PubMed] [Google Scholar]
- 24.Kamiya R, Asakura S. J Mol Biol. 1976;108:513–518. doi: 10.1016/s0022-2836(76)80133-7. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya R, Asakura S. J Mol Biol. 1976;106:167–186. doi: 10.1016/0022-2836(76)90306-5. [DOI] [PubMed] [Google Scholar]
- 26.Alam M, Oesterhelt D. J Mol Biol. 1987;194:495–499. doi: 10.1016/0022-2836(87)90677-2. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa E, Kamiya R, Asakura S. J Mol Biol. 1982;160:609–621. doi: 10.1016/0022-2836(82)90318-7. [DOI] [PubMed] [Google Scholar]
- 28.Westerlund-Wikstrom B. Int J Med Microbiol. 2000;290:223–230. doi: 10.1016/S1438-4221(00)80119-8. [DOI] [PubMed] [Google Scholar]
- 29.van Bommel KJC, Shinkai S. Langmuir. 2002;18:4544–4548. [Google Scholar]
- 30.Livage J, Henry M, Sanchez C. Prog Solid State Chem. 1988;18:259–341. [Google Scholar]
- 31.Cihlár J. Colloids Surf A. 1993;70:239–251. [Google Scholar]
- 32.Ikeda T, Kamiya R, Yamaguchi S. J Bacteriol. 1983;153:506–510. doi: 10.1128/jb.153.1.506-510.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SCHAEFER DW. Science. 1989;243:1023–1027. doi: 10.1126/science.243.4894.1023. [DOI] [PubMed] [Google Scholar]
- 34.Fowler CE, Shenton W, Stubbs G, Mann S. Adv Mater. 2001;13:1266–1269. [Google Scholar]
- 35.Yuwono VM, Hartgerink JD. Langmuir. 2007;23:5033–5038. doi: 10.1021/la0629835. [DOI] [PubMed] [Google Scholar]
- 36.Brinker CJ. J Non-Cryst Solids. 1988;100:31–50. [Google Scholar]
- 37.Wenzl S, Deutzmann R, Hett R, Hochmuth E, Sumper M. Angew Chem Int Ed. 2004;43:5933–5936. doi: 10.1002/anie.200461236. [DOI] [PubMed] [Google Scholar]
- 38.Sumper M, Brunner E, Lehmann G. FEBS Lett. 2005;579:3765–3769. doi: 10.1016/j.febslet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Kröger N, Deutzmann R, Sumper M. Science. 1999;286:1129–1132. doi: 10.1126/science.286.5442.1129. [DOI] [PubMed] [Google Scholar]
- 40.Kumara MTM, Subra, Tripp, Brian C. J Nanosci Nanotechnol. 2007;7:2260–2272. doi: 10.1166/jnn.2007.641. [DOI] [PubMed] [Google Scholar]
- 41.Proft T, Baker E. Cell Mol Life Sci. 2009;66:613–635. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao B, Xu H, Mao C. Angew Chem Int Ed. 2011;50:6264–6268. doi: 10.1002/anie.201102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.