Abstract
Although exercise improves anxiety in humans, it is controversial whether exercise is anxiolytic in rodents. We tested the hypothesis that stress influences the effect of exercise on anxiety-like and defensive behaviors. To explore the neurobiological mechanisms of exercise, we also examined whether exercise alters gene expression for the stress-related peptide galanin. Rats were housed in the presence or absence of a running wheel for 21 d. A subset of these rats were (1) not injected or received a single high, dose of the β-carboline FG7142 (inverse agonist at the benzodiazepine receptor site) immediately prior to testing or (2) were injected repeatedly with vehicle or FG7142 during the last 10 d of exercise. On day 22, anxiety-like and defensive behaviors were measured in the elevated plus maze, shock probe defensive burying, and defensive withdrawal tests. Locus coeruleus prepro-galanin mRNA was measured by in situ hybridization. Exercise and sedentary rats that were not injected exhibited similar behavior in all tests, whereas FG7142 injected immediately prior to the test battery produced intense avoidance and immobility consistent with an anxiety-like response. However, exercise produced anxiolytic-like and active defensive behaviors in the test battery relative to the sedentary condition in rats injected repeatedly with vehicle or FG7142. Exercise also increased prepro-galanin mRNA in the locus coeruleus relative to sedentary controls. These data suggest that the emergence of enhanced adaptive behavior after chronic voluntary exercise is influenced by stress. Our data support a role for galanin in the beneficial consequences of wheel running.
Keywords: anxiety, exercise, galanin, locus coeruleus, stress, wheel running
1. Introduction
Physical activity improves anxiety symptoms in healthy people and medical patients [1–3]. However, it is controversial whether wheel running – a common experimental means to voluntarily permit exercise – indicates treatment potential in rodent models of anxiety. For example, although chronic wheel running produces anxiolytic-like effects in some experiments [4–11], both null [12] and anxiogenic effects have also been reported [13–16]. Research designed to resolve this discrepancy is critical because it will help to determine the value of rodent models to study the anxiolytic potential of exercise and its underlying neurobiology.
A potential source for divergence in this literature may result because of the moderating influence of stress. Discrepant findings under baseline conditions may result because non-manipulated variables (e.g., environmental aversiveness of the testing environment, handling and housing conditions) operate as stressors and interact with the effects of exercise. Lending support to this hypothesis, wheel running exerts a clear anxiolytic-like profile after exposure to stress. For instance, wheel running reduces the behavioral toll of several acute stressors, including intense illumination [4], social defeat [17], tail shock [18, 19], the serotonergic receptor agonist metachlorophenylpiperazine [20], the selective serotonin reuptake inhibitor fluoxetine [21], and uncontrollable footshock [22, 23]. Wheel running also buffers the behavioral deficits induced by stress-based models of psychopathology, including chronic mild stress [24], maternal deprivation [25], and social defeat [26]. Further, a role for exercise in stress-resilience has been documented for several neurobiological, neuroendocrine, and neuroimmune responses and in other behavioral paradigms [for review see 27, 28, 29]. Thus, it is expected that the anxiolytic actions of exercise are consistently observed when stress is experimentally manipulated because exercise interacts with stress to produce an anxiolytic-like response during testing.
Another potential source for divergence in this literature may result because enhanced defensive behavior (e.g., heightened sensory processing, immobility, flight, defensive threat/attack behaviors) in wheel runners competes with the display of anxiolytic-like behavior. Indeed, ‘anxiogenic’ responding in wheel runners was suggested to result because they exhibit enhanced defensive behaviors in tests of anxiety [16, 30]. However, this hypothesis is yet to be examined using tests that are optimized to detect active defensive behaviors, while also assessing behaviors specific to anxiety. Structured tests of threat, such as the shock probe defensive burying test, permit detection of defensive behaviors that otherwise are undetected or uninitiated in other, standard tests of anxiety [for reveiws see 31, 32]. Active defensive behaviors are theorized to be preserved across species and perturbed in anxiety disorders [33, 34] and these behaviors are also altered by anxiolytic drugs [35, 36]. Thus, understanding the effects of exercise on active defensive behaviors is of relevance for understanding and treating anxiety.
The molecular mechanisms underlying the affective consequences of voluntary exercise are not well understood [37]. Research in our laboratory over the past decade supports the hypothesis that wheel running mitigates the effects of stress by norepinephrine-galanin mediated mechanisms that involve the locus coeruleus. The locus coeruleus is an important noradrenergic nucleus that mediates stress and anxiety [38]. The majority of locus coeruleus neurons also contain the peptide galanin [39–42]. Wheel running increases prepro-galanin mRNA in the locus coeruleus [43–45] and dampens stress-induced norepinephrine release in a region the locus coeruleus targets (i.e.., frontal cortex) [46] relative to sedentary conditions. Plasma galanin is increased after an acute bout of exercise in humans [47], supporting the potential clinical relevance of exercise-induced regulation of galanin. Chronic stress and a model of mental pathology also increase the expression of prepro-galanin mRNA in the locus coeruleus relative to controls [41, 48], which suggests that alterations in locus coeruleus galanin occur as a common molecular adaptation to compensate for the toll of stress or psychopathology.
The primary aim of the present experiments was to evaluate the influence of voluntary wheel running on anxiety-like and defensive behavior as a function of stress. We tested the hypothesis that chronic wheel running would produce anxiolytic-like behaviors in rats that were exposed to stress. We also hypothesized that repeated wheel running would not reliably influence anxiety-like behavior in rats that were not exposed to stress. Anxiety-like and defensive behaviors were measured in an array of behavioral tests to characterize the effects of exercise, including the elevated plus maze, shock probe defensive burying, and defensive withdrawal tests. The secondary aim was to verify that exercise upregulates galanin gene expression, as expected from previous experiments using different behavioral manipulations [43–45].
2. Material and methods
2.1. Subjects
Seventy-seven male Sprague-Dawley rats (200–250 g; Harlan, Prattville, AL) were used at approximately 2 months of age at the beginning of testing. Rats had ad libitum access to food and water and were individually housed in clear polycarbonate cages (50 × 30 × 30 cm) with wood chip bedding. Rats were housed under constant temperature (23 ± 1°C) and lighting (12:12 reverse light:dark) with lights off at 7 or 9 am. Rats were allowed to habituate to the animal facility for at least 5 d before the initiation of any experimental procedures. All rats were weighed on experimental day 1, 11, and 21. Rats receiving chronic injections were also weighed on experimental day 16 to ensure accurate drug dosing. All procedures were carried out in accordance with the National Institute of Health guide for the care and use of laboratory animals and formal approval to conduct the experiments was obtained from the University of Georgia Animal Care and Use Committee.
2.2. General experimental methods
2.2.1. Experiment 1: Validation of a lack of an effect of chronic exercise on anxiety-like behavior at baseline (i.e., no stressor exposure)
Rats were randomly selected and assigned to either exercise or sedentary conditions on experimental day 1 and remained under these conditions for 21 d until wheels were locked just after lights off on experimental day 22. Half of the rats were then randomly assigned to receive no stress (i.e., were not injected to test the hypothesis of a null effect of exercise in the absence of stress), and the remaining half was assigned to receive an single, high dose (30 mg/kg i.p.) of the β-carboline compound FG7142 immediately prior to behavioral testing (to validate the sensitivity of our tests of anxiety). The design of experiment 1 was as follows: sedentary/0x FG (n = 10), exercise/0x FG (n = 10), sedentary/1x FG (n = 8), exercise/1x FG (n = 10). Rats were tested in the test battery (elevated plus maze, shock probe defensive burying test, defensive withdrawal test) on experimental day 22.
2.2.2. Experiment 2: Effects of chronic exercise on anxiety-like behavior after exposure to repeated injection or pharmacological stress
A separate group of rats were randomly selected and assigned to either exercise or sedentary conditions on experimental day 1 and remained under these conditions for 21 d until wheels were locked just after lights off on experimental day 22. On experimental day 12, half of the rats were randomly assigned to receive daily, repeated intraperitoneal injections of vehicle (10 d on experimental days 12–21) or chronic FG7142 (7.5 mg/kg × 10 d on experimental days 12–21). The design of experiment 2 was as follows: sedentary/10x Vehicle (n = 10), exercise/10x Vehicle (n = 10), sedentary/10x FG (n = 9), exercise/10x FG (n = 10). Rats were tested in the test battery (elevated plus maze, shock probe defensive burying test, defensive withdrawal test) on experimental day 22.
2.2.3. Experiment 3: Effects of exercise on galanin gene expression in the locus coeruleus of rats that were exposed to either no injection stress or repeated injections of FG7142
As a positive control to verify previous findings from this laboratory that exercise upregulates galanin gene expression in the locus coeruleus [43–45], in situ hybridization was performed in the brains of all unstressed rats. Directly after testing in experiment 1, rats that were not exposed to stress were rapidly decapitated and brains were harvested and frozen. In order to determine the generalizability of exercise-induced upregulation of galanin when potentially stressful behavioral manipulations are involved, brains were similarly harvested from rats repeatedly injected with FG7142 from experiment 2.
2.3 Exercise
The homecage of rats assigned to sedentary conditions was without a wheel, whereas the exercise condition had a stainless steel running wheel (Mini Mitter, Bend, OR) that permitted 24-hr free access. Wheel rotations were measured for each subject by an electromagnetic counter and were recorded by an experimenter at the same time each day (2–3 hr post the onset of the dark phase of the light: dark cycle). Daily distance ran was determined by multiplying the number of wheel rotations by the wheel circumference (105 cm).
2.4. Drugs
The β-carboline FG7142 (partial inverse agonist at the benzodiazepine site of the GABAA receptor; Tocris Bioscience, Ellisville, MI) was selected because it mimics the effects of stress and produces robust anxiety across several species [for review see 49]. FG7142 was prepared fresh daily by suspending the drug in vehicle (distilled H2O containing 1 drop Tween80/5 mL) and vortexting. Injections were administered in a volume of 1 mL/kg in a room separate from the animal holding room 2–3 hr after the onset of the dark phase of the light: dark cycle. Doses were selected based on previous research that reported anxiety-like effects [50–52]. For experiments requiring acute administration of FG7142, rats were injected and immediately underwent behavioral testing. Substantiating that the effects of acute FG7142 are behaviorally active for the entire test battery, previous evidence in the rodent shows that FG7142 quickly (as early as 10 min after) and lastingly (up to 1 hr after injection) alters behavior [50, 53–58]. For experiments requiring chronic administration of FG7142, our injection paradigm did not influence the behavioral acquisition or maintenance of wheel running (see Fig. 1). Rats were also visually inspected for seizures 1 hr post each repeated FG7142 injection to verify that the doses employed were subconvulsant [59, 60].
Figure 1. Daily distance ran in a running wheel increased across experimental days in a similar manner for all treatment groups.
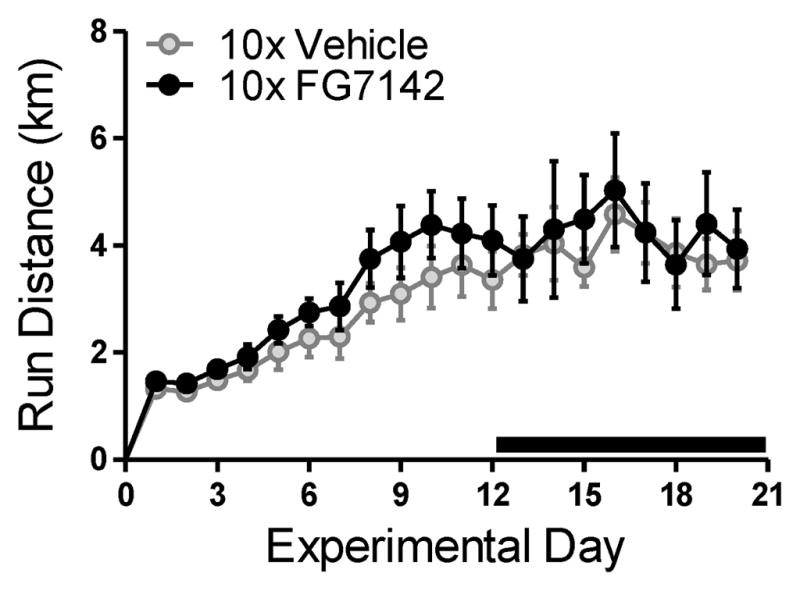
Note that the bold line indicates the experimental days on which repeated (10x) injections of vehicle or FG7142 occurred. Data are mean ± SEM (n = 9–10).
2.5. Behavioral testing
Testing occurred during the dark phase of the light: dark cycle (started ~2 hrs after lights off and ended ~2 hrs before lights on). Rats were tested in the defensive withdrawal, shock probe defensive burying, and elevated plus maze tests, in that order, such that testing lasted about 50 min. Pilot experiments confirmed that serial testing produced acceptable baseline responding (i.e., non-injected controls exhibited about 10–15% of the time spent on the open arms of the elevated plus maze, 15–30% of the time spent immobile and burying in the shock probe defensive burying test, and 15–20 m traveled in the defensive withdrawal test). In particular, the single shock received in the defensive burying test likely did not alter subsequent elevated plus maze behavior, as baseline responding in the maze in rats that were shocked (present report) is similar to data previously reported by the investigators from rats that were not shocked and of the same strain [61]. Although the possibility of carry-over-effects from serial testing cannot be ruled out [62–65], the evidence indicated above suggests that the influence of serial testing in the present report is minimal. Background noise of 79 dB was emitted during testing by a white-noise generator. Behavioral equipment was wiped clean with Vimoba disinfectant (Quip Laboratories, Wilmington, DE) and allowed to dry between subjects.
2.5.1. Elevated plus maze
The apparatus was a wooden “+”-shaped maze elevated 50 cm from the floor and consisted of two opposite open arms (45 × 9 cm), two opposite closed arms (45 × 9 × 38 cm), and a central platform (9 × 9 cm). Illumination in the apparatus (~15 lux) was generated by placing a 15 W bulb ~1 m above the central platform. Testing was performed as previously described [61]. Each rat was placed in the center of the maze facing opposite the experimenter and towards an open arm of the maze. Behavior was video recorded for 5 min. An experimenter blind to group assignment, quietly remained in the testing room behind a divider and scored behavior. Measured behaviors were open and closed arm time and entries.
2.5.2. Shock probe defensive burying test
The test cage (50 × 30 × 60 cm; polycarbonate) was filled with 5 cm of fresh bedding (Sani Chips, Harlan, Prattville, AL) and contained a shock probe that extended 6 cm into the cage and 2 cm above the bedding. The probe was a glass rod (1 cm diameter) wrapped with 2 alternating copper wires (18 G wires spaced 5 loops/cm) that were connected to a shock generator (Coulbourne Instruments, Whitehall, PA). Illumination in the apparatus (~15 lux) was generated by a 15 W bulb that was centered ~1 m above the floor of the cage. Upon testing, rats were placed in the cage at a position that was farthest from the probe and in a direction opposite the probe. After receipt of a single shock (3 mA), the shock generator was turned off and behavior was recorded for 15 min, as described previously [66]. An experimenter blind to group assignment quietly remained in the testing room and scored behavior. A video record was also obtained for each rat for behavioral analysis at a later date due to the complicated nature of scoring the wealth of relevant behavior in this test. Measured behaviors included the onset of initial probe contact and burying, time spent engaging in burying, immobility, and rearing behaviors, shock reactivity based on a four-point scale used previously [67] and the frequency of probe bites and non-shock probe returns [see 32, 68].
2.5.3. Defensive withdrawal test
The test chamber (ENV 515-16, Med Associates, St. Albans, VT) was a clear polycarbonate cage (44.5 × 44 × 30 cm) that contained a dark enclosure that was impenetrable to visible light (22 × 12.5 × 15 cm with a 6 × 7 cm door; Cyro Acrylite GP, Rideout Plastics, San Diego, CA). Ultraviolet beam interruptions were used to track the coordinate position and movement of the rat. Illumination in the light portion of the apparatus (250 lux) was generated by a 40 W bulb located 70 cm above the floor of the chamber. Each rat was placed in the center of the apparatus, facing opposite the experimenter, and allowed to explore the chamber for 15 min. Behavior was automatically collected (ENV-520, SOF-810, Med Associates) for latency to enter and time spent in the dark enclosure, frequency of dark enclosure transitions, and distance traveled in the entire test chamber, as described previously [69].
2.6. Tissue sectioning
Sedentary and exercised rats (N=39) from the no-injection and chronic FG7142 groups were decapitated immediately after the last behavioral test. Brains were rapidly harvested, blocked (at a coronal plane caudal to thalamus), frozen on dry ice, and stored at −80°C until cryostat sectioning (−22°C Microm; Waldorf, Germany). Tissue was cut into 12 μm coronal sections, collected between −9.8 to 10.04 mm from bregma, and subsequently thaw-mounted onto gelatin-coated glass microscope slides (2 sections/slide), which were stored at −80°C until further processing. Anatomical location was also verified in adjacent sections using .1% thionin stain and a rat brain atlas [70].
2.7. In situ hybridization and densitometry
Tissue was processed as previously described [71]. For pretreatment, tissue was fixed in 4% formaldehyde in .12M phosphate buffered saline (PBS), rinsed in PBS, soaked in .25% acetic anhydride in .1M triethanolamine HCl and .9% NaCl, dehydrated in a series of EtOH washes, delipidated in chloroform, and washed in EtOH. An oligonucleotide probe (Human galanin: 5′-G AAG GTA GCC AGC GCT GTT CAG GGT CCA GCC TCT CTT CTC CTT T - 3′; Oligos etc, Wilsonville, OR) was labeled at the 3′ end with 35S-dATP (1 mCi; Perkin Elmer, Boston, MA), tailing buffer, CoCl2, and terminal deoxynucleotransferase (Roche, Indianapolis, IN). Unbound radionucelotide was removed using column separation (Micro Bio-Spin P30 in Tris, Bio-Rad, Hercules, CA) and bound radionucelotide was stabilized using 1M dithiothreitol. Tissue from rats in all experimental groups was processed concurrently in the same assay. Sections were covered with radiolabeled probe in hybridization buffer (25% formamide, 72mM NaCl, 3.2mM Tris HCl, .0032mM EDTA, .001% sodium pyrophosphate, .004% sodium dodecyl sulfate, .002 mg/mL heparin sulfate, and 2% dextran sulfate) and incubated for 24 hrs at 37°C. Sections underwent a series of washes in 1% SSC and 2% SSC-formamide (50:50) at 40°C and room temperature as well as in distilled H2O and EtOH. Sections were allowed to dry and subsequently opposed to 35S-sensitive film (Kodak BioMax MR, Rochester, NY) for 14 d. Films were developed in Kodak GBX fixer and developer and air dried.
Film images were captured under optimized conditions using a light table (Northern Light D95, Imaging Research Inc., Piscataway, NJ) and digital camera equipped with a macro lens (Nikon D5000, Micro-NIKKOR 55mmf/2.8 lens, Melville, NY). Images were processed on a Macintosh computer (Apple, Inc., Cupertino, CA) using NIH Image (Bethesda, MD, http://rsb.info.nih.gov/nih-image/). Images of the locus coeruleus were selected and measured using a uniform area of the dorsal portion of the locus coeruleus. Mean grayscale brightness values were obtained from 2–4 sections per subject. Densitometry was performed on original images that were in no way digitally manipulated. Example photomicrographs were uniformly transformed across groups to a color scale using NIH Image.
2.8. Statistics
Intraclass correlation coefficients using Cronbach α were calculated to verify strong inter-rater reliability in behavioral coding from a random subset of videos, which ranged from α = .84 – 1. A two-way (drug x time) or three-way (exercise x drug x time) analysis of variance (ANOVA) was performed with time point as the repeated measure to assess running distance or body weight and distance traveled during the defensive withdrawal test, respectively. Greenhouse-Geisser corrections were applied to repeated factors violating sphericity [72]. Cronbach α (model II intraclass correlation) was calculated to determine internal consistency of body weight and running distance across the experiments. Separate 2 (exercise) × 2 (drug) ANOVAs were performed for all other behavioral measures to evaluate the effects of exercise. Bonferroni post-hoc tests were performed for significant interaction effects. Partial eta squared (η2) effect size calculations were performed for each ANOVA to gauge the amount of variance our manipulations accounted for in the dependent measures evaluated. Using Cohen’s standards, η2 values above .01, .06, and .14 are commonly considered small, medium, and large effects, respectively [73–75]. To assess the effects of exercise on locus coeruleus prepro-galanin mRNA expression, separate t-tests were performed in rats that received no injections and repeated injection of FG7142. To generate statistical power needed to avoid a type II error, linear regression analysis was performed with data obtained from exercise rats that were exposed to no injection stress and chronic FG7142 groups (matched for prior housing and testing experience) to gauge the relationship between running distance and galanin message. All analyses were performed using SPSS statistical software (SPSS Incorporated, Chicago, IL).
3. Results
3.1. Experiment 1
3.1.1. Wheel running and body weight increased across time in rats that were not injected or injected with FG7142 immediately prior to testing
Distance ran in wheels increased linearly across experimental days and was internally consistent across time (F1.14, 20.47 = 26.32, p < .01; η2= .61; Cronbach α = .96; Data not shown). Wheel running was not affected by drug treatment (p > .05; Data not shown). Average distance ran during week one, two, and three of running was 1.62 ± .17, 3.32 ± .41, and 4.53 ± .64 km/d (Data not shown), respectively. All rats gained weight across experimental days (F1.31, 44.62 = 831.80, p < .01; η2= .96; Cronbach α = .78; Data not shown). Exercised rats maintained a lower body weight relative to sedentary rats (F1.31, 44.62 = 44.40, p < .01; η2= .57; Data not shown) on experimental day 11 (p < .01) and day 22 (p < .01), but were initially similar in body weight on experimental day 1 (p > .05).
3.1.2. Acute FG7142 increased anxiogenic behavior in a battery of tests when given immediately prior to testing
In the elevated plus maze, injection of an acute, high dose of FG7142 immediately prior to testing did not reliably alter the amount of time spent on the open arms of the maze (p = .09; Table 1), but reduced the frequency of open arm entries (F1, 34 = 6.43, p < .05; η2 = .16; Table 1) relative to the no injection condition. In addition, injection of acute FG7142 immediately prior to testing also increased the amount of time spent on the closed arms of the maze (F1, 34 = 6.32, p < .05; η2 = .16; Table 1) and reduced the frequency of closed arm entries relative to the no injection condition (F1, 34 = 29.10, p < .01; η2 = .46; Table 1).
Table 1.
Anxiety-like behavior of rats with a history of voluntary exercise or sedentary conditions that were not exposed to an experimental stressor or received an acute injection of the β-carboline FG7142.
| Sedentary 0x FG5 |
Sedentary 1x FG |
Exercise 0x FG |
Exercise 1x FG |
|
|---|---|---|---|---|
| Elevated plus maze | ||||
| Open arm time (s) | 31.75 ± 10.33 | 10.85 ± 3.39ns | 30.37 ± 6.83 | 24.81 ± 6.29ns |
| Closed arm time (s) | 251.31 ± 12.34 | 276.03 ± 4.51# | 248.82 ± 6.79 | 266.77 ± 6.72# |
| Open arm entries (freq) | 3.70 ± 1.23 | .75 ± .25# | 3.60 ± .82 | 2.20 ± .63# |
| Closed arm entries (freq) | 13.60 ± 2.21 | 5.25 ± 1.39## | 17.20 ± 1.03 | 7.60 ± 1.65## |
| Shock probe defensive burying | ||||
| Burying time (s) | 130.59 ± 38.98 | 4.15 ± 2.39## | 79.85 ± 30.28 | 37.73 ± 14.58## |
| Immobility time (s) | 136.69 ± 63.39 | 334.85 ± 38.82## | 181.15 ± 42.84 | 378.87 ± 77.34## |
| Probe bites (freq) | .20 ± .20 | .00 ± .00 | 1.00 ± .62 | .10 ± .10 |
| Rearing time (s) | 155.09 ± 26.10 | 32.08 ± 6.40## | 117.83 ± 16.61 | 26.73 ± 7.56## |
| Non-shock probe returns (freq) | 2.50 ± 1.36 | .13 ± .13 | 2.90 ± 1.75 | .40 ± .22 |
| Shock reactivity (scale) | 1.90 ± .10 | 1.88 ± .35 | 2.30 ± .21 | 2.00 ± .00 |
| Latency to initiate probe contact (s) | 64.30 ± 41.06 | 16.88 ± 3.86 | 27.30 ± 7.36 | 65.89 ± 33.88 |
| Latency to initiate burying (s) | 256.60 ± 108.53 | 684.75 ± 134.73 | 285.60 ± 105.21 | 265.56 ± 120.80 |
| Defensive withdrawal | ||||
| Distance traveled 0–5 min (m) | 5.33 ± 1.28 | 2.41 ± .57## | 5.27 ± .96 | 2.97 ± .86## |
| Distance traveled 6–10 min (m) | 6.30 ± 1.38 | 1.14 ± .81## | 6.20 ± .86 | .47 ± .13## |
| Distance traveled 11–15 min (m) | 5.19 ± 1.40 | .94 ± .73## | 4.98 ± .96 | .97 ± .64## |
| Dark box time (s) | 565.45 ± 78.70 | 807.53 ± 42.41## | 585.01 ± 31.21 | 740.55 ± 87.46## |
| Dark box transitions (freq) | 18.50 ± 3.81 | 2.63 ± 1.35## | 20.40 ± 2.66 | 2.40 ± .82## |
| Latency to enter dark box (s) | 20.94 ± 3.61 | 48.60 ± 11.87## | 20.98 ± 3.50 | 31.76 ± 5.85## |
Data are mean ± S.E.M. (n = 8–10);
p < .01,
p < .05 vs. No injection (0x FG7142);
p = .09 vs. No injection.
In the shock probe defensive burying test, injection of an acute, high dose of FG7142 immediately prior to testing reduced the amount of time spent burying (F1, 33 = 9.15, p < .01; η2 = .22; Table 1) and rearing (F1, 33 = 37.49, p < .01; η2 = .53; Table 1) and concomitantly increased the amount of time spent immobile (F1, 33 = 11.38, p < .01; η2 = .26; Table 1) relative to the no injection condition. Acute injection of FG7142 did not alter any other measure in the shock probe defensive burying test relative to the no injection condition (p > .05; Table 1).
In the defensive withdrawal test, injection of an acute, high dose of FG7142 immediately prior to testing reduced the distance traveled across the entire duration of the test relative to the no injection condition (F1.62, 56.62 = 5.58, p < .01; η2 = .14; Table 1). In addition, injection of FG7142 immediately prior to testing increased the time spent in the dark box (F1, 34 = 8.82, p < .01; η2 = .01; Table 1) and latency to enter the dark box (F1, 34 = 8.93, p < .01; η2 = .05; Table 1) and reduced the frequency of dark box transitions (F1, 34 = 43.57, p < .01; η2 = .15; Table 1) relative to the no injection condition.
3.1.3. Exercise failed to alter anxiety-like behavior across a battery of tests in rats not exposed to stress or exposed to intense stress via injection of FG7142 immediately prior to testing
Exercise did not alter any measure of anxiety-like or defensive behavior in elevated plus maze, shock probe defensive burying, and defensive withdrawal tests relative to sedentary conditions in rats that were not injected or injected with a high dose of FG7142 immediately prior to testing (p > .05; Table 1).
3.2. Experiment 2
3.2.1. Wheel running and body weight increased across time in rats repeatedly injected with vehicle or FG7142
Distance ran in wheels increased linearly across experimental days and was internally consistent across time (F2, 36 = 23.45, p < .01; η2= .61; Cronbach α = .95; Fig. 1). Wheel running was not affected by drug treatment (p > .05; Fig. 1). Average distance ran during week one, two, and three of running was 1.92 ± .14, 3.78 ± .40, and 4.12 ± .44 km/d, respectively. All rats gained weight across experimental days (F1.40, 40.63 = 261.12, p < .01; η2= .96; Cronbach α = .77; Data not shown). Exercised rats maintained a lower body weight relative to sedentary rats (F1.40, 40.63 = 13.46, p < .01; η2= .32; Data not shown) on experimental day 11 (p < .01) and day 22 (p < .01), but were initially similar in body weight on experimental day 1 (p > .05). Rats that received repeated FG7142 were no different in weight from rats that received repeated vehicle (p > .05; Data not shown).
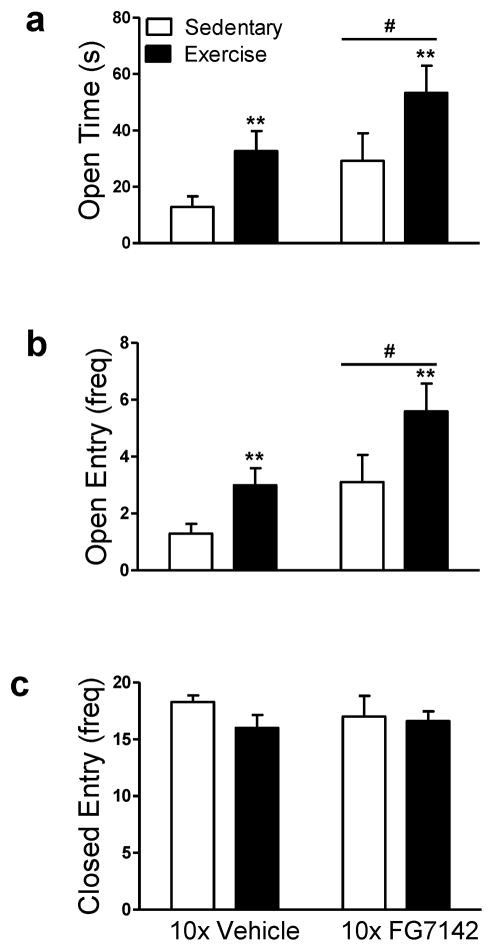
3.2.2. Exercise produced anxiolytic-like behavior in the elevated plus maze in rats repeatedly injected with vehicle or FG7142
In rats that received repeated injections of vehicle or FG7142, exercise increased the time spent on the open arms of the maze (F1, 35 = 7.78, p < .01; η2 = .18; Fig. 2a) and frequency of open arm entries (F1, 35 = 7.81, p < .01; η2 = .18; Fig. 2b) relative to sedentary controls. Exercise reduced the time spent on the closed arms of the maze (F1, 35 = 6.89, p < .01; η2 = .16; Data not shown), but did not alter the frequency of closed arm entries relative to sedentary conditions in rats that received repeated injections of vehicle or FG7142 (p > .05; Fig. 2c).
Figure 2. Exercise produces anxiolytic-like behavior in the elevated plus maze in rats exposed to repeated injection or FG7142.
Exercised rats that were repeatedly injected (10x) with vehicle or FG7142 exhibit increased (a) open arm time and (b) more open arm entries compared to sedentary rats. Rats repeatedly injected with FG7142 exhibit increased (a) open arm time and (b) open arm entries relative to vehicle-treated rats. (c) Neither exercise manipulation nor repeated injection stress altered (c) closed arm entries. Data are mean ± SEM (n = 9–10). **p < .01 vs. Sedentary; ##p < .01, #p < .05 vs. Vehicle.
Main effects of repeated FG7142 were also detected for time spent on the open (F1, 35 = 5.53, p < .05; η2 = .14; Fig. 2a) and closed arms of the maze (F1, 35 = 5.27, p < .05; η2 = .13; Data not shown) as well as the frequency of open arm entries (F1, 35 = 8.66, p < .01; η2 = .20; Fig. 2b).
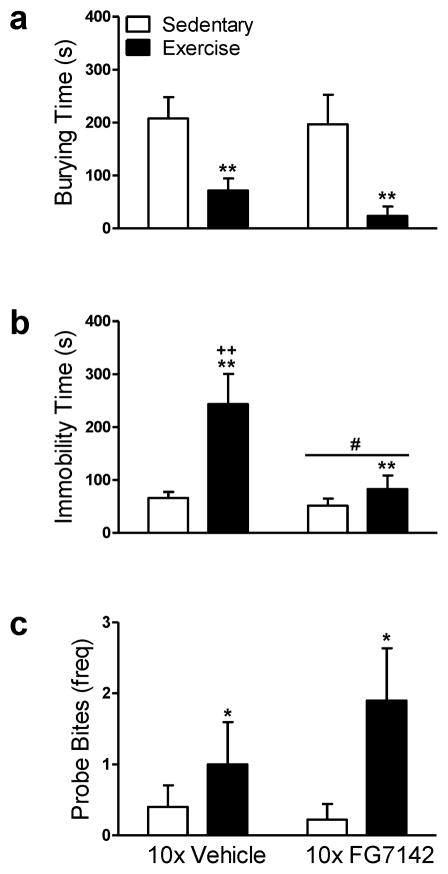
3.2.3. Exercise increased anxiolytic-like behaviors in the shock probe defensive burying test in rats repeatedly injected with vehicle or FG7142
In rats that received repeated injections of vehicle or FG7142, exercise reduced the amount of time spent burying (F1, 35 = 18.20, p < .01; η2 = .34; Fig. 3a) and rearing (F1, 35 = 6.64, p < .01; η2 = .16; Data not shown) and concomitantly increased the amount of time spent immobile (F1, 35 = 9.82, p < .01; η2 = .22; Fig. 3b) compared to sedentary conditions. Further, the effects of exercise on immobility time were abolished by repeated FG7142 administration (F1, 35 = 4.78, p < .05; η2 = .12; Fig. 3b). Exercise increased the frequency of probe bites (F1, 35 = 4.76, p < .05; η2 = .18; Fig. 3c) and non-shock probe returns (F1, 35 = 4.79, p < .05; η2 = .12; Data not shown) relative to sedentary conditions in rats that received repeated injections of vehicle or FG7142. Shock reactivity, onset of initial probe contact, or time to initiate burying was comparable between exercise and sedentary rats (p > .05; Data not shown).
Figure 3. Exercise increases both anxiolytic-like and active defensive behaviors in the shock probe defensive burying test in rats exposed to repeated injection or FG7142.
Exercised rats that were repeatedly injected (10x) with vehicle or FG7142 spent less (a) time burying and more (b) time immobile while simultaneously exhibiting a greater frequency of (c) probe bites compared to sedentary rats. Repeated FG7142 abolished the increase in (b) time spent immobile in exercise rats. Rats repeatedly injected with FG7142 exhibit reduced (b) time spent immobile relative to vehicle-treated rats. Data are mean ± SEM (n = 9–10). **p < .01, *p < .05 vs. Sedentary; #p < .05 vs. Vehicle; ++p < .01 vs. All other groups.
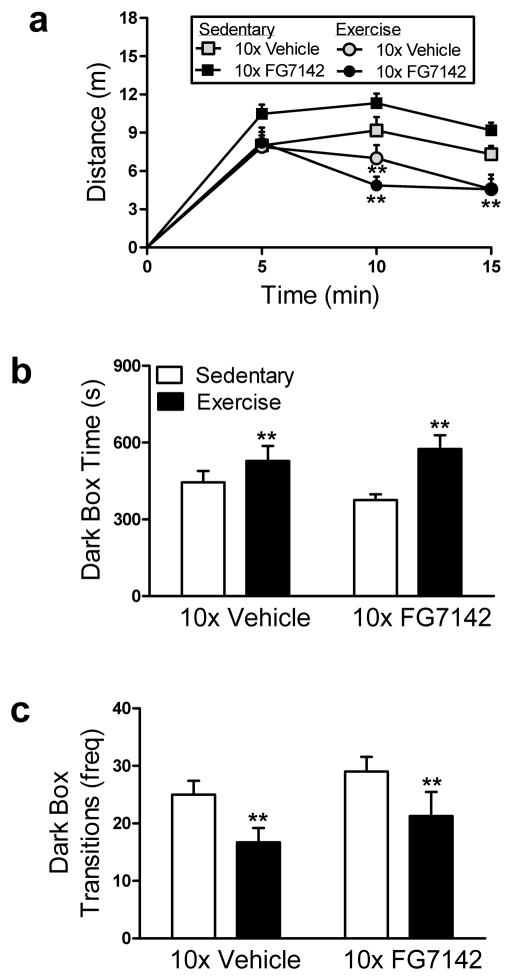
3.2.4. Exercise facilitated locomotor habituation and defensive withdrawal in rats repeatedly injected with vehicle or FG7142
In rats that received repeated injections of vehicle or FG7142, exercise reduced the amount of distance traveled (F1.62, 56.62 = 5.58, p < .01; η2= .14; Fig. 4a) in the defensive withdrawal test in a manner that was time-dependent compared to sedentary conditions. Exercised rats that were repeatedly injected with either vehicle or FG7142 were no different from sedentary counterparts in distance traveled at 5 min (p > .05; Fig. 4a), but exhibited reduced distance traveled at both 10 (p < .01; Fig. 4a) and 15 min (p < .01; Fig. 4a) of the test. In addition, exercise increased the amount of time spent in the dark box (F1, 35 = 8.70, p < .01; η2 = .20; Fig. 4b) and reduced the frequency of dark box transitions (F1, 35 = 6.89, p < .01; η2 = .17; Fig. 4c) relative to sedentary conditions in rats that received repeated injections of vehicle or FG7142. Exercise did not alter the latency to enter the dark box relative to the sedentary group in rats that received repeated injections of vehicle or FG7142 (p > .05; Data not shown).
Figure 4. Exercise facilitates locomotor habituation and defensive withdrawal in rats exposed to repeated injection or FG7142.
Exercised rats that were repeatedly injected (10x) with vehicle or FG7142 exhibited similar initial (0–5 min) (b) distance traveled, but thereafter (6–15 min) exhibited reduced distance traveled compared to sedentary rats. Exercised rats that were repeatedly injected with vehicle or FG7142 exhibit increased (a) time spent in the dark box and fewer (b) dark box transitions compared to sedentary rats. Data are mean ± SEM (n = 9–10). **p < .01 vs. Sedentary.
3.3. Experiment 3
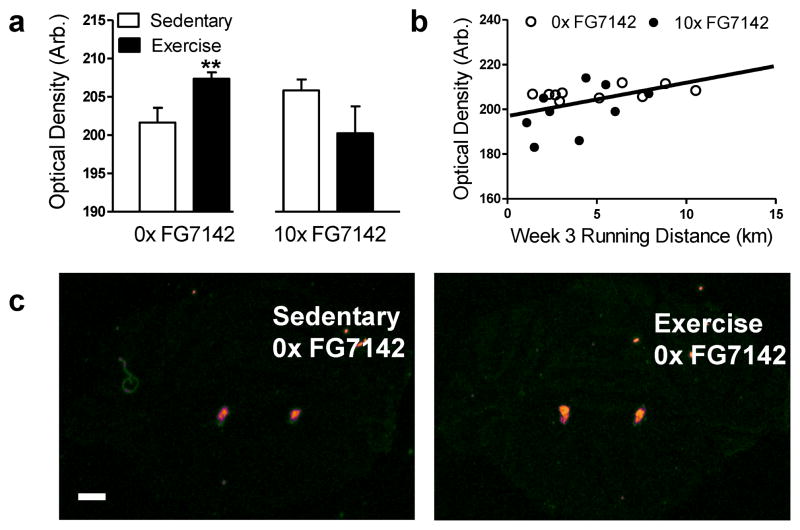
3.3.1. Exercise increases prepro-galanin mRNA expression in locus coeruleus, which is positively correlated with the amount of running on an exercise wheel
Exercise increased prepro-galanin mRNA expression in the locus coeruleus relative to sedentary conditions in rats that were not injected (t18 = −2.73, p < .01; Fig. 5a). However, exercise did not reliably alter prepro-galanin mRNA expression in the locus coeruleus relative to sedentary conditions in rats that were repeatedly injected with FG7142 (p > .05; Fig. 5a). Regression analysis revealed a positive correlation between prepro-galanin mRNA in the locus coeruleus and distance ran during week three (β = .44, t17 = 2.03, p = .05; R2 = .20; Fig. 5b), but not during week one or two (p > .05; Fig. Data not shown), which suggests that the increased variance (coefficient of variation, cv = 33%, 48%, and 60% during week one, two, and three, respectively) in running during week three contributes to the positive correlation between running distance and locus coeruleus prepro-galanin mRNA.
Figure 5. Exercise increased prepro-galanin mRNA expression in the locus coeruleus.
Exercise rats that were not injected (0x FG7142) exhibit increased (a) optical density for prepro-galanin mRNA in the locus coeruleus compared to sedentary counterparts. Suggesting that long durations of running are needed to increase galanin gene expression, the (b) optical density for prepro-galanin mRNA in the locus coeruleus was positively correlated with distance ran at 3 weeks. (c) The representative photomicrographs show 35S-oligonucleotide binding directed towards prepro-galanin mRNA in the brain of rats that were not injected and either forced to remain in sedentary conditions (left) or allowed access to a running wheel (right) for three weeks. Sections were collected at −10.04 mm from bregma. Scale bar indicates 1 mm. Data are mean ± SEM (n = 9–10). **p < .01 vs. Sedentary 0x FG7142.
Discussion
The present study demonstrates that voluntary wheel running elicited anxiolytic-like behavior in rats with a history of stress relative to their sedentary counterparts in several behavioral tests. However, under baseline stress conditions (i.e., no experimental stressor administered), exercise failed to differentially alter anxiety-like behavior. Additionally, regardless of whether rats exercised intense avoidance and immobility, at the loss of other defensive behaviors, was generated by severe stress induced by an acute, high dose of the β-carboline FG7142 administered immediately prior to testing. Further, exercise increased the expression of prepro-galanin mRNA in the locus coeruleus in rats that were not exposed to an experimental stressor.
The present data indicate alterations in emotion-related behavior rather than locomotion or pain sensitivity, as exercise and sedentary rats were no different in several measures of these variables (i.e., number of closed arm entries in the elevated plus maze, onset of initial probe contact or shock reactivity in the shock probe defensive burying test, or latency to enter the dark box in the defensive withdrawal test). Our data generated in rats that were exposed to stress (i.e., repeated injection with vehicle or FG7142) are consistent with other reports that show exercise exerts anxiolytic potential after stressor exposure [17–26]. Of note, chronic injections alone appear to be the critical factor leading to the appearance of the anxiolytic-like effect of voluntary exercise. Perhaps the ability to run after the stress of injection was the critical factor allowing the anxiolytic-like effect of exercise to be revealed. Although this possibility cannot be conclusively determined from our data set, this interpretation is supported by previous research showing that stress increases wheel running in a manner that is ameliorated by the anxiolytic drug diazepam [76]. Further, our data generated under baseline conditions of stress are in line with previous findings that show exercise does not alter anxiety-like behavior in the unstressed rodent [9, 12, 30, 77–82]. However, anxiolytic-like effects are also reported in exercised rodents that were not exposed to an experimental stressor [5, 6, 10, 11, 13]. We expect that the inconsistent effects of wheel running in the literature on baseline levels of anxiety-like behavior result from differing levels of stress (e.g., due to handling or other environmental variables) and/or other factors (e.g., genetics) that influence stress reactivity. Alternatively, no effect of exercise in the non-stressed (and highly stressed) rat could mean that a longer wheel access is necessary to demonstrate the stress reducing effects of voluntary exercise. Supporting this, wheel running that lasts 6 wks, but not less, is necessary to see the stress-reducing effects of exercise on “learned helplessness” behaviors (i.e., exaggerated freezing and shuttle box escape deficit after uncontrollable stress) [18, 19, 22, 83]. However, many previous reports reveal that 3 weeks of wheel running (as used in the present experiment) or less produces anxiolytic-like [4, 5, 8, 20, 24, 26, 84], anxiogenic [13–15], and null [77, 81] effects in several other tests of anxiety, suggesting that this duration of exercise is sufficient to detect its influence on anxiety-related behaviors using a variety of paradigms.
The present report showed that a high dose of the β-carboline FG7142 (30 mg/kg i.p. × 1 d) produced intense immobility and avoidance in the shock probe defensive burying and defensive withdrawal tests regardless of whether rats ran on a wheel, which could imply that the beneficial effects of exercise may not be sufficient to overcome intense stressors. The lack of an acute vehicle group in experiment 1 limits our ability to determine whether injection/handling or drug per se produced the anxiogenic effect. In other words, because rats from the no-stress control group were not injected the difference between acute FG7142 and non-injected groups is likely inflated. However, we expect that the anxiety-like behaviors detected in experiment 1 are most likely attributable to the high dose of FG7142 because of the extensive previous evidence that acute FG7142 mimics the effects of stress on behavioral, physiological, neuroendocrine, neuroimmune, and neurobiological responses and produces robust anxiety-like behavior [for review see 49]. Regardless of the source of the anxiogenic effects in the acute FG7142 injected rats, the present design suited the primary goal of the present report, which was to evaluate whether the emotional consequences of exercise are influenced by stress. The group assignment used in the present report permitted detection of a null effect of exercise in the absence of stress in non-injected rats as well as validation of the sensitivity of our tests of anxiety using the anxiogenic FG7142 manipulation. Additionally, it is important to note that we did not observe increases in anxiety-like behavior in rats given repeated injections of FG7142, whereas rats that received a single injection in Experiment 1 showed increased anxiety-like behavior. It is possible that the behavioral profile of rats given a single verses repeated injections of FG7142 differed because they experienced different levels of stress during testing (i.e., drug was present in sufficient plasma concentrations to be behaviorally active during testing in rats receiving a single inject of FG7142, but not for those receiving repeated injections prior to testing). Further, this group difference could be due to compensatory changes in brain signaling systems after repeated administration of FG7142 (e.g., upregulation of the GABAA receptor complex and/or the β adrenergic receptor), as supported by prior neurochemical and behavioral evidence [59, 60, 85, 86]. Future research is needed to systematically evaluate how FG7142 alters the brain galanin system, as this is the first report to our knowledge that examined brain galanin expression after FG7142 administration.
The increased frequency of probe bites by exercised rodents in the shock probe test may represent either exploratory behavior, which is consistent with an anxiolytic-like effect of exercise, or a form of aggressive behavior. Considering anxiety and aggression are inversely related and that the neural systems that regulate these two behaviors can be distinct [87, 88], it is reasonable that under different circumstances anxiolytic-like effects of exercise may increase aggressive behavior. In line with this interpretation, exercised rats were previously reported to be more aggressive and displayed a greater degree of biting and struggling during handling by an experimenter than sedentary rats [16]. However, wheel running reduced another form of aggression (i.e., conspecific aggression) in a manner that was dependent on whether rats had access to a wheel or whether they were locked [89]. Further, a line of mice that were bred for high running capacity exhibited elevated predatory aggression towards crickets, but reduced maternal and intermale aggression compared to control lines [90]. These findings suggest that the effects of wheel running on aggression are complex and possibly dependent on whether aggression is offensive or defensive.
The shock probe defensive burying test was sensitive to detect an enhanced learning capacity of runners, as indicated by the greater frequency of probe returns and bites in exercised rats after the probe was turned off. This suggests that exercised rats had an enhanced drive to re-approach the probe after the initial shock and, thus, had the opportunity to learn that (1) the probe was no longer electrified and (2) there would be no negative consequence of further probe contacts or bites. Indeed, improved learning after exercise has been extensively documented in humans and non-human animals in both fear and non-fear dependent paradigms [8, 9, 11, 37, 45, 84, 91–97].
Exercise increased galanin gene expression in the locus coeruleus in rats that were not exposed to an experimental stressor (see Fig. 5c). Prior research from our laboratory shows that 3–4 weeks of wheel running increases the expression of prepro-galanin mRNA in the locus coeruleus in rats that were exposed to other experimental manipulations that may involve varying degrees of stress [43–45]. The present result thus confirms the generalizability of exercise-induced galanin gene expression across a variety of paradigms. Further, in the present report we reproduced correlation data that suggests chronic exercise is driving the enhancement in locus coeruleus galanin [43, 98]. In particular, we show that increases in prepro-galanin mRNA in the locus coeruleus are statistically correlated with increases in the distance ran on a wheel during the third week of running, but not before then. However, in the present report no exercise-induced increase in locus coeruleus galanin gene expression was observed in rats that were repeatedly injected with FG7142. This result does not preclude the possibility that the galanin peptide itself was increased by exercise in this group or that mRNA levels were maximal (i.e. at a ceiling) or subject to negative feedback caused by galanin levels exceeding some threshold. Direct measures of galanin peptide will be required to test these hypotheses in future experiments. Furthermore, future research using galanin receptor antagonists and agonists will be needed to determine whether galanin is necessary and/or sufficient for the anxiolytic effect of exercise. Supporting the idea that stress and exercise interact to alter the genetic expression of galanin, we have previously shown that wheel runners exhibit increased expression of prepro-galanin mRNA expression in the locus coeruleus after acute footshock [46] and that forced exercise (a stress-maintained behavior) produced this same effect [99]. Elevated galaninergic tone in the locus coeruleus may function as a counter-regulatory mechanism that serves to dampen noradrenergic activity in wheel runners. Indeed, exercise attenuates stress-induced norepinephrine release in the frontal cortex [46]. Galanin has been shown to inhibit the activity of locus coeruleus norepinephrine neurons [100–103] and to reduce norepinephrine release in target sites in a manner that is dependent on the galanin receptor [104, 105]. Collectively, these data support the possibility that wheel running mitigates the effect of stress via norepinephrine-galanin mediated mechanisms.
Additional brain regions that are targeted by the locus coeruleus may be involved in generating the anxiolytic-like and stress-buffering capacity offered by wheel running. Brain mapping studies for immediate early gene expression have already begun to identify such regions and show that wheel running attenuates stress-induced elevations of cFos in numerous structures relevant to the study of stress and anxiety, including the prelimbic and infralimbic cortex, lateral septum, subiculum, bed nucleus of the stria terminalis, paraventricular nucleus of the hypothalamus, striatum, preoptic area, dorsal medial hypothalamus, dorsal raphe, cuneiform nucleus, and locus coeruleus [18, 19, 106, 107]. In addition, brain mapping studies that utilize markers that accumulate over time (e.g., the truncated, splice variant of ΔFosB; 35–37 kD size) may help to identify anatomical locations that are responsible for the long-term neural adaptations required to express the affective benefits of wheel running.
5. Conclusions
A prolonged, voluntary exercise regimen produced anxiolytic-like effects in rats that also had a history of repeated stress, but failed to produce these effects in exercised rats tested under baseline conditions of stress or intense stress elicited by a high dose of a β-carboline. These data support the idea that chronic exercise exerts anxiolytic-potential in a manner that depends on the presence or absence of stress. Wheel running increased galanin gene expression in the locus coeruleus, suggesting galanin plays a role in exercise-mediated regulation of stress responsivity. Our data caution against interpreting exercise-induced increases in defensive behavior as anxiogenic, and are consistent with the conclusion that a chronic exercise regimen produces beneficial effects on anxiety.
Highlights.
Exercise does not reliably alter anxiety-like and defensive behaviors at baseline
Anxiolytic potential of exercise emerges after mild-to-moderate intensity stress
Locus coeruleus galanin is elevated by exercise & correlates with wheel running
A role for galanin in the beneficial consequences of wheel running is supported
Acknowledgments
This research was supported by National Institute of Drug Abuse (NIDA) grant DA027535A (to Philip V. Holmes) and NIDA Diversity Supplement (to DA027535AS1 for Natale R. Sciolino).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services. Physical activity guidelines advisory committee report. 2008. [DOI] [PubMed] [Google Scholar]
- 2.Herring MP, Jacob ML, Suveg C, Dishman RK, O’Connor PJ. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychotherapy and Psychosomatics. 2012;81:21–8. doi: 10.1159/000327898. [DOI] [PubMed] [Google Scholar]
- 3.Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Archives of Internal Medicine. 2010;170:321–31. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- 4.Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behavioral Brain Research. 2009;197:31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Research. 2008;1199:148–58. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder E, Droste SK, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behavioral Brain Research. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Dishman RK, Dunn AL, Youngstedt SD, Davis JM, Burgess ML, Wilson SP, et al. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiology and Behavior. 1996;60:699–705. doi: 10.1016/0031-9384(96)00102-3. [DOI] [PubMed] [Google Scholar]
- 8.Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behavioral Brain Research. 2010;207:321–31. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Experimental Neurology. 2010;224:106–13. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Gorton LM, Vuckovic MG, Vertelkina N, Petzinger GM, Jakowec MW, Wood RI. Exercise effects on motor and affective behavior and catecholamine neurochemistry in the MPTP-lesioned mouse. Behavioral Brain Research. 2010;213:253–62. doi: 10.1016/j.bbr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkins ME, Bucci DJ. BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiology of Learning and Memory. 2010;94:278–84. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietropaolo S, Feldon J, Alleva E, Cirulli F, Yee BK. The role of voluntary exercise in enriched rearing: a behavioral analysis. Behavioral Neuroscience. 2006;120:787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- 13.Grace L, Hescham S, Kellaway LA, Bugarith K, Russell VA. Effect of exercise on learning and memory in a rat model of developmental stress. Metabolic Brain Disease. 2009;24:643–57. doi: 10.1007/s11011-009-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20:364–76. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- 16.Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Research. 2004;1019:84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 17.Lancel M, Droste SK, Sommer S, Reul JM. Influence of regular voluntary exercise on spontaneous and social stress-affected sleep in mice. European Journal of Neuroscience. 2003;17:2171–9. doi: 10.1046/j.1460-9568.2003.02658.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Research. 2005;1033:164–78. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. Journal of Neuroscience. 2003;23:2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behavioral Neuroscience. 2008;122:943–8. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood BN, Strong PV, Brooks L, Fleshner M. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology (Berlin) 2008;199:209–22. doi: 10.1007/s00213-008-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behavioral Neuroscience. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- 23.Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, et al. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Research Bulletin. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- 24.Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, et al. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behavioral Brain Research. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniam J, Morris MJ. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: Role of hippocampus. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 26.De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, et al. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010;35:374–87. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- 28.Sothmann MS, Buckworth J, Claytor RP, Cox RH, White-Welkley JE, Dishman RK. Exercise training and the cross-stressor adaptation hypothesis. Exercise and Sport Sciences Reviews. 1996;24:267–87. [PubMed] [Google Scholar]
- 29.Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exercise and Sport Sciences Reviews. 2011;39:140–9. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Capdevila S, Portell-Cortes I, Torras-Garcia M, Coll-Andreu M, Costa-Miserachs D. Effects of long-term voluntary exercise on learning and memory processes: dependency of the task and level of exercise. Behavioral Brain Research. 2009;202:162–70. doi: 10.1016/j.bbr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Blanchard RJ, Blanchard DC. Bringing natural behaviors into the laboratory: a tribute to Paul MacLean. Physiology and Behavior. 2003;79:515–24. doi: 10.1016/s0031-9384(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 32.De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. European Journal of Pharmacology. 2003;463:145–61. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- 33.Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neuroscience and Biobehavioral Reviews. 2001;25:205–18. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 34.Blanchard DC, Hynd AL, Minke KA, Minemoto T, Blanchard RJ. Human defensive behaviors to threat scenarios show parallels to fear- and anxiety-related defense patterns of non-human mammals. Neuroscience and Biobehavioral Reviews. 2001;25:761–70. doi: 10.1016/s0149-7634(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 35.Griebel G, Blanchard DC, Jung A, Lee JC, Masuda CK, Blanchard RJ. Further evidence that the mouse defense test battery is useful for screening anxiolytic and panicolytic drugs: effects of acute and chronic treatment with alprazolam. Neuropharmacology. 1995;34:1625–33. doi: 10.1016/0028-3908(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 36.Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacology Biochemistry and Behavior. 1981;15:619–26. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- 37.Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–56. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 38.Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, et al. Role of brain norepinephrine in the behavioral response to stress. Progress in Neuropsychopharmacology and Biological Psychiatry. 2005;29:1214–24. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–46. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- 40.Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, et al. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. Journal of Neuroscience. 1986;6:3640–54. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes PV, Crawley JN. Olfactory bulbectomy increases prepro-galanin mRNA levels in the rat locus coeruleus. Molecular Brain Research. 1996;36:184–8. doi: 10.1016/0169-328x(95)00295-4. [DOI] [PubMed] [Google Scholar]
- 42.Holets VR, Hokfelt T, Rokaeus A, Terenius L, Goldstein M. Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience. 1988;24:893–906. doi: 10.1016/0306-4522(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 43.Holmes PV, Yoo HS, Dishman RK. Voluntary exercise and clomipramine treatment elevate prepro-galanin mRNA levels in the locus coeruleus in rats. Neuroscience Letters. 2006;408:1–4. doi: 10.1016/j.neulet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 44.Reiss JI, Dishman RK, Boyd HE, Robinson JK, Holmes PV. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Res. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 45.Van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behavioral Neuroscience. 2004;118:1378–90. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- 46.Soares J, Holmes PV, Renner KJ, Edwards GL, Bunnell BN, Dishman RK. Brain noradrenergic responses to footshock after chronic activity-wheel running. Behavioral Neuroscience. 1999;113:558–66. doi: 10.1037//0735-7044.113.3.558. [DOI] [PubMed] [Google Scholar]
- 47.Legakis IN, Mantzouridis T, Saramantis A, Phenekos C, Tzioras C, Mountokalakis T. Human galanin secretion is increased upon normal exercise test in middle-age individuals. Endocrine Research. 2000;26:357–64. doi: 10.3109/07435800009066173. [DOI] [PubMed] [Google Scholar]
- 48.Holmes PV, Blanchard DC, Blanchard RJ, Brady LS, Crawley JN. Chronic social stress increases levels of preprogalanin mRNA in the rat locus coeruleus. Pharmacology Biochemistry and Behavior. 1995;50:655–60. doi: 10.1016/0091-3057(94)00334-3. [DOI] [PubMed] [Google Scholar]
- 49.Evans AK, Lowry CA. Pharmacology of the beta-carboline FG-7,142, a partial inverse agonist at the benzodiazepine allosteric site of the GABA A receptor: neurochemical, neurophysiological, and behavioral effects. CNS Drug Reviews. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C, et al. Anxiogenic properties of an inverse agonist selective for alpha3 subunit-containing GABA A receptors. British Journal of Pharmacology. 2005;144:357–66. doi: 10.1038/sj.bjp.0706056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Degroot A, Nomikos GG. Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. European Journal of Neuroscience. 2004;20:1059–64. doi: 10.1111/j.1460-9568.2004.03556.x. [DOI] [PubMed] [Google Scholar]
- 52.Sink KS, Segovia KN, Sink J, Randall PA, Collins LE, Correa M, et al. Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142. European Neuropsychopharmacology. 2010;20:112–22. doi: 10.1016/j.euroneuro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans AK, Lowry CA. Pharmacology of the beta-carboline FG-7,142, a partial inverse agonist at the benzodiazepine allosteric site of the GABA A receptor: neurochemical, neurophysiological, and behavioral effects. CNS Drug Rev. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohmer JG, Di Scala G, Sandner G. Behavioral analysis of the effects of benzodiazepine receptor ligands in the conditioned burying paradigm. Behav Brain Res. 1990;38:45–54. doi: 10.1016/0166-4328(90)90023-8. [DOI] [PubMed] [Google Scholar]
- 55.Brose N, O’Neill RD, Boutelle MG, Anderson SM, Fillenz M. Effects of an anxiogenic benzodiazepine receptor ligand on motor activity and dopamine release in nucleus accumbens and striatum in the rat. J Neurosci. 1987;7:2917–26. doi: 10.1523/JNEUROSCI.07-09-02917.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Degroot A, Nomikos GG. Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. Eur J Neurosci. 2004;20:1059–64. doi: 10.1111/j.1460-9568.2004.03556.x. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology. 2004;29:59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- 58.Johnston AL, File SE. Sodium phenobarbitone reverses the anxiogenic effects of compounds acting at three different central sites. Neuropharmacology. 1989;28:83–8. doi: 10.1016/0028-3908(89)90072-5. [DOI] [PubMed] [Google Scholar]
- 59.Peris J, Scott JD. FG7142 causes opposite changes in [3H]GABA release from nigrocollicular regions. Pharmacology Biochemistry and Behavior. 1993;44:333–8. doi: 10.1016/0091-3057(93)90470-e. [DOI] [PubMed] [Google Scholar]
- 60.Pritchard GA, Galpern WR, Lumpkin M, Miller LG. Chronic benzodiazepine administration. VIII. Receptor upregulation produced by chronic exposure to the inverse agonist FG-7142. Journal of Pharmacology and Experimental Therapeutics. 1991;258:280–5. [PubMed] [Google Scholar]
- 61.Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacological Research. 2011 doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–17. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 63.Blokland A, Ten Oever S, van Gorp D, van Draanen M, Schmidt T, Nguyen E, et al. The use of a test battery assessing affective behavior in rats: order effects. Behav Brain Res. 2012;228:16–21. doi: 10.1016/j.bbr.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 64.Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Voikar V, Vasar E, Rauvala H. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav. 2004;3:27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- 66.Echevarria DJ, Hernandez A, Diogenes A, Morilak DA. Administration of the galanin antagonist M40 into lateral septum attenuates shock probe defensive burying behavior in rats. Neuropeptides. 2005;39:445–51. doi: 10.1016/j.npep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Treit D, Pesold C. Septal lesions inhibit fear reactions in two animal models of anxiolytic drug action. Physiology and Behavior. 1990;47:365–71. doi: 10.1016/0031-9384(90)90155-w. [DOI] [PubMed] [Google Scholar]
- 68.Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. Journal of Neuroendocrinology. 2008;20:1115–37. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Primeaux SD, Holmes PV. Role of aversively motivated behavior in the olfactory bulbectomy syndrome. Physiology and Behavior. 1999;67:41–7. doi: 10.1016/s0031-9384(99)00027-x. [DOI] [PubMed] [Google Scholar]
- 70.Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- 71.Murray PS, Groves JL, Pettett BJ, Britton SL, Koch LG, Dishman RK, et al. Locus coeruleus galanin expression is enhanced after exercise in rats selectively bred for high capacity for aerobic activity. Peptides. 2010;31:2264–8. doi: 10.1016/j.peptides.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- 73.Cohen J. Statistical power analysis for the behavioral sciences. 2. New York: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- 74.Levine TR, Hullett CR. Eta squared, partial eta squared, and misreporting of effect size in communication research. Human Communication Research. 2002;28:612–25. [Google Scholar]
- 75.Pierce CA, Block RA, Aguinis H. Cautionary Note on Reporting Eta-Squared Values From Multifactor ANOVA Designs. Educational and Psychological Measurement. 2004;64:916–24. [Google Scholar]
- 76.Uchiumi K, Aoki M, Kikusui T, Takeuchi Y, Mori Y. Wheel-running activity increases with social stress in male DBA mice. Physiol Behav. 2008;93:1–7. doi: 10.1016/j.physbeh.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Hopkins ME, Bucci DJ. Interpreting the effects of exercise on fear conditioning: the influence of time of day. Behavioral Neuroscience. 2010;124:868–72. doi: 10.1037/a0021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pietropaolo S, Sun Y, Li R, Brana C, Feldon J, Yee BK. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav Brain Res. 2008;192:42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 79.Cacciaglia R, Krause-Utz A, Vogt MA, Schmahl C, Flor H, Gass P. Voluntary exercise does not ameliorate context memory and hyperarousal in a mouse model for post-traumatic stress disorder (PTSD) World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.583270. [DOI] [PubMed] [Google Scholar]
- 80.Garcia-Mesa Y, Lopez-Ramos JC, Gimenez-Llort L, Revilla S, Guerra R, Gruart A, et al. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J Alzheimers Dis. 2011;24:421–54. doi: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- 81.Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 82.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–65. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 83.Greenwood BN, Strong PV, Brooks L, Fleshner M. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology (Berl) 2008;199:209–22. doi: 10.1007/s00213-008-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dubreucq S, Marsicano G, Chaouloff F. Emotional consequences of wheel running in mice: Which is the appropriate control? Hippocampus. 2010 doi: 10.1002/hipo.20778. [DOI] [PubMed] [Google Scholar]
- 85.Taylor SC, Johnston AL, Wilks LJ, Nicholass JM, File SE, Little HJ. Kindling with the beta-carboline FG7142 suggests separation between changes in seizure threshold and anxiety-related behaviour. Neuropsychobiology. 1988;19:195–201. doi: 10.1159/000118460. [DOI] [PubMed] [Google Scholar]
- 86.Stanford SC, Baldwin HA, File SE. Effects of a single or repeated administration of the benzodiazepine inverse agonist FG7142 on behaviour and cortical adrenoceptor binding in the rat. Psychopharmacology (Berl) 1989;98:417–24. doi: 10.1007/BF00451698. [DOI] [PubMed] [Google Scholar]
- 87.Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Frontiers in Behavioral Neuroscience. 2010;4:12. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 89.Hoffmann P, Thoren P, Ely D. Effect of voluntary exercise on open-field behavior and on aggression in the spontaneously hypertensive rat (SHR) Behavioral and Neural Biology. 1987;47:346–55. doi: 10.1016/s0163-1047(87)90461-4. [DOI] [PubMed] [Google Scholar]
- 90.Gammie SC, Hasen NS, Rhodes JS, Girard I, Garland T., Jr Predatory aggression, but not maternal or intermale aggression, is associated with high voluntary wheel-running behavior in mice. Hormones and Behavior. 2003;44:209–21. doi: 10.1016/s0018-506x(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 91.Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behavioral Neuroscience. 2004;118:1123–7. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- 92.Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacology Biochemistry and Behavior. 2006;84:306–12. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 93.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 94.Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cellular and Molecular Neurobiology. 2010;30:493–503. doi: 10.1007/s10571-009-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP. Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6J mice. Neurobiology of Aging. 1985;6:17–24. doi: 10.1016/0197-4580(85)90066-1. [DOI] [PubMed] [Google Scholar]
- 97.Van Hoomissen J, Kunrath J, Dentlinger R, Lafrenz A, Krause M, Azar A. Cognitive and locomotor/exploratory behavior after chronic exercise in the olfactory bulbectomy animal model of depression. Behavioral Brain Research. 2011;222:106–16. doi: 10.1016/j.bbr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 98.Eisenstein SA, Holmes PV. Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats. Pharmacol Biochem Behav. 2007;86:607–15. doi: 10.1016/j.pbb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 99.O’Neal HA, Van Hoomissen JD, Holmes PV, Dishman RK. Prepro-galanin messenger RNA levels are increased in rat locus coeruleus after treadmill exercise training. Neuroscience Letters. 2001;299:69–72. doi: 10.1016/s0304-3940(00)01780-8. [DOI] [PubMed] [Google Scholar]
- 100.Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, Hokfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64:861–74. doi: 10.1016/0306-4522(94)00450-j. [DOI] [PubMed] [Google Scholar]
- 101.Xu ZQ, Tong YG, Hokfelt T. Galanin enhances noradrenaline-induced outward current on locus coeruleus noradrenergic neurons. Neuroreport. 2001;12:1779–82. doi: 10.1097/00001756-200106130-00052. [DOI] [PubMed] [Google Scholar]
- 102.Seutin V, Verbanck P, Massotte L, Dresse A. Galanin decreases the activity of locus coeruleus neurons in vitro. European Journal of Pharmacology. 1989;164:373–6. doi: 10.1016/0014-2999(89)90481-0. [DOI] [PubMed] [Google Scholar]
- 103.Sevcik J, Finta EP, Illes P. Galanin receptors inhibit the spontaneous firing of locus coeruleus neurones and interact with mu-opioid receptors. European Journal of Pharmacology. 1993;230:223–30. doi: 10.1016/0014-2999(93)90806-s. [DOI] [PubMed] [Google Scholar]
- 104.Kehr J, Yoshitake T, Wang FH, Wynick D, Holmberg K, Lendahl U, et al. Microdialysis in freely moving mice: determination of acetylcholine, serotonin and noradrenaline release in galanin transgenic mice. Journal of Neuroscience Methods. 2001;109:71–80. doi: 10.1016/s0165-0270(01)00403-4. [DOI] [PubMed] [Google Scholar]
- 105.Yoshitake T, Reenila I, Ogren SO, Hokfelt T, Kehr J. Galanin attenuates basal and antidepressant drug-induced increase of extracellular serotonin and noradrenaline levels in the rat hippocampus. Neuroscience Letters. 2003;339:239–42. doi: 10.1016/s0304-3940(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 106.Campeau S, Nyhuis TJ, Sasse SK, Kryskow EM, Herlihy L, Masini CV, et al. Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. Journal of Neuroendocrinology. 2010;22:872–88. doi: 10.1111/j.1365-2826.2010.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003;120:269–81. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]