Chemokine-induction activity of LL-37 in monocytes is dependent on Src-family kinase Lyn.
Keywords: monocyte, chemokine, signaling pathways
Abstract
Cathelicidin LL-37 is a multifunctional, immunomodulatory and antimicrobial host-defense peptide of the human immune system. Here, we identified the role of SFKs in mediating the chemokine induction activity of LL-37 in monocytic cells. LL-37 induced SFK phosphorylation; and chemical inhibitors of SFKs suppressed chemokine production in response to LL-37 stimulation. SFKs were required for the downstream activation of AKT, but Ca2+-flux and MAPK induction were SFK-independent. Through systematic siRNA knockdown of SFK members, a requirement for Lyn in mediating LL-37 activity was identified. The involvement of Lyn in cathelicidin activities was further confirmed using Lyn-knockout mouse BMDMs. The role of SFKs and Lyn was also demonstrated in the activities of the synthetic cationic IDR peptides, developed as novel, immunomodulatory therapeutics. These findings elucidate the common molecular mechanisms mediating the chemokine induction activity of natural and synthetic cationic peptides in monocytic cells and identify SFKs as a potential target for modulating peptide responses.
Introduction
LL-37 is a cathelicidin family host-defense peptide produced in humans [1, 2], and it has a close homologue in mice CRAMP (67% identity). LL-37 is primarily made by hematopoietic and epithelial cells and is released from its precursor human cathelicidin antimicrobial protein 18 by proteolytic cleavage. The mature peptide is cationic and amphipathic, with a charge of +6 at physiological pH and a length of 37 aa residues. LL-37 is only weakly antimicrobial under physiological conditions but has a broad spectrum of immunomodulatory activities, including stimulation of chemokine secretion by monocytes, as well as other cell types [3–9].
Natural cathelicidin peptides have been used as general templates for the production of synthetic derivatives with potent immunomodulatory properties, and a number of such compounds have entered clinical trials for their anti-infective activity [10]. Optimization of the properties of such peptides for therapeutic applications remains a major challenge, and we have recently developed peptides with enhanced activities through in vitro screening of iterative peptide libraries for chemokine induction in human blood mononuclear cells. This resulted in the development of peptide IDR-1002 with enhanced protective activities in mouse models of bacterial infections [11] and IDR-HH2 with adjuvant properties [12]. Further understanding of the signaling mechanisms mediating the chemokine induction activities of such synthetic peptides will facilitate future development and optimization of this class of therapeutics.
The signaling mechanisms mediating the activities of natural and synthetic immunomodulatory peptides are complex, and although many signal transducers have been identified, substantial variation occurs across different cell types, and our understanding of the signaling pathways remains incomplete. For example, the direct chemotactic activities of LL-37 in leukocytes are mediated via FPRL1 with the downstream induction of Ca2+ flux, whereas mast cell chemotaxis involves other as-yet-uncharacterized GPCRs [13, 14]. The antiapoptotic effects of LL-37 on neutrophils are mediated by the P2X7 receptor, GPCRs, and MAPKs [15, 16]. In contrast, chemokine secretion in LL-37-stimulated monocytes was shown to be independent of P2X7 or FPRL1 [17] but dependent on other GPCRs [18] and to require cytoskeletal integrity, intracellular uptake of the peptide, interactions with GAPDH [17], and activation of several pathways, including MAPK, AKT, and NF-κB [18]. Another influential receptor in LL-37 signaling is EGFR, which is transactivated by LL-37 to mediate IL-8 induction in epithelial cells, keratinocyte migration, and wound-healing activity [19–21]. Here, we considered the possibility that some of these biological activities of LL-37 might be transduced through the SFKs, which are downstream of several of the above-mentioned receptors, a possibility that was reinforced by pathway over-representation analysis of differential gene expression in human monocytes in response to LL-37 (unpublished results).
Tyrosine phosphorylation plays a critical role in a vast array of signal transduction pathways in mammalian cells. Src was the first tyrosine kinase to be discovered, based on the studies of the Rous sarcoma virus oncogene v-Src [22, 23]. Since then, a total of nine SFKs has been characterized: Blk, Fgr, Fyn, Hck, Lck, Lyn, Src, Yes, and Yrk [24]. All members of the family have a conserved, modular structure, consisting of SH3, SH2, and C-terminal catalytic domains [25], and are regulated through a reversible phosphorylation of two conserved tyrosine residues [26]. SFKs can be activated by a host of receptors, including GPCRs [27], receptor tyrosine kinases, such as EGFR and other growth factor receptors [28], and integrins that mediate cell adhesion and migration [29, 30]. SFKs participate in many cellular signaling networks, primarily involving ITAM motif receptor and adaptor proteins, and SFK phosphorylation of ITAM motifs creates binding sites for SH2 domain-containing signal transducers, which is followed by their activation and downstream propagation of the signal. Thus, SFK signaling networks are involved in the regulation of universal cellular processes, such as growth, proliferation, survival, adhesion, and migration, as well as in the regulation of many specialized activities of cells of the immune system [31, 32].
Previously, pharmacological inhibition of SFKs was shown to repress the LL-37-induced release of CXCL8/IL-8 in human airway smooth muscle cells [5]; however, to our knowledge, the role of the Src family in LL-37 activity was not explored further. Here, we establish the role of SFKs in LL-37 signaling and chemokine-induction activities in monocytic cells, identify Lyn as the Src family member involved using systematic siRNA knockdown and knockout studies, and demonstrate the role of SFKs in the downstream activation of AKT. The role of SFKs in the chemokine-induction activities of synthetic peptides was also demonstrated.
MATERIALS AND METHODS
Reagents
Peptides LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES), IDR-1002 (VQRWLIVWRIRK-NH2), and IDR-HH2 (VQLRIRVAVIRA-NH2) were synthesized using F-Moc chemistry at the Nucleic Acid/Protein Synthesis Unit or at the Brain Research Center (University of British Columbia, Vancouver, Canada), purified to >90% purity using RT-HPLC and analyzed by mass spectrometry. Inhibitors SU6656 and PP2 were purchased from Biosource International (Camarillo, CA, USA) or Calbiochem (San Diego, CA, USA) and resuspended in DMSO (Sigma-Aldrich, Oakville, ON, Canada). The final concentrations of DMSO in cell culture never exceeded 0.02% (v/v), and all experiments included DMSO vehicle controls. The inhibitors and peptides were checked against cytotoxic effects, using the WST-1 cell viability reagent (Roche Applied Sciences, Mannheim, Germany) or the LDH release cytotoxicity detection kit (Roche Applied Sciences). LPS from Pseudomonas aeruginosa strain H103 was purified using the Darveau-Hancock method [33].
Cell isolation and culture
The isolation of blood mononuclear cells was performed from the venous blood of healthy volunteers, collected into heparin-containing Vacutainer tubes (BD Biosciences, Franklin Lakes, NJ, USA), in accordance with the guidelines of the University of British Columbia Research Ethics Board. The blood was diluted 1:1 in PBS, pH 7.4 (Invitrogen, Burlington, ON, Canada), and separated by density gradient centrifugation over Ficoll-Paque Plus (GE Healthcare, Baie d'Urfe, Quebec, Canada). Mononuclear cell layers were collected; washed twice in PBS; seeded at 1 × 106 cells/ml in RPMI 1640 with 10% (v/v) heat-inactivated FCS, 2 mM L-glutamine, and 1 mM sodium pyruvate (all from Invitrogen); and maintained at 37°C and 5% CO2.
Human THP-1 cells (TIB-202; American Type Culture Collection, Manassas, VA, USA) were cultured in the same media and conditions for up to six passages. The cells seeded at 1 × 106 cells/ml were treated with PMA (60 ng/mL; Sigma-Aldrich) overnight to induce differentiation into plastic-adherent, macrophage-like cells and rested for 24 h before stimulation. MonoMac6 cells were maintained in the same media and conditions with further addition of 0.1 mM nonessential amino acids and 10 μg/ml human insulin (both from Invitrogen). Mouse BMDMs were prepared from the BM of WT and Lyn−/− C57BL/6J mice, using 7-day culture in high-glucose DMEM with 20% FCS, 2 mM l-glutamine, and 1 mM sodium pyruvate (all from Invitrogen), and supplemented with L-conditioned media (supernatant of cell line L-929). The Lyn−/− strain was described previously [34]; the mice were age- and sex-matched; and all of the experiments were in accordance with the Animal Care Ethics Guidelines of the University of British Columbia.
Western blotting
Cells were washed in ice-cold PBS with 1 mM vanadate (Sigma-Aldrich) and lysed in 10 mM Tris, 150 mM NaCl, 2 mM EDTA, 1% v/v Triton X-100, pH 7.4, supplemented with 1 mM PMSF and protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Protein concentration in the lysates was quantified using the BCA protein assay kit (Pierce, Thermo Scientific, Nepean, ON, Canada). Sample buffer (5×), containing 250 mM Tris, pH 6.8, 10% (w/v) SDS, 30% (v/v) glycerol, 0.5 M DTT, and 0.1% bromophenol blue, was added, and the lysates were denatured at 97°C for 7 min. The lysates were resolved on an 8% SDS-PAGE gel, followed by transfer at 95 V for 1 h to PVDF membranes (BioRad, Hercules, CA, USA). The membranes were blocked for 1 h in 5% (w/v) BSA in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% v/v Tween-20) and then incubated overnight at 4°C with antibodies against phospho-Src [Y416, rabbit polyclonal; the product note indicates “may cross-react with other Src family members (Lyn, Fyn, Lck, Yes, and Hck) when phosphorylated at equivalent sites”], phospho-CREB (S133, clone 87G3; product note indicates “this antibody also recognizes the phosphorylated form of the CREB-related factor ATF1”), phospho-AKT (S473, clone 193H12), and β-actin (all from Cell Signaling, Danvers, MA, USA) or GAPDH (Fitzgerald, Acton, MA, USA) in TBST with 1% (w/v) BSA or 5% (w/v) nonfat milk. The membranes were washed in TBST and developed with HRP-conjugated goat anti-rabbit IgG (Cell Signaling) or HRP-conjugated sheep anti-mouse IgG (GE Healthcare) and the chemiluminescence detection system (GE Healthcare).
siRNA gene silencing
Knockdown of Src, Lyn, Fyn, and Fgr was performed using Accell siRNA (Dharmacon, Thermo Fisher Scientific, Lafayette, CO, USA): THP-1 cells were treated with the targeting or nontargeting control Accell siRNA at 1 μM in Accell media (Dharmacon) for 96 h, differentiated with PMA overnight (60 ng/mL, Sigma-Aldrich), washed, and rested for 24 h. Knockdown of Hck and Yes was performed by lipid transfection: THP-1 cells were PMA-differentiated as previously and transfected with 100 nM targeting or nontargeting control siRNA using Dharmafect-2 (Dharmacon) overnight. All of the cells were stimulated with LL-37 (20 or 50 μg/mL) for 4 h. The siRNA treatments were checked for cytotoxicity using the LDH release colorimetric kit (Roche Applied Sciences) and the WST-1 cell proliferation reagent (Roche Applied Sciences). Knockdown efficiencies were confirmed by qRT-PCR, and the knockdowns of Lyn and Src were demonstrated further at the protein levels by Western blotting with the anti-Lyn (clone C13F9) and anti-Src (clone 36D10) mAb (Cell Signaling), followed by anti-rabbit HRP (Cell Signaling).
qRT-PCR
RNA was isolated using the RNeasy mini kit (Qiagen, Toronto, ON, Canada) or the MagNA Pure LC RNA isolation kit (Roche Applied Sciences), according to the manufacturers' protocols. Gene expression was analyzed by qRT-PCR using a SuperScript III Platinum Two-Step qRT-PCR kit with SYBR Green (Invitrogen) in the PRISM 7000 sequence detection system (Applied Biosystems, Carlsbad, CA, USA). Fold changes were determined by the comparative cycle threshold method and normalized against mRNA levels of the β2-microglobulin or GAPDH housekeeping gene. The primers were designed using the Roche online primer design tool: qpcr.probefinder.com.
Flow cytometry
The data were collected on FACSCalibur and analyzed using CellQuest Pro software (Becton Dickinson, Franklin Lakes, NJ, USA). For analysis of protein phosphorylation, the stimulated cells were fixed in 2% (w/v) paraformaldehyde in PBS for 15 min, permeabilized in PhosphoPerm Buffer III (BD Biosciences) on ice for 20 min, and washed in 0.5% (w/v) BSA in PBS. The cells were stained for 45 min for phospho-p38 (T180/Y182, antibody clone 3D7), phospho-p44/42 MAPK (T202/Y204, clone E10), phospho-CREB (S133, clone 87G3), and phospho-AKT (S473, clone 193H12; all antibodies from Cell Signaling). Following washing, the cells were stained further with goat anti-rabbit IgG Alexa Fluor 647 (Invitrogen) at 2 μg/ml or with goat anti-mouse IgG Alexa Fluor 488 (Sigma-Aldrich) at 0.8 μg/ml for 30 min. Alternatively, the cells were stained with anti-phospho-p65 NF-κB (S529, clone K10-895.12.50, BD Biosciences). All of the cells were counterstained with anti-CD14 Alexa Fluor 488 or 647 (clone M5E2) or anti-CD14-PE (clone HCD14; all from BioLegend, San Diego, CA, USA). For Ca-flux analysis, the cells were resuspended in HBSS, with 1 mM CaCl2, 1 mM MgCl2, and 1% (v/v) FCS (Invitrogen), at 1 × 107 cells/ml and loaded with Fluo-4 calcium-sensitive fluorescent dye (Invitrogen) at 2.5 μM for 20 min at 37°C. The cells were washed twice and analyzed by flow cytometry: baseline fluorescence of the cells in the fluorescence 1 channel was recorded for 1 min, followed by stimulation with LL-37 or with ionomycin (Calbiochem) as a positive control.
ELISA
The ELISAs were performed using anti-human CCL2/MCP-1 antibody, clones 5D3-F7 and 2H5 (eBioscience, San Diego, CA, USA); anti-human CXCL8/IL-8, clones 893A6G8 and 790A28G2 (Invitrogen); and anti-human TNF-α, clones mAb1 and mAb11 (eBioscience), followed by avidin-HRP (eBioscience), as per the manufacturers ' protocols. The ELISAs were developed with the tetramethylbenzidine liquid substrate system (Sigma-Aldrich) and imaged with the PowerWave ×340 plate-reader (BioTek Instruments, Winooski, VT, USA). Cytokine quantification was done against serial dilutions of recombinant cytokines (from R&D Systems, Minneapolis, MN, USA, or eBioscience).
Statistical analyses
Statistical analyses used Prism 4.0 software (GraphPad Software, La Jolla, CA, USA) with two-tailed Student's t test for comparisons of two datasets and ANOVA with Bonferroni's or Dunnett's post hoc tests for multiple comparisons.
RESULTS
SFK inhibitors suppress LL-37 activity in blood mononuclear cells
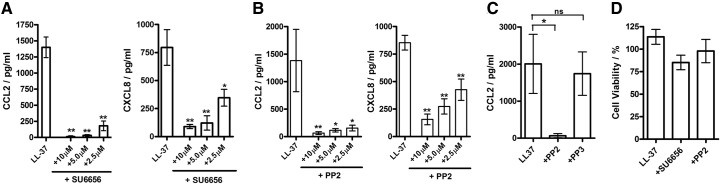
To establish the role of SFKs in LL-37 immunomodulatory activity, human blood mononuclear cells were pretreated with specific SFK chemical inhibitors SU6656 or PP2 for 1 h [35–37], stimulated with LL-37 at 20 μg/ml for a further 18 h, and analyzed for secretion of chemokines CCL2/MCP-1 and CXCL8/IL-8, which are induced in CD14+ monocytes but not lymphocytes or DCs at this LL-37 concentration [18]. The inhibitors caused an almost complete suppression of CCL2 and a strong suppression of CXCL8 production in response to LL-37 stimulation (Fig. 1A and B). The control compound PP3, which is chemically related to PP2 but does not bind SFKs [37], did not have any inhibitory activity, indicating that the effect of the PP2 inhibitor was SFK-specific (Fig. 1C). Neither the peptide nor the inhibitors showed significant cytotoxicity within the concentration range used in these experiments (Fig. 1D), and no inhibition of LPS-induced TNF-α production was seen, further confirming lack of nonspecific effects on other cellular activities (data not shown). All of the experiments using chemical inhibitors also included DMSO vehicle controls at levels identical to the inhibitor treatments, and no DMSO toxicity or effects on chemokine production were detected (data not shown). Overall, these findings suggested a role for SFKs in LL-37 signaling and immunomodulatory activity in blood mononuclear cells.
Figure 1. The suppression of LL-37-induced chemokine response by chemical inhibitors of SFKs in human blood mononuclear cells.
The cells were stimulated with 20 μg/ml LL-37 for 18 h, with the inhibitors added 1 h prior to the beginning of the stimulation at indicated concentrations. (A–C) LL-37-induced CCL2/MCP1 and CXCL8/IL-8 production was suppressed by SFK inhibitors (A) SU6656 and (B) PP2, (C) but not by the negative control compounds PP3. (D) LL-37 (20 μg/ml) and SFK inhibitors (10 μM) had minimal cytotoxicity over 18 h of treatment, as indicated by WST-1 cell viability assay. Bars represent means ± sem from three to five independent experiments. *P < 0.05; **P < 0.01 using ANOVA with Dunnett's multiple comparison post-test.
LL-37-induced, activating phosphorylation of SFKs in human monocytic cells
To confirm the role of SFKs in LL-37 signaling, human monocytic MonoMac6 cells were similarly stimulated with LL-37 and analyzed for SFK phosphorylation by Western blotting. Increased, activating SFK phosphorylation, at the residue equivalent to Y416 of Src, was detected at 10 min of stimulation (Fig. 2A). The effect was statistically significant, as shown by quantification of blots from five independent experiments (Fig. 2B). We further confirmed that the peptide was nontoxic to the cells at the concentration used in the experiments and over 18 h of stimulation, using the WST-1 cell viability assay (data not shown). As the anti-Src antibody cross-reacts with several SFK members when phosphorylated at an equivalent site (Src, Lyn, Fyn, Lck, Yes, and Hck), at this point, it was unknown which of the family members were specifically involved.
Figure 2. LL-37-induced SFK phosphorylation in monocytic cells.
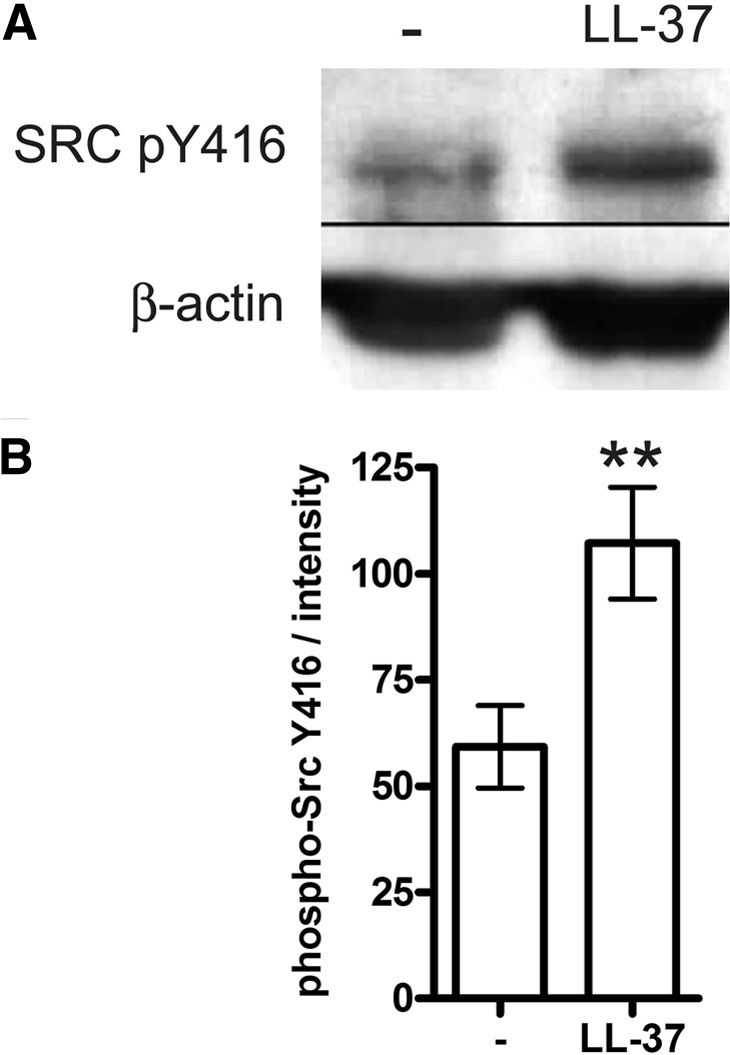
MonoMac6 cells were stimulated with LL-37 at 25 μg/ml for 10 min and blotted with the antibody for phospho (p)-Src Y416, which cross-reacts with other SFKs when phosphorylated at the equivalent residue. (A) Representative Western blot, showing induction of SFK phosphorylation in LL-37-stimulated cells. (B) Blot intensities quantified using ImageJ 1.43u software and normalized against the intensity of the loading control. The data are from five independent experiments. Bars show means ± sem. **P < 0.01 using paired t test.
Activation of AKT downstream of SFKs in LL-37-stimulated monocytic cells
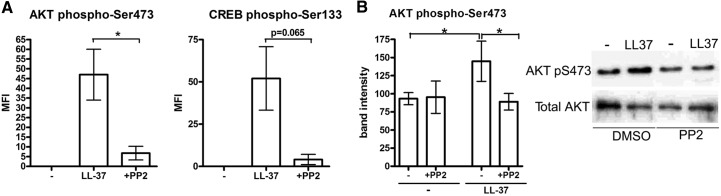
The signaling pathways activated downstream of SFKs in LL-37-stimulated monocytes were explored. Human blood mononuclear cells were pretreated with SFK inhibitor PP2, stimulated with LL-37 as previously, and analyzed for phosphorylation of AKT (S473), CREB (S133), and MAPKs by flow cytometry, gating on CD14+ monocytes. Significant inhibition of AKT phosphorylation was seen in the PP2-treated cells, indicating that the LL-37-induced activation of AKT was SFK-dependent (Fig. 3A). There was also a strong trend toward inhibition of CREB phosphorylation by PP2, suggesting a possible upstream role of SFKs in the activation of this transcription factor (P=0.065; Fig. 3A). In contrast, the LL-37-induced phosphorylation of ERK1/2 and p38 MAPKs was not affected by SFK inhibition (Supplemental Fig. 1A), and SFK activation was also not required for the LL-37-mediated induction of Ca2+ flux (Supplemental Fig. 1B).
Figure 3. The role of SFKs upstream of AKT in the signaling pathway activated by LL-37 in human monocytic cells.
(A) SFK inhibitor PP2 suppressed LL-37-induced activation of AKT in primary monocytes. Blood mononuclear cells, pretreated with inhibitor PP2 at 10 μM, or an equivalent concentration of DMSO vehicle control for 1 h were stimulated with LL-37 at 25 μg/ml for 30 min and analyzed by flow cytometry for phospho-AKT S473 or phospho-CREB S133. Mean fluorescence intensity (MFI) of CD14+ monocytes following background subtraction of the mean fluorescence intensity of unstimulated monocytes is shown. Bars represent means ± sem from four independent experiments. *P < 0.05 using paired t test. (B) SFK inhibitor PP2 suppressed LL-37-induced activation of AKT in monocytic cells MonoMac6. The cells, pretreated with inhibitor PP2 or an equivalent concentration of DMSO vehicle control for 1 h, were stimulated with LL-37 at 25 μg/ml for 30 min and analyzed for phospho-AKT S473 by Western blotting. Band intensities were quantified using ImageJ 1.43u software and normalized against the intensity of total AKT or β-actin-loading control; bars show means ± sem from four experiments. *P < 0.05 using paired ANOVA.
The role of SFKs in the LL-37-mediated activation of AKT was confirmed further by Western blotting using MonoMac6 monocytes. The cells were pretreated with PP2 and stimulated with LL-37 as previously, and the LL-37-induced AKT phosphorylation was suppressed consistently by PP2 pretreatment, as demonstrated by quantification of blots from four experiments (Fig. 3B).
LL-37 activity in human monocytic cells was partly dependent on Lyn
To identify the specific SFK members involved in LL-37 signaling activity in monocytic cells, six family members known to be expressed in this cell type [24, 32] were transiently knocked down one at a time using siRNA in a monocyte cell line. Each knockdown was repeated in three to five independent experiments, confirmed by qRT-PCR, and was, on average, 74% effective. The knockdown of Lyn and Src was confirmed further at the protein level by Western blotting (Fig. 4A).
Figure 4. LL-37 chemokine induction activity in human monocytic cells was dependent on Lyn.
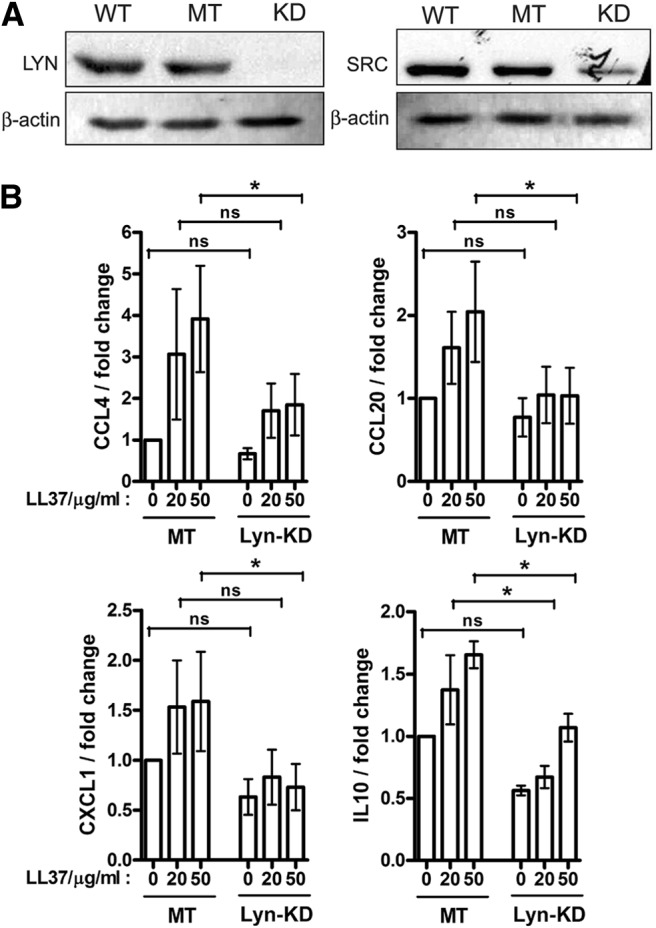
(A) Confirmation of Lyn and Src knockdown in monocytic THP-1 cells by Western blotting of WT, mock-transfected (MT), and knockdown (KD) cell lysates, using β-actin as the loading control. (B) Reduced induction of CCL4, CCL20, CXCL1, and IL-10 in the Lyn-knockdown (Lyn-KD) compared with control mock-transfected THP-1 cells at 4 h of LL-37 stimulation (20 and 50 μg/ml). qRT-PCR data were normalized against the β2-microglubulin housekeeping gene and expressed as fold change relative to unstimulated, mock-transfected samples. Bars represent means ± sem from five independent experiments; statistical analysis by two-way ANOVA indicated that Lyn-knockdown impacted on the production of all four cytokines (P<0.05); and follow-up comparisons of individual datasets were performed with Bonferroni's multiple comparison post hoc test (*P<0.05).
The siRNA knockdown and mock-treated control cells were stimulated with LL-37 for 4 h and analyzed for induction of chemokines and cytokine gene expression CCL4, CCL20, IL-10, and CXCL1 by qRT-PCR, as these were previously shown to be the most consistently induced genes in response to LL-37 in this cell type [17]. The knockdown of Src, Fyn, Fgr, Hck, or Yes (one at a time) did not result in a statistically significant inhibition of LL-37 responses (Supplemental Fig. 2). However, the knockdown of Lyn affected LL-37 responses, resulting in a significant reduction in the induction of all four cytokine and chemokine genes, indicating the role of Lyn in LL-37 signaling activity (Fig. 4B).
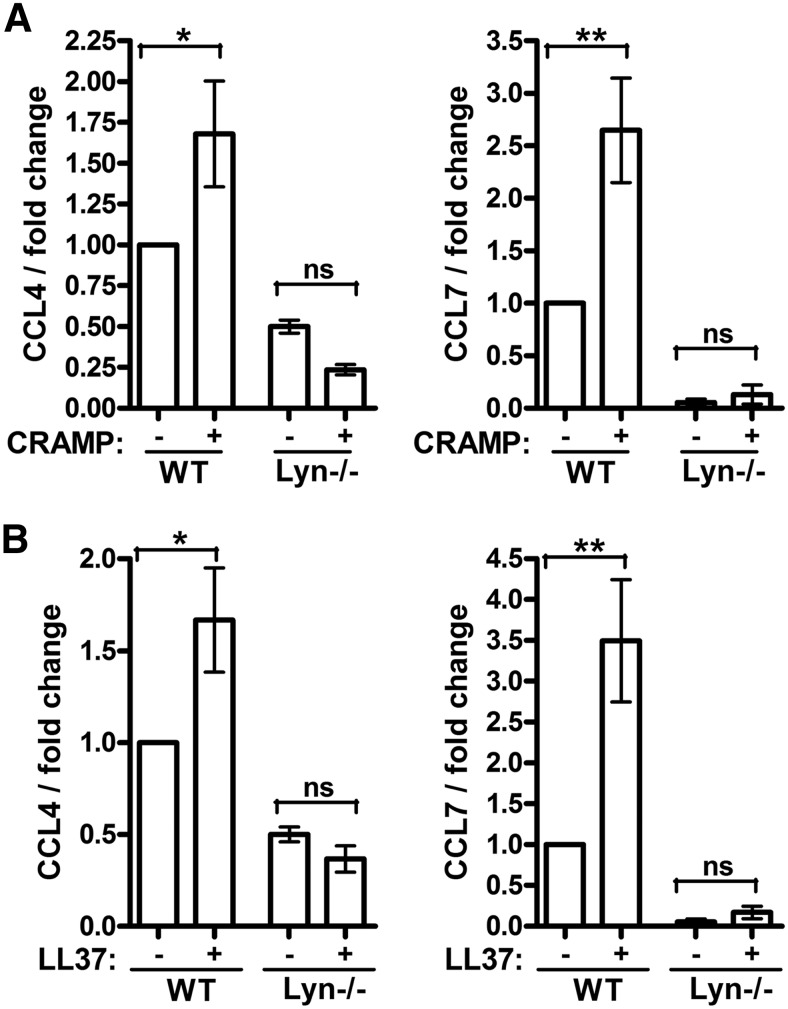
To further test the role of Lyn in the signaling and immunomodulatory activity of host-defense peptides, WT and Lyn−/− BMDM cells from mice were stimulated with the mouse cathelicidin peptide CRAMP (a 67% identical homologue of LL-37) and LL-37 and analyzed for chemokine gene expression by qRT-PCR. Induction of CCL4 and CCL7 in response to CRAMP and LL-37 was reduced significantly in Lyn−/− cells (Fig. 5A and B; significant differences between the untreated and peptide-stimulated cells were seen only for the WT cells, whereas for the Lyn−/− cells, the difference was nonsignificant, and in fact, the trend in the data was toward lower CCL4 transcript levels in the peptide-stimulated, as compared with untreated, Lyn−/− cells; analysis by ANOVA). Overall, these data support a requirement for Lyn in the signaling activity of cathelicidin peptides in monocytic cells. Baseline expression of the chemokines was also reduced in the knockout cells, indicating that basal levels of the chemokine expression in unstimulated cells were also dependent on Lyn.
Figure 5. Requirement for Lyn in the activity of cathelicidin peptides on mouse macrophages.
BMDM cells from WT and Lyn−/− mice were stimulated with (A) mouse cathelicidin CRAMP or (B) LL-37 at 50 μg/ml for 24 h and analyzed for CCL4 and CCL7 induction by qRT-PCR. The data were normalized against the GAPDH housekeeping gene and expressed as fold change relative to unstimulated WT sample. Bars represent means ± sem from three animals. *P < 0.05; **P < 0.01 using ANOVA with Bonferroni's multiple comparison post hoc test.
Antiendotoxic activity of LL-37 is independent of SFKs
In addition to its chemokine-induction activity on monocytic cells, LL-37 is known to inhibit proinflammatory responses to endotoxin, and this is mediated through direct endotoxin binding and through modulation of signaling responses downstream of TLR4 [38, 39]. As the SFK Lyn was recently shown to act as a negative regulator of TLR4 signaling [40], the role of SFKs in the antiendotoxic activity of LL-37 was tested. Blood mononuclear cells were pretreated with SFK inhibitors, stimulated with LPS and LL-37, and analyzed for TNF-α secretion and for the activation of signaling mediators p65 NF-κB and p38 MAPK. SFK inhibition had no effect on the antiendotoxic activities of LL-37 (Supplemental Fig. 3), indicating that the antiendotoxic properties of the peptide are SFK-independent.
Synthetic therapeutic peptides signal through SFK-dependent signaling pathways
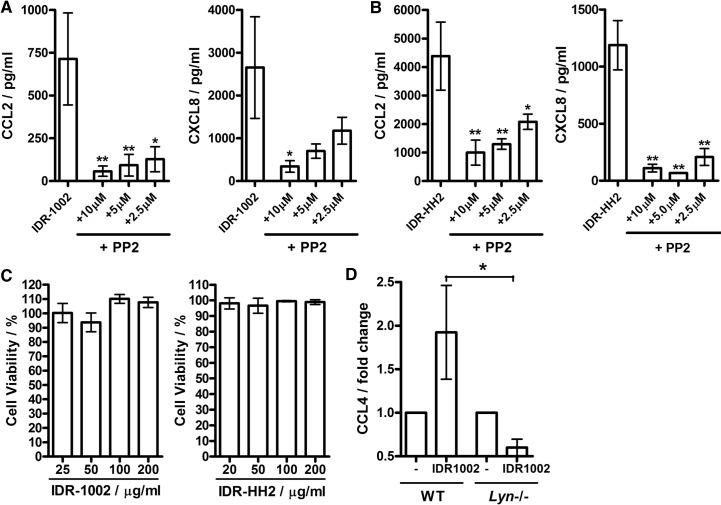
To test the role of SFKs in the activities of synthetic IDR peptides, blood mononuclear cells were pretreated with SFK inhibitor PP2, stimulated with IDR-1002 and IDR-HH2, and analyzed for chemokine secretion. Strong inhibition of the response was observed in the presence of the SFK inhibitor (Fig. 6A), demonstrating the role for SFKs in chemokine induction by IDR peptides, which had minimal cytotoxicity at the concentrations used in the study (Fig. 6B). Furthermore, responses to IDR-1002 were also impaired in Lyn−/− BMDM cells (Fig. 6C). This suggests that SFKs are a common mediator of the immunomodulatory properties of natural and synthetic cationic peptides in monocytic cells.
Figure 6. The role of SFKs in the signaling activity of synthetic therapeutic peptides IDR-1002 and IDR-HH2.
(A and B) SFK inhibitor PP2 suppressed IDR-induced chemokine responses in blood mononuclear cells. The cells were stimulated with 100 μg/ml peptides (A) IDR-1002 or (B) IDR-HH2 for 18 h with the inhibitors added 1 h prior to the beginning of the stimulation at the indicated concentrations. (C) IDR peptides showed minimal cytotoxicity over the 20- to 200-μg/ml concentration range, measured using a LDH release assay at 24 h of stimulation. (D) Requirement for Lyn in the chemokine-induction activity of IDR-1002 on mouse macrophages: BMDM cells from WT and Lyn−/− mice were stimulated with IDR-1002 at 100 μg/ml for 24 h and analyzed for CCL4 induction by qRT-PCR. The data were normalized against the GAPDH housekeeping gene. Bars represent means ± sem from three to five biological replicates. *P < 0.05; **P < 0.01 using (A and B) ANOVA with Dunnett's multiple comparison post hoc test or (D) t-test.
DISCUSSION
This work established the role of SFKs in the chemokine-induction activities of LL-37 in monocytic cells, demonstrated the role of SFKs in the downstream activation of AKT, and has identified Lyn as the main SFK member involved, using siRNA knockdown and knockout studies. We also demonstrated the role of SFKs in the activities of synthetic cationic peptides, developed as potential therapeutics for anti-infective and adjuvant properties. Overall, these findings expand our understanding of the common signaling pathways mediating the chemokine-induction activities of natural and synthetic cationic peptides.
LL-37 uses multiple receptors that are known to have the potential to activate SFKs, including GPCRs [27], and receptor tyrosine kinases, such as EGFR [28]. The chemokine-induction activities of LL-37 in monocytes require GPCRs [18], are independent of FPRL1 or P2X7 [17], and also depend on the intracellular localization of the peptide and its interaction with GAPDH [17]. Although the mechanisms linking GPCRs and GAPDH to LL-37 signaling are at present poorly understood, it is worth noting that cross-talk of GPCRs with SFKs has been widely documented in other signaling pathways [27]. Furthermore, in addition to its function as a glycolytic enzyme, GAPDH regulates nonmetabolic cellular processes, such as vesicular trafficking, apoptosis, and autophagy [41, 42], and some of these activities require GAPDH association and phosphorylation by SFKs [43, 44].
Whereas we have established a major role for SFKs in AKT activation and chemokine induction in LL-37-stimulated monocytes, the signaling pathways upstream and downstream of SFKs and AKT are likely complex. PI3Ks may play an intermediate role in the SFK-dependent AKT activation, as PI3K activity is required for chemokine induction in response to LL-37 in monocytes [18] and as the PI3K-to-AKT signaling module has been reported in other signaling pathways downstream of SFKs [45, 46]. Whereas transcription factor CREB can be induced directly by AKT [47], our data indicated that other pathways may also be involved in its LL-37-dependent activation. Thus, SFK inhibitors strongly suppressed the induction of AKT, whereas inhibition of CREB did not reach statistical significance (P=0.065; Fig. 3A), suggesting that other SFK-independent mechanisms were partially responsible for its activation. Overall, it is important to emphasize that the linear signaling pathways discussed here and in most similar studies are highly useful but oversimplified models and should be more accurately represented as signaling networks, with multiple complex interconnections among all of the cellular signaling mediators. Such signaling networks integrate information from a multitude of extracellular stimuli and allow precise modulation of cellular biological response. Modeling of such signaling networks using systems biology approaches will be required for a more complete understanding of chemokine induction in response to LL-37 stimulation in monocytes [18].
The functions of the different members of the Src family in LL-37 signaling were investigated using siRNA knockdown in human cells, followed by studies with Lyn−/− murine macrophages. Knockdown of Lyn affected cytokine and chemokine gene expression in response to LL-37, although its effects were less profound compared with pharmacological inhibition of Src family activity, indicating a requirement for Lyn, with possible partial redundancy and/or compensation by other SFK members. Redundancy and overlap in function between SFKs have been reported in many biological processes, including macrophage phagocytosis [48, 49], and the signaling by FcRs, cytokine receptors, and the BCR complex [49–51]. Nevertheless, the fact that responses to cathelicidin stimulation were altered in human Lyn-knockdown and mouse Lyn−/− systems suggests that the involvement of SFKs, in general, and Lyn, in particular, in the signaling activity of cathelicidin peptides was conserved across both species. Furthermore, the requirement for SFKs also extended to synthetic IDR peptides, and we demonstrated that Lyn was the family member involved at least for IDR-1002. The IDR peptides share many biological activities of LL-37 but no direct similarity in sequence or structure apart from their cationic nature [10].
Overall, this study has demonstrated the common role of SFKs and Lyn in the chemokine induction activity of LL-37 and synthetic cationic peptides in monocytic cells. The study broadens our knowledge of the biochemical mechanisms mediating the immunomodulatory properties of natural and synthetic peptides, which may lead to better therapeutic intervention in disorders associated with altered peptide function or facilitate development of synthetic peptides with optimized therapeutic activities.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the financial support from the Foundation for the National Institutes of Health and the Bill and Melinda Gates Foundation through two separate program grants from the Grand Challenges in Global Health Initiative and from the CIHR (to R.E.W.H. and K.W.H.). A.N. was a CIHR and MSFHR postdoctoral fellow. N.C.J.F. was the recipient of studentships from the Natural Sciences and Engineering Research Council and the MSFHR. R.E.W.H. is a Canada Research Chair in Microbiology, and K.W.H. is a Canada Research Chair in Immunology.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- AKT
- PKB
- BMDM
- bone marrow-derived macrophage
- CIHR
- Canadian Institutes for Health Research
- CRAMP
- cathelicidin-related antimicrobial peptide
- FPRL1
- FPR-like 1
- IDR
- innate defense regulator
- Lyn−/−
- Lyn-deficient
- MSFHR
- Michael Smith Foundation for Health Research
- P2X7
- P2X purinoceptor 7
- qRT-PCR
- quantitative RT-PCR
- SFK
- Src family tyrosine kinase
- SH
- Src homology
- siRNA
- small interfering RNA
- WST-1
- 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt
AUTHORSHIP
A.N., J.P., P.C., N.C.J.F., R.F., and A.R. performed the experiments; R.E.W.H., K.W.H., and A.N. supervised the work; and A.N. and R.E.W.H. wrote the paper.
DISCLOSURES
R.E.W.H. has cofounded companies for the exploitation of host-defense peptides as antimicrobials (Migenix, Vancouver, BC, Canada) and immunomodulators (Inimex Pharmaceuticals, Vancouver, BC, Canada). In both, he is a minor shareholder.
REFERENCES
- 1. Zanetti M. (2004) Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75, 39–48 [DOI] [PubMed] [Google Scholar]
- 2. Nijnik A., Hancock R. E. W. (2009) The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 16, 41–47 [DOI] [PubMed] [Google Scholar]
- 3. Scott M. G., Davidson D. J., Gold M. R., Bowdish D., Hancock R. E. (2002) The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 169, 3883–3891 [DOI] [PubMed] [Google Scholar]
- 4. Tjabringa G. S., Aarbiou J., Ninaber D. K., Drijfhout J. W., Sorensen O. E., Borregaard N., Rabe K. F., Hiemstra P. S. (2003) The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 171, 6690–6696 [DOI] [PubMed] [Google Scholar]
- 5. Zuyderduyn S., Ninaber D. K., Hiemstra P. S., Rabe K. F. (2006) The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J. Allergy Clin. Immunol. 117, 1328–1335 [DOI] [PubMed] [Google Scholar]
- 6. Filewod N. C., Pistolic J., Hancock R. E. (2009) Low concentrations of LL-37 alter IL-8 production by keratinocytes and bronchial epithelial cells in response to proinflammatory stimuli. FEMS Immunol. Med. Microbiol. 56, 233–240 [DOI] [PubMed] [Google Scholar]
- 7. Cosseau C., Devine D. A., Dullaghan E., Gardy J. L., Chikatamarla A., Gellatly S., Yu L. L., Pistolic J., Falsafi R., Tagg J., Hancock R. E. (2008) The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect. Immun. 76, 4163–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braff M. H., Hawkins M. A., Di Nardo A., Lopez-Garcia B., Howell M. D., Wong C., Lin K., Streib J. E., Dorschner R., Leung D. Y., Gallo R. L. (2005) Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J. Immunol. 174, 4271en]4278 [DOI] [PubMed] [Google Scholar]
- 9. Niyonsaba F., Ushio H., Nagaoka I., Okumura K., Ogawa H. (2005) The human β-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 175, 1776–1784 [DOI] [PubMed] [Google Scholar]
- 10. Easton D. M., Nijnik A., Mayer M. L., Hancock R. E. (2009) Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 27, 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nijnik A., Madera L., Ma S., Waldbrook M., Elliott M. R., Easton D. M., Mayer M. L., Mullaly S. C., Kindrachuk J., Jenssen H., Hancock R. E. (2010) Synthetic cationic peptide IDR-1002 provides protection against bacterial infections through chemokine induction and enhanced leukocyte recruitment. J. Immunol. 184, 2539–2550 [DOI] [PubMed] [Google Scholar]
- 12. Kindrachuk J., Jenssen H., Elliott M., Townsend R., Nijnik A., Lee S. F., Gerdts V., Babiuk L. A., Halperin S. A., Hancock R. E. (2009) A novel vaccine adjuvant comprised of a synthetic innate defence regulator peptide and CpG oligonucleotide links innate and adaptive immunity. Vaccine 27, 4662–4671 [DOI] [PubMed] [Google Scholar]
- 13. De Y., Chen Q., Schmidt A. P., Anderson G. M., Wang J. M., Wooters J., Oppenheim J. J., Chertov O. (2000) LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192, 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niyonsaba F., Iwabuchi K., Someya A., Hirata M., Matsuda H., Ogawa H., Nagaoka I. (2002) A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106, 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagaoka I., Tamura H., Hirata M. (2006) An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J. Immunol. 176, 3044–3052 [DOI] [PubMed] [Google Scholar]
- 16. Barlow P. G., Li Y., Wilkinson T. S., Bowdish D. M., Lau Y. E., Cosseau C., Haslett C., Simpson A. J., Hancock R. E., Davidson D. J. (2006) The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J. Leukoc. Biol. 80, 509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mookherjee N., Lippert D. N., Hamill P., Falsafi R., Nijnik A., Kindrachuk J., Pistolic J., Gardy J., Miri P., Naseer M., Foster L. J., Hancock R. E. (2009) Intracellular receptor for human host defense peptide LL-37 in monocytes. J. Immunol. 183, 2688–2696 [DOI] [PubMed] [Google Scholar]
- 18. Mookherjee N., Hamill P., Gardy J., Blimkie D., Falsafi R., Chikatamarla A., Arenillas D. J., Doria S., Kollmann T. R., Hancock R. E. (2009) Systems biology evaluation of immune responses induced by human host defence peptide LL-37 in mononuclear cells. Mol. Biosyst. 5, 483–496 [DOI] [PubMed] [Google Scholar]
- 19. Tokumaru S., Sayama K., Shirakata Y., Komatsuzawa H., Ouhara K., Hanakawa Y., Yahata Y., Dai X., Tohyama M., Nagai H., Yang L., Higashiyama S., Yoshimura A., Sugai M., Hashimoto K. (2005) Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 175, 4662–4668 [DOI] [PubMed] [Google Scholar]
- 20. Carretero M., Escamez M. J., Garcia M., Duarte B., Holguin A., Retamosa L., Jorcano J. L., Rio M. D., Larcher F. (2008) In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J. Invest. Dermatol. 128, 223–236 [DOI] [PubMed] [Google Scholar]
- 21. Yin J., Yu F. S. (2010) LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Invest. Ophthalmol. Vis. Sci. 51, 1891–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin G. S. (2001) The hunting of the Src. Nat. Rev. Mol. Cell. Biol. 2, 467–475 [DOI] [PubMed] [Google Scholar]
- 23. Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. (1976) DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature 260, 170–173 [DOI] [PubMed] [Google Scholar]
- 24. Korade-Mirnics Z., Corey S. J. (2000) Src kinase-mediated signaling in leukocytes. J. Leukoc. Biol. 68, 603–613 [PubMed] [Google Scholar]
- 25. Boggon T. J., Eck M. J. (2004) Structure and regulation of Src family kinases. Oncogene 23, 7918–7927 [DOI] [PubMed] [Google Scholar]
- 26. Roskoski R., Jr. (2005) Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 331, 1–14 [DOI] [PubMed] [Google Scholar]
- 27. Luttrell D. K., Luttrell L. M. (2004) Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene 23, 7969–7978 [DOI] [PubMed] [Google Scholar]
- 28. Bromann P. A., Korkaya H., Courtneidge S. A. (2004) The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23, 7957–7968 [DOI] [PubMed] [Google Scholar]
- 29. Playford M. P., Schaller M. D. (2004) The interplay between Src and integrins in normal and tumor biology. Oncogene 23, 7928–7946 [DOI] [PubMed] [Google Scholar]
- 30. Lowell C. A., Berton G. (1999) Integrin signal transduction in myeloid leukocytes. J. Leukoc. Biol. 65, 313–320 [DOI] [PubMed] [Google Scholar]
- 31. Abram C. L., Lowell C. A. (2008) The diverse functions of Src family kinases in macrophages. Front. Biosci. 13, 4426–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lowell C. A. (2004) Src-family kinases: rheostats of immune cell signaling. Mol. Immunol. 41, 631–643 [DOI] [PubMed] [Google Scholar]
- 33. Darveau R. P., Hancock R. E. (1983) Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol. 155, 831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hibbs M. L., Tarlinton D. M., Armes J., Grail D., Hodgson G., Maglitto R., Stacker S. A., Dunn A. R. (1995) Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell 83, 301–311 [DOI] [PubMed] [Google Scholar]
- 35. Geahlen R. L., Handley M. D., Harrison M. L. (2004) Molecular interdiction of Src-family kinase signaling in hematopoietic cells. Oncogene 23, 8024–8032 [DOI] [PubMed] [Google Scholar]
- 36. Blake R. A., Broome M. A., Liu X., Wu J., Gishizky M., Sun L., Courtneidge S. A. (2000) SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol. Cell. Biol. 20, 9018–9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., Connelly P. A. (1996) Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 [DOI] [PubMed] [Google Scholar]
- 38. Mookherjee N., Brown K. L., Bowdish D. M., Doria S., Falsafi R., Hokamp K., Roche F. M., Mu R., Doho G. H., Pistolic J., Powers J. P., Bryan J., Brinkman F. S., Hancock R. E. (2006) Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 176, 2455–2464 [DOI] [PubMed] [Google Scholar]
- 39. Kandler K., Shaykhiev R., Kleemann P., Klescz F., Lohoff M., Vogelmeier C., Bals R. (2006) The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int. Immunol. 18, 1729–1736 [DOI] [PubMed] [Google Scholar]
- 40. Keck S., Freudenberg M., Huber M. (2010) Activation of murine macrophages via TLR2 and TLR4 is negatively regulated by a Lyn/PI3K module and promoted by SHIP1. J. Immunol. 184, 5809–5818 [DOI] [PubMed] [Google Scholar]
- 41. Sirover M. A. (1999) New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta 1432, 159–184 [DOI] [PubMed] [Google Scholar]
- 42. Colell A., Ricci J. E., Tait S., Milasta S., Maurer U., Bouchier-Hayes L., Fitzgerald P., Guio-Carrion A., Waterhouse N. J., Li C. W., Mari B., Barbry P., Newmeyer D. D., Beere H. M., Green D. R. (2007) GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 129, 983–997 [DOI] [PubMed] [Google Scholar]
- 43. Tisdale E. J., Artalejo C. R. (2007) A GAPDH mutant defective in Src-dependent tyrosine phosphorylation impedes Rab2-mediated events. Traffic 8, 733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tisdale E. J., Artalejo C. R. (2006) Src-dependent aprotein kinase C iota/lambda (aPKCiota/lambda) tyrosine phosphorylation is required for aPKCiota/lambda association with Rab2 and glyceraldehyde-3-phosphate dehydrogenase on pre-golgi intermediates. J. Biol. Chem. 281, 8436–8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hennessy B. T., Smith D. L., Ram P. T., Lu Y., Mills G. B. (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 4, 988–1004 [DOI] [PubMed] [Google Scholar]
- 46. Franke T. F., Kaplan D. R., Cantley L. C., Toker A. (1997) Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275, 665–668 [DOI] [PubMed] [Google Scholar]
- 47. Du K., Montminy M. (1998) CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 273, 32377–32379 [DOI] [PubMed] [Google Scholar]
- 48. Lowell C. A., Soriano P., Varmus H. E. (1994) Functional overlap in the src gene family: inactivation of hck and fgr impairs natural immunity. Genes Dev. 8, 387–398 [DOI] [PubMed] [Google Scholar]
- 49. Fitzer-Attas C. J., Lowry M., Crowley M. T., Finn A. J., Meng F., DeFranco A. L., Lowell C. A. (2000) Fcγ receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J. Exp. Med. 191, 669–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kobayashi N., Kono T., Hatakeyama M., Minami Y., Miyazaki T., Perlmutter R. M., Taniguchi T. (1993) Functional coupling of the src-family protein tyrosine kinases p59fyn and p53/56lyn with the interleukin 2 receptor: implications for redundancy and pleiotropism in cytokine signal transduction. Proc. Natl. Acad. Sci. USA 90, 4201–4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saijo K., Schmedt C., Su I. H., Karasuyama H., Lowell C. A., Reth M., Adachi T., Patke A., Santana A., Tarakhovsky A. (2003) Essential role of Src-family protein tyrosine kinases in NF-κB activation during B cell development. Nat. Immunol. 4, 274–279 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.