P-glycoprotein activity in the thymus provides a mechanism whereby protease inhibitors effectively suppress HIV-1 replication in thymocytes
Keywords: HIV-1, thymus, children, multidrug resistance 1, T cells
Abstract
The thymus harbors HIV-1 and supports its replication. Treatment with PI-containing ART restores thymic output of naïve T cells. This study demonstrates that CXCR4-using WT viruses are more sensitive to PI in fetal thymcocytes than mature T cells with average IC50 values for two PIs, RTV and IDV, of 1.5 nM (RTV) and 4.4 nM (IDV) in thymocytes versus 309.4 nM (RTV) and 27.3 nM (IDV) in mature T cells. P-gp activity, as measured using Rh123 efflux and quantitation of P-gp mRNA, increased with thymocyte maturation into CD4 and CD8 lineage T cells. P-gp activity is developmentally regulated in the thymus. Thymocytes developed increased levels of P-gp activity as maturation from DP to SP CD4 or CD8 T cells occurred, although CD4 T cells acquired activity more rapidly. Reduced P-gp activity in thymocytes is one mechanism for effectiveness of PI therapy in suppressing viral replication in the thymus and in reconstitution of naïve T cells, particularly among children receiving PI-containing ART.
Introduction
The thymus, the primary lymphoid organ involved in T cell maturation and differentiation, is an important tissue target for HIV-1 infection [1, 2]. Thymocytes are susceptible to infection in vivo and ex vivo, predominantly by viruses that use the CXCR4 coreceptor, providing a milieu for emergence of these viruses in vivo [3–6]. As a result, HIV-1 infection leads to decreased thymic output and aberrant T cell maturation [7, 8]. When combination ART controls viral replication, thymic output is restored, particularly in children who have greater age-related thymic capacity [9–11]. Many HIV-infected children and adults treated with PI-containing ART display viral rebound with drug-resistant HIV-1 variants yet continue to sustain gains in naïve T cell numbers [10, 12–15]. One possible explanation for this paradox is that PI-resistant viruses are attenuated for replication in the thymus [16–19]. Alternatively, increased PI intacellular concentrations within thymocytes compared with peripheral CD4 T cells could contribute to more effective clearance of WT virus from the thymus.
PIs are substrates for the ATP-binding cassette efflux drug transporters, of which P-gp is the predominant efflux pump that actively lowers intracellular drug levels for a broad range of substrates [20–25]. High P-gp activity within CD4 T cells leads to lower intracellular PI levels, contributing to viral rebound [24, 26, 27]. Lymphocytes of differing cellular phenotype and differentiation stage vary in their P-gp activity [24, 28–32]. For example, NK cells and CTLs display the highest levels of P-gp activity, suggesting a specific immunological function for P-gp within cytolytic cells [24, 28, 29, 31–34]. Within blood CD4 T cells, high levels of CXCR4 expression correlate with high levels of P-gp activity [35], and naïve CD45RA T cells have higher levels of P-gp activity when compared with memory CD45RO T cells [24, 28, 29, 31–34]. It is likely that P-gp activity impacts PI activity within thymic tissues as well, but no studies have directly examined P-gp activity or its impact on effectiveness of PIs in inhibiting viral replication within thymocytes. This study examines the effect of PI on viral replication within thymocytes and examines the effect of P-gp activity on viral clearance within the thymus. P-gp activity was evaluated in human thymocyte subsets at distinct maturational stages in the presence or absence of PIs, RTV or IDV, known P-gp substrates [36]. The studies were designed to explain, in part, the selective role for PI therapy in restoring thymic cellular output in the presence of ongoing replication of drug-resistant viruses.
MATERIALS AND METHODS
Thymocytes and PBMC cultures
This study was approved by the Institutional Review Board for Human Research at All Children's Hospital (St. Petersburg, FL, USA). Normal, postnatal thymuses were obtained from children 5 days to 4 months of age undergoing corrective cardiac surgery, or fetal thymus tissues from donors 20–24 weeks of gestation were obtained from Advanced Bioscience Resources (Alameda, CA, USA). Thymic single-cell suspensions were prepared and suspended in IMDM, supplemented with 1100 μg/ml delipidized BSA (BD Biosciences, San Jose, CA, USA), 85 μg/ml transferrin (BD Biosciences), 2 mM L-glutamine, 25 U/ml penicillin, and 25 μg/ml streptomycin (thymocyte culture medium), as described previously [6]. PBMCs were isolated from blood from normal, HIV-seronegative volunteers and cultured, as described previously [37]. Each experiment was done in triplicate wells using a different donor.
IC50 by PIs for HIV-1 replication in thymocytes or PBMCs
A replication-competent rCXCR-4-using virus, designated pretherapy HIV, with a pLAI.4 backbone and the gag-pol allele of a pretherapy virus from a pediatric patient [37], was used to infect fetal thymocytes and PBMCs. Ex vivo HIV-1 infection of thymocytes was performed as described previously [6] with slight modifications. Briefly, freshly prepared, nonstimulated thymocytes were infected with pretherapy HIV (MOI=0.01) for 2 h at 37°C and then cultured at 5 × 105 cells/well in 200 μl thymocyte culture medium supplemented with IL-2 (20 U/ml) and IL-4 (20 ng/ml) in the absence or presence of 0.0001–1 μM RTV or IDV (AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD, USA) in a 96-well V-bottom plate. RTV and IDV were dissolved as a stock solution in DMSO at a concentration of 13.8 mM and in H2O at a concentration of 14 mM, respectively. Following PHA stimulation, PBMCs were infected with pretherapy HIV (MOI=0.01) and cultured at 2 × 105 cells/well in 200 μl PBMC media in the absence or presence of 0.01–100 μM RTV or IDV in a 96-well round-bottom plate. Thymocytes and PBMCs were cultured for 10 days in a humidified 5% CO2 atmosphere with a 10% media change every 2 days. PI was maintained in the cultures throughout the course of infection. Supernatant p24 antigen levels were determined by the HIV-1 p24 antigen assay ELISA (PerkinElmer Life Sciences, Boston, MA, USA), according to the manufacturer's protocol. IC50 values were calculated with a nonlinear regression analysis using the GraphPad Prism software package (GraphPad Software, San Diego, CA, USA).
Rh123 efflux assay and flow cytometric analysis
Rh123 dye efflux assay was used to evaluate P-gp activity and its efflux kinetics, as described previously [24], with minor modifications. Briefly, thymocytes were loaded with Rh123 (Invitrogen, Molecular Probes, Carlsbad, CA, USA) in thymocyte culture medium for 30 min at 37°C. Excess Rh123 was removed by centrifugation, and then cells were allowed to efflux the dye in fresh thymocyte culture medium for 2 h at 37°C in a humidified 5% CO2 atmosphere. Cells were then stained with a cocktail of fluorochrome-conjugated mAb consisting of anti-CD1a-APC (Immunotech, Marseille, France) and anti-CD3-Pacific Blue, anti-CD4-APC-Cy7, anti-CD8-AmCyan, anti-CD27-PE, and anti-CD45RA-PE-Cy7 (BD Biosciences). To assess the effect of RTV and IDV on the Rh123 efflux, Rh123-loaded thymocytes were incubated in the presence or absence of 0.1, 1, or 10 μM RTV or IDV for 2 h at 37°C and then stained with anti-CD3-Pacific Blue. Verapamil (5 μM), a known P-gp inhibitor [38], and 0.07% DMSO or H2O (the concentration used as 10 μM RTV or IDV diluents) were used as controls. Dead cells were excluded by initial gating of the thymocyte population by a combination of a forward-scatter plot and 7-amino-actinomycin D staining plot of nonviable cells. Flow data acquisition was performed on a BD LSR II System (BD Biosciences). All flow data were analyzed using the FlowJo software, version 4.3 (Tree Star, Ashland, OR, USA).
Cell sorting and P-gp mRNA analysis by real-time RT-PCR
P-gp mRNA expression levels in thymocyte subpopulations were analyzed by real-time PCR. In these experiments, thymic single-cell suspensions were sorted for CD3–, CD3low, and CD3high thymocytes using anti-CD3-Pacific Blue and BD FACSAria I (BD Biosciences). Sorted thymocyte subsets were >95% pure. Total RNA from sorted thymocytes was isolated using the RNeasy mini kit (Qiagen, Valencia, CA, USA) and on-column DNase digestion with the RNase-Free DNase Set (Qiagen) and reverse-transcribed into cDNA using the RT2 First Strand Kit (SuperArray Bioscience, Frederick, MD, USA), according to the manufacturer's protocol. Quantitative analysis of P-gp mRNA was performed using RT2 SYBR Green/ROX qPCR master mix and RT2 qPCR primers for P-gp and GAPDH (SuperArray Bioscience) on an ABI Prism 7900 HT sequence detection system (Applied Biosystems, Foster City, CA, USA). PCR amplification was conducted with an initial 10-min step at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. To calculate mRNA expression levels by the standard curve method, standard curves for P-gp and GAPDH were generated by preparing a five-point series of tenfold dilutions of cDNA template synthesized using XpressRef Universal Total RNA (SuperArray Bioscience). The data were analyzed using the SDS 2.3 software (Applied Biosystems), and P-gp expression levels were normalized to the corresponding GAPDH levels.
Statistical analysis
Statistical data analysis was performed using the GraphPad Prism software. An unpaired Student's t test was used to verify the significance of obtained data. Values of P < 0.05 were considered statistically significant.
RESULTS
Effects of PIs on HIV-1 replication in thymocytes and PBMCs
Replication in PBMC in the absence of PI by a CXCR4-tropic, pretherapy HIV-1 variant has been well characterized in previous studies [37]. HIV-1 replication in thymocytes in the absence of PI produced mean (±sd) supernatant p24 levels of 103 ± 11 ng/ml in five independent experiments, similar to replication levels in PBMC. To determine susceptibility to PI, IC50 for RTV or IDV for pretherapy virus in fetal thymocytes and PBMCs was determined. An average IC50 value in three independent donors was 1.5 nM for RTV or 4.4 nM IDV in thymocytes, which was substantially lower than IC50 values of 309.4 nM or 27.3 nM, respectively, in PBMCs (Table 1). Replication by pretherapy virus in culture was more sensitive to RTV or IDV in thymocytes compared with PBMCs.
Table 1. PI Sensitivity of Pre-Therapy Virus in Thymocytes and PBMC.
| RTV IC50 (nM) | IDV IC50 (nM) | |
|---|---|---|
| Thymocytes | 1.5 ± 1.6 | 4.4 ± 1.1 |
| PBMC | 309.4 ± 11.8 | 27.3 ± 6.8 |
Rh123 efflux and steady-state levels of P-gp mRNA in human CD3–, CD3low, and CD3high thymocytes
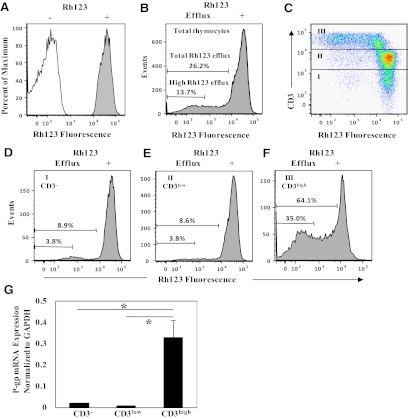
To investigate the mechanism of increased sensitivity of pretherapy HIV-1 to PIs within the thymus, efflux transporter activity in human thymocytes was determined by measuring efflux of Rh123. Complete Rh123 efflux was equivalent to the MFI of thymocytes in the absence of Rh123 loading (Fig. 1A). In the absence of Rh123 efflux, cells maintained high-level fluorescence intensity equivalent to levels immediately after Rh123 loading (Fig. 1A). High Rh123 efflux was defined as MFI equivalent to unloaded cells (a fluorescence shift of nearly 2 logs), and total Rh123 efflux was defined as the average MFI shift following Rh123 loading (Fig. 1A). Typical proportions of total and high Rh123 efflux in thymocytes were 26.2% and 13.7%, respectively (Fig. 1B). Proportions of total and high Rh123 efflux cells in thymocytes ranged from 20% to 27% and 9% to 14%, respectively, in three independent experiments.
Figure 1. Rh123 efflux and P-gp mRNA expression in CD3–, CD3low, and CD3high thymocytes.
(A) The open and filled histograms represent autofluorescence in thymocytes prior to Rh123 loading and uptake-fluorescence in Rh123-loaded thymocytes, respectively. (B) Histogram shows Rh123 efflux in live-gated total thymocytes. High efflux was defined as fluorescence intensity equivalent to unloaded cells, and total efflux was defined as the fluorescence intensity shift following Rh123 loading. (C) Rh123 versus CD3 fluorescence in live-gated thymocytes is shown by dot plots. Rh123 efflux and cell surface-staining with mAb were performed as described in Materials and Methods. Fractions I, II, and III indicate CD3–, CD3low, and CD3high thymocyte populations, respectively. (D–F) Rh123 efflux in the CD3–-, CD3low-, and CD3high-gated thymocytes is shown by histograms. The numbers in the histograms show the proportions of total and high efflux in each cell subset. Similar results were obtained in three independent experiments. (G) qRT-PCR for P-gp mRNA expression in CD3–, CD3low, and CD3high thymocytes. Thymic single-cell suspensions were sorted for CD3–, CD3low, and CD3high thymocytes. The expression levels of P-gp were quantified by real-time PCR and normalized to the corresponding GAPDH levels as described in Materials and Methods. Bars represent mean values ± sd of three separate experiments. (*P<0.05).
P-gp activity in thymocytes evaluated by fluorescence plots of Rh123 and CD3 indicated that the highest levels of P-gp activity were localized in thymocytes that expressed high levels of CD3 (CD3high; Fig. 1C). Analysis of Rh123 efflux gated on CD3–, CD3low, or CD3high thymocyte populations showed that the proportion of CD3high thymocytes with greatest Rh123 efflux was 64.1% compared with CD3– (8.9%) or CD3low (8.6%) thymocytes (Fig. 1, D–F). Similar results were obtained in three independent experiments. To determine whether Rh123 efflux was related to the P-gp mRNA levels, CD3–, CD3low, and CD3high thymocytes were sorted by flow cytometry, and steady-state levels of P-gp mRNA in each thymocyte subset were quantified using real-time RT-PCR and normalized to GAPDH mRNA levels. P-gp mRNA levels in CD3high thymocytes were ∼30-fold greater than in CD3– or CD3low thymocytes (P=0.02; Fig. 1G). Overall, CD3high thymocytes exhibited higher levels of P-gp mRNA, as well as Rh123 efflux (P-gp activity), than CD3– or CD3low thymocytes.
Effects of RTV and IDV on Rh123 efflux in thymocytes
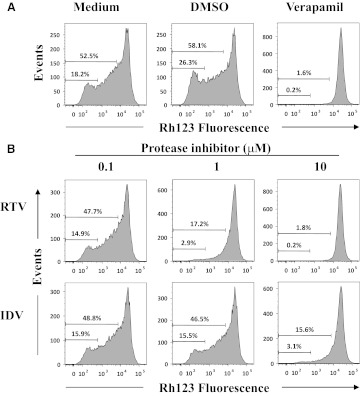
RTV and IDV are substrates for P-gp efflux within T cells [20–22, 24–27, 36]. Effects of PIs on P-gp efflux activity were evaluated by incubating Rh123-loaded thymocytes in the presence or absence of RTV or IDV and measuring inhibition of Rh123 efflux among CD3high thymocytes. The percentages of P-gp+ cells in CD3high thymocytes treated with medium were 52.5%, with no inhibitory effect on Rh123 efflux by 0.07% DMSO or 0.07% H2O (data not shown) used as vehicle controls (Fig. 2A). In comparison, 5 μM verapamil, a P-gp inhibitor [38], prevented Rh123 efflux in >98% of CD3high thymocytes (Fig. 2A). RTV or IDV significantly reduced Rh123 efflux in a dose-dependent manner (Fig. 2B). Results from two experiments showed that cells displaying total Rh123 efflux declined from an average of 48.6% to 18.0% and 2.0% at RTV concentrations of 0.1, 1, and 10 μM, respectively, and declines from 50.1% to 47.8% and 16.5% occurred at IDV concentrations of 0.1, 1, and 10 μM, respectively. The proportions of high Rh123 efflux cells were reduced correspondingly. RTV blocked Rh123 efflux about tenfold more efficiently than IDV and was as efficient at verapamil in inhibiting Rh123 efflux.
Figure 2. RTV and IDV effects on Rh123 efflux on CD3high thymocytes.
Rh123-loaded thymocytes were incubated for 2 h at 37°C in the presence of (A) medium alone, 0.07% DMSO (vehicle control), or 5 μM verapamil or (B) PI (0.1, 1, or 10 μM) and then stained with anti-CD3-Pacific Blue on ice. Histograms of Rh123 fluorescence in the live and CD3high-gated thymocytes are shown. The numbers in the histograms show the proportions of total and high Rh123 efflux. Data are representative of two independent experiments.
Rh123 efflux in human thymocyte subsets
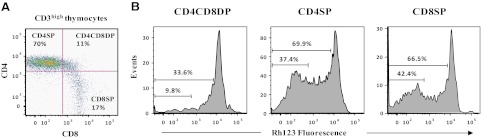
CD3high thymocytes consist of cell populations at different developmental stages. To examine P-gp activity across stages of thymocyte differentiation, Rh123 efflux from CD4+CD8+ DP and CD4+ or CD8+ SP cells within the CD3high thymocyte population was analyzed. The majority of CD3high thymocytes includes CD4+ SP cells, with fewer CD8+ SP or CD4+ CD8+ DP (Fig. 3A). Analysis of Rh123 fluorescence in each subset revealed that CD4SP and CD8SP cells displayed greater Rh123 efflux than CD4CD8DP cells (Fig. 3B). Similar results were obtained in three independent experiments. These results demonstrated increased P-gp activity, as DP thymocytes mature to CD4SP and CD8SP thymocytes.
Figure 3. Rh123 efflux in CD3high CD4CD8DP, CD4SP, and CD8SP thymocyte subsets.
(A) Expression of CD4 and CD8 was analyzed in the gate of CD3high. (B) Histograms show Rh123 efflux in the CD4SP, CD4CD8DP, and CD8SP thymocytes with a high surface expression of CD3. The numbers in the histograms show the proportions of total and high Rh123 efflux within each cell subset. Similar results were obtained in three independent experiments.
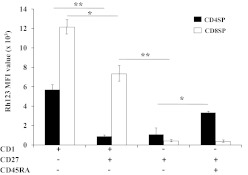
As thymocytes mature, CD1, expressed during the early stages of thymopoiesis, is down-regulated, and CD27 and CD45RA are sequentially up-regulated, as thymocytes transition from CD1–CD27+CD45RA– to CD1–CD27+CD45RA+ T cells [1]. Application of multicolor flow cytometry simultaneously assessed Rh123 efflux within CD4SP or CD8SP cells at different developmental stages based on CD1, CD27, and CD45RA expression in three independent donors (Fig. 4). Rh123 MFI of CD4SP cells with a CD1+CD27–CD45RA– phenotype was lower than Rh123 MFI in the CD8SP with the same phenotype (P=0.0134), indicating that Rh123 efflux activity was significantly higher in the CD4SP than CD8SP thymocytes when CD4CD8DP thymocytes differentiated to CD1+CD27–CD45RA– CD4SP and CD8SP thymocytes. As thymocytes expressed increased levels of CD27, Rh123 MFI significantly decreased in CD4SP cells (P=0.0096) and in CD8SP cells (P=0.0324). In CD8SP cells, thymocyte maturation was associated with a significant decrease in Rh123 MFI, as CD1 expression is down-regulated (P=0.0091). As CD4SP cells matured and expressed CD45RA, Rh123 MFI increased almost fourfold compared with less-mature CD1–CD27+CD45RA– CD4 lineage cells (P=0.0306). Most mature CD4SP thymocytes had greater Rh123 MFI than CD8SP (P=0.0029), indicating that the proportion of CD8SP thymocytes with Rh123 efflux activity surpassed that of the CD4SP thymocytes with Rh123 efflux activity when CD4SP and CD8SP lineages acquired CD45RA. These results showed that P-gp activity varied according to thymocyte differentiation stage and lineage.
Figure 4. Rh123 MFI in CD3high CD4SP and CD8SP lineage subsets during the late stages of thymopoiesis.
CD3high CD4SP and CD8SP thymocytes were classified into four distinct stages of T-lymphocyte development by the expression of CD1, CD27, and CD45RA. Bars represent average Rh123 MFI values ± sd of three independent experiments. Cells with low-efflux activity retain Rh123 (high Rh123 MFI), whereas high-efflux activity has low Rh123 MFI. Statistical difference in Rh123 efflux between thymocyte subpopulations was determined using the unpaired Student's t test. (*P<0.05; **P<0.01).
DISCUSSION
HIV-1 variants that use the CXCR4 coreceptor replicate well in thymocytes and peripheral blood T cells [19, 35, 37]. Our study shows that PIs are capable of inhibiting ex vivo viral replication in immature and mature T cells but with a surprising difference in IC50 values for PIs in different target cells. The viral inhibitory activity by PIs is substantially greater in thymocytes than in PBMCs, although X4 virus with WT gag-PR replicated to similar levels in thymocytes and PBMCs in the absence of PIs. The results indicate that PIs more effectively suppress HIV-1 replication in human thymocytes than in PBMCs, raising the possibility that intracellular concentration of the PI is higher in thymocytes. As ABC drug efflux transporters, particularly P-gp, play a critical role in regulating the intracellular concentration of PI, examining P-gp activity within thymocytes could explain the difference in viral replication within the two cell types.
Rh123 efflux is an accepted measure of P-gp activity ex vivo and is used to compare differences between P-gp substrates and P-gp activity in different cell types [24]. The highest levels of Rh123 efflux appear in CD3high thymocytes, corresponding to steady-state levels of P-gp mRNA. Rh123 efflux is reduced by competitive substrates such as verapamil [38]. As expected, verapamil effectively reduced Rh123 efflux in CD3high thymocytes. RTV and IDV reduced Rh123 efflux in a dose-dependent manner. As in other cell types [20, 24, 25], RTV was more effective than IDV at reducing Rh123 efflux from thymocytes.
P-gp plays a fundamental role in cellular functions, such as proliferation, differentiation, and survival [20, 39–41]. P-gp transporter protein is differentially expressed and its activity varies in different cell types and tissues, such as subpopulations of human peripheral blood and bone marrow cells [20, 25, 28–32, 41, 42]. In spite of the critical role that P-gp plays in regulating immune function and cellular differentiation, very few studies evaluate P-gp activity in normal human thymic tissues [32]. Although P-gp mRNA was low within CD3– and CD3low early thymocytes, thymocyte maturation in the thymus medulla, and gain in CD3 expression, steady-state levels of P-pg mRNA increased dramatically, suggesting that P-gp expression is developmentally regulated within the thymus.
As subsets of CD3high thymocytes progress through thymic differentiation in the medulla, more mature SP cells—CD4 and CD8—showed distinctly higher levels of a P-gp activity than the more immature CD4 CD8 DP cells. These results indicate that thymocyte maturation parallels acquisition of P-gp activity and that late-stage thymocytes, identified based on the expression of CD1, CD27, and CD45RA [1], had increased levels of P-gp activity with maturation. Previous studies have shown that down-regulation of CD1 corresponds to the generation of T cell function within the thymus [43]. Our findings indicate that acquisition of P-gp activity parallels the acquisition of T cell function within the thymus, although with differences in the kinetics within CD4SP and CD8SP cells. CD4SP lineage thymocytes acquired P-gp activity earlier in maturation than CD8SP lineage cells. However, when both lineages acquired CD45RA, the frequency of CD8SP thymocytes with high Rh123 efflux activity surpassed CD4SP high Rh123 efflux thymocytes, raising the possibility of a physiological role of P-gp in the events controlling late stages of differentiation of normal CD4+ and CD8+ T lymphocytes in the thymus. Some degree of variability in Rh123 efflux was observed among the different individual thymuses examined, perhaps as a result of ABCB1 polymorphisms [25, 35, 44, 45]. However, the cellular factors and pathways that drive P-gp expression and activation remain to be determined.
Early- and late-stage thymocytes are susceptible to HIV-1 infection, as early-stage thymocytes are infected by X4 viruses, and late-stage thymocytes are infected by R5 viruses [3, 46]. The results of the Rh123 efflux studies correlated with the findings of increased IC50 in thymocytes compared with PBMCs. Susceptibility of late-stage thymocytes coincides with the acquisition of P-gp activity, which may in turn impact PI activity and viral replication. Because of the differences in culture conditions and cell preparation needed to examine thymocytes and PBMCs ex vivo, it is difficult to directly compare P-gp activity in the thymocytes and post-thymic T cells obtained from blood samples. However, taken together, the relative stage of maturation and activation clearly impacts P-gp activity and also intracellular concentrations of PI within different T cell and thymic subsets [26, 47]. Our observations support other studies using mature T cells that show PIs are inhibitors of P-gp, and RTV especially possesses high affinity for and competitive interaction with P-gp, diminishing efflux of the PI [20, 21, 24, 25, 48, 49].
HIV-1 variants replicating in the thymus predominantly use CXCR4 as their coreceptor [3, 4, 50]. We have shown recently that the thymus plays a key role in the evolution of CXCR4 variants found in the thymus, blood, and tissues [5]. Studies using the SCID-hu (Thy/Liv) mouse model showed that the thymus can harbor CXCR4 and CCR5 HIV-1 variants, indicating that mature thymocytes might be a latent HIV reservoir [51, 52]. Transcriptionally quiescent CD3high CD27+ thymocytes, which express high Rh123 efflux activity, may be a pharmacological sanctuary site for HIV-1. The results of this study show that P-gp activity varies according to thymocyte maturational stage. The thymus harbors a complex mixture of cell types, so it would be impractical from an experimental standpoint to measure intracellular PI levels within thymocytes at different stages of maturation. However, this study shows that low levels of P-gp activity are likely to result in higher intracellular concentration of PI, which inhibits virus and enhances naïve T cell reconstitution, particularly in HIV-infected children with high levels of thymic output.
ACKNOWLEDGMENTS
Research was supported in part by Public Health Service R01 awards HD032259, AI065265, AI028571, and AI047723; Stephany W. Holloway University Chair for AIDS Research, Laura McClamma Fellowship, and the Center for Research for Pediatric Immune Deficiency at the University of Florida; the Pediatric Clinical Research Center of All Children's Hospital and the University of South Florida; and the Maternal Child Health Bureau, R60 MC 00003-01, Department of Health and Human Services, Resources and Services Administration. The authors are grateful to Dr. Christel Uittenbogaart for her guidance and assistance in setting up the thymic culture system. We also thank Ms. Amy Baldwin for her help in preparing this manuscript and Steve Pomeroy for his help with viral culture experiments.
Footnotes
- ABC
- ATP-binding cassette
- APC
- allophycocyanin
- ART
- antiretroviral therapy
- DP
- double-positive
- IDV
- indinavir
- MFI
- median fluorescence intensity
- P-gp
- P-glycoprotein
- PI
- protease inhibitor
- qPCR
- quantitative PCR
- Rh123
- rhodamine 123
- RTV
- ritonavir
- SP
- single-positive
- WT
- wild type
AUTHORSHIP
S.H. conceived of and designed the study, performed most experiments, collected and analyzed data, and wrote the manuscript. S.K.H. assisted in performing IC50 studies in PBMCs. M.M. assisted S.H. in flow cytometry to assess P-gp activity measurement. M.M.G. oversaw the conception and study design and contributed to writing and revising the manuscript. J.W.S. oversaw the entire project, including conception and study design and data management, and contributed to writing and revising the manuscript.
REFERENCES
- 1. Ho Tsong Fang R., Colantonio A. D., Uittenbogaart C. (2008) The role of the thymus in HIV infection: a 10 year perspective. AIDS 22, 171–184 [DOI] [PubMed] [Google Scholar]
- 2. Ladi E., Yin X., Chtanova T., Robey E. A. (2006) Thymic microenvironments for T cell differentiation and selection. Nat. Immunol. 7, 338–343 [DOI] [PubMed] [Google Scholar]
- 3. Kitchen S. G., Zack J. A. (1997) CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J. Virol. 71, 6928–6934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedroza-Martins L., Gurney K. B., Torbett B. E., Uittenbogaart C. H. (1998) Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J. Virol. 72, 9441–9452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salemi M., Burkhardt B. R., Gray R. R., Ghaffari G., Sleasman J. W., Goodenow M. M. (2007) Phylodynamics of HIV-1 in lymphoid and non-lymphoid tissues reveals a central role for the thymus in emergence of CXCR4-using quasispecies. PLoS ONE 2, e950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uittenbogaart C. H., Anisman D. J., Jamieson B. D., Kitchen S., Schmid I., Zack J. A., Hays E. F. (1996) Differential tropism of HIV-1 isolates for distinct thymocyte subsets in vitro. AIDS 10, F9–F16 [DOI] [PubMed] [Google Scholar]
- 7. Douek D. C., McFarland R. D., Keiser P. H., Gage E. A., Massey J. M., Haynes B. F., Polis M. A., Haase A. T., Feinberg M. B., Sullivan J. L., Jamieson B. D., Zack J. A., Picker L. J., Koup R. A. (1998) Changes in thymic function with age and during the treatment of HIV infection. Nature 396, 690–695 [DOI] [PubMed] [Google Scholar]
- 8. Kourtis A. P., Ibegbu C., Nahmias A. J., Lee F. K., Clark W. S., Sawyer M. K., Nesheim S. (1996) Early progression of disease in HIV-infected infants with thymus dysfunction. N. Engl. J. Med. 335, 1431–1436 [DOI] [PubMed] [Google Scholar]
- 9. Douek D. C., Koup R. A., McFarland R. D., Sullivan J. L., Luzuriaga K. (2000) Effect of HIV on thymic function before and after antiretroviral therapy in children. J. Infect. Dis. 181, 1479–1482 [DOI] [PubMed] [Google Scholar]
- 10. Sleasman J. W., Nelson R. P., Goodenow M. M., Wilfret D., Hutson A., Baseler M., Zickerman J., Pizzo P. A., Mueller B. U. (1999) Immunoreconstitution after ritonavir therapy in children with human immunodeficiency virus infection involves multiple lymphocyte lineages. J. Pediatr. 134, 597–606 [DOI] [PubMed] [Google Scholar]
- 11. Yin L., Rodriguez C. A., Hou W., Potter O., Caplan M. J., Goodenow M. M., Sleasman J. W. (2008) Antiretroviral therapy corrects HIV-1-induced expansion of CD8+ CD45RA+ CD27– CD11a(bright) activated T cells. J. Allergy Clin. Immunol. 122, 166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chavan S., Bennuri B., Kharbanda M., Chandrasekaran A., Bakshi S., Pahwa S. (2001) Evaluation of T cell receptor gene rearrangement excision circles after antiretroviral therapy in children infected with human immunodeficiency virus. J. Infect. Dis. 183, 1445–1454 [DOI] [PubMed] [Google Scholar]
- 13. Deeks S. G., Barbour J. D., Martin J. N., Swanson M. S., Grant R. M. (2000) Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficiency virus infection. J. Infect. Dis. 181, 946–953 [DOI] [PubMed] [Google Scholar]
- 14. Essajee S. M., Kim M., Gonzalez C., Rigaud M., Kaul A., Chandwani S., Hoover W., Lawrence R., Spiegel H., Pollack H., Krasinski K., Borkowsky W. (1999) Immunologic and virologic responses to HAART in severely immunocompromised HIV-1-infected children. AIDS 13, 2523–2532 [DOI] [PubMed] [Google Scholar]
- 15. Rodriguez C. A., Koch S., Goodenow M. M., Sleasman J. W. (2008) Clinical implications of discordant viral and immune outcomes following protease inhibitor containing antiretroviral therapy for HIV-infected children. Immunol. Res. 40, 271–286 [DOI] [PubMed] [Google Scholar]
- 16. Kitchen S. G., Killian S., Giorgi J. V., Zack J. A. (2000) Functional reconstitution of thymopoiesis after human immunodeficiency virus infection. J. Virol. 74, 2943–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pettoello-Mantovani M., Kollmann T. R., Raker C., Kim A., Yurasov S., Tudor R., Wiltshire H., Goldstein H. (1997) Saquinavir-mediated inhibition of human immunodeficiency virus (HIV) infection in SCID mice implanted with human fetal thymus and liver tissue: an in vivo model for evaluating the effect of drug therapy on HIV infection in lymphoid tissues. Antimicrob. Agents Chemother. 41, 1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stoddart C. A., Joshi P., Sloan B., Bare J. C., Smith P. C., Allaway G. P., Wild C. T., Martin D. E. (2007) Potent activity of the HIV-1 maturation inhibitor bevirimat in SCID-hu Thy/Liv mice. PLoS ONE 2, e1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoddart C. A., Liegler T. J., Mammano F., Linquist-Stepps V. D., Hayden M. S., Deeks S. G., Grant R. M., Clavel F., McCune J. M. (2001) Impaired replication of protease inhibitor-resistant HIV-1 in human thymus. Nat. Med. 7, 712–718 [DOI] [PubMed] [Google Scholar]
- 20. Delph Y. (2002) P-glycoprotein and HIV. GMHC Treat. Issues 16, 6–10 [PubMed] [Google Scholar]
- 21. Gutmann H., Fricker G., Drewe J., Toeroek M., Miller D. S. (1999) Interactions of HIV protease inhibitors with ATP-dependent drug export proteins. Mol. Pharmacol. 56, 383–389 [DOI] [PubMed] [Google Scholar]
- 22. Huisman M. T., Smit J. W., Schinkel A. H. (2000) Significance of P-glycoprotein for the pharmacology and clinical use of HIV protease inhibitors. AIDS 14, 237–242 [DOI] [PubMed] [Google Scholar]
- 23. Lee C. G., Ramachandra M., Jeang K. T., Martin M. A., Pastan I., Gottesman M. M. (2000) Effect of ABC transporters on HIV-1 infection: inhibition of virus production by the MDR1 transporter. FASEB J. 14, 516–522 [DOI] [PubMed] [Google Scholar]
- 24. Lucia M. B., Rutella S., Leone G., Vella S., Cauda R. (2001) HIV-protease inhibitors contribute to P-glycoprotein efflux function defect in peripheral blood lymphocytes from HIV-positive patients receiving HAART. J. Acquir. Immune Defic. Syndr. 27, 321–330 [DOI] [PubMed] [Google Scholar]
- 25. Sankatsing S. U., Beijnen J. H., Schinkel A. H., Lange J. M., Prins J. M. (2004) P glycoprotein in human immunodeficiency virus type 1 infection and therapy. Antimicrob. Agents Chemother. 48, 1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janneh O., Jones E., Chandler B., Owen A., Khoo S. H. (2007) Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J. Antimicrob. Chemother. 60, 987–993 [DOI] [PubMed] [Google Scholar]
- 27. Jones K., Bray P. G., Khoo S. H., Davey R. A., Meaden E. R., Ward S. A., Back D. J. (2001) P-glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance? AIDS 15, 1353–1358 [DOI] [PubMed] [Google Scholar]
- 28. Chaudhary P. M., Mechetner E. B., Roninson I. B. (1992) Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood 80, 2735–2739 [PubMed] [Google Scholar]
- 29. Drach D., Zhao S., Drach J., Mahadevia R., Gattringer C., Huber H., Andreeff M. (1992) Subpopulations of normal peripheral blood and bone marrow cells express a functional multidrug resistant phenotype. Blood 80, 2729–2734 [PubMed] [Google Scholar]
- 30. Klimecki W. T., Futscher B. W., Grogan T. M., Dalton W. S. (1994) P-glycoprotein expression and function in circulating blood cells from normal volunteers. Blood 83, 2451–2458 [PubMed] [Google Scholar]
- 31. Ludescher C., Pall G., Irschick E. U., Gastl G. (1998) Differential activity of P-glycoprotein in normal blood lymphocyte subsets. Br. J. Haematol. 101, 722–727 [DOI] [PubMed] [Google Scholar]
- 32. Pilarski L. M., Paine D., McElhaney J. E., Cass C. E., Belch A. R. (1995) Multidrug transporter P-glycoprotein 170 as a differentiation antigen on normal human lymphocytes and thymocytes: modulation with differentiation stage and during aging. Am. J. Hematol. 49, 323–335 [DOI] [PubMed] [Google Scholar]
- 33. Chong A. S., Markham P. N., Gebel H. M., Bines S. D., Coon J. S. (1993) Diverse multidrug-resistance-modification agents inhibit cytolytic activity of natural killer cells. Cancer Immunol. Immunother. 36, 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupta S., Kim C. H., Tsuruo T., Gollapudi S. (1992) Preferential expression and activity of multidrug resistance gene 1 product (P-glycoprotein), a functionally active efflux pump, in human CD8+ T cells: a role in cytotoxic effector function. J. Clin. Immunol. 12, 451–458 [DOI] [PubMed] [Google Scholar]
- 35. Owen A., Chandler B., Bray P. G., Ward S. A., Hart C. A., Back D. J., Khoo S. H. (2004) Functional correlation of P-glycoprotein expression and genotype with expression of the human immuno-deficiency virus type 1 coreceptor CXCR4. J. Virol. 78, 12022–12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee C. G., Gottesman M. M., Cardarelli C. O., Ramachandra M., Jeang K. T., Ambudkar S. V., Pastan I., Dey S. (1998) HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry 37, 3594–3601 [DOI] [PubMed] [Google Scholar]
- 37. Ho S. K., Coman R. M., Bunger J. C., Rose S. L., O′Brien P., Munoz I., Dunn B. M., Sleasman J. W., Goodenow M. M. (2008) Drug-associated changes in amino acid residues in Gag p2, p7NC, and p6Gag/p6Pol in human immunodeficiency virus type 1 (HIV-1) display a dominant effect on replicative fitness and drug response. Virology 378, 272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toffoli G., Simone F., Corona G., Raschack M., Cappelletto B., Gigante M., Boiocchi M. (1995) Structure-activity relationship of verapamil analogs and reversal of multidrug resistance. Biochem. Pharmacol. 50, 1245–1255 [DOI] [PubMed] [Google Scholar]
- 39. Johnstone R. W., Ruefli A. A., Smyth M. J. (2000) Multiple physiological functions for multidrug transporter P-glycoprotein? Trends Biochem. Sci. 25, 1–6 [DOI] [PubMed] [Google Scholar]
- 40. Loo T. W., Clarke D. M. (2005) Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J. Membr. Biol. 206, 173–185 [DOI] [PubMed] [Google Scholar]
- 41. Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 84, 7735–7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van der Valk P., van Kalken C. K., Ketelaars H., Broxterman H. J., Scheffer G., Kuiper C. M., Tsuruo T., Lankelma J., Meijer C. J. L. M., Pinedo H. M., Scheper R. J. (1990) Distribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues. Analysis with 3 monoclonal antibodies recognizing different epitopes of the P-glycoprotein molecule. Ann. Oncol. 1, 56–64 [PubMed] [Google Scholar]
- 43. Res P., Blom B., Hori T., Weijer K., Spits H. (1997) Downregulation of CD1 marks acquisition of functional maturation of human thymocytes and defines a control point in late stages of human T cell development. J. Exp. Med. 185, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Y., Hu M. (2000) P-glycoprotein and bioavailability-implication of polymorphism. Clin. Chem. Lab. Med. 38, 877–881 [DOI] [PubMed] [Google Scholar]
- 45. Marzolini C., Paus E., Buclin T., Kim R. B. (2004) Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin. Pharmacol. Ther. 75, 13–33 [DOI] [PubMed] [Google Scholar]
- 46. Zaitseva M. B., Lee S., Rabin R. L., Tiffany H. L., Farber J. M., Peden K. W., Murphy P. M., Golding H. (1998) CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J. Immunol. 161, 3103–3113 [PubMed] [Google Scholar]
- 47. Janneh O., Anwar T., Jungbauer C., Kopp S., Khoo S. H., Back D. J., Chiba P. (2009) P-Glycoprotein, multidrug resistance-associated proteins and human organic anion transporting polypeptide influence the intracellular accumulation of atazanavir. Antivir. Ther. 14, 965–974 [DOI] [PubMed] [Google Scholar]
- 48. Drewe J., Gutmann H., Fricker G., Torok M., Beglinger C., Huwyler J. (1999) HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem. Pharmacol. 57, 1147–1152 [DOI] [PubMed] [Google Scholar]
- 49. Merry C., Barry M. G., Mulcahy F., Ryan M., Heavey J., Tjia J. F., Gibbons S. E., Breckenridge A. M., Back D. J. (1997) Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS 11, F29–F33 [DOI] [PubMed] [Google Scholar]
- 50. Berkowitz R. D., Beckerman K. P., Schall T. J., McCune J. M. (1998) CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J. Immunol. 161, 3702–3710 [PubMed] [Google Scholar]
- 51. Brooks D. G., Kitchen S. G., Kitchen C. M., Scripture-Adams D. D., Zack J. A. (2001) Generation of HIV latency during thymopoiesis. Nat. Med. 7, 459–464 [DOI] [PubMed] [Google Scholar]
- 52. Aldrovandi G. M., Feuer G., Gao L., Jamieson B., Kristeva M., Chen I. S., Zack J. A. (1993) The SCID-hu mouse as a model for HIV-1 infection. Nature 363, 732–736 [DOI] [PubMed] [Google Scholar]