Abstract
Glucuronidation mediated by UDP-glucuronosyltransferases (UGTs) is a significant metabolic pathway that facilitates efficient elimination of numerous endo- and xenobiotics including phenolics. UGT genetic deficiency and polymorphisms or inhibition of glucuronidation by concomitant use of drugs are associated with inherited physiological disorders or drug induced toxicities. Moreover, extensive glucuronidation can be a barrier to oral bioavailability as the first-pass glucuronidation (or premature clearance by UGTs) of orally administered agents usually results in the poor oral bioavailability and lack of efficacies. This review focused on the first-pass glucuronidation of phenolics including natural polyphenols and pharmaceuticals. The complexity of UGT-mediated metabolism of phenolics is highlighted with species-, gender-, organ- and isoform-dependent specificity, as well as functional compensation between UGT1A and 2B subfamily. In addition, recent advances are discussed with respect to the mechanisms of enzymatic actions including the important properties such as binding pocket size and phosphorylation requirements.
Keywords: Phenolics, Polyphenols, Glucuronidation, Bioavailability, UGTs
1 Introduction
Phenolics are a class of chemical compounds consisting of one or more hydroxyl group(s) (-OH) bonded directly to an aromatic ring. Phenolic compounds such as flavonoids (including flavones, isoflavones, flavonols, flavanones, chalcones and catechins), stilbenes, coumarins, and quinones are widely distributed in the nature, especially in plant kingdom. For example, significant amounts of isoflavones (e.g., genistein and daidzein) are found in soybeans, catechins (e.g., EGCG) in green teas, and stilbene (e.g., resveratrol) in the skin of red grapes. Those phenolics are also referred to dietary polyphenols as they possess multiple aromatic hydroxyl groups. Polyphenols are regularly consumed by human being in the forms of fruits, vegetables, spices, and herbs. In addition to the natural phenolics, synthetic phenolics are used as drugs, and are found across many therapeutic categories such as nonsteroidal anti-inflammatory drugs (acetaminophen), anti-estrogen agents (raloxifene), immunosuppressants (mycophenolic acid), catechol-O-methyl transferase inhibitors (entacapone) and decongestants (phenylephrine).
The natural polyphenols are currently only supplied as dietary supplements, many of which are enjoying increased market acceptance in the developed countries. The “claimed” health benefits of dietary phenolics are extensively reviewed.1–3 The reported pharmacological activities for this class of compounds mainly include antioxidant, anti-inflammatory and anti-cancer.1–3 Although dietary phenolics show diverse pharmacological potentials, their poor oral bioavailabilities impede the further development of these chemicals as therapeutic agents.4 For example, the in vivo plasma concentrations of flavonoids are typically in the range of 0.01 to 0.1 μM, significantly less than the IC50 or EC50 values of 5 to 50 μM commonly reported for their anticancer and other effects in vitro.5
The causes of the poor oral bioavailability of phenolics had been explored for decades by this and other laboratories. 6 – 8 Studies using microsomal, cellular, and animal models consistently indicate that rapid conjugation, especially glucuronidation in the intestine and liver, is primarily responsible for the poor bioavailability of phenolics, although other factors such as stability and solubility might also be involved sometimes.6 Glucuronidation is mediated by UDP-glucuronosyltransferases (UGTs) (EC 2.4.1.17), and together with cytochrome P450 enzymes (CYPs), they represented more than 80% of the metabolic pathways.9 Hence, glucuronidation is increasingly recognized as an important clearance pathway in addition to that of P450 enzymes.
Study of UGTs that are responsible for phenolic glucuronidation is also necessitated by the prevalence of UGT variants that are functionally different from the wide-types. Currently, functional UGT polymorphisms are systematically identified, and newly identified variants are updated in the UGT web site at http://www.pharmacogenomics.pha.ulaval.ca/sgc/ugt_alleles/. Most notably, UGT1A1 is a highly polymorphic isoform. Deficiency in its expression and/or activity may lead to genetic and acquired diseases such as Crigler-Najjar or Gilbert’s syndromes and jaundice (hyperbilirubinemia).10 Although glucuronidation generally detoxifies chemicals and results in pharmacologically inactive products, the exceptions were also observed for several compounds such as ezetimibe and morphine, whose glucuronides are equivalent or more potent than the parent compounds (also see section 10).11,12 It is also interesting to note that the glucuronides of some natural phenolics (e.g., daidzein and genistein) retain rather weaker (compared to the free aglycones) biological activities including estrogen receptor binding and natural killer cell activation.13 Glucuronidation of phenolics often occurs at the nucleophilic -OH group attached to the aromatic ring (also refers to O-glucuronidation). For molecules with more than one hydroxyl group, multiple glucuronide isomers are often generated. 10
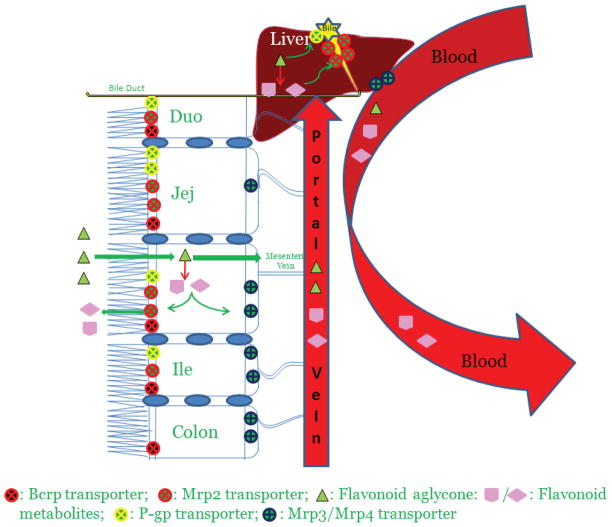
Phenolic glucuronides are much more hydrophilic comparing to their parent compounds, and their transport from intracellular to extracellular compartments requires the aids of efflux transporters. This creates the interplay between UGT enzymes and efflux transporters. As a result, glucuronide excretion (or production) is not always determined by UGT metabolism alone, and efflux transport occasionally could be the rate-limiting step governing the overall efficiency of glucuronidation in vivo. 7,8 The role of efflux transporters in the disposition of phenolic glucuronides has been reviewed. 7,8,14 Excreted glucuronides in the bile (from hepatocytes) and in the intestinal lumen (from enterocytes) may undergo enterohepatic and enteric recirculation or recycling, due to β-glucuronidase- or bacterial-catalyzed hydrolysis and subsequent intestinal and/or colon re-absorption of the parent drug. Consequently, first-pass metabolism and disposition does not result in complete drug elimination. Rather, their apparent terminal elimination half-lives are significantly prolonged (~ 6–8 hours) despite of their poor bioavailabilities in vivo (< 5%).4,7,8 The complex disposition via glucuronidation pathway involving the interplay of UGTs and efflux transporters is illustrated in Figure 1, using flavonoids as an example, for more detailed discussion one can refer to the recent reviews. 7,8
Figure 1.
Flow diagram depicting absorption and metabolism of flavonoids in rat intestine and liver. When taken orally, the aglycones shown here as triangles get absorbed in the enterocytes and undergo extensive phase II metabolism via various UGT isoforms. This first pass metabolism significantly reduces the flavonoid aglycone concentration reaching the systemic circulation thereby causing poor oral bioavailability. The flavonoid conjugates thus formed, being bulky and hydrophilic in nature require efflux transporters to get out of the cell on the luminal side by Mrp2 or on the serosal side by Mrp3 and 4. Flavonoid aglycone may escape the intestinal metabolism and make its way into the liver through the portal vein. Similar to intestine, flavonoid aglycone is rapidly and extensively glucuronidated by various UGT isoforms in liver and the conjugates are excreted either into the bile (major pathway) via Mrp2 transporters or into the systemic circulation (minor pathway) by membrane-bound Mrp3 and Mrp4 transporters. The bile along with flavonoid conjugates is then emptied in the upper part of the duodenum. The flavonoid conjugates thus excreted from liver and from the upper part of the intestine (duodenum and jejunum) make their way into the colon. The bacterial microflora present in the lower part of the small intestine and colon hydrolyzes these conjugates back into flavonoid aglycone which gets absorbed through the colon back into the systemic circulation. These recycling mechanisms called enteric and enterohepatic recycling thereby improve apparent plasma half-life of flavonoids in rats.
Phenolics represent a large class of compounds including natural-occurring polyphenols and synthetic drugs. The main objective of this paper is to review the phenolic compounds with poor oral bioavailability resulted from extensive glucuronidation in the gut and/or liver (i.e., first-pass glucuronidation). It is noteworthy that intestinal and/or hepatic first-pass extraction via glucuronidation could limit the oral bioavailabilities of phenolics. These results were mostly based on studies conducted using a number of in vitro/in situ intestinal/hepatic models (e.g., microsomes, Caco-2, HepG2, hepatocytes, intestine perfusion and isolated liver perfusion). The theoretical basis for these models and their ability to predict the fraction of dose absorbed/metabolic clearance in humans are extensively discussed. 15,16 The complexity of UGT-mediated metabolism of phenolics is highlighted with the species-, gender-, organ- and isoform-dependent specificity, as well as the functional compensation between UGT1A and 2B subfamily. To update the most recent UGT isoform information, we also briefly reviewed the UGT enzymology including the tissue distribution (probed by mRNA level), topology, and molecular structure. Since the enzyme is the key player in cellular glucuronidation (others include the efflux transporters mediating the excretion of glucuronides), the updates should help us understand better about UGT functions. In particular, recent advances are discussed in the areas of enzymatic mechanism with emphasis on properties such as the (large) size of the binding pocket and phosphorylation states of the UGT isoform.
2 Distribution of UGTs in GI tract and liver
Human UGTs are classified into four families: UGT1, UGT2, UGT3, and UGT8, on the basis of amino acid sequence identity. 17 The most important drug-conjugating UGTs belong to UGT1 and UGT2 families. The human UGT1A gene cluster, located on chromosome 2q37, spans approximately 200 kb. It contains 13 distinct individual promoters/first exons and shared exons 2–5. Each exon 1 spliced to the same exons 2–5 is regarded as a unique gene which translates to the corresponding active UGT1A isoform excluding the pseudogenes (i.e., UGT1A2p, UGT1A11p, UGT1A12p and UGT1A13p). On the other hand, the UGT2B subfamily isoforms are encoded individually and each consists of six exons clustered on chromosome 4q13.17
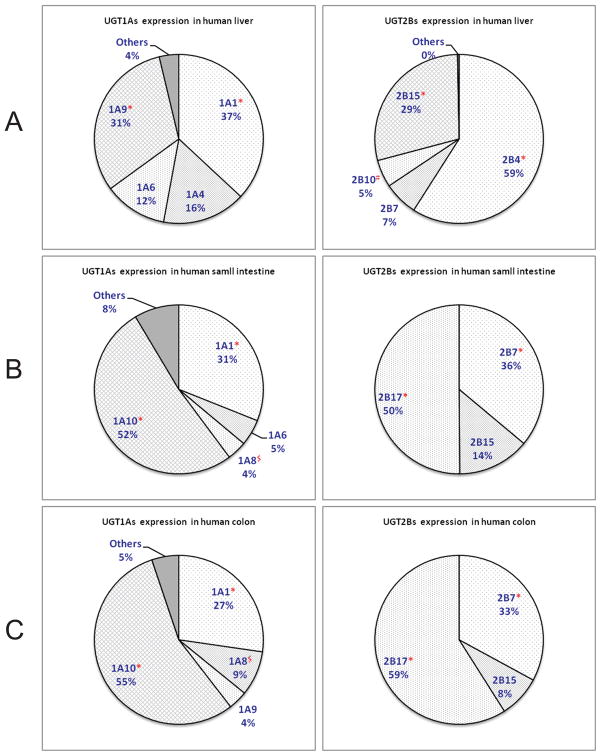
Human liver, as the major metabolic organ, are reported to express a panel of UGT isoforms, including UGT1A1, 1A3, 1A4, 1A5, 1A6, 1A8, 1A9, 2B4, 2B7, 2B10, 2B11, 2B15, and 2B17. 10, 18, 19 Recently, mRNA expression level of UGT isoforms in various human tissues was determined and quantitatively compared using an exhaustive (RT-PCR) method.20 The results (Figure 2) were generally consistent with previous findings, although the authors acknowledged some minor differences due to high inter-individual variability and perhaps low amplification efficiency. 20 Compared to UGT1As, UGT2Bs are much more abundantly expressed in the liver. Among UGT2Bs, UGT2B4 has the highest expression, followed closely by UGT2B15, which, respectively, had ~9 and 4~ times greater expression than UGT2B7 (Figure 2). Among UGT1A isoforms, 1A1 and 1A9 are most abundantly expressed isoforms in the liver. By contrast, UGT1A10 and 2B17 were found predominantly in the intestines and colon. It is interesting to note that UGT1A7 is expressed only in the proximal tissues of the gastrointestinal tract, such as the esophagus and stomach.20 However, one should be more careful when interpreting data using the mRNA expression level, since one study showed that poor or no correlations were observed between the protein and mRNA levels of UGT isoforms.21
Figure 2.
Relative mRNA distribution level between human UGT1A isoforms (left) and between 2B isoforms (right) in human liver (A), small intestine (B) and colon (C).20 * The two most abundant isoforms in the organs. # The unique isoform in liver. $ The unique isoform in GI tract.
A panel of 10 Ugt1as (Ugt1a1, 1a2, 1a3, 1a4, 1a5, 1a6, 1a7, 1a8, 1a9, and 1a10) are expressed in rats.17,22 Rat 2b subfamily consists of six members: Ugt2b1, 2b2, 2b3, 2b6, 2b8, and 2b12. The mRNA expression of rat Ugt1a isoforms is more predominant in both liver and intestine in comparison to other tissues.23 Ugt isoforms in rat liver include Ugt1a1, 2b1, 2b2, 2b3, 2b6, and 2b12. In contrast to the Ugt1 family, a few Ugt2b subfamily members are found in rat intestine. Ugt isoforms in rat intestine mainly include Ugt1a1, 1a2, 1a6, 1a7, and 2b8. 23
In mice, the Ugt1a subfamily contains 14 first exons, coding nine enzymes (Ugt1a1, 2, 5, 6a, 6b, 7c, 8, 9, and 10) and five pseudogenes (Ugt1a3, 4, 7a, 7b, and 11). 17,22 The seven Ugt2b genes in mice include Ugt2b1, 2b5, 2b34, 2b35, 2b36, 2b37, and 2b38. UGT gene expression profiles in mice were determined by Buckley and Klaassen.24 All 2b members, as well as Ugt1a1, 1a5, 1a6, 1a9, are highly expressed in mouse liver. Several Ugt isoforms were expressed in the gastrointestinal tract, including Ugt1a6, 1a7c, 2a3, 2b34, and 2b35. 24
2.1. Gender-specific distribution of UGTs
UGT expression pattern was gender specific in human, rat and mouse. Gallagher et al. showed that men exhibited an approximately 4-fold higher level of UGT2B17 expression than women (p = 0.007), 25 which is consistent with the fact that human liver microsomes (HLM) from men had a higher level of glucuronidation activity than HLM from women against three UGT2B17 substrates. For rats, some UGT isoforms were detected at higher levels in female than male rats with big difference in liver and smaller ones in other tissues.23 For example, the Ugt1a5, 2b1 and 2b2 mRNA levels in liver of female rats were, respectively, ~ 35%, ~50% and ~ 60% higher than those in male rats. In mice, male-predominant expression was observed for Ugt2b1 in the liver; female-predominant expression was observed for Ugt1a1 and Ugt1a5 in the liver.24
3 Topology of UGTs in ER membrane
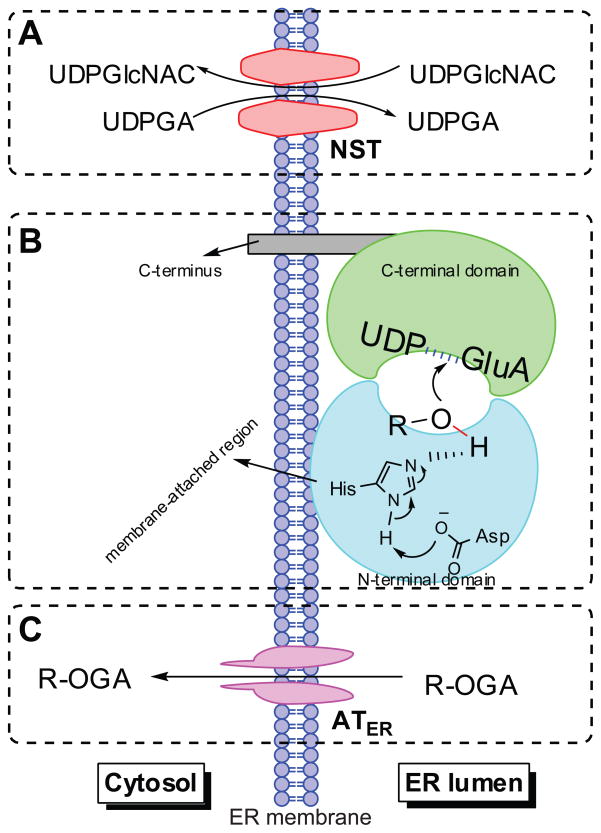
UGT active site faces the lumen of endoplasmic reticulum (ER) where the conjugation occurs, different from CYP enzymes, which face the cytosolic side.26 While the lipophilic compounds usually can passively permeate through ER membrane to access the active site of the enzyme, UDPGA is transported into the ER lumen using nucleotide sugar transporters (NSTs) (Figure 3A). 27 NSTs act as antiporters requiring the counter-transport of UDP-N-acetyl glucosamine (UDPGlcNAC), which is a known positive, allosteric modulator of UGTs. 28,29 The translocation of the formed glucuronide(s) to the cytosol domain appeared to be mediated by ER-localized organic anion transporters (ATER), which do not need ATP, but transport organic anions through ER membrane by facilitated diffusion (Figure 3C).30
Figure 3.
Schematic representation of human UGT topology. UGTs consist of two domains (panel B) and are predicted to function as dimers or oligomers. The amino-terminal domain binds the aglycone and the carboxy-terminal domain binds the UDPGA cofactor; the catalytic site is placed between the two domains. Most of the enzyme mass is located on the luminal side of the endoplasmic reticulum and the carboxy-terminal tail is on the cytosolic side of the membrane. Transport of the cofactor UDPGA into the ER lumen requires the nucleotide sugar transporters (NSTs) (panel A), which act as antiporters counter-transporting the UDP-N-acetyl glucosamine (UDPGlcNAC) out of ER lumen. Whereas, the translocation of the formed glucuronide(s) to the cytosol is suggested to be mediated by ER-localized organic anion transporters (ATER), which do not need ATP, but transport organic anions through ER membranes by facilitated diffusion (panel C). R-OH: phenolics. R-OGA: glucuronide.
As shown in Figure 3B, a UGT monomer consists of the N-terminal aglycone substrate binding domain (or half) and the C-terminal UDP-glucuronic acid binding domain, and the latter contains one transmembrane fragment and a cytosolic tail. The membrane attached region in N-terminal domain is thought to facilitate the entry of aglycone to the active site.31,32 Single UGT enzyme has been demonstrated to operate by forming dimeric structures (either homodimers or heterodimers).31,32 The tetramers of UGT dimers may also be formed to generate diglucuronide of bilirubin, benezo(α)pyrene and chrysene-3,6-quinols, as suggested from the radiation target analysis.33 It was reasoned that, in the formation of diglucuronide, dimmers may loosely interact in ER membrane to form tetramers, which generate a compartment between two dimers in which monoglucuronides reach high enough levels to facilitate diglucuronide formation.33 Whether these enzymes actually form such dimeric or oligomeric structures within the ER membrane is unproven, more work is needed to characterize these homo- and hetero-oligomers and their functional implications.
4 Structure and Catalytic mechanisms
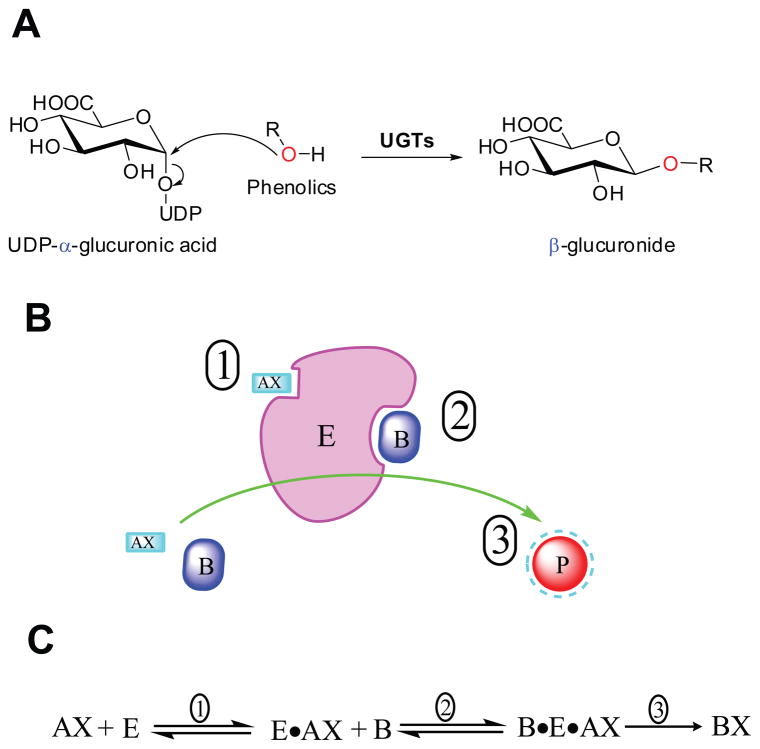
Human UGTs belong to family 1 of glycosyltransferases (GTs) according to the CAZY database (http://www.cazy.org). Enzymes in GT1 adopt GT-B fold (i.e., consist of two α/β/α or Rossmann fold domains) and an inverting catalytic mechanism (Figure 4A). Approximately 50% of the GT1 family share a highly conserved motif in the C-terminal domain, denoted as the UGT defining sequence or the UGT signature motif.34, 35 Unlike mammalian UGTs, which are membrane-bound proteins, most plant or bacterial GT1 enzymes are soluble.36 Because of the exceeding difficulties in purifying them in active form, there is no complete three-dimensional structure of any mammalian UGTs. Recently, a partial crystal structure (C-terminal or UDPGA binding domain) of human UGT2B7 was determined and found to be consistent with other GT-B structures.37 In contrast to human UGTs, several complete 3D crystal structures of UGTs derived from other organisms have been reported, including five from plants and seven from bacteria.38–42
Figure 4.
Panel A human UGTs are inverting glycosyltransferases, like all other members of the GT1 family, the configuration of anomeric carbon is changed from α (in UDPGA) to β (in glucuronide). Panel B/C: schematic illustration of compulsory ordered binding UDPGA (first) and aglycone (next) to UGTs for reaction with glucuronide formed. E: UGTs; AX: UDP-GA; A: UDP; AB: aglycone; P or BX: glucuronide.
Numerous studies have been conducted to explore the nature of UDPGA or aglycone binding to mammalian UGTs in last decades. Understanding of the UDPGA binding has been significantly advanced with the aid of crystal structure of UGT2B7 C-terminal. The UDPGA binding site is formed mainly by the residues from the signature motif in C-terminal domain.32,43,44 The sugar donor binding pocket is composed of three clusters: the residues interacting with 1) nucleotide, 2) diphosphate, or 3) glucuronic acid. 32,43,44 It was noted that mutations at one of the residues interacting with the nucleotides had less effects on enzyme function than those interacting with diphosphate or glucuronic acid moieties.
In contrast to the sound understanding of UDPGA binding, knowledge about the residues interacting with the (acceptor) substrate is rather limited, mostly due to the lack of a N-terminal crystal structure. Various biochemical methods such as chemical modification, and application of technologies such photo-affinity labeling and site-directed mutagenesis had identified some key amino acids that govern the substrate specificity of the enzymes. Among these, the mutagenesis and activity assays suggested Phe117 of UGT1A9 participates in 1-naphthol binding.45 The four amino acid motif of UGT1A10 [F(90)-M(91)-V(92)-F(93)] was identified as a key determinant of the binding of phenolic substrates.46,47 On the other hand, the presence of an aromatic amino acid residue at position 33 was important for the activity and substrate specificity of both UGT2B4 and 2B7.48
4.1. Homology structure models of UGTs
For a better understanding of the UGT-substrate binding and of the reaction mechanism, scientists have simulated human UGT structures via homology modeling (or comparative modeling) using glycosyltransferases from plants or bacteria with solved crystal structures. Presently, four 2-domain homology models have been published for human UGTs: three for the human UGT1A1,49–51 and one for the human UGT1A9.52
4.1.1. Sequence alignment
Due to their low sequence identity (~13%), human UGT1A1 sequence were aligned to the plant UGT templates with the aids of predicted secondary structures.49–51 Major portions of the human UGT sequences were highly conserved and easily matched to the plant UGTs. However, there are two regions presumed to make contact with the (acceptor) substrates are highly variable: the Nα3 helix and a region between Nα5 and Nβ6 that was defined as “loop 5” by Laakkonen and Finel. 51 In contrast to plant UGTs which have one relatively short helix Nα3, the Nα3 of human UGTs was divided into three helices (designated as Nα3-1 Nα3-2 and Nα3-3). It was reasoned that Nα3-1 may more properly lied at the interface between the N- and C-domains, and Nα3-2 should be matched to the Nα3 of plant UGTs. 51 The helices Nα3-1 and Nα3-3 were not modeled in the work of Locuson and Tracy,50 and were shown as the random coils in the final generated structures. However, this sequence aligning approach was not favored by Li and Wu, who match the Nα3-1 (and a part of Nα3-2) to plant Nα3.49 The helix Nα3-1 was predicted to isolate the reaction site from bulk water, and move concertedly to allow the exit of the products; whereas it was thought that helix Nα3-3 packs to Nα4 and Nα5-2. 51 In terms of “loop 5”, bacterial UGTs (1iir or 1rrv) were used as the extra templates in earlier works. 50,51 Since the predicted helical pattern immediately upstream of Nβ6 agrees well with the bacterial UGT proteins but not with the plant UGTs, Li and Wu relied more on sequence similarity in handing alignment for “loop 5”. 49 It is recognized that the secondary structure of this area is the most divergent not only among the 5 plant UGTs with known structures, but also when compared to and between other GT-B fold GTs.53
4.1.2. Substrate binding implications from the UGT1A1 homology models
The substrate binding pocket was almost entirely formed by the N-terminal residues, although some C-terminal residues also contributed to the formation of the pocket. 49–51 The pocket was primarily formed by LoopN1, Nα1, Nα3-2, LoopN4, Nα5-1, Nα5-2, Loop C1 and Loop C5,49–51 which was consistent with the topological arrangement of β strands (3-2-1-4-5-6-7) of the enzymes. Nβ2, Nβ3, Nβ6 and Nβ7 twisted far away from the core Nβ1 where the catalytic histidine is situated. The residues predicted to be in contact with aglycones were mainly hydrophobic, suggesting that hydrophobicity is one key substrate recognition characteristic for the enzyme. The latter agrees well with the QSAR regression models. 54
The volume of UGT1A1 model binding pocket is estimated to be 700 Å3, which is ~ 2 times as large as the big substrates such as bilirubin (~400 Å3) or ~4 times as large as the smaller flavonol myricetin (~170 Å3), respectively. The large aglycone-binding domain, as also supported by the CoMFA contours that do not show any steric disfavoring areas,55 might serve as the molecular basis for the generation of multiple metabolites from a single substrate (e.g, multi-hydroxyl flavonoids), because the sufficient space can permit multiple binding modes (distinct orientations) of the acceptor substrate for region-specific catalysis.
4.2. Catalytic mechanism
Similar to most of plant GT1 enzymes, human UGTs (except UGT1A4 and 2B10) use a serine-protease like mechanism for O-catalysis, whereby the catalytic histidine (located at the beginning of Nα2) functions to deprotonate -OH group, which at the same time is stabilized by a neighboring aspartic acid (so called “charge relay”). The deprotonated oxygen subsequently attacks anomeric carbon (C1) of glucuronic acid moiety in UDPGA. 56,57 UGT1A4 and 2B10 do not have the equivalent histidine (replaced by proline and leucine, respectively), tend to catalyze N-glucuronidation using a distinct catalytic mechanism.56,57 The N-nucleophiles are suggested to develop a formal positive charge during the N-glucuronidation, and thus require a negatively charged (i.e., aspartic acid) residue to stabilize the transition state. 56,57
It is well recognized that UGT metabolism followed compulsory ordered bi bi (i.e., two substrates and two products) mechanism.58 UDPGA binds first to the enzyme, followed by the binding of aglycone (Figure 4B/C). The compulsory mechanism was suggested to explain the substrate inhibition, which is increasingly seen in the kinetic profiling of UGTs-mediated metabolism. 58 Binding of the aglycone substrate to the enzyme-UDP complex (E•A) led to a nonproductive dead-end complex that slows the completion of the catalytic cycle. On the other hand, the substrate inhibition pattern was also accounted by the two-site binding models,59 which stated that binding of the substrate to a allosteric site resulted in a decrease of intrinsic catalytic rate constant (characterized by β < 1).60 Apparently, more works are needed to resolve the controversy of causes of the substation inhibition kinetics.
5 Regulation of UGTs by phosphorylation
UGT gene expression is known to be regulated by a number of transcription factors including hepatocyte HNF1 and HNF2, Ah receptor, and nuclear receptors (NR). 61,62 The contribution of those regulators to the large inter-individual variation of hepatic UGT levels has been discussed.63 In recent years, it is becoming increasing evident that UGT enzymes are also regulated via phosphorylation mediated by protein kinase C (PKC) or Src tyrosine kinase (SrcTK). 64, 65 Phosphorylation at serine/threonine by PKC is required for UGT1A activity, whereas tyrosine phosphorylation regulates UGT2B activity. 66–68 The phosphorylation sites for UGT isoforms are summarized in Table 1 and their locations in UGT structure (UGT1A1 homology model 51) are shown in Figure 5. It is not surprising that the phosphorylation sites all appear on the surface of the protein. In addition to the activity abolishment, it is also found that the PKC–mediated phosphorylation in UGT regulates substrate specificity; mutation of PKC sites in UGT1A7 demonstrated that S432G-UGT1A7 caused a major shift in the enzyme’s pH 8.5 optimum to 6.4 with new substrate preferences, including 17β-estradiol.65 Alteration of the substrate selection by phosphrylatoin was also observed for UGT2B7. Non-Src phosphorylated 2B7 metabolizes both 4-OH-estradiol and 4-OH-estrone, while Src-dependent phosphorylation of 2B7 allows metabolism of the former chemical, but not the latter.69
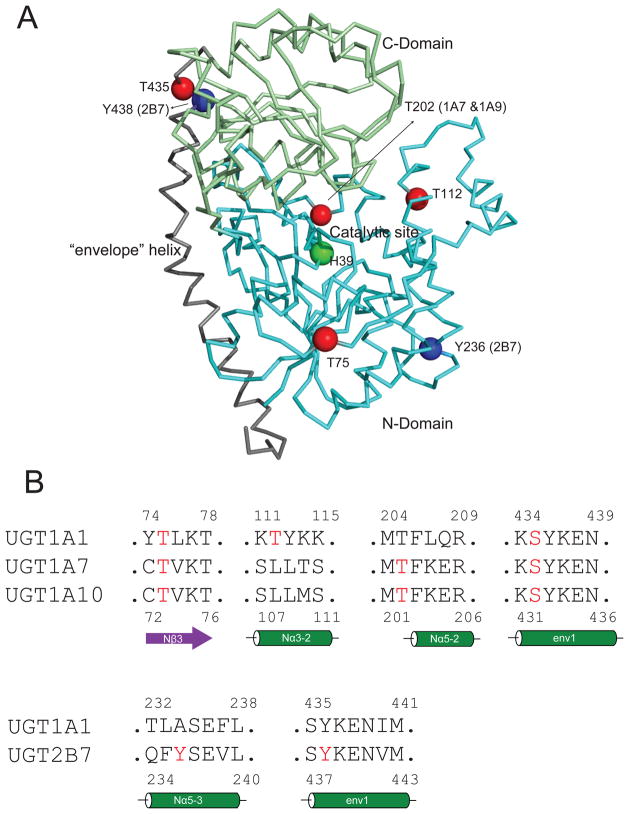
Table 1.
Phosphorylation sites of UGT isoforms and their locations in UGT homology structure
| UGTs | Phosphorylation sites | Location in the UGT1A1 homology model51 |
|---|---|---|
| UGT1A166 | T75 | middle of Nβ3 |
| T112 | early in Nα3-2 | |
| S435 | early in env 1 | |
| UGT1A767 | T73 | middle of Nβ3* |
| T202 | before Nα5-2* | |
| S432 | early in env 1* | |
| UGT1A1067,68 | T73 | middle of Nβ3* |
| T202 | before Nα5-2* | |
| S432 | early in env 1* | |
| UGT2B765,69 | Y236 | middle in Nα5-3* |
| Y438 | early in env 1* |
Locations estimated by matching the relevant residues to UGT1A1 homology model.
Figure 5.
Panel A locations of phosphorylation sites in UGT 3D structure (UGT1A1 homology model 51). Green sphere: catalytic residue (histidine 39); red spheres: protein kinase C (PKC) mediated phosphorylation sites; blue spheres: Src tyrosine kinase (SrcTK) mediated phosphorylation sites. Panel B: alignment of UGT sequences, showing the phosphorylation amino acids (in red) in secondary structures. Sequence alignment was performed using Clustal W (http://www.clustal.org/)
Inhibition of UGT phosphorylation by PKC inhibitor(s) may represent a novel mechanism for drug-drug interactions. 70 PKC-mediated inhibition of human UGT1A6 using different PKC inhibitors was characterized; PKC delta inhibitors could interfere with UGT1A6-mediated glucuronidation of several substrates.70 In addition, drug uptake and in vivo efficacy can be modulated via phosphorylation, which is discussed in section 18.
6 Glucuronidation of flavonoids
6.1. Flavones
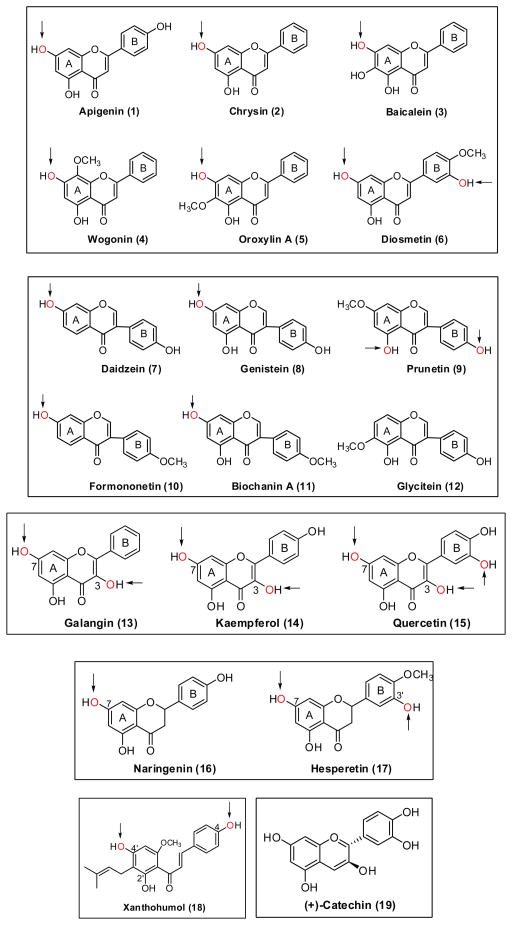
Intestinal and/or hepatic disposition of flavones using apigenin (1, Fig. 6) as the model compound was systematically performed in Caco-2 and rat intestine perfusion models. 71–74 The experimental results are summarized in Table 2. In Caco-2 71,73, apigenin glucuronide was substantially excreted at high concentrations (≥ 25 μM), whereas the excretion of sulfate was significant at low concentrations (≤ 10 μM). Unlike the sulfate which was mainly effluxed apically, the glucuronide was preferentially excreted basolaterally. MK-571 (a multidrug resistance-related proteins (MRPs) inhibitor), when applied, significantly decreased the excretion of both apigenin conjugates, but was also found to inhibit glucuronidation of apigenin at a high concentration (≥ 25 μM). In addition, the authors determined how transport and metabolism change as a function of days-post-seeding. The excretion rates of metabolite were found to be similar from 21 to 24 days post-seeding. UGT1A6 was demonstrated to be a major contributor to glucuronidation of apigenin in Caco-2 cells using the small interfering RNA technique (siRNA).75 In the rat intestine perfusion with bile cannulation,71,72 significant amount of conjugates (mainly glucuronide, ~40% of absorbed apigenin) were found in both perfusate and bile, suggesting that first-pass glucuronidation is critically important in determining its oral bioavailability. Furthermore, apigenin was more rapidly glucuronidated in both human and mouse liver microsomes; the rate of glucuronidation was much faster than that of sulfation.76
Figure 6.
Chemical structures of flavones (1–6), isoflavones (7–12), flavonols (13–15), flavanones (16–17), chalcone (18) and catechin (19). Arrows indicate the favorite position(s) for glucuronidation.
Table 2.
Summary of disposition of apigenin (1, Fig. 6) via glucuronidation using Caco-2 and intestinal perfusion models
| Apigenin | |
|---|---|
| Caco-2 Model 71–73 | |
| Permeability (Papp) | > 1.7 ×10−5 cm/s |
| Cell lysate glucuronidation | Hyperbolic kinetics curve with Km > 25 μM and Vm ~ 250 pmol/min/mg |
| AL excreted glucuronide | 3.42 nmol a |
| BL excreted glucuronide | 5.92 nmol a |
| Rat Perfusion Model 71,72 | |
| P*eff | > 1.5 |
| P*w | > 7 |
| % absorbed | ~ 30% (476 nmol) b |
| Mgut/Mab | 33% b |
| Mbile/Mab | 7% b |
| Intestinal excreted# | 150 nmol b |
| Biliary excreted# | 30 nmol b |
| Intestinal microsomes glucuronidation | Substrate inhibition curve with Vmax > 16 nmol/min/mg |
| Liver microsomes glucuronidation | Substrate inhibition curve with Vmax > 10 nmol/min/mg |
| Regional glucuronidation | jejunum>duodenum>ileum>colon |
| Regional excretion | jejunum>duodenum>ileum>colon |
maximal amount per 30min;
at the end 2 hour of experiment;
perfusion experiment was performed at 35 μM of apigenin and flow rate of 0.382 ml/min. The total amount of compound perfused over a 30-min for each segment was 401 nmol.
Walle et al. evaluated the metabolic fate of chrysin (2, Fig. 6) using Caco-2 and HepG2 cells. 77,78 The absorptive transport (from apical to basolateral side) showed apparent permeability (Papp) value of 6.9 × 3 10−6 cm/s, indicating that the compound is fairly permeable. Following apical loading of chrysin (20 μM), as much as 90% of conjugate (with equivalent glucuronide and sulfate) appeared on the apical side, which is consistent with the efficient metabolism by UGT1A6 (highly expressed in Caco-2 cells) showing Km = 12 μM and Vmax = 2.2 nmol/min/mg. It was noted that the time-course of glucuronidation of chrysin in Hep G2 cells appeared quite similar to that in Caco-2 cells under the same experimental conditions. 78
First pass glucuronidation was much more significant than other pathways such as sulfation and P450 mediated hydroxylation in determining in vivo fate of baicalein (3, Fig. 6).79,80 In the study of Akao et al.,81 a large amount (30.4% recovery) of baicalein glucuronide, but no baicalein, was detected in the intestinal lumens of germ-free rats 4 h after oral administration of the parent compound (12.1 mg/kg). Extensive metabolism of baicalein and rapid efflux of its glucuronide by intestinal epithelium cells were also confirmed in rats using the in situ jejunal loop technique and in vitro jejunal everted sac experiments,81 as well as in Caco-2 cells.82,83 Glucuronidation of baicalein is catalyzed by multiple human UGT isoforms with dominant contributions from UGT1A8 and 1A9. 79
In the study of Zhou et al.,84 wogonin (4, Fig. 6) and oroxylin A (5, Fig. 6) were shown to be glucuronidated by multiple UGT1As, with major contributions from UGT1A3 and UGT1A7-1A10. A comparison of the kinetic parameters and profiles suggested that UGT1A9 is likely the main isoform responsible for the hepatic metabolism using human liver microsomes. In contrast, a combination of UGT1As with a major contribution from UGT1A10 contributed to their intestinal metabolism. The same group of investigators 85 also showed that UGT isoform-specific metabolism could describe their metabolism rates and profiles in human liver and intestinal microsomes based on the correlation analysis. After po treatment of the rat with diosmetin (6, Fig. 6), a rapid glucuronidation takes place and that the compound circulates as glucuronides, whereas no free diosmetin is present in blood and urine.86 Four different glucuronides in blood and the two major ones (diosmetin-7,3′-diglucuronide and 3′-glucuronide) were identified.86
6.2. Isoflavones
In rat perfusion model, Wang and coworkers 87 measured excreted glucuronides for six isoflavone analogs (7–12, Fig. 6) in four intestinal segments (duodenum, jejunum, ileum and colon). They also tried to correlate the excretion rates with the glucuronidation parameters (i.e., glucuronidation rates at 10 μM, CLint, Vmax and Km) which were derived from UGT reaction using intestinal microsomes. The resulting poor correlations indicated that excretion rates were not controlled by any of the microsomes-derived kinetic parameters.
Extensive first-pass metabolism of genistein (8, Fig. 6) is one of the main reasons for its poor bioavailability. In rats, the main metabolites are genistein-7-O-glucuronide and genistein-4′-O-sulfate. 88 In humans, genistein-7-O-glucuronide is the major metabolite (about 90%), whereas genistein-4′-O-sulfate (< 10%) is the minor metabolite. 89, 90 Disposition parameters of genistein in Caco-2 and rodent intestine perfusion models are summarized in Table 3. In Caco-2 cells, 91 glucuronide was the main metabolite for genistein, which was preferably excreted to the basolateral side. In rat perfusion model, 92 genistein was rapidly absorbed with a P*eff value of > 1.5 and was conjugated instantly. The conjugates were substantially excreted to intestine lumen and via the bile, although the fraction of excreted versus absorbed amount was dependent on the experimental conditions such as perfusate concentration and region of the intestine perfused (e.g., jejunum vs. colon) (Table 3). 71,72,92 In mouse perfusion model,93 the excretion rate of genistein glucuronide (4.5 nmol/30min/10 cm) was comparable to sulfate excretion in mouse small intestine, but glucuronide excretion was significantly lower than sulfate excretion in mouse colon.
Table 3.
Summary of disposition of genistein (8, Fig. 6) via glucuronidation using Caco-2 and intestinal perfusion models
| Genistein | |
|---|---|
| Caco-2 Model 71,72 | |
| Permeability (P) | > 3.0 ×10−5 cm/s |
| Cell lysate glucuronidation | Vm ~ 25 pmol/min/mg |
| AL excreted glucuronide | 0.87 nmol a |
| BL excreted glucuronide | 1.68 nmol a |
| Four-site Rat Perfusion Model 72,92 | |
| P*eff | > 1.5 |
| P*w | > 7 |
| % absorbed | ~ 28% (448 nmol)b; 77%* |
| Mgut/Mab | 13% b; 30% (jej, 100 μM)$; 40.3%* |
| Mbile/Mab | 11% b; 6.4%* |
| Intestinal excreted# | 61 nmol b |
| Biliary excreted# | 50 nmol b |
| Intestinal microsomes glucuronidation | Vmax = 3.2 nmol/min/mg; CLint = 0.48 ml/min/mg |
| Liver microsomes glucuronidation | Vmax = 3.7 nmol/min/mg; CLint = 0.16 ml/min/mg |
| Regional glucuronidation | jejunum>duodenum>ileum>colon |
| Regional excretion | jejunum>duodenum>ileum>colon |
| Mouse intestine Perfusion Model 93 | |
| Intestinal glucuronides excreted | 4.5 nmol/30 min/10 cm (small intestine) |
maximal amount per 30min;
at the end 2 hour of experiment;
perfusion experiment was performed at 35 μM of genistein.
One-site rat intestine perfusion using 10 μM of genistein.
Rat jejunum perfusion using 100 μM of genistein
Intestinal and/or hepatic disposition of formononetin (10, Fig. 6) and biochanin A (11, Fig. 6) were studied and compared using Caco-2 and rodent intestine perfusion models. 91,94,95 The experimental results are summarized in Table 4. In Caco-2 transport study, the two compounds showed similar apparent permeabilities. The glucuronides of both compounds were rapidly formed and excreted preferentially to basolateral side. And, the excretion rates of glucuronides were much slower than those of apigenin or genistein (Tables 2–4). Compared to formononetin, biochanin A was better absorbed and was more rapidly glucuronidated in rat perfusion model. 94 However, the excretion of its glucuronide was much slower than that of formononetin glucuronide, suggesting that the efflux transporters favor the transport of formononetin glucuronide over biochanin A glucuronide. In the mouse perfusion study of formononetin,95 the amounts of sulfates excreted in mouse intestine (8–11 nmol/30 min/10 cm) were significantly higher than those for rats, whereas the amounts of glucuronides excreted (7–10 nmol/30 min/10 cm) were comparable. The authors 95 suggested that mouse perfusion model might be more advantageous over the rat intestinal perfusion model for flavonoid disposition studies, in that both sulfates and glucuronides are excreted, as shown in humans.
Table 4.
Summary of disposition of formononetin (10, Fig. 6) and biochanin A (11, Fig. 6) via glucuronidation using Caco-2 and intestinal perfusion models
| Formononetin | Biochanin A | |
|---|---|---|
| Caco-2 Model 94 | ||
| Permeability (Papp) | 1.7 ×10−5 cm/s | 1.6 ×10−5 cm/s |
| Cell lysate glucuronidation | N/A | N/A |
| AL excreted glucuronide | 0.60 nmol a | 0.49 nmol a |
| BL excreted glucuronide | 1.50 nmol a | 0.64 nmol a |
| Rat intestine Perfusion Model 94 | ||
| P*eff | > 1.0 | 2.5 |
| % absorbed | 39% | 70% |
| Mgut/Mab | 29% | 7% |
| Mbile/Mab | 46% | 12% |
| Intestinal excreted# | 31 nmol | 12 nmol |
| Biliary excreted# | 51 nmol | 20 nmol |
| Intestinal microsomes glucuronidation | Km = 5~8.4 μM and CLint = 0.14 ml/min/mg | Km = 2.7~7 μM and CLint = 0.4 ml/min/mg |
| Liver microsomes glucuronidation | CLint = 0.041 ml/min/mg | CLint = 0.12 ml/min/mg |
| Regional glucuronidation | duodenum >ileum >colon > jejunum | duodenum > jejunum >ileum >colon |
| Regional excretion | duodenum >ileum> jejunum > colon | duodenum > ileum > jejunum >colon |
| Mouse intestine Perfusion Model 95 | ||
| P*eff | > 2.5 | N/A |
| Intestinal glucuronides excreted | 7–10 nmol/30 min/10 cm | N/A |
maximal amount per 30min;
at the end 4 hour of experiment; all perfusion experiments were performed at 10 μM of flavonoids and a flow rate of 0.181 ml/min. The total amount of compound perfused over a 30-min for each segment was 57 nmol.
6.3. Flavonols
Glucuronidation is the major metabolic pathway (accounted for more than 70% of the metabolism) for galangin (13, Fig. 6) in hepatocytes. 96 When characterized using liver microsomes, the CLint values for the two major glucuronides conjugated at the 7- and 3-positions were 155 ± 30 and 427 ± 26 μl/min/mg protein, respectively. This highly efficient glucuronidation was suggested to be catalyzed mainly by the UGT1A9 isoform, with contributions from UGT1A1 and UGT2B15.96 Barrington et al. studied the absorption and metabolism of galangin using Caco-2 model,97 three glucuronides and one sulfate were identified in the apical and basolateral media. The total amount of three mono-glucuronides was much higher than that of sulfate, and showed preferential basolateral efflux as demonstrated by the apical to basolateral ratio below 0.6. The three mono-glucuronides, 3-O-, 5-O- and 7-O-glucuronides accounted, respectively, 61.5%, 7.5%, and 31% of the glucuronides secreted apically.
The oral bioavailability of kaempferol (14, Fig. 6) in rats was reported to be ~ 2%.98 The low bioavailability of kaempferol is attributed in part to extensive first-pass metabolism by glucuronidation and other metabolic pathways, based on in vitro phase I and phase II metabolism.98 After oral intake of kaempferol in human, 3-O-glucuronide of kaempferol was found to be the predominant metabolite in plasma.99 In Caco-2 cells,97 glucuronidation of kaempferol predominated over its sulfation, as the total excreted amount of glucuronides (with two major ones conjugated at 3-OH and 7-OH, respectively) was more than 2 times of that of sulfate. The glucuronides showed preferential basolateral transport as demonstrated by an apical to basolateral ratio below 0.3. In the microsomal study, kaempferol was extensively metabolized by UGT1A9 with two kaempferol glucuronides (3-O- and 7-O-glucuronides) being detected after incubation.100
Quercetin (15, Fig. 6) probably is one of the most widely studied flavonoids for its biological effects.101 Quercetin glucuronide readily appeared in blood circulation in both human and rat plasma after oral administration of quercetin. 102, 103 The presence of a quercetin monoglucuronide in the urine of a volunteer after consumption of Ginkgo biloba tablets was demonstrated.104 Chen et al.105 evaluated the regioselectivity of two UGT isoforms, UGT1A3 and 1A9 towards quercetin (with 5 available glucuronidation sites). UGT1A3 was shown to have the highest glucuronidation efficiency for the 3′-OH group, followed by 3-, 4′- and 7-OH. The catalytic efficiency order for UGT1A9 was 3->7->3′->4′-OH, different from that of UGT1A3. Similarly, in the study of Oliveira and Watson, four monoglucuronides of quercetin were generated by UGT1A9.100 When incubating quercetin with UGT1A1, 90% 3′-O-glucuronide but only 10% 4′-O-glucuronide were produced, suggesting the enzyme’s high preference for the 3′-OH group.106
6.4. Flavanones
Absolute bioavailability of oral naringenin (16, Fig. 6) was only 4% in rabbits.107 The contribution of UGTs to first pass metabolism of naringenin had been demonstrated in various studies. For instance, only naringenin glucuronide, but not naringin or naringenin was detected in blood and urine after administration of 20 ml grapefruit juice per kilogram to six healthy adults. 108 Likewise, predominant presence of glucuronide conjugates over the aglycone was observed in rats under oral doses of naringenin (30–270 mg/kg).109 Xu et al. studied the disposition of naringenin via glucuronidation pathway using rat intestine perfusion model. 110 A large amount of glucuronide was excreted to intestinal lumen and bile, as summarized in Table 5. The amount of glucuronide excreted to bile is ~2 times of that of glucuronide excreted to intestinal lumen, suggesting a more significant role of liver in disposition of this class of flavonoids. Glucuronidation of naringenin in intestine is region-dependent following the order of jejunum > ileum > colon.
Table 5.
Summary of disposition of naringenin (16, Fig. 7) via glucuronidation using rat intestinal perfusion model
| Naringenin | |
|---|---|
| Four-site Rat Perfusion Model 110 | |
| % absorbed | 49% |
| Mgut/Mab | 11.8% |
| Mbile/Mab | 22.3% |
| Intestinal excreted# | 27 nmol |
| Biliary excreted# | 51 nmol |
| Jejunum microsomes glucuronidation | Km = 3.62 μM; CLint = 2.07 nmol/min/mg |
| Ileum microsomes glucuronidation | Km = 2.54 μM; CLint = 0.78 nmol/min/mg |
| Colon microsomes glucuronidation | Km = 21.22 μM; CLint = 0.31 nmol/min/mg |
| Liver microsomes glucuronidation | Km = 13.41 μM; CLint = 0.98 nmol/min/mg |
| Regional glucuronidation | jejunum >ileum>colon |
| Regional excretion | duodenum >jejunum >ileum>colon |
maximal amount per 30min; perfusion experiment was performed at 10 μM of naringenin and a flow rate of 0.191 ml/min.
3′-O-glucuronide and 7-O-glucuronide are the major hesperetin (17, Fig. 6) metabolites found in vivo.111–113 In Caco-2 transport study, hesperetin 7-O-glucuronide was excreted at a high rate of 14.3 ± 3.7 pmol/min/monolayer after an apical dose of 10 μM hesperetin. 114 Glucuronidation of hesperetin was characterized using human recombinant UGT enzymes.115 UGT1A1, UGT1A7, UGT1A8, UGT1A9, and UGT1A3 are the major enzymes catalyzing hesperetin glucuronidation. UGT1A3 only generated 7-O-glucuronide, whereas UGT1A7 produced mainly 3′-O-glucuronide.115
6.5. Chalcones
Xanthohumol (18, Fig. 6) is a prenylated chalcone, present in beer.116 No xanthohumol is detected in plasma after oral administration of xanthohumol. 117 Xanthohumol and its metabolites are excreted mainly in feces within 24 h of administration.117 Two major glucuronides of xanthohumol (glucuronidated at 4′-OH and 4-OH) were found when incubated it with either rat or human liver microsomes.118 Xanthohumol was efficiently glucuronidated by UGT1A8, 1A9 and 1A10, followed by UGT1A1, 1A7 and 2B7.119 Xanthohumol was also shown to bind to cytosolic proteins in intestinal epithelial cells which might also affect its oral bioavailability.120
6.6. Catechins
Metabolism of catechins (19, Fig. 6) including glucuronidation was extensively reviewed by Feng,121 to which one can refer for details. In the study of Crespy et al., glucuronidation of catechins was shown to be much less efficient compared to flavonols (e.g., quercetin).122
7 Stilbenes
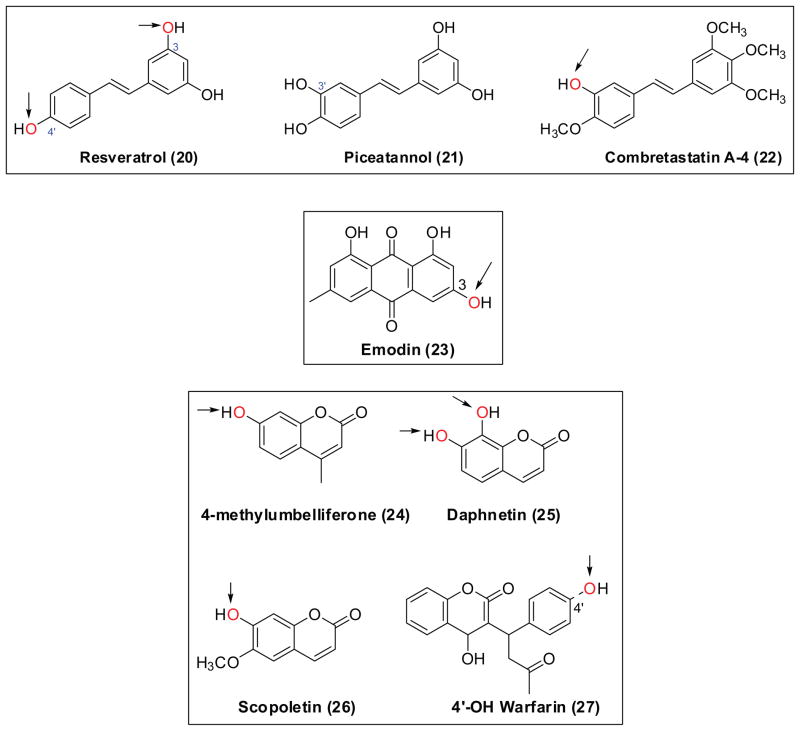
Resveratrol (20, Fig. 7), present in grape and wine, has beneficial effects against cancer and protective effects on the cardiovascular system. Bioavailability of resveratrol in human is very low, only trace amounts of unconjugated resveratrol (< 5 ng/ml) could be detected in plasma after oral administration of 25 mg resveratrol. 123 On the contrary, a moderate bioavailability (38%) was reported in rats.124 The susceptibility of resveratrol to first pass glucuronidation had been revealed in many studies. Human liver UGTs actively glucuronidate resveratrol with the 3-O-glucuronide being the predominant product, and the cis-isomer was glucuronidated at a faster rate than the trans-isomer. 125,126 Likewise, resveratrol is efficiently glucuronidated in the human gastrointestinal tract and in Caco-2 cells. 126,127 In an isolated rat small intestine model, 96.5% of absorbed resveratrol was converted to its glucuronide conjugate.128 In Caco-2 transport study, resveratrol showed a fair Papp value of ~ 7 × 10−6 cm/s, unexpectedly, the metabolism of resveratrol in Caco-2 cells involved mainly sulfation and, to a much less extent, glucuronidation.129
Figure 7.
Chemical structures of stilbenes (20–22), quinone (23), and coumarins (24–27). Arrows indicate the favorite position(s) for glucuronidation. UGTs mediate glucuronidation of 4′-hydroxylwarfarin (27), which is the product of CYP450 enzymes.
Piceatannol (21, Fig. 7), is 3′-hydroxyl resveratrol. Resveratrol might serve as a pro-drug for the production of piceatannol, since resveratrol is metabolized in vitro to piceatannol via cytochrome P450 enzymes.130 Roupe and co-workers 131 have revealed two piceatannol glucuronides in rat plasma after intravenous administration of 10 mg/kg piceatannol. Piceatannol was extensively metabolized in the human liver to three conjugates (monoglucuronides).132 The main UGT isoforms catalyzing the glucuronidation of this compound are UGT1A1, 1A8 and 1A10.132
Combretastatin A-4 (22, Fig. 8) has been described as a potent tubulin polymerization inhibitor, and is currently being evaluated in multiple clinical trials as a treatment for solid tumors.133 Glucuronidation is the main metabolic pathway that contributes to the clearance of this compound. 134 UGT1A9 was primarily responsible for the in vitro glucuronidation of combretastatin A-4 with a Vmax value of 12.78 ± 0.29 nmol/min/mg protein and a Km value of 6.98 ± 0.65 μM. UGT1A6 was also a significant contributor to combretastatin A-4 glucuronidation (Vmax = 3.95 ± 0.13 nmol/min/mg protein and S50 = 44.80 ± 3.54 μM).134
Figure 8.
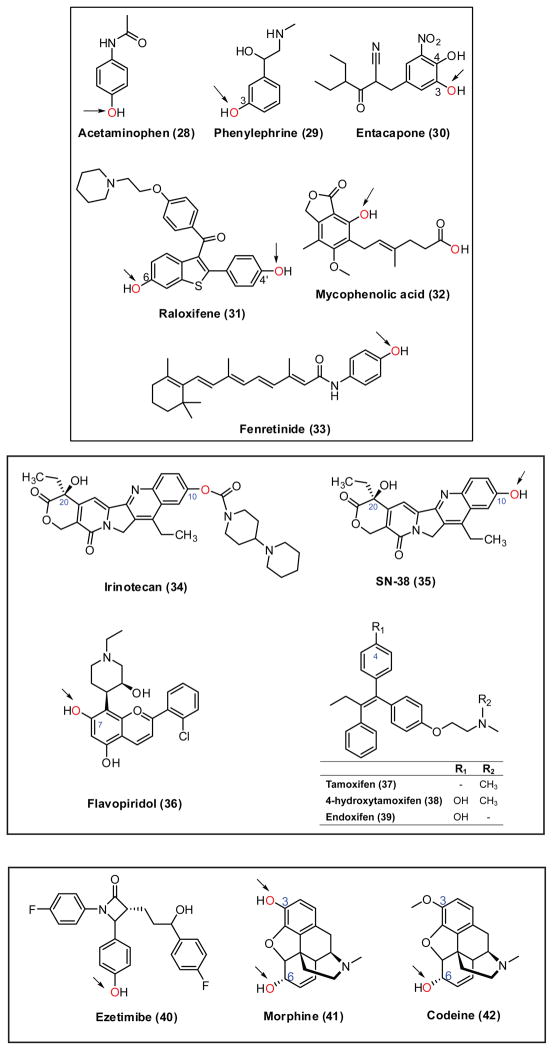
Chemical structures of phenolic drugs [i.e., acetaminophen (28), phenylephrine (29), entacapone (30), raloxifene (31), mycophenolic acid (32) and fenretinide (33)], irinotecan (34), SN38 (35), flavopiridol (36), tamoxifen and its CYP metabolites (37–39), ezetimibe (40), morphine (41), and codeine (42). Arrows indicate the favorite position(s) for glucuronidation.
8 Anthraquinone
Emodin (23, Fig. 7) is a major active anthraquinone present in the rhubarb.135 Oral administration of emodin to rabbits resulted in a very low serum concentration.136 In an isolated rat small intestine model, emodin glucuronide (8.69%) and sulfate (1.84%) were detected at the vascular side, whereas the glucuronide (5.23%) and sulfate (1.08%) moieties were also found in the luminal perfusate.137 Liu et al.138 showed that rapid metabolism by UGTs is the major reason why emodin has poor bioavailability. Interestingly, glucuronidation rates of emodin obtained using liver microsomes from various experimental animals (i.e., mice, rats, guinea pigs, dogs) of the same gender correlated well with those in human liver microsomes.138
9 Coumarins
Oral bioavailability of 4-methylumbelliferone (24, Fig. 7) in rats is less than 1.5%.139 The total body plasma clearance of 4-methylumbelliferone was accounted for mostly by the hepatic conjugative metabolism.139 In the isolated perfused livers, cumulative biliary excretion of the glucuronide was extensive (= 25 μmol), after a 30 μmol dose of 4-methylumbelliferone.140 4-methylumbelliferone is known to be metabolized by multiple human UGT isoforms, especially by UGT1A6, 1A7 and 1A10.141
Daphnetin (25, Fig. 7) has been developed as an oral medicine for the treatment of coagulation disorders and rheumatoid arthritis in China.142 By far, there is only one report about the glucuronidation of daphnetin,142 which demonstrated that 7-O- and 8-O-glucuronides were almost exclusively generated by UGT1A9 and UGT1A6, respectively. Furthermore, the kinetic characterization studies, chemical inhibition experiments, and correlation analysis were performed to demonstrate that human UGT1A9 and UGT1A6 were major isoforms involved in the daphnetin glucuronidation in human intestine and liver microsomes. 142
Bioavailability of scopoletin (26, Fig. 7) appeared to be low in human after the ingestion of Noni Fruit Extract. 143 The role of glucuronidation in disposition of scopelein is yet to be confirmed using animal models, although this compound is shown to be metabolized rapidly by UGT1A3, 1A6 and 1A9. 58
Warfarin (27, Fig. 7) is a widely prescribed anticoagulant. UGT-mediated metabolism of warfarin has been reviewed.144 Glucuronidation acts as a secondary (sequential) process in metabolism of warfarin, whereby the hydroxywarfarins (products of cytochrome P450s) are glucuronidated. The glucuronides (mainly 4′-OH-warfarin-4′-O-glucuronide) accounted for up to 13% of all metabolites in human.144
10 Phenolic Drugs
10.1. Acetaminophen
Acetaminophen (28, Fig. 8), a widely used over-the-counter analgesic and antipyretic, is subjected to extensive first-pass glucuronidation. Glucuronidation accounts for 40% to two-thirds of the metabolism in human.145 Acetaminophen glucuronide excreted to bile accounts for ~7% of the administered acetaminophen dose (100 mg/kg) in rats and ~10% in the isolated perfused rat liver at the equivalent acetaminophen dose.146 UGT1A1, 1A6, 1A9 and 2B15 contribute significantly to its glucuronidation.147 Glucuronidation of acetaminophen was also observed in Caco-2 cell microsomes.148 However, in Caco-2 transport study, acetaminophen glucuronide was not formed, but apical and basolateral efflux of sulfate was observed.149 This is explained by that during the permeability experiments, the intracellular acetaminophen concentration in Caco-2 cells may not be high enough for sufficient glucuronidation. While sulfotransferases often have much higher affinity for the substrates and can operate at low substrate concentrations.149
10.2. Phenylephrine
Phenylephrine (29, Fig. 8) is 1-adrenergic receptor agonist, and commonly used as decongestant. This drug generally causes fewer side effects, owing to its selectively binding to the desirable target. However, the effectiveness of oral phenylephrine is compromised by the poor and varied bioavailability (< 38%).150 This issue of bioavailability is resulted from the extensive pre-systemic metabolism, particularly, ~59% of the administered compound is found to be in phase II conjugated forms (i.e., 12% glucuronide and 47% sulfate).151–153
10.3. Entacapone
Entacapone (30, Fig. 8) functions as a catechol-O-methyl transferase (COMT) inhibitor. It is used in the treatment of Parkinson’s disease. The most important metabolic pathway of this compound is glucuronidation. 154 The glucuronides of entacapone and its main phase I metabolite, entacapone(Z)-isomer, have been found to represent over 95% of all urinary metabolites in humans.154 Entacapone is glucuronidated primarily by UGT1A7, 1A8, 1A9 and 1A10.155 Although less efficient, UGT1A1 generated two entacapone glucuronide isomers at 3-OH and 4-OH positions, respectively.156 Glucuronidation of entacapone and its analogs were also evaluated using rat liver microsomes, and substituents on the backbone had a remarkable effect on the enzyme kinetic parameters.157
10.4. Raloxifene
Raloxifene (31, Fig. 8), a selective estrogen receptor modulator, is used for the treatment of osteoporosis. The absolute bioavailability of raloxifene is only 2%. 158 Human intestinal glucuronidation limited the hepatic exposure of raloxifene that underwent bio-activation in the liver. 158 In Caco-2, raloxifene has low absorptive permeabilities (PAB = 0.39 × 10−6 − 4.1 × 10−6, 1.5–30 μM), when compared to flavonoids (e.g., apigenin and genistein) (Tables 2–3). The total clearance value was up to 21 μl/h for glucuronides.159
10.5. Mycophenolic acid
Mycophenolic acid (32, Fig. 8) is a potent immunosuppressant by inhibiting inosine monophosphate dehydrogenase (IMPDH) and preventing proliferation of activated T- and B-lymphocytes. Mycophenolic acid undergoes high level of glucuronidation,160 UGT1A8 and UGT1A10, were especially active in glucuronidation of mycophenolic acid.161 The amount of mycophenolic acid recycled in the body was estimated to be only 29.1% of the total amount absorbed.162 Specific UGT genotypes significantly alter mycophenolic acid pharmacokinetic profiles after an oral dose of mycophenolate mofetil (mofetil-formulated prodrug).163 Interestingly, targeted inhibition of glucuronidation by the kinase inhibitors markedly enhanced the uptake and efficacy of mycophenolic acid in mice, because kinase-mediated phosphorylation is required for the mouse Ugts activity.164
10.6. Fenretinide
Fenretinide (33, Fig. 8) is a retinoic acid analogue, currently used in clinical trials in oncology. 165 Fenretinide shows very poor and varied oral bioavailability, possibly due to a variety of factors including solubility, permeability and extensive metabolism.166 In addition to significant oxidative metabolism by CYP2C8, fenretinide was found to be glucuronidated rapidly by UGT1A1, 1A3 and 1A6. 166
10.7. Anti-cancer phenolics that are mainly cleared by UGTs
10.7.1. Irinotecan
Irinotecan (CPT-11) (34, Fig. 8) is a widely used anticancer drug, especially for the treatment of colorectal cancer. Irinotecan is activated by the hydrolysis to form SN-38 (35, Fig. 8), an inhibitor of topoisomerase I. SN-38 is primarily eliminated via glucuronidation pathway mediated by UGT1A1. Clinical application of irinotecan had seen serious toxicities (e.g., neutropenia, diarrhea and asthenia), which largely arise from the inefficient or reduced glucuronidation of SN-38. 167 Generic polymorphisms in UGT1A1 (e.g., UGT1A1*28 variant) was linked to increased irinotecan toxicity.168 The most common UGT1A1*28 allele (promoter (TA)6/7TAA mutation) markedly decreases UGT1A1 expression, resulting in seriously impaired glucuronidation via UGT1A1.169 In order to prevent serious toxicity and assist in selection of initial dose, US Food and Drug Administration (FDA) recommended UGT1A1 genotyping of patients prior to the irinotecan treatment in 2005. 167
10.7.2. Flavopiridol
Flavopiridol (36, Fig. 8) is a cyclin-dependent kinase inhibitor for the treatment of chronic lymphocytic leukemia.170 Glucuronidation is the major mechanism for hepatic clearance of flavopiridol.171,172 Clinical toxicity (e.g., diarrhea) accompanied with this drug appeared to be inversely correlated with the magnitude of its glucuronidation. 172 Both 7-O- and 5-O-glucuronides were observed after incubation of flavopiridol with human liver microsomes. However, formation rate of 7-O-glucuronide was 50 times higher than that of 5-O-glucuronide. UGT1A9 and UGT1A1 were demonstrated to be responsible for the formation of 7-O- and 5-O-glucuronide, respectively, via kinetics determination, inhibition and reaction phenotyping studies. 173, 174 Surprisingly, the common polymorphisms in UGT1A9 (i.e., I399C>T and-118T(9>10)) did not result in altered UGT1A9 activities measured using flavopiridol.175
10.7.3. Tamoxifen
Tamoxifen (37, Fig. 8) is an antagonist of the estrogen receptor that is used for the treatment and prevention of breast cancer in women. In contrast to its limited activity, the phase I metabolites of tamoxifen, 4-hydroxytamoxifen (38, Fig. 8) and endoxifen (39, Fig. 8) showed very potent antiestrogenic effect.176 4-hydroxytamoxifen and endoxifen are mainly cleared via glucuronidation. 177–179 Both N- and O-glucuronidation occurred for 4-hydroxytamoxifen in human liver microsomes, but only O-glucuronidating activity was observed for endoxifen. UGT1A4 appeared to be the only enzyme that catalyzed the formation of N+-glucuronide from 4-hydroxytamoxifen. 180 By contrast, UGT1A8, 1A10 and 2B7 exhibited significant O-glucuronidating activity against trans-4-hydroxytamoxifen and trans-endoxifen, whereas UGT1A10 alone showed the highest activity against cis-4-hydroxytamoxifen and cis-endoxifen.179 In a recent study,181 UGT variants (UGT2B7268Tyr and UGT1A8173Ala/277Tyr) showed no or 2–5 folds decreased activity against the trans isomers of either 4-hydroxytamoxifen and endoxifen. The functional polymorphisms were suggested to be important in inter-individual variability in tamoxifen metabolism and response to the tamoxifen therapy.
10.8. Pharmacologically active glucuronides
10.8.1. Ezetimibe
Ezetimibe (40, Fig. 10) is a selective cholesterol absorption inhibitor, which potently inhibits the absorption of biliary and dietary cholesterol from the small intestine by binding to the sterol transporter (Niemann-Pick C1 Like 1 or NPC1L1). 182 Ezetimibe undergoes extensive glucuronidation which is primarily mediated by UGT1A1 and 1A3, 12,183 and its glucuronide binds with higher affinity for NPC1L1 than ezetimibe to prevent the cholesterol absorption.184,185 Although the deficiency of UGT1A activity did not alter the cholesterol-lowering effect of ezetimibe in rats at therapeutic doses,186 UGT1A1*28 (but not UGT1A1*6) allele was suggested to affect the pharmacokinetics of ezetimibe.187 The in intro glucuronidation kinetics of ezetimibe was characterized using human liver microsomes,188 the determined apparent Km and Vm values were, respectively, 1.51 μM and 2.86 nmol/min/mg protein.
Figure 10.
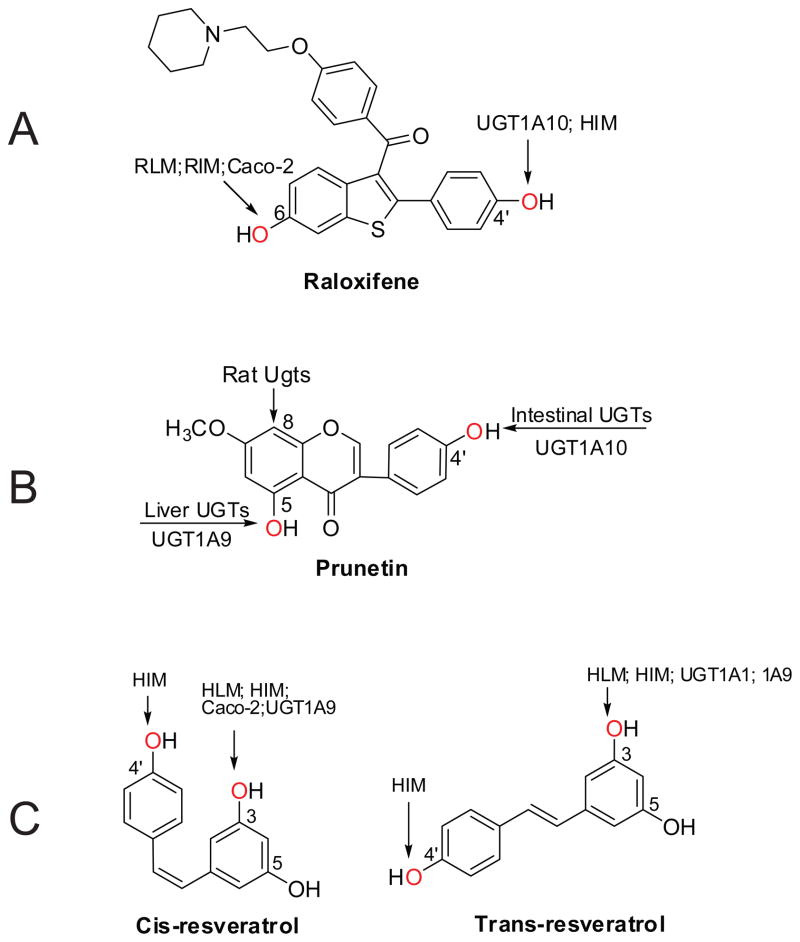
Summary of region-, organ-, or isoform-specifc glucuronidation of prunetin (A) 198, raloxifene (B) 159,199 and resveratrol (C).125,126 HLM, human liver microsomes; HIM, human intestine microsomes; RLM, rat liver microsomes; RIM, rat intestine microsomes. Arrows indicate the position(s) for glucuronidation.
10.8.2. Morphine
Morphine (41, Fig. 8), the prototypical opioid analgesic, is widely used for the relief of acute and chronic pain. Two glucuronide isomers, 3- and 6-glucuronide are often generated from morphine by UGTs. 6-glucuronide has been shown to be a superior opioid agonist than the parent compounds,189 whereas 3-glucuronide is not. Morphine-6-glucuronide (M6G) is currently undergoing phase III clinical trials in patients with postoperative pain.190. It was concluded in a recent review that M6G is an attractive alternative to morphine in the treatment of severe postoperative pain. 191 Earlier study reported that only UGT2B7 was involved in the glucuronidation of morphine to M6G.192 However, a more recent study suggested that apart from UGT2B7, UGT1A1 and 1A8 also contributed to the morephine-6-glucuronidation in vivo.193
10.8.3. Codeine
Codeine or 3-methylmorphine (42, Fig. 8) is considered as a pro-drug. It is primarily metabolized in vivo to the active compounds morphine and codeine-6-glucuronide (C6G).194 Formation of C6G is catalyzed by UGT2B4 and 2B7.195 However, the genetic variant, UGT2B7*2 (H268Y) did not affect the glucuronidation of codeine.195 On the other hand, using the in vitro-in vivo extrapolation approach, 1.60- to 3.66-fold increases in AUC of codeine were predicted with the inhibition of UGT2B4- or 2B7-catalyzed codeine glucuronidation by the known UGT2B7 inhibitors (i.e., dextropropoxyphene, fluconazole, ketoconazole and methadone).196
11 Gender-dependent glucuronidation
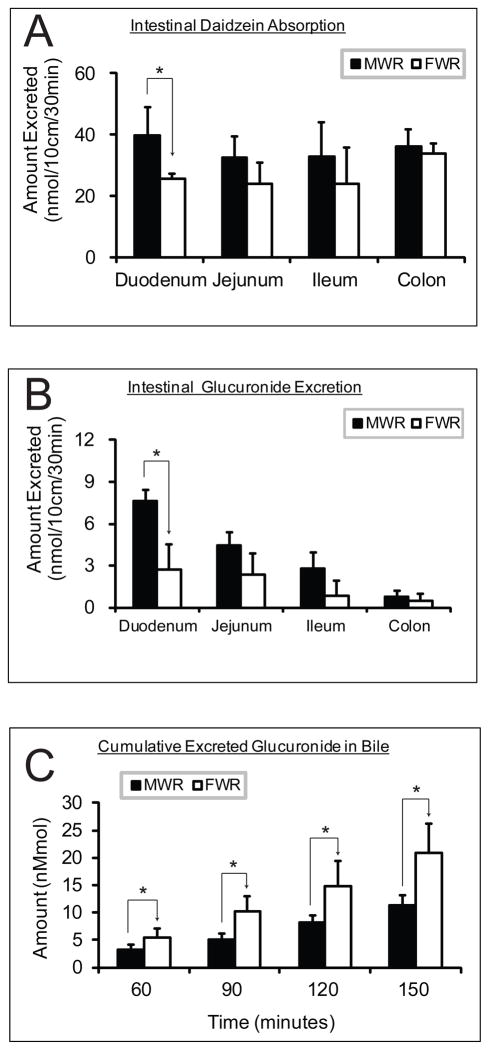
Liu et al. 138 studied the disposition of emodin using rat intestine perfusion model, excretion rates of emodin-3-O-glucuronide were significantly different (p < 0.05) in four regions of the intestine and were higher in males than in females (p < 0.01). Similarly, we found duodenal excretion of glucuronide was significantly higher in male than female rats for the isoflavone daidzein (7, Fig. 6) (p < 0.05), which coincided with a higher absorption of the parent compound in duodenum (Figure 9A/B). In contrast, biliary excretion of the glucuronide is significantly higher in female than male rats (p < 0.05) (Figure 9C). Gender differences were also apparent in glucuronidation of acetaminophen, with intrinsic clearance (CLint) values significantly higher in male compared with female ferret livers.200 In mice model, the biliary excretion rate constant was 7-fold higher in males than in females for acetaminophen glucuronide.197
Figure 9.
Disposition of daidzein (see Fig. 6 for chemical structure) in four-site rat intestine perfusion model with bile cannulation, showing the comparison of aglycone absorption (A), intestinal (B) and biliary (C) glucuronide excretion between male and female Wistar rats. MWR, male Wistar rats; FWR, female Wistar rats; * indicates significant difference with p < 0.05; n=4.
12 Species- dependent glucuronidation
Species difference in glucuronidation activity was observed with human jejunum microsomes higher than rat intestinal microsomes for all hydroxyl-flavones except for 3,7-dihydroxyflavone.204 Glucuronidation of the isoflavone prunetin (9, Fig. 6) (CLint) in human intestinal microsomes was 3- to 6-fold higher than that in rat intestinal microsomes, but was similar in liver microsomes.198 In addition, rat Ugts might be able to produce an unusually prunetin C-glucuronide.198 Emodin glucuronidation in liver microsomes was species dependent among mice, rats, guinea pigs, dogs, and humans,138 and Km values varied 5.7-fold (3.2–18.2 μM) in males and 2.8-fold (4.6–13.0 μM) in females. The male intrinsic clearance (CLint) values differed by 5-fold (27.6–138.3 ml/h/mg protein), and female CLint values differed by 4.3-fold (24.3–103.5 ml/h/mg protein). Raloxifene has a much lower bioavailability in humans (2%) than in rats (39%), which can be largely explained by the glucuronidation difference in intestine. Clint value for raloxifene glucuronidation was 7.5-fold158 or 2–5 fold199 higher in the human intestine as compared to rats. Also, it was shown that UGT1A10 (highly expressed in human intestine) is very proficient in glucuronidating raloxifene (Figure 10A).199 Glucuronidation of acetaminophen was relatively slow in ferret livers compared with livers from all other species except cat.200
13 Intestinal versus hepatic glucuronidation
In rats or mice, intestine probably plays a more significant role than liver in first-pass disposition of flavonoids via glucuronidation. 72 Glucuronidation of flavonoids using rat intestinal microsomes often times show higher catalytic efficiency than that using rat liver microsomes. In human, intestinal disposition may also be more important than hepatic disposition, because human intestinal microsomes were more efficient than liver microsomes in glucuronidating phenolic compound such as prunetin and raloxifene. 198,201
Regioselective glucuronidation differences were observed between human intestine and liver. Human intestine microsomes preferred to generate 4′-O-glucuronide for prunetin, while 5-O-glucuronide was mainly formed by human liver microsomes (Figure 10B). In stereo- and region- glucuronidation studies of resveratrol,125,126 experiments using human intestine microsomes generated 3-O- and 4′-O-glucuronide for both cis-resveratrol and trans-resveratrol, whereas those using human liver microsomes tend to form 3-O-glucuronides only (Figure 10C).
14 Isoform-specific glucuronidation
The UGT isoforms that are responsible for glucuronidating phenolics are generally from UGT1A subfamily, especially, 1A1, 1A3, and 1A7~1A10.62,85 Substrate specificity of the six main contributors often exhibits significant overlaps.
For prunetin, UGT1A7, 1A8, and 1A9 were mainly responsible for the formation of 5-O-glucuronide, whereas UGT1A1, 1A8, and 1A10 were mainly responsible for the formation of 4′-O-glucuronide. 198 UGT1A10 was also shown to be responsible metabolizing 4′-OH of raloxifene (Figure 10A). UGT1A1 appears to be more selective on certain position of a phenolic molecule. For example, UGT1A1 predominantly metabolized the 3′-OH group of flavones or flavonols106 and 3-OH of trans-resveratrol (Figure 10C). 125,126 The strict regiospecificity was also observed with UGT1A9, which predominantly catalyzed glucuronidation at the 3-OH group of either trans- or cis-resveratrol (Figure 12C).
Even though that the quantitative UGT protein level in the intestine and liver is unknown, this lab 84,85 made attempts to correlate the isoform- with organ-specific glucuronidation rates considering the predominant isoforms only (intestine: UGT1A1, 1A8 and 1A10; liver: UGT1A1 and 1A9). Interestingly, good correlations were observed in these studies. The results suggest that the differences in intestinal and hepatic glucuronidation might be ascribed to the presence of the organ-specific UGT isoforms (intestine: UGT1A8 and 1A10; liver: UGT1A9).
15 Structure-glucuronidation relationship
The significances pertaining structure-glucuronidation relationship are emphasized by Wong et al.202 Briefly, the knowledge can usually be used to (but not limited to): (a) predict glucuronidation-mediated drug interactions that include both xenobiotics or endogenous compounds; (b) screen for compounds that are exclusively metabolized by a particular UGT isoform, which might be utilized as probe substrates for a particular UGT isoform; and (c) assist in the biosynthesis of flavonoid (or other compounds) glucuronides conjugated at desired position(s) for pharmaceutical and/or analytical purpose.
The existence of structure-glucuronidation relationship for flavonoids was first noted in the study of Chen et al.,91 which showed that the intestinal glucuronidation is slower in isoflavones without an additional A-ring substitution (electro-donating groups:-OH or –OCH3). Zuo et al. further evaluated the glucuronidation of mono- and di-hydroxyflavones using intestinal microsomes and S9 fraction.203,204 Glucuronidation activity of 6- and 3′-mono-hydroxyflavones was much greater than that of 3-, 4′-, 7- and 2′-HF, with 5-HF to be the lowest.203 Increasing the number of hydroxyl groups on A- or B-ring (except for 4′-OH) would enhance the glucuronidation activity of flavones, whereas adding a 3-OH group on C-ring might not.204 Furthermore, the existence of a hydroxyl group at the 3′ position may enhance the glucuronidation activity of flavonoids.204 Recently, a 3D-QSAR model was established for UGT1A9 to predict the 3-O-glucuronidation of flavonols.55 The authors showed that it is necessary to model the regiospecific glucuronidation separately in order to determine or predict the glucuronidation rates of flavonoids, most of which possess more than one glucuronidation site. 55
16 Compensation between UGT1As and 2Bs
A recent study205 showed that flavonoids (i.e., apigenin and genistein) are efficiently metabolized by Ugt1a-deficient Gunn rats at a comparable or even higher level, in contrast to control Wistar rats. The equivalent or increased glucuronidation in Gunn rats was ascribed to the compensatory up-regulation of intestinal Ugt2bs and hepatic anion efflux transporters.205 This was the first report to show that activities of other Ugt isoforms (2b isoforms) had changed (i.e., elevated) to compensate for Ugt1a deficiency.
17 Deglucuronidation by β-glucuronidase
For the majority of dietary polyphenols, it remains unclear that their glucuronide still retain the biological functions in vivo. However, accumulating evidences suggest that β-glucuronidase -mediated deglucuronidation can occur in vivo, which converts the glucuronides back to free aglycones. This process was proposed to assist in the uptake, transport of the polar metabolite, and more importantly make the inactive metabolite active. 206,207 Deglucuronidation had been frequently reported in the gut contributing to the enteric/enterohepatic recycling,7,8,71 where the glucuronides excreted from enterocytes can be hydrolyzed by intestinal bacteria that have β-glucuronidase. Hepatic and/or renal deglucuronidation might also contribute to disposition of acetaminophen.208 In the study of O’Leary et al.,209 HepG2 cells can absorb and turnover quercetin glucuronides, and β-glucuronidase activity could modulate the intracellular biological activities of dietary antioxidant flavonoids.
Shimoi et al. systematically investigated the β-glucuronidase activity in inflammation. 206,210,211 Supernatants obtained from the neutrophils stimulated with ionomycin/cytochalasin B hydrolyzed luteolin (5,7,3′,4′-tetrahydroxyflavone) monoglucuronide to free luteolin, suggesting that the β-glucuronidase was secreted from the stimulated neutrophils.210,211 The β-glucuronidase activity in rat and mouse plasma also increased after iv injection of lipopolysaccharide (LPS). 206 Therefore, there is a possibility that the inactive glucuronides of exogenous compounds formed in vivo can be converted back to the active parent compounds at the sites of inflammation.212 In addition, Caco-2 cells also showed a significant level of β-glucuronidase activity, suggesting that expression level of β-glucuronidase is higher in this cancerous cell. 206 This finding and its metabolic implications remain to be confirmed. Nevertheless, the deglucuronidation process is less noted in Caco-2 transport experiment, 83 probably because the formed glucuronide is rapidly pumped out of the cells by efflux transporters. The efficient removal of glucuronide might preclude the occurrence of reverse reaction to a significant extent.
18 Possible strategies to improve bioavailability
First, inhibitors of UGT phosphorylation may be applied to reduce the magnitude of first-pass glucuronidation, thus improving the oral bioavailability. A study by Basu et al.164 represented the first work to show that targeted inhibition of glucuronidation could lead to enhanced drug (i.e., mycophenolic acid) uptake and efficacy. The authors successfully utilized curcumin (a protein kinase C inhibitor) to down-regulate UGT phosphorylation reversibly, thus, suppress glucuronidation; and demonstrated between a 6- and 9-fold improvement in free-drug (mycophenolic acid) uptake and therapeutic efficacy. 164 However, when using this approach, one needs to consider the duration of the inhibitory effect (or the body residence time of the inhibitor), the prolonged inhibition of UGT activity might also result in reduced total body clearance, a potential safety concern.
As discussed earlier, glucuronidation is rather sensitive to the substrate structure. The significant changes in glucuronidation activity were seen even with a single hydroxyl or methoxyl group addition or deletion (section 15). Therefore, structural modification of compounds of interest would allow modulation of their metabolism in vivo, thus enhance their bioavailabilities. For example, methylated flavonoids have greatly improved intestinal absorption and metabolic stability, compared to the unmethylated polyphenols, resveratrol and quercetin.213
Lastly, colon seems to be a desired target for phenolic compound delivery to enhance the systemic bioavailability.214 In the rodent perfusion studies, colon is often the organ with good absorption (a comparable or even higher level than upper intestine), but minimal or none glucuronidation and glucuronide excretion. 72,93,94,110 However, this approach to improve bioavailability by circumventing the intestinal glucuronidation has not been reported so far, perhaps because the compounds are also rapidly metabolized in the liver.
19 Concluding remarks
First-pass glucuronidation in the intestine and liver is one of major barriers limiting the oral bioavailability of phenolic compounds. UGTs (a large family of enzymes) catalyze the glucuronidation of phenolics in species-, gender-, organ-, and isoform-dependent manner. Furthermore, it was shown recently that Ugt1as deficiency in Gunn rats is compensated by the increases in Ugt2bs activities, which indeed complicates the attempts to bypass first-pass metabolism by suppressing the activity of a single or even multiple UGT isoforms. Based on the literatures, a temporal inhibition of phosphorylation (reversibly) and the structural modification of the substrates represent two possible directions, in order to modulate in vivo glucuronidation, thus increase the oral bioavailability of phenolics. The latter requires a better understanding of substrate-UGTs interaction, which requires further advancements in UGT structure and enzymology.
Acknowledgments
This work was supported by grants from the National Institutes of Health [GM070737] to MH.
Abbreviations used
- UGTs
UDP-glucuronosyltransferases
- GTs
glycosyltransferases
- GT1
family 1 of glycosyltransferases
- CYPs
Cytochrome P450
- UDPGA
uridine diphosphoglucuronic acid
- ER
endoplasmic reticulum
- UDPGlcNAC
UDP-N-acetyl glucosamine
- NSTs
nucleotide sugar transporters
- ATER
ER-localized organic anion transporters
- NR
nuclear receptors
- PKC
protein kinase C
- SrcTK
Src tyrosine kinase
- HNF-1
hepatocyte nuclear factor
- IMPDH
inosine monophosphate dehydrogenase
- FDA
Food and Drug Administration
- NPC1L1
Niemann-Pick C1 Like 1
- M6G
Morphine-6-glucuronide
- C6G
codeine-6-glucuronide
- LPS
lipopolysaccharide
- NSAIDs
Non-steroidal Anti-inflammatory Drugs
- MRP
multidrug resistance-related protein
- Papp
apparent permeability
- COMT
catechol-O-methyl transferase
References
- 1.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90(2–3):157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 2.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 3.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(8):1001–43. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 4.Hu M. Commentary: bioavailability of flavonoids and polyphenols: call to arms. Mol Pharm. 2007;4(6):803–6. doi: 10.1021/mp7001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131(4 Suppl):1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 6.Gao S, Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev Med Chem. 2010;10(6):550–67. doi: 10.2174/138955710791384081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong EJ, Liu X, Jia X, Chen J, Hu M. Coupling of conjugating enzymes and efflux transporters: impact on bioavailability and drug interactions. Curr Drug Metab. 2005;6(5):455–68. doi: 10.2174/138920005774330657. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Hu M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin Drug Metab Toxicol. 2007;3(3):389–406. doi: 10.1517/17425255.3.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emoto C, Murayama N, Rostami-Hodjegan A, Yamazaki H. Methodologies for Investigating Drug Metabolism at the Early Drug Discovery Stage: Prediction of Hepatic Drug Clearance and P450 Contribution. Curr Drug Metab. 2010;11(8):678–85. doi: 10.2174/138920010794233503. [DOI] [PubMed] [Google Scholar]
- 10.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 11.Ritter JK. Roles of glucuronidation and UDP-glucuronosyltransferases in xenobiotic bioactivation reactions. Chem Biol Interact. 2000;129(1–2):171–93. doi: 10.1016/s0009-2797(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 12.Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44(5):467–94. doi: 10.2165/00003088-200544050-00002. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J Nutr. 1999;129(2):399–405. doi: 10.1093/jn/129.2.399. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Zuo Z, Lin G. Intestinal and hepatic glucuronidation of flavonoids. Mol Pharm. 2007;4(6):833–45. doi: 10.1021/mp700077z. [DOI] [PubMed] [Google Scholar]
- 15.Ehrhardt C, Kim K. Drug Absorption Studies: In Situ, In Vitro and In Silico Models. AAPS press; 2008. [Google Scholar]
- 16.Miners JO, Mackenzie PI, Knights KM. The prediction of drug-glucuronidation parameters in humans: UDP-glucuronosyltransferase enzyme-selective substrate and inhibitor probes for reaction phenotyping and in vitro-in vivo extrapolation of drug clearance and drug-drug interaction potential. Drug Metab Rev. 2010;42(1):189–201. doi: 10.3109/03602530903210716. [DOI] [PubMed] [Google Scholar]
- 17.Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenetics Genomics. 2005;15:677–685. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- 18.Fisher MB, Paine MF, Strelevitz TJ, Wrighton SA. The role of hepatic and extrahepatic UDP-glucuronosyltransferases in human drug metabolism. Drug Metab Rev. 2001;33(3–4):273–297. doi: 10.1081/dmr-120000653. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab Dispos. 2008;36(8):1461–4. doi: 10.1124/dmd.108.021428. [DOI] [PubMed] [Google Scholar]
- 20.Ohno S, Nakajin S. Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos. 2009;37(1):32–40. doi: 10.1124/dmd.108.023598. [DOI] [PubMed] [Google Scholar]
- 21.Izukawa T, Nakajima M, Fujiwara R, Yamanaka H, Fukami T, Takamiya M, Aoki Y, Ikushiro S, Sakaki T, Yokoi T. Quantitative analysis of UDP-glucuronosyltransferase (UGT) 1A and UGT2B expression levels in human livers. Drug Metab Dispos. 2009;37(8):1759–68. doi: 10.1124/dmd.109.027227. [DOI] [PubMed] [Google Scholar]
- 22.Owens IS, Basu NK, Banerjee R. UDP-glucuronosyltransferases: gene structures of UGT1 and UGT2 families. Methods Enzymol. 2005;400:1–22. doi: 10.1016/S0076-6879(05)00001-7. [DOI] [PubMed] [Google Scholar]
- 23.Shelby MK, Cherrington NJ, Vansell NR, Klaassen CD. Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metab Dispos. 2003;31(3):326–33. doi: 10.1124/dmd.31.3.326. [DOI] [PubMed] [Google Scholar]
- 24.Buckley DB, Klaassen CD. Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos. 2007;35(1):121–7. doi: 10.1124/dmd.106.012070. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher CJ, Balliet RM, Sun D, Chen G, Lazarus P. Sex Differences in UDP-Glucuronosyltransferase 2B17 Expression and Activity. Drug Metab Dispos. 2010;38(12):2204–2209. doi: 10.1124/dmd.110.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magdalou J, Fournel-Gigleux S, Ouzzine M. Insights on membrane topology and structure/function of UDP-glucuronosyltransferases. Drug Metab Rev. 2010;42(1):154–61. doi: 10.3109/03602530903209270. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi T, Sleeman JE, Coughtrie MWH, Burchell B. Molecular and functional characterization of microsomal UDP-glucuronic acid uptake by members of the nucleotide sugar transporter (NST) family. Biochem J. 2005;400:281–289. doi: 10.1042/BJ20060429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossuyt X, Blanckaert N. Mechanism of stimulation of microsomal UDP-glucurosyltransferase by UDP-N-acetylglucosamine. Biochem J. 1995;305:321–328. doi: 10.1042/bj3050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakim D, Dannenberg AJ. How does the microsomal membrane regulate UDP-glucuronosyltransferases? Biochem Pharmacol. 1992;43(7):1385–93. doi: 10.1016/0006-2952(92)90192-l. [DOI] [PubMed] [Google Scholar]
- 30.Csala M, Staines AG, Banhegyi G, Mandl J, Coughtrie MWH, Burchell B. Evidence for multiple glucuronide transporters in rat liver microsomes. Biochem Pharmacol. 2004;68:1353–1362. doi: 10.1016/j.bcp.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 31.Finel M, Kurkela M. The UDP-glucuronosyltransferases as oligomeric enzymes. Curr Drug Metab. 2008;9:70–76. doi: 10.2174/138920008783331158. [DOI] [PubMed] [Google Scholar]
- 32.Radominska-Pandya A, Ouzzine M, Fournel-Gigleux S, Magdalou J. Structure of UDP-glucuronosyltransferases in membranes. Methods Enzymol. 2005;400:116–47. doi: 10.1016/S0076-6879(05)00008-X. [DOI] [PubMed] [Google Scholar]
- 33.Bock KW, Köhle C. Topological aspects of oligomeric UDP-glucuronosyltransferases in endoplasmic reticulum membranes: advances and open questions. Biochem Pharmacol. 2009;77(9):1458–65. doi: 10.1016/j.bcp.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Bowles D, Lim EK, Poppenberger B, Vaistij FE. Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol. 2006;57:567–597. doi: 10.1146/annurev.arplant.57.032905.105429. [DOI] [PubMed] [Google Scholar]
- 35.Paquette S, Moller BL, Bak S. On the origin of family 1 plant glycosyltransferases. Phytochemistry. 2003;62:399–413. doi: 10.1016/s0031-9422(02)00558-7. [DOI] [PubMed] [Google Scholar]
- 36.Ross J, Li Y, Lim E, Bowles DJ. Higher plant glycosyltransferases. Genome Biol. 2001;2(2):REVIEWS3004. doi: 10.1186/gb-2001-2-2-reviews3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miley MJ, Zielinska AK, Keenan JE, Bratton SM, Radominska-Pandya A, Redinbo MR. Crystal structure of the cofactor-binding domain of the human phase II drug-metabolism enzyme UDP-glucuronosyltransferase 2B7. J Mol Biol. 2007;369(2):498–511. doi: 10.1016/j.jmb.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao H, He X, Achnine L, Blount JW, Dixon RA, Wang X. Crystal structures of a multifunctional triterpene/flavonoid glycosyltransferase from Medicago truncatula. Plant Cell. 2005;17:3141–3154. doi: 10.1105/tpc.105.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Offen W, Martinez-Fleites C, Yang M, Kiat-Lim E, Davis BG, Tarling CA, Ford CM, Bowles DJ, Davies GJ. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 2006;25:1396–1405. doi: 10.1038/sj.emboj.7600970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Modolo LV, Escamilla-Trevino LL, Achnine L, Dixon RA, Wang X. Crystal Structure of Medicago truncatula UGT85H2 - Insights into the Structural Basis of a Multifunctional (Iso)flavonoid Glycosyltransferase. J Mol Biol. 2007;370:951–963. doi: 10.1016/j.jmb.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 41.Brazier-Hicks M, Offen WA, Gershater MC, Revett TJ, Lim EK, Bowles DJ, Davies GJ, Edwards R. Characterization and engineering of the bifunctional N- and O-glucosyltransferase involved in xenobiotic metabolism in plants. Proc Natl Acad Sci. 2007;104:20238–20243. doi: 10.1073/pnas.0706421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modolo LV, Li L, Pan H, Blount JW, Dixon RA, Wang X. Crystal structures of glycosyltransferase UGT78G1 reveal the molecular basis for glycosylation and deglycosylation of (iso)flavonoids. J Mol Biol. 2009;392(5):1292–302. doi: 10.1016/j.jmb.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Radominska-Pandya A, Czernik PJ, Little JM, Battaglia E, Mackenzie PI. Structural and functional studies of UDP-glucuronosyltransferases. Drug Metab Rev. 1999;31:817–899. doi: 10.1081/dmr-100101944. [DOI] [PubMed] [Google Scholar]
- 44.Radominska-Pandya A, Bratton SM, Redinbo MR, Miley MJ. The crystal structure of human UDP-glucuronosyltransferase 2B7 C-terminal end is the first mammalian UGT target to be revealed: the significance for human UGTs from both the 1A and 2B families. Drug Metab Rev. 2010;42(1):128–39. doi: 10.3109/03602530903209049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itäaho K, Laakkonen L, Finel M. How many and which amino acids are responsible for the large activity differences between the highly homologous UDP-glucuronosyltransferases (UGT) 1A9 and UGT1A10? Drug Metab Dispos. 2010;38(4):687–696. doi: 10.1124/dmd.109.031229. [DOI] [PubMed] [Google Scholar]
- 46.Xiong Y, Bernardi D, Bratton S, Ward MD, Battaglia E, Finel M, Drake RR, Radominska-Pandya A. Phenylalanine 90 and 93 are localized within the phenol binding site of human UDP-glucuronosyltransferase 1A10 as determined by photoaffinity labeling, mass spectrometry, and site-directed mutagenesis. Biochemistry. 2006;45(7):2322–32. doi: 10.1021/bi0519001. [DOI] [PubMed] [Google Scholar]
- 47.Starlard-Davenport A, Xiong Y, Bratton S, Gallus-Zawada A, Finel M, Radominska-Pandya A. Phenylalanine(90) and phenylalanine(93) are crucial amino acids within the estrogen binding site of the human UDP-glucuronosyltransferase 1A10. Steroids. 2007;72(1):85–94. doi: 10.1016/j.steroids.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barre L, Fournel-Gigleux S, Finel M, Netter P, Magdalou J, Ouzzine M. Substrate specificity of the human UDP-glucuronosyltransferase UGT2B4 and UGT2B7. Identification of a critical aromatic amino acid residue at position 33. FEBS J. 2007;274(5):1256–64. doi: 10.1111/j.1742-4658.2007.05670.x. [DOI] [PubMed] [Google Scholar]
- 49.Li C, Wu Q. Adaptive evolution of multiple-variable exons and structural diversity of drug metabolizing enzymes. BMC Evol Biol. 2007;7:69. doi: 10.1186/1471-2148-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locuson CW, Tracy TS. Comparative modelling of the human UDPglucuronosyltransferases: insights into structure and mechanism. Xenobiotica. 2007;37(2):155–68. doi: 10.1080/00498250601129109. [DOI] [PubMed] [Google Scholar]
- 51.Laakkonen L, Finel M. A molecular model of the human UGT1A1, its membrane orientation and the interactions between different parts of the enzyme. Mol Pharmacol. 2010;77(6):931–939. doi: 10.1124/mol.109.063289. [DOI] [PubMed] [Google Scholar]
- 52.Fujiwara R, Nakajima M, Yamamoto T, Nagao H, Yokoi T. In silico and in vitro approaches to elucidate the thermal stability of human UDP-glucuronosyltransferase (UGT) 1A9. Drug Metab Pharmacokinet. 2009;24(3):235–244. doi: 10.2133/dmpk.24.235. [DOI] [PubMed] [Google Scholar]
- 53.Osmani SA, Bak S, Møller BL. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry. 2009;70(3):325–347. doi: 10.1016/j.phytochem.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Sorich MJ, Smith PA, McKinnon RA, Miners JO. Pharmacophore and quantitative structure activity relationship modelling of UDP-glucuronosyltransferase 1A1 (UGT1A1) substrates. Pharmacogenetics. 2002;12(8):635–645. doi: 10.1097/00008571-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Wu B, Morrow J, Singh R, Zhang S, Hu M. 3D-QSAR Studies on UGT1A9-mediated 3-O-Glucuronidation of Natural Flavonols Using a Pharmacophore-based CoMFA Model. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.175356. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patana A-S, Kurkela M, Finel M, Goldman A. Mutation analysis in UGT1A9 suggests a relationship between substrate and catalytic residues in UDP-glucuronosyltransferases. Protein Eng Des Sel. 2008;21:537–543. doi: 10.1093/protein/gzn030. [DOI] [PubMed] [Google Scholar]
- 57.Kerdpin O, Mackenzie PI, Bowalgaha K, Finel M, Miners JO. Influence of N-terminal domain histidine and proline residues on the substrate selectivties of human UDP-glucuronosyltransferase (UGT) 1A1, 1A6, 1A9, 2B7 and 2B10. Drug Metab Dispos. 2009;37(9):1948–1955. doi: 10.1124/dmd.109.028225. [DOI] [PubMed] [Google Scholar]
- 58.Luukkanen L, Taskinen J, Kurkela M, Kostiainen R, Hirvonen J, Finel M. Kinetic characterization of the 1A subfamily of recombinant human UDP-glucuronosyltransferases. Drug Metab Dispos. 2005;33(7):1017–1026. doi: 10.1124/dmd.105.004093. [DOI] [PubMed] [Google Scholar]
- 59.Zhou J, Tracy TS, Remmel RP. Glucuronidation of dihydrotestosterone and trans-androsterone by recombinant UDP-glucuronosyltransferase (UGT) 1A4: evidence for multiple UGT1A4 aglycone binding sites. Drug Metab Dispos. 2010;38(3):431–40. doi: 10.1124/dmd.109.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Houston JB, Galetin A. Modelling atypical CYP3A4 kinetics: principles and pragmatism. Arch Biochem Biophys. 2005;433(2):351–60. doi: 10.1016/j.abb.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Mackenzie PI, Gregory PA, Gardner-Stephen DA, Lewinsky RH, Jorgensen BR, Nishiyama T, Xie W, Radominska-Pandya A. Regulation of UDP glucuronosyltransferase genes. Curr Drug Metab. 2003;4(3):249–57. doi: 10.2174/1389200033489442. [DOI] [PubMed] [Google Scholar]
- 62.Zhou J, Zhang J, Xie W. Xenobiotic nuclear receptor-mediated regulation of UDP-glucuronosyl-transferases. Curr Drug Metab. 2005;6(4):289–98. doi: 10.2174/1389200054633853. [DOI] [PubMed] [Google Scholar]
- 63.Bock KW. Functions and transcriptional regulation of adult human hepatic UDP-glucuronosyl-transferases (UGTs): mechanisms responsible for interindividual variation of UGT levels. Biochem Pharmacol. 2010;80(6):771–7. doi: 10.1016/j.bcp.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 64.Basu NK, Kole L, Basu M, Chakraborty K, Mitra PS, Owens IS. The major chemical-detoxifying system of UDP-glucuronosyltransferases requires regulated phosphorylation supported by protein kinase C. J Biol Chem. 2008;283(34):23048–61. doi: 10.1074/jbc.M800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitra PS, Basu NK, Basu M, Chakraborty S, Saha T, Owens IS. Regulated phosphorylation of a major UDP-glucuronosyltransferase isozyme by tyrosine kinases dictates endogenous substrate-selection for detoxification. J Biol Chem. 2010 doi: 10.1074/jbc.M110.165126. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basu NK, Kole L, Owens IS. Evidence for phosphorylation requirement for human bilirubin UDP-glucuronosyltransferase (UGT1A1) activity. Biochem Biophys Res Commun. 2003;303(1):98–104. doi: 10.1016/s0006-291x(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 67.Basu NK, Korava M, Garza A, Kubota S, Saha T, Mitra PS, Banerjee R, Rivera J, Owens IS. Phosphorylation of UDP-glucuronosyltransferase regulates substrate specificity. Proc Natl Acad Sci USA. 2005;102:6285–6290. doi: 10.1073/pnas.0407872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basu NK, Kubota S, Meselhy MR, Ciotti M, Chowdhury B, Hartori M, Owens IS. Gastrointestinally distributed UDP-glucuronosyltransferase 1A10, which metabolizes estrogens and non-steroidal anti-inflammatory drugs, depends upon phosphorylation. J Biol Chem. 2004;279:28320–28329. doi: 10.1074/jbc.M401396200. [DOI] [PubMed] [Google Scholar]
- 69.Mitra PS, Basu NK, Owens IS. Src supports UDP-glucuronosyltransferase-2B7 detoxification of catechol estrogens associated with breast cancer. Biochem Biophys Res Commun. 2009;382(4):651–6. doi: 10.1016/j.bbrc.2009.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volak LP, Court MH. Role for protein kinase C delta in the functional activity of human UGT1A6: implications for drug-drug interactions between PKC inhibitors and UGT1A6. Xenobiotica. 2010;40(5):306–18. doi: 10.3109/00498251003596817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30(4):370–7. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 72.Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther. 2003;304(3):1228–35. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 73.Hu M, Chen J, Lin H. Disposition of Flavonoids via Recycling: Mechanistic Studies of Disposition of Apigenin in the Caco-2 Cell Culture Model. J Pharmacol Exp Ther. 2003;307(1):314–21. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- 74.Ng SP, Wong KY, Zhang L, Zuo Z, Lin G. Evaluation of the first-pass glucuronidation of selected flavones in gut by Caco-2 monolayer model. J Pharm Pharm Sci. 2004;20;8(1):1–9. [PubMed] [Google Scholar]
- 75.Liu X, Tam VH, Hu M. Disposition of flavonoids via enteric recycling: determination of the UDP-glucuronosyltransferase isoforms responsible for the metabolism of flavonoids in intact Caco-2 TC7 cells using siRNA. Mol Pharm. 2007;4(6):873–82. doi: 10.1021/mp0601190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai H, Boocock DJ, Steward WP, Gescher AJ. Tissue distribution in mice and metabolism in murine and human liver of apigenin and tricin, flavones with putative cancer chemopreventive properties. Cancer Chemother Pharmacol. 2007;60(2):257–66. doi: 10.1007/s00280-006-0368-5. [DOI] [PubMed] [Google Scholar]
- 77.Walle UK, Galijatovic A, Walle T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem Pharmacol. 1999;58(3):431–8. doi: 10.1016/s0006-2952(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 78.Galijatovic A, Otake Y, Walle UK, Walle T. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells. Xenobiotica. 1999;29(12):1241–56. doi: 10.1080/004982599237912. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L, Lin G, Zuo Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm Res. 2007;24(1):81–9. doi: 10.1007/s11095-006-9126-y. [DOI] [PubMed] [Google Scholar]
- 80.Xing J, Chen X, Zhong D. Absorption and enterohepatic circulation of baicalin in rats. Life Sci. 2005;78(2):140–6. doi: 10.1016/j.lfs.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 81.Akao T, Sakashita Y, Hanada M, Goto H, Shimada Y, Terasawa K. Enteric excretion of baicalein, a flavone of Scutellariae Radix, via glucuronidation in rat: involvement of multidrug resistance-associated protein 2. Pharm Res. 2004;21(11):2120–6. doi: 10.1023/b:pham.0000048205.02478.b5. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L, Lin G, Kovács B, Jani M, Krajcsi P, Zuo Z. Mechanistic study on the intestinal absorption and disposition of baicalein. Eur J Pharm Sci. 2007;31(3–4):221–31. doi: 10.1016/j.ejps.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 83.Sun H, Zhang L, Chow EC, Lin G, Zuo Z, Pang KS. A catenary model to study transport and conjugation of baicalein, a bioactive flavonoid, in the Caco-2 cell monolayer: demonstration of substrate inhibition. J Pharmacol Exp Ther. 2008;326(1):117–26. doi: 10.1124/jpet.108.137463. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Q, Zheng Z, Xia B, Tang L, Lv C, Liu W, Liu Z, Hu M. Use of isoform-specific UGT metabolism to determine and describe rates and profiles of glucuronidation of wogonin and oroxylin A by human liver and intestinal microsomes. Pharm Res. 2010;27(8):1568–83. doi: 10.1007/s11095-010-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang L, Ye L, Singh R, Wu B, Lv C, Zhao J, Liu Z, Hu M. Use of glucuronidation fingerprinting to describe and predict mono- and dihydroxyflavone metabolism by recombinant UGT isoforms and human intestinal and liver microsomes. Mol Pharm. 2010;7(3):664–79. doi: 10.1021/mp900223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boutin JA, Meunier F, Lambert PH, Hennig P, Bertin D, Serkiz B, Volland JP. In vivo and in vitro glucuronidation of the flavonoid diosmetin in rats. Drug Metab Dispos. 1993;21(6):1157–66. [PubMed] [Google Scholar]
- 87.Wang SW, Chen J, Jia X, Tam VH, Hu M. Disposition of flavonoids via enteric recycling: structural effects and lack of correlations between in vitro and in situ metabolic properties. Drug Metab Dispos. 2006;34(11):1837–48. doi: 10.1124/dmd.106.009910. [DOI] [PubMed] [Google Scholar]
- 88.King RA, Broadbent JL, Head RJ. Absorption and excretion of the soy isoflavone genistein in rats. J Nutr. 1996;126:176–182. doi: 10.1093/jn/126.1.176. [DOI] [PubMed] [Google Scholar]
- 89.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 90.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131 (Suppl 4):1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 91.Chen J, Lin H, Hu M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 2005;55(2):159–69. doi: 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- 92.Chen J, Wang S, Jia X, Bajimaya S, Lin H, Tam VH, Hu M. Disposition of flavonoids via recycling: comparison of intestinal versus hepatic disposition. Drug Metab Dispos. 2005;33(12):1777–84. doi: 10.1124/dmd.105.003673. [DOI] [PubMed] [Google Scholar]
- 93.Zhu W, Xu H, Wang SW, Hu M. Breast cancer resistance protein (BCRP) and sulfotransferases contribute significantly to the disposition of genistein in mouse intestine. AAPS J. 2010;12(4):525–36. doi: 10.1208/s12248-010-9209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jia X, Chen J, Lin H, Hu M. Disposition of flavonoids via enteric recycling: enzyme-transporter coupling affects metabolism of biochanin A and formononetin and excretion of their phase II conjugates. J Pharmacol Exp Ther. 2004;310(3):1103–13. doi: 10.1124/jpet.104.068403. [DOI] [PubMed] [Google Scholar]
- 95.Jeong EJ, Jia X, Hu M. Disposition of formononetin via enteric recycling: metabolism and excretion in mouse intestinal perfusion and Caco-2 cell models. Mol Pharm. 2005;2(4):319–28. doi: 10.1021/mp0498852. [DOI] [PubMed] [Google Scholar]
- 96.Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos. 2002;30(5):576–81. doi: 10.1124/dmd.30.5.576. [DOI] [PubMed] [Google Scholar]
- 97.Barrington R, Williamson G, Bennett RN, Davis BD, Brodbelt JS, Kroon PA. Absorption, Conjugation and Efflux of the Flavonoids, Kaempferol and Galangin, Using the Intestinal CACO-2/TC7 Cell Model. J Funct Foods. 2009;1(1):74–87. doi: 10.1016/j.jff.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barve A, Chen C, Hebbar V, Desiderio J, Saw CL, Kong AN. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm Drug Dispos. 2009;30(7):356–65. doi: 10.1002/bdd.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DuPont MS, Day AJ, Bennett RN, Mellon FA, Kroon PA. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur J Clin Nutr. 2004;58(6):947–54. doi: 10.1038/sj.ejcn.1601916. [DOI] [PubMed] [Google Scholar]
- 100.Oliveira EJ, Watson DG. In vitro glucuronidation of kaempferol and quercetin by human UGT-1A9 microsomes. FEBS Lett. 2000;471(1):1–6. doi: 10.1016/s0014-5793(00)01355-7. [DOI] [PubMed] [Google Scholar]
- 101.Williamson G, Barron D, Shimoi K, Terao J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic Res. 2005;39(5):457–69. doi: 10.1080/10715760500053610. [DOI] [PubMed] [Google Scholar]
- 102.Janisch KM, Williamson G, Needs P, Plumb GW. Properties of quercetin conjugates: modulation of LDL oxidation and binding to human serum albumin. Free Radical Res. 2004;38:877–884. doi: 10.1080/10715760410001728415. [DOI] [PubMed] [Google Scholar]
- 103.Moon JH, Tsushida T, Nakahara K, Terao J. Identification of quercetin 3-O-beta-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic Biol Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 104.Wang FM, Yao TW, Zeng S. Disposition of quercetin and kaempferol in human following an oral administration of Ginkgo biloba extract tablets. Eur J Drug Metab Pharmacokinet. 2003;28(3):173–7. doi: 10.1007/BF03190482. [DOI] [PubMed] [Google Scholar]
- 105.Chen YK, Chen SQ, Li X, Zeng S. Quantitative regioselectivity of glucuronidation of quercetin by recombinant UDP-glucuronosyltransferases 1A9 and 1A3 using enzymatic kinetic parameters. Xenobiotica. 2005;35(10–11):943–54. doi: 10.1080/00498250500372172. [DOI] [PubMed] [Google Scholar]
- 106.Davis BD, Brodbelt JS. Regioselectivity of human UDP-glucuronosyl-transferase 1A1 in the synthesis of flavonoid glucuronides determined by metal complexation and tandem mass spectrometry. J Am Soc Mass Spectrom. 2008;19(2):246–256. doi: 10.1016/j.jasms.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsiu SL, Huang TY, Hou YC, Chin DH, Chao PD. Comparison of metabolic pharmacokinetics of naringin and naringenin in rabbits. Life Sci. 2002;70(13):1481–9. doi: 10.1016/s0024-3205(01)01491-6. [DOI] [PubMed] [Google Scholar]
- 108.Fuhr U, Kummert AL. The fate of naringin in humans: a key to grapefruit juice-drug interactions? Clin Pharmacol Ther. 1995;58(4):365–73. doi: 10.1016/0009-9236(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 109.Ma Y, Li P, Chen D, Fang T, Li H, Su W. LC/MS/MS quantitation assay for pharmacokinetics of naringenin and double peaks phenomenon in rats plasma. Int J Pharm. 2006;307(2):292–9. doi: 10.1016/j.ijpharm.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 110.Xu H, Kulkarni KH, Singh R, Yang Z, Wang SW, Tam VH, Hu M. Disposition of naringenin via glucuronidation pathway is affected by compensating efflux transporters of hydrophilic glucuronides. Mol Pharm. 2009;6(6):1703–15. doi: 10.1021/mp900013d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manach C, Morand C, Gil-Izquierdo A, Bouteloup-Demange C, Rémésy C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr. 2003;57:235–242. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 112.Matsumoto H, Ikoma Y, Sugiura M, Yano M, Hasegawa Y. Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J Agric Food Chem. 2004;52:6653–6659. doi: 10.1021/jf0491411. [DOI] [PubMed] [Google Scholar]
- 113.Brett GM, Hollands W, Needs PW, Teucher B, Dainty JR, Davis BD, Brodbelt JS, Kroon PA. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br J Nutr. 2009;101:664–675. doi: 10.1017/S000711450803081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brand W, van der Wel PA, Rein MJ, Barron D, Williamson G, van Bladeren PJ, Rietjens IM. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab Dispos. 2008;36:1794–1802. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- 115.Brand W, Boersma MG, Bik H, Hoek-van den Hil EF, Vervoort J, Barron D, Meinl W, Glatt H, Williamson G, van Bladeren PJ, Rietjens IM. Phase II metabolism of hesperetin by individual UDP-glucuronosyltransferases and sulfotransferases and rat and human tissue samples. Drug Metab Dispos. 2010;38(4):617–25. doi: 10.1124/dmd.109.031047. [DOI] [PubMed] [Google Scholar]
- 116.Gerhäuser C. Beer constituents as potential cancer chemopreventive agents. Eur J Cancer. 2005;41(13):1941–54. doi: 10.1016/j.ejca.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 117.Avula B, Ganzera M, Warnick JE, Feltenstein MW, Sufka KJ, Khan IA. High-performance liquid chromatographic determination of xanthohumol in rat plasma, urine, and fecal samples. J Chromatogr Sci. 2004;42(7):378–82. doi: 10.1093/chromsci/42.7.378. [DOI] [PubMed] [Google Scholar]
- 118.Yilmazer M, Stevens JF, Buhler DR. In vitro glucuronidation of xanthohumol, a flavonoid in hop and beer, by rat and human liver microsomes. FEBS Lett. 2001;491(3):252–6. doi: 10.1016/s0014-5793(01)02210-4. [DOI] [PubMed] [Google Scholar]
- 119.Ruefer CE, Gerhäuser C, Frank N, Becker H, Kulling SE. In vitro phase II metabolism of xanthohumol by human UDP-glucuronosyltransferases and sulfotransferases. Mol Nutr Food Res. 2005;49(9):851–6. doi: 10.1002/mnfr.200500057. [DOI] [PubMed] [Google Scholar]
- 120.Pang Y, Nikolic D, Zhu D, Chadwick LR, Pauli GF, Farnsworth NR, van Breemen RB. Binding of the hop (Humulus lupulus L.) chalcone xanthohumol to cytosolic proteins in Caco-2 intestinal epithelial cells. Mol Nutr Food Res. 2007;51(7):872–9. doi: 10.1002/mnfr.200600252. [DOI] [PubMed] [Google Scholar]
- 121.Feng WY. Metabolism of green tea catechins: an overview. Curr Drug Metab. 2006;7(7):755–809. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- 122.Crespy V, Nancoz N, Oliveira M, Hau J, Courtet-Compondu MC, Williamson G. Glucuronidation of the green tea catechins, (−)-epigallocatechin-3-gallate and (−)-epicatechin-3-gallate, by rat hepatic and intestinal microsomes. Free Radic Res. 2004;38(9):1025–31. doi: 10.1080/10715760410001728424. [DOI] [PubMed] [Google Scholar]
- 123.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54(1):7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 124.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302(1):369–73. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 125.Aumont V, Krisa S, Battaglia E, Netter P, Richard T, Mérillon JM, Magdalou J, Sabolovic N. Regioselective and stereospecific glucuronidation of trans- and cis-resveratrol in human. Arch Biochem Biophys. 2001;393(2):281–9. doi: 10.1006/abbi.2001.2496. [DOI] [PubMed] [Google Scholar]
- 126.Brill SS, Furimsky AM, Ho MN, Furniss MJ, Li Y, Green AG, Bradford WW, Green CE, Kapetanovic IM, Iyer LV. Glucuronidation of trans-resveratrol by human liver and intestinal microsomes and UGT isoforms. J Pharm Pharmacol. 2006;58(4):469–79. doi: 10.1211/jpp.58.4.0006. [DOI] [PubMed] [Google Scholar]
- 127.Sabolovic N, Humbert AC, Radominska-Pandya A, Magdalou J. Resveratrol is efficiently glucuronidated by UDP-glucuronosyltransferases in the human gastrointestinal tract and in Caco-2 cells. Biopharm Drug Dispos. 2006;27(4):181–9. doi: 10.1002/bdd.498. [DOI] [PubMed] [Google Scholar]
- 128.Kuhnle G, Spencer JP, Chowrimootoo G, Schroeter H, Debnam ES, Srai SK, Rice-Evans C, Hahn U. Resveratrol is absorbed in the small intestine as resveratrol glucuronide. Biochem Biophys Res Commun. 2000;272(1):212–7. doi: 10.1006/bbrc.2000.2750. [DOI] [PubMed] [Google Scholar]
- 129.Kaldas MI, Walle UK, Walle T. Resveratrol transport and metabolism by human intestinal Caco-2 cells. J Pharm Pharmacol. 2003;55(3):307–12. doi: 10.1211/002235702612. [DOI] [PubMed] [Google Scholar]
- 130.Piver B, Fer M, Vitrac X, Merillon JM, Dreano Y, Berthou F, Lucas D. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem Pharmacol. 2004;68(4):773–82. doi: 10.1016/j.bcp.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 131.Roupe K, Teng XW, Fu X, Meadows GG, Davies NM. Determination of piceatannol in rat serum and liver microsomes: pharmacokinetics and phase I and II biotransformation. Biomed Chromatogr. 2004;18(8):486–91. doi: 10.1002/bmc.342. [DOI] [PubMed] [Google Scholar]
- 132.Miksits M, Maier-Salamon A, Vo TP, Sulyok M, Schuhmacher R, Szekeres T, Jäger W. Glucuronidation of piceatannol by human liver microsomes: major role of UGT1A1, UGT1A8 and UGT1A10. J Pharm Pharmacol. 2010;62(1):47–54. doi: 10.1211/jpp.62.01.0004. [DOI] [PubMed] [Google Scholar]
- 133.Delmonte A, Sessa C. AVE8062: a new combretastatin derivative vascular disrupting agent. Expert Opin Investig Drugs. 2009;18(10):1541–8. doi: 10.1517/13543780903213697. [DOI] [PubMed] [Google Scholar]
- 134.Aprile S, Del Grosso E, Grosa G. Identification of the human UDP-glucuronosyltransferases involved in the glucuronidation of combretastatin A-4. Drug Metab Dispos. 2010;38(7):1141–6. doi: 10.1124/dmd.109.031435. [DOI] [PubMed] [Google Scholar]
- 135.Srinivas G, Babykutty S, Sathiadevan PP, Srinivas P. Molecular mechanism of emodin action: transition from laxative ingredient to an antitumor agent. Med Res Rev. 2007;27:591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- 136.Liang JW, Hsiu SL, Wu PP, Chao PD. Emodin pharmacokinetics in rabbits. Planta Med. 1995;61(5):406–8. doi: 10.1055/s-2006-958125. [DOI] [PubMed] [Google Scholar]
- 137.Teng ZH, Zhou SY, Yang RT, Liu XY, Liu RW, Yang X, Zhang BL, Yang JY, Cao DY, Mei QB. Quantitation assay for absorption and first-pass metabolism of emodin in isolated rat small intestine using liquid chromatography-tandem mass spectrometry. Biol Pharm Bull. 2007;30(9):1628–33. doi: 10.1248/bpb.30.1628. [DOI] [PubMed] [Google Scholar]
- 138.Liu W, Tang L, Ye L, Cai Z, Xia B, Zhang J, Hu M, Liu Z. Species and gender differences affect the metabolism of emodin via glucuronidation. AAPS J. 2010;12(3):424–36. doi: 10.1208/s12248-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morita K, Sugiyama Y, Hanano M. Pharmacokinetic study of 4-methylumbelliferone in rats: influence of dose on its first-pass hepatic elimination. J Pharmacobiody. 1986;9(2):117–24. doi: 10.1248/bpb1978.9.117. [DOI] [PubMed] [Google Scholar]
- 140.Zamek-Gliszczynski MJ, Hoffmaster KA, Humphreys JE, Tian X, Nezasa K, Brouwer KL. Differential involvement of Mrp2 (Abcc2) and Bcrp (Abcg2) in biliary excretion of 4-methylumbelliferyl glucuronide and sulfate in the rat. J Pharmacol Exp Ther. 2006;319(1):459–67. doi: 10.1124/jpet.106.101840. [DOI] [PubMed] [Google Scholar]
- 141.Uchaipichat V, Mackenzie PI, Guo XH, Gardner-Stephen D, Galetin A, Houston JB, Miners JO. Human udp-glucuronosyltransferases: isoform selectivity and kinetics of 4-methylumbelliferone and 1-naphthol glucuronidation, effects of organic solvents, and inhibition by diclofenac and probenecid. Drug Metab Dispos. 2004;32(4):413–23. doi: 10.1124/dmd.32.4.413. [DOI] [PubMed] [Google Scholar]
- 142.Liang SC, Ge GB, Liu HX, Zhang YY, Wang LM, Zhang JW, Yin L, Li W, Fang ZZ, Wu JJ, Li GH, Yang L. Identification and characterization of human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of daphnetin. Drug Metab Dispos. 2010;38(6):973–80. doi: 10.1124/dmd.109.030734. [DOI] [PubMed] [Google Scholar]
- 143.Issell B, Franke A, Fielding R. Pharmacokinetic Study of Noni Fruit Extract. Journal of Dietary Supplements. 2008;5(4):373–382. doi: 10.1080/19390210802519671. [DOI] [PubMed] [Google Scholar]
- 144.Jones DR, Moran JH, Miller GP. Warfarin and UDP-glucuronosyltransferases: writing a new chapter of metabolism. Drug Metab Rev. 2010;42(1):53–9. doi: 10.3109/03602530903209395. [DOI] [PubMed] [Google Scholar]
- 145.Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10:291S–298S. doi: 10.1111/j.1365-2125.1980.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiong H, Turner KC, Ward ES, Jansen PL, Brouwer KL. Altered hepatobiliary disposition of acetaminophen glucuronide in isolated perfused livers from multidrug resistance-associated protein 2-deficient TR(−) rats. J Pharmacol Exp Ther. 2000;295(2):512–8. [PubMed] [Google Scholar]
- 147.Mutlib AE, Goosen TC, Bauman JN, Williams JA, Kulkarni S, Kostrubsky S. Kinetics of acetaminophen glucuronidation by UDP-glucuronosyltransferases 1A1, 1A6, 1A9 and 2B15. Potential implications in acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2006;19(5):701–9. doi: 10.1021/tx050317i. [DOI] [PubMed] [Google Scholar]
- 148.Abid A, Bouchon I, Siest G, Sabolovic N. Glucuronidation in the Caco-2 human intestinal cell line: induction of UDP-glucuronosyltransferase 1*6. Biochem Pharmacol. 1995;50:557–561. doi: 10.1016/0006-2952(95)00162-s. [DOI] [PubMed] [Google Scholar]
- 149.Siissalo S, Laine L, Tolonen A, Kaukonen AM, Finel M, Hirvonen J. Caco-2 cell monolayers as a tool to study simultaneous phase II metabolism and metabolite efflux of indomethacin, paracetamol and 1-naphthol. Int J Pharm. 2010;383(1–2):24–9. doi: 10.1016/j.ijpharm.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 150.Stockis A, Deroubaix X, Jeanbaptiste B, Lins R, Allemon AM, Laufen H. Relative Bioavailability of Carbinoxamine and Phenylephrine from a Retard Capsule after Single and Repeated Dose Adminstration in Healthy Subjects. Arzneimittelforschung. 1995;45(9):1009–12. [PubMed] [Google Scholar]
- 151.Ibrahim KE, Midgley JM, Crowley JR, Williams CM. The Mammalian Metabolism of R-(−)-m-Synephrine. Journal of Pharmacy and Pharmacology. 1983;35(3):144–147. doi: 10.1111/j.2042-7158.1983.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 152.Gumbhir K. Pharmaceutical Sciences. Vol. 216. University of Missouri-Kansas City; 1993. An Investigation of Pharmacokinetics of Phenylephrine and its Metabolites in Humans. [Google Scholar]
- 153.Hengstmann JH, Goronzy J. Pharmacokinetics of 3H-Phenylephrine in Man. European Journal of Clinical Pharmacology. 1982;21:335–341. doi: 10.1007/BF00637623. [DOI] [PubMed] [Google Scholar]
- 154.Wikberg T, Vuorela A, Ottoila P, Taskinen J. Identification of major urinary metabolites of the catechol-O-methyltransferase inhibitor entacapone in rats and humans. Drug Metab Dispos. 1993;21:81–92. [PubMed] [Google Scholar]
- 155.Luukkanen L, Taskinen J, Kurkela M, Kostiainen R, Hirvonen J, Finel M. Kinetic characterization of the 1A subfamily of recombinant human UDP-glucuronosyltransferases. Drug Metab Dispos. 2005;33(7):1017–26. doi: 10.1124/dmd.105.004093. [DOI] [PubMed] [Google Scholar]
- 156.Lautala P, Ethell BT, Taskinen J, Burchell B. The specificity of glucuronidation of entacapone and tolcapone by recombinant human UDP-glucuronosyltransferases. Drug Metab Dispos. 2000;28(11):1385–9. [PubMed] [Google Scholar]
- 157.Lautala P, Kivimaa M, Salomies H, Elovaara E, Taskinen J. Glucuronidation of entacapone, nitecapone, tolcapone, and some other nitrocatechols by rat liver microsomes. Pharm Res. 1997;14(10):1444–8. doi: 10.1023/a:1012133008134. [DOI] [PubMed] [Google Scholar]
- 158.Dalvie D, Kang P, Zientek M, Xiang C, Zhou S, Obach RS. Effect of intestinal glucuronidation in limiting hepatic exposure and bioactivation of raloxifene in humans and rats. Chem Res Toxicol. 2008;21(12):2260–71. doi: 10.1021/tx800323w. [DOI] [PubMed] [Google Scholar]
- 159.Jeong EJ, Lin H, Hu M. Disposition mechanisms of raloxifene in the human intestinal Caco-2 model. J Pharmacol Exp Ther. 2004;310(1):376–85. doi: 10.1124/jpet.103.063925. [DOI] [PubMed] [Google Scholar]
- 160.Vietri M, Pietrabissa A, Mosca F, Pacifici GM. Mycophenolic acid glucuronidation and its inhibition by non-steroidal anti-inflammatory drugs in human liver and kidney. Eur J Clin Pharmacol. 2000;56(9–10):659–64. doi: 10.1007/s002280000227. [DOI] [PubMed] [Google Scholar]
- 161.Mackenzie PI. Identification of uridine diphosphate glucuronosyltransferases involved in the metabolism and clearance of mycophenolic acid. Ther Drug Monit. 2000;22(1):10–3. doi: 10.1097/00007691-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 162.Jiao Z, Ding JJ, Shen J, Liang HQ, Zhong LJ, Wang Y, Zhong MK, Lu WY. Population pharmacokinetic modelling for enterohepatic circulation of mycophenolic acid in healthy Chinese and the influence of polymorphisms in UGT1A9. Br J Clin Pharmacol. 2008;65(6):893–907. doi: 10.1111/j.1365-2125.2008.03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lévesque E, Delage R, Benoit-Biancamano MO, Caron P, Bernard O, Couture F, Guillemette C. The impact of UGT1A8, UGT1A9, and UGT2B7 genetic polymorphisms on the pharmacokinetic profile of mycophenolic acid after a single oral dose in healthy volunteers. Clin Pharmacol Ther. 2007;81(3):392–400. doi: 10.1038/sj.clpt.6100073. [DOI] [PubMed] [Google Scholar]
- 164.Basu NK, Kole L, Basu M, McDonagh AF, Owens IS. Targeted inhibition of glucuronidation markedly improves drug efficacy in mice - a model. Biochem Biophys Res Commun. 2007;360(1):7–13. doi: 10.1016/j.bbrc.2007.05.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Illingworth N, Boddy A, Daly A, Veal G. Characterization of the metabolism of fenretinide by human liver microsomes, cytochrome P450 enzymes and UDP-glucuronosyltransferases. Br J Pharmacol. 2010 doi: 10.1111/j.1476-5381.2010.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Adamson PC, Pitot HC, Balis FM, Rubin J, Murphy RF, Poplack DG. Variability in the oral bioavailability of all-trans-retinoic acid. J Natl Cancer Inst. 1993;85(12):993–6. doi: 10.1093/jnci/85.12.993. [DOI] [PubMed] [Google Scholar]
- 167.O’Dwyer PJ, Catalano RB. Uridine diphosphate glucuronosyltransferase (UGT) 1A1 and irinotecan: practical pharmacogenomics arrives in cancer therapy. J Clin Oncol. 2006;24:4534–8. doi: 10.1200/JCO.2006.07.3031. [DOI] [PubMed] [Google Scholar]
- 168.Fujita K, Sparreboom A. Pharmacogenetics of irinotecan disposition and toxicity: a review. Curr Clin Pharmacol. 2010;5(3):209–17. doi: 10.2174/157488410791498806. [DOI] [PubMed] [Google Scholar]
- 169.Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci U S A. 1998;95:8170–8174. doi: 10.1073/pnas.95.14.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kelland LR. Flavopiridol, the first cyclin-dependent kinase inhibitor to enter the clinic: current status. Expert Opin Investig Drugs. 2000;9(12):2903–11. doi: 10.1517/13543784.9.12.2903. [DOI] [PubMed] [Google Scholar]
- 171.Jäger W, Zembsch B, Wolschann P, Pittenauer E, Senderowicz AM, Sausville EA, Sedlacek HH, Graf J, Thalhammer T. Metabolism of the anticancer drug flavopiridol, a new inhibitor of cyclin dependent kinases, in rat liver. Life Sci. 1998;62(20):1861–73. doi: 10.1016/s0024-3205(98)00152-0. [DOI] [PubMed] [Google Scholar]
- 172.Innocenti F, Stadler WM, Iyer L, Ramírez J, Vokes EE, Ratain MJ. Flavopiridol metabolism in cancer patients is associated with the occurrence of diarrhea. Clin Cancer Res. 2000;6(9):3400–5. [PubMed] [Google Scholar]
- 173.Hagenauer B, Salamon A, Thalhammer T, Kunert O, Haslinger E, Klingler P, Senderowicz AM, Sausville EA, Jäger W. In vitro glucuronidation of the cyclin-dependent kinase inhibitor flavopiridol by rat and human liver microsomes: involvement of UDP-glucuronosyltransferases 1A1 and 1A9. Drug Metab Dispos. 2001;29(4 Pt 1):407–14. [PubMed] [Google Scholar]
- 174.Ramírez J, Iyer L, Journault K, Bélanger P, Innocenti F, Ratain MJ, Guillemette C. In vitro characterization of hepatic flavopiridol metabolism using human liver microsomes and recombinant UGT enzymes. Pharm Res. 2002;19(5):588–94. doi: 10.1023/a:1015341726183. [DOI] [PubMed] [Google Scholar]
- 175.Ramírez J, Liu W, Mirkov S, Desai AA, Chen P, Das S, Innocenti F, Ratain MJ. Lack of association between common polymorphisms in UGT1A9 and gene expression and activity. Drug Metab Dispos. 2007;35(12):2149–53. doi: 10.1124/dmd.107.015446. [DOI] [PubMed] [Google Scholar]
- 176.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310(3):1062–75. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 177.Ogura K, Ishikawa Y, Kaku T, Nishiyama T, Ohnuma T, Muro K, Hiratsuka A. Quaternary ammonium-linked glucuronidation of trans-4-hydroxytamoxifen, an active metabolite of tamoxifen, by human liver microsomes and UDP-glucuronosyltransferase 1A4. Biochem Pharmacol. 2006;71(9):1358–69. doi: 10.1016/j.bcp.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 178.Zheng Y, Sun D, Sharma AK, Chen G, Amin S, Lazarus P. Elimination of antiestrogenic effects of active tamoxifen metabolites by glucuronidation. Drug Metab Dispos. 2007;35(10):1942–8. doi: 10.1124/dmd.107.016279. [DOI] [PubMed] [Google Scholar]
- 179.Sun D, Sharma AK, Dellinger RW, Blevins-Primeau AS, Balliet RM, Chen G, Boyiri T, Amin S, Lazarus P. Glucuronidation of active tamoxifen metabolites by the human UDP glucuronosyltransferases. Drug Metab Dispos. 2007;35(11):2006–14. doi: 10.1124/dmd.107.017145. [DOI] [PubMed] [Google Scholar]
- 180.Sun D, Chen G, Dellinger RW, Duncan K, Fang JL, Lazarus P. Characterization of tamoxifen and 4-hydroxytamoxifen glucuronidation by human UGT1A4 variants. Breast Cancer Res. 2006;8(4):R50. doi: 10.1186/bcr1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Blevins-Primeau AS, Sun D, Chen G, Sharma AK, Gallagher CJ, Amin S, Lazarus P. Functional significance of UDP-glucuronosyltransferase variants in the metabolism of active tamoxifen metabolites. Cancer Res. 2009;69(5):1892–900. doi: 10.1158/0008-5472.CAN-08-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Davis HR, Veltri EP. Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J Atheroscler Thromb. 2007;14(3):99–108. doi: 10.5551/jat.14.99. [DOI] [PubMed] [Google Scholar]
- 183.Ghosal A, Hapangama N, Yuan Y, Achanfuo-Yeboah J, Iannucci R, Chowdhury S, Alton K, Patrick JE, Zbaida S. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe (Zetia) Drug Metab Dispos. 2004;32(3):314–20. doi: 10.1124/dmd.32.3.314. [DOI] [PubMed] [Google Scholar]
- 184.van Heek M, Farley C, Compton DS, Hoos L, Alton KB, Sybertz EJ, Davis HR., Jr Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Br J Pharmacol. 2000;129(8):1748–54. doi: 10.1038/sj.bjp.0703235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O’neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc Natl Acad Sci U S A. 2005;102(23):8132–7. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yamamoto T, Ito K, Honma M, Takada T, Suzuki H. Cholesterol-lowering effect of ezetimibe in uridine diphosphate glucuronosyltransferase 1A-deficient (Gunn) rats. Drug Metab Dispos. 2007;35(9):1455–8. doi: 10.1124/dmd.107.015628. [DOI] [PubMed] [Google Scholar]
- 187.Bae JW, Choi CI, Lee JH, Jang CG, Chung MW, Lee SY. Effects of UDP-glucuronosyltransferase polymorphisms on the pharmacokinetics of ezetimibe in healthy subjects. Eur J Clin Pharmacol. 2010 doi: 10.1007/s00228-010-0899-x. (in press) [DOI] [PubMed] [Google Scholar]
- 188.Chang JH, Yoo P, Lee T, Klopf W, Takao D. The role of pH in the glucuronidation of raloxifene, mycophenolic acid and ezetimibe. Mol Pharm. 2009;6(4):1216–27. doi: 10.1021/mp900065b. [DOI] [PubMed] [Google Scholar]
- 189.Kilpatrick GJ, Smith TW. Morphine-6-glucuronide: actions and mechanisms. Med Res Rev. 2005;25(5):521–44. doi: 10.1002/med.20035. [DOI] [PubMed] [Google Scholar]
- 190.Joshi GP. Morphine-6-glucuronide, an active morphine metabolite for the potential treatment of post-operative pain. Curr Opin Investig Drugs. 2008;9(7):786–99. [PubMed] [Google Scholar]
- 191.van Dorp EL, Morariu A, Dahan A. Morphine-6-glucuronide: potency and safety compared with morphine. Expert Opin Pharmacother. 2008;9(11):1955–61. doi: 10.1517/14656566.9.11.1955. [DOI] [PubMed] [Google Scholar]
- 192.Stone AN, Mackenzie PI, Galetin A, Houston JB, Miners JO. Isoform selectivity and kinetics of morphine 3-and 6-glucuronidation by human udp-glucuronosyltransferases: evidence for atypical glucuronidation kinetics by UGT2B7. Drug Metab Dispos. 2003;31(9):1086–9. doi: 10.1124/dmd.31.9.1086. [DOI] [PubMed] [Google Scholar]
- 193.Ohno S, Kawana K, Nakajin S. Contribution of UDP-glucuronosyltransferase 1A1 and 1A8 to morphine-6-glucuronidation and its kinetic properties. Drug Metab Dispos. 2008;36(4):688–94. doi: 10.1124/dmd.107.019281. [DOI] [PubMed] [Google Scholar]
- 194.Vree TB, van Dongen RT, Koopman-Kimenai PM. Codeine analgesia is due to codeine-6-glucuronide, not morphine. Int J Clin Practa. 2000;54(6):395–8. [PubMed] [Google Scholar]
- 195.Court MH, Krishnaswamy S, Hao Q, Duan SX, Patten CJ, Von Moltke LL, Greenblatt DJ. Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab Dispos. 2003;31(9):1125–33. doi: 10.1124/dmd.31.9.1125. [DOI] [PubMed] [Google Scholar]
- 196.Raungrut P, Uchaipichat V, Elliot DJ, Janchawee B, Somogyi AA, Miners JO. In vitro-in vivo extrapolation predicts drug-drug interactions arising from inhibition of codeine glucuronidation by dextropropoxyphene, fluconazole, ketoconazole, and methadone in humans. J Pharmacol Exp Ther. 2010;334(2):609–18. doi: 10.1124/jpet.110.167916. [DOI] [PubMed] [Google Scholar]
- 197.Lee JK, Abe K, Bridges AS, Patel NJ, Raub TJ, Pollack GM, Brouwer KL. Drug Metab Dispos. 2009;37(9):1916–21. doi: 10.1124/dmd.109.026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Joseph TB, Wang SW, Liu X, Kulkarni KH, Wang J, Xu H, Hu M. Disposition of flavonoids via enteric recycling: enzyme stability affects characterization of prunetin glucuronidation across species, organs, and UGT isoforms. Mol Pharm. 2007;4(6):883–94. doi: 10.1021/mp700135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jeong EJ, Liu Y, Lin H, Hu M. Species- and disposition model-dependent metabolism of raloxifene in gut and liver: role of UGT1A10. Drug Metab Dispos. 2005;33(6):785–94. doi: 10.1124/dmd.104.001883. [DOI] [PubMed] [Google Scholar]
- 200.Court MH. Acetaminophen UDP-glucuronosyltransferase in ferrets: species and gender differences, and sequence analysis of ferret UGT1A6. J Vet Pharmacol Ther. 2001;24(6):415–22. doi: 10.1046/j.1365-2885.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 201.Mizuma T. Intestinal glucuronidation metabolism may have a greater impact on oral bioavailability than hepatic glucuronidation metabolism in humans: a study with raloxifene, substrate for UGT1A1, 1A8, 1A9, and 1A10. Int J Pharm. 2009;378(1–2):140–1. doi: 10.1016/j.ijpharm.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 202.Wong YC, Zhang L, Lin G, Zuo Z. Structure-activity relationships of the glucuronidation of flavonoids by human glucuronosyltransferases. Expert Opin Drug Metab Toxicol. 2009;5(11):1399–1419. doi: 10.1517/17425250903179300. [DOI] [PubMed] [Google Scholar]
- 203.Zhang L, Lin G, Zuo Z. Position preference on glucuronidation of mono-hydroxylflavones in human intestine. Life Sci. 2006;78(24):2772–80. doi: 10.1016/j.lfs.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 204.Wong YC, Zhang L, Lin G, Zuo Z. Intestinal first-pass glucuronidation activities of selected dihydroxyflavones. Int J Pharm. 2009;366(1–2):14–20. doi: 10.1016/j.ijpharm.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 205.Wang SW, Kulkarni KH, Tang L, Wang JR, Yin T, Daidoji T, Yokota H, Hu M. Disposition of flavonoids via enteric recycling: UDP-glucuronosyltransferase (UGT) 1As deficiency in Gunn rats is compensated by increases in UGT2Bs activities. J Pharmacol Exp Ther. 2009;329(3):1023–31. doi: 10.1124/jpet.108.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shimoi K, Nakayama T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005;400:263–72. doi: 10.1016/S0076-6879(05)00015-7. [DOI] [PubMed] [Google Scholar]
- 207.Lee-Hilz YY, Stolaki M, van Berkel WJ, Aarts JM, Rietjens IM. Activation of EpRE-mediated gene transcription by quercetin glucuronides depends on their deconjugation. Food Chem Toxicol. 2008;46(6):2128–34. doi: 10.1016/j.fct.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 208.Bohnenstengel F, Kroemer HK, Sperker B. In vitro cleavage of paracetamol glucuronide by human liver and kidney beta-glucuronidase: determination of paracetamol by capillary electrophoresis. J Chromatogr B Biomed Sci Appl. 1999;721(2):295–9. doi: 10.1016/s0378-4347(98)00464-2. [DOI] [PubMed] [Google Scholar]
- 209.O’Leary KA, Day AJ, Needs PW, Mellon FA, O’Brien NM, Williamson G. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003;65(3):479–91. doi: 10.1016/s0006-2952(02)01510-1. [DOI] [PubMed] [Google Scholar]
- 210.Shimoi K, Saka N, Kaji K, Nozawa R, Kinae N. Metabolic fate of luteolin and its functional activity at focal site. Biofactors. 2000;12(1–4):181–6. doi: 10.1002/biof.5520120129. [DOI] [PubMed] [Google Scholar]
- 211.Shimoi K, Saka N, Nozawa R, Sato M, Amano I, Nakayama T, Kinae N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab Dispos. 2001;29(12):1521–4. [PubMed] [Google Scholar]
- 212.Mochizuki M, Kajiya K, Terao J, Kaji K, Kumazawa S, Nakayama T, Shimoi K. Effect of quercetin conjugates on vascular permeability and expression of adhesion molecules. Biofactors. 2004;22(1–4):201–4. doi: 10.1002/biof.5520220142. [DOI] [PubMed] [Google Scholar]
- 213.Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos. 2006;34(10):1786–92. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 214.Patel M, Shah T, Amin A. Therapeutic opportunities in colon-specific drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 2007;24(2):147–202. doi: 10.1615/critrevtherdrugcarriersyst.v24.i2.20. [DOI] [PubMed] [Google Scholar]