Abstract
Background:
Published data indicate that thyroid stimulating hormone receptor (TSHR) activities are associated with osteoporosis in some patients.
Aim:
This study aimed to elucidate whether a given polymorphism of the TSHR gene is associated with osteoporosis.
Materials and Methods:
One hundred and fifty subjects with osteoporosis were recruited in this study. The diagnosis of osteoporosis was performed with quantitative ultrasound system. The TSHR gene polymorphism was examined by polymerase chain reaction–restriction fragment length polymorphism.
Results:
The results showed a nucleotide substitution in the first position of codon 36 of the TSHR gene. The nucleotide substitution was from G to C, leading to a 36D → 36H change (D36H) in the predicted amino acid sequence of the receptor. The change did not show significance between healthy subjects and patients with osteoporosis (P > 0.05). On the other hand, we identified another single nucleotide polymorphism that is a C-to-G substitution at codon 727 (GAC to GAG); its frequency was significantly higher in patients with osteoporosis than that in healthy subjects. Using logistic regression analysis, significant correlation was revealed between the genotype D727E and the serum levels of TSH, or the quantitative ultrasound value of the calcaneal bone.
Conclusions:
The present study suggests that the genotype D727E of the TSHR, but not the genotype D36H, may be a genetic risk factor for osteoporosis.
Keywords: Gene polymorphism, Osteoporosis, Restriction fragment length polymorphism, RFLP, Thyrotropin receptor
Introduction
Osteoporosis, defined as an inappropriate decrease in bone mass, is a common disease affecting the majority of the populations with older age. It is featured as the gradual reduction in bone strength with advancing age, particularly in women after menopause.[1] Age is the strongest factor in osteoporosis; however, the density of bones is quite various among individuals with the same age.[2] Apparently, apart from old age, a number of other factors, such as genetic factors,[3] hormone levels,[4,5] and environmental factors[6] play a role in the development of osteoporosis.
Thyroid hormones affect bone metabolism. There is an increased bone remodeling, with the net effect on bone resorption in hyperthyroidism; the induced bone remodeling can be generally reversible in most patients once the hyperthyroidism is treated and thyroid status returns to normal provided to maintain the serum levels of thyroid stimulating hormone (TSH) during the treatment.[7,8] The fact implies that the receptor of TSH (TSHR) is also important in maintaining the proper bone metabolism based on that TSH plays a key role in thyroid growth and function.[9] The TSHR is also expressed on bone cells and is directly involved in the bone cell metabolism.[10] The gene of the TSHR, located at chromosome 14q31, encodes a protein of 764 amino acids with a large glycosylated ectodomain of 395 residues that are encoded by 9 exons.[11] The remaining 349 residues, encoded by the 10th exon, constitute the seven-transmembrane domain and intracytoplasmic tail.[11] Gene polymorphism is related to genetic variation and adaptation; it usually functions to retain certain physiologic or pathologic features in a population living in a varied environment. Some gene polymorphisms may be associated with certain diseases, such as an association between the genotype D727E and serum levels of TSH has been noted.[12] However, whether this allele of the TSHR gene is associated with osteoporosis is to be further understood.
Abnormality of TSHR expression may affect the activities of osteoclasts.[13] TSH can manipulate the precursors of osteoblasts and osteoclasts, suppress the precursors to develop to osteoclasts as well as activate osteoclasts.[14] Yet, whether the TSHR gene polymorphism is involved in osteoporosis is to be further understood. Thus, we carried out a study on assessing the TSHR gene polymorphism in 300 persons of age 46–80 years. The results show that a single nucleotide substitution at locus 727, the D727E, in the TSHR gene is associated with osteoporosis.
Materials and Methods
The study was approved by the Human Research Ethics Committee at the Guangzhou Medical University. Informed consent was obtained from each subject.
Study subjects
One hundred and fifty male patients with osteoporosis were randomly recruited from Guangdong province of China. The selective criteria included the following: (i) living at the same residential address ≥ 10 years; (ii) no identical relative relationship between each other; (iii) age: 46–80 years. The exclusive criteria included the following: (i) disorders in major organs (including the thyroid gland, liver, kidney, heart, lung, gastrointestinal tract, or blood); (ii) using drugs in the recent 3 months known to influence bone and calcium metabolism, such as vitamin D, bisphosphonate, or estrogen. The demographic data are presented in Table 1.
Table 1.
Demographic data
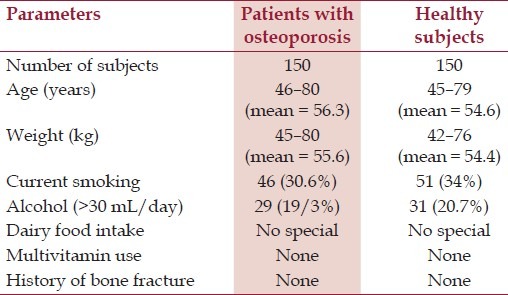
The healthy control group, including a total of 150 men (45–79 years of age), were recruited into the study. Subjects with Paget's disease, hyperparathyroidism, rheumatoid arthritis, ankylosing spondyloarthritis, endocrine disease, fracture history, and those receiving any treatment known to affect bone metabolism, such as biophosphonate, corticosteroids, thiazide diuretics, calcium, fluoride, calcitonin, and vitamin D, were excluded from the study.
Each participant underwent the calcaneal bone density measurement. A Hologic Sahara quantitative ultrasound (QUS) device (Hologic, Inc, Bedford, MA, USA) was employed for the calcaneal QUS measurement following standard operating procedures defined in the manufacturers’ manuals. The broadband ultrasound attenuation (BUA, decibels per megahertz) and speed of sound (SOS, meters per second) in a fixed region of interest in the central calcaneal zone were measured by this equipment.
Polymerase chain reaction–restriction fragment length polymorphism
The genomic DNA was extracted from whole blood samples using the reported method.[15] The polymorphisms of TSHR were detected by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) analysis.[16] The primers for the D36H polymorphic locus were: forward: 5’-ATTTCGGAGGATGGAGAAATA-3’: reverse: 5’-GTCTGCGTACTGGGCGGTAA-3’. D727E: forward: 5’-CCATTCCTCT ATGCTATTTTCAC-3’; reverse: 5’-CCGTTTGCATATACTCTTCTG-3’ (NCBI: NM_000369.2). PCR was performed through 35 cycles by the following conditions: denaturation at 95°C for 60 s, annealing at 50°C for 60 s, and extension at 72°C for 60 s. The PCR product was 207 bp for D36H, 265 bp for D727E. After amplification the PCR product was digested with fast digest restriction endonucleases, Bsp1286I and NlaIII (New England Biolabs Ltd., Pickering, ON, Canada) separately for 5 min. The digested products were analyzed in 1.5% agarose gel stained with ethidium bromide.
The judgment of gene polymorphism of D36H: The amplified gene sequence of D36H was 207 bp (from site 117 to 323) in the present study. Bsp1286I can recognize the mutation of G→C on site 263. Thus, PCR results showing one band of 207 bp indicate a homozygote GG with no G→C mutation. PCR results showing 147 and 60 bp two bands indicate a homozygote CC with G→C mutation. PCR results showing 207, 147, and 60 bp three bands indicate a heterozygote GC with G→C mutation [Figure 1].
Figure 1.
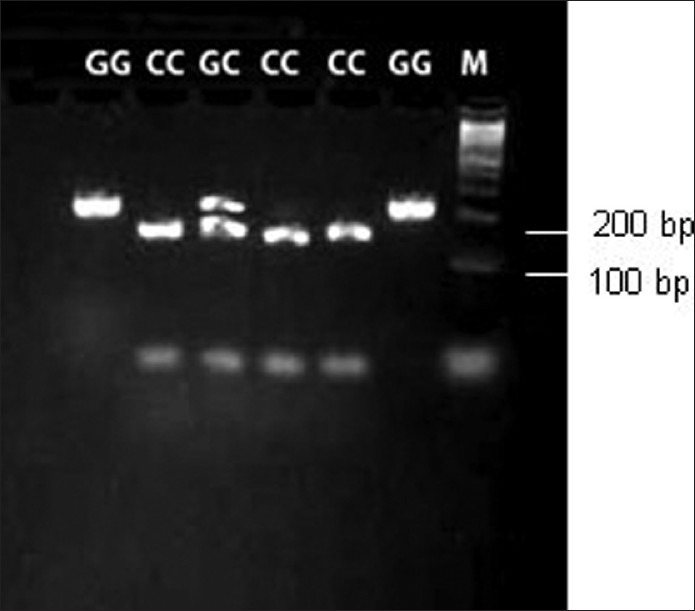
Polymerase chain reaction (PCR) products at D36H site. PCR products were cut by Bsp 1286I endonuclease and separated by agarose gel electrophoresis. “M” indicates the molecular marker
The judgment of gene polymorphism of D727E: The amplified gene sequence of D727E was 265 bp (from site 2179 to 2443) in the present study. NlaIII can recognize the transversion of C→G (GAC to GAG) on sites 2262 and 2283. If the point mutation occurs on site 2283, there will not be a second site for NlaIII. Thus, PCR results showing three bands of 160, 84, and 21 bp indicate a homozygote CC with no G→C mutation. PCR results showing 147 and 60 bp two bands indicate a homozygote CC with C→G mutation. PCR results showing 181 and 84 bp two bands indicate a homozygote GG with C→G mutation. PCR results showing 181, 160, 84, and 21 bp indicate a heterozygote GC with C→G mutation [Figure 2].
Figure 2.
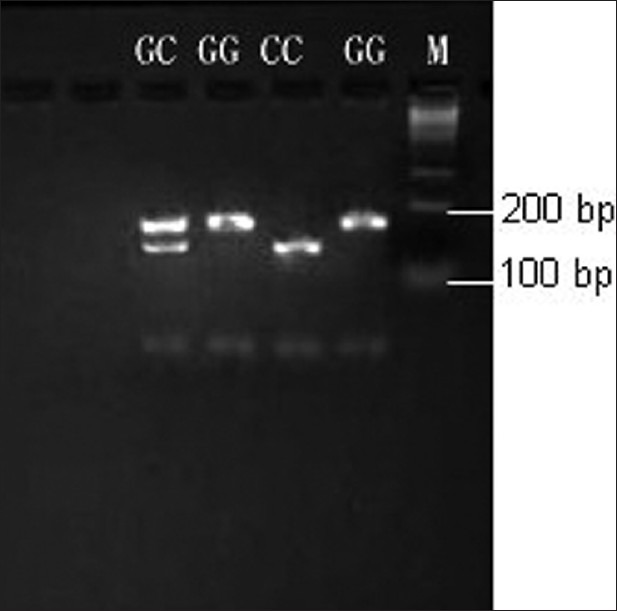
Polymerase chain reaction (PCR) products at D727E site. PCR products were cut by NlaIII endonuclease and separated by agarose gel electrophoresis
Measurement of broadband ultrasound attenuation
The BUA (a parameter that describes the energy loss of an ultrasound wave as it passes through a medium) was measured in the heel bone for all the subjects using a Sahara clinical bone sonometer (Hologic Inc., Bedford, MA, USA) following the manufacturer's instructions.
Determining the serum levels of TSH
The sera were obtained from each subject. The measurements of serum TSH were performed by the Clinical Chemistry Laboratory of our Hospital using commercial reagent kits (Abazyme, MA, USA) following the manufacturer's instruction.
Statistics
Data were expressed as mean ± SD. Differences between the two groups were assessed by Student's t test or analysis of variance if more than two groups. The frequency of genotype and allele gene was counted in all the groups and assessed by Chi-square test. Logistic stepwise regression assay was employed to analyze the association between TSHR gene polymorphism and serum levels of TSH.
Results
The distribution of TSHR gene polymorphism, frequency, and composite genotypes
The genotype screens resulted in 2 genotypes, GG and GC, at locus 36 of the TSHR gene among the recruited subjects with the frequency of 94.3% (GG) and 5.7% (GC, or D36H), respectively, in patients with osteoporosis; in healthy subjects, the frequency of genotypes at locus 36 was 95% (GG) and 5% (GC), respectively. No statistical difference was identified between osteoporosis group and healthy control groups (P > 0.05). The distribution of D36H genotypes was in line with Hardy-Weinberg equilibrium among the results.
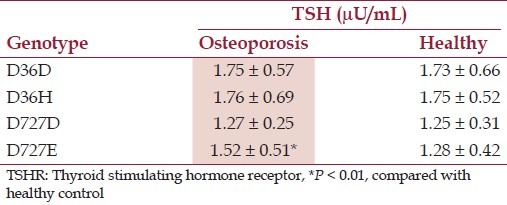
At locus 727 of the TSHR gene, 2 genotypes, GG and GC, were identified. In patients with osteoporosis, the frequency of genotype GG was 90.7% and GC (or D727E) was 9.3%. In healthy subjects, the frequency of genotype GG was 94.5% and GC was 5.5%. The frequency of D727E genotype was significantly higher in osteoporosis group as compared with that in the healthy control group (P < 0.05). The distribution of D727E genotype was in line with Hardy–Weinberg equilibrium among the results.
Correlation assay between TSHR genotypes and serum levels of TSH or calcaneal bone BUA
Tables 2 and 3 present the TSHR genotype D36H and D727E, calcaneal bone BUA, and serum levels of TSH.
Table 2.
TSHR genotype and calcaneal bone BUA
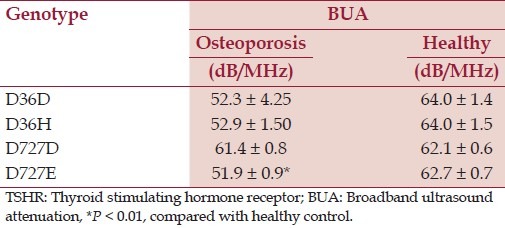
Table 3.
TSHR genotype and serum levels of TSH
Discussion
As early as 1995, a Swedish research group first reported the human TSHR on the first nucleotide (G→C) of the 36th codon of the gene sequence that resulted in histidine replacing aspartic acid.[17] Next, the TSHR gene D727E was reported; TSHR with such genotype has high responsibility to TSH stimulation. The gene polymorphism of D727E was described shortly after. An association between D727E and serum levels of TSH was reported afterward.[12] Further studies confirmed this phenomenon, such as the genotype of D727E can strengthen cyclic 3’,5’-adenosine monophosphate (cAMP)'s responsibility;[18] it is well established that increasing cAMP can regulate the expression of some key genes involved in bone metabolism.[19]
TSHR is also expressed by osteoblasts.[20] The fact implies that TSH can modulate the metabolism in the bone in situ. Published data indicate that TSHR–cAMP pathway plays a role in activating human osteoblasts under physiologic conditions.[21] It is suggested that overexpressing TSHR can suppress the activities of osteoclasts even in lacking TSH.[13] Experimental data from TSHR gene knockout mice show that TSH manipulates the precursors of osteoblast and osteoclast, suppressing the precursors to develop to osteoclasts as well as activating osteoclasts.[14] Studies with animal models demonstrate that the insufficient TSHR homozygotes result in the reduction of bone mineral substance or osteoporosis.[22] The fact further demonstrates an important role of TSHR in bone metabolism. A direct association between TSH and osteoporosis has been reported,[23] in which the expression of TSHR plays a critical role in the activities of osteoblasts and osteoclasts.[22]
The present data show that the polymorphisms of the TSHR gene, the genotypes of D36H and D727E, the frequency of observed genotypes, among which the genotype of D36H does not show a statistical difference in comparing the data from an osteoporosis group with a healthy control group. On the other hand, the TSHR D727E genotype analysis shows statistical significance between patients with osteoporosis and healthy subjects. Importantly, the frequency of the TSHR gene D727E genotype has statistical positive correlation with the calcaneal bone BUA value in both osteoporosis group and healthy control group. The fact implies that the TSHR gene mutation is associated with osteoporosis that needs further work to elucidate. Based on the present data and literature review, we may envisage the effect of TSHR gene polymorphism on osteoporosis via two approaches. The first approach is that the D727E substitution directly regulates the activities of the TSHR and TSH on osteoblasts and osteoclasts. The second approach is that when the serum levels of TSH are low, D727E polymorphism causes the osteogenesis system only weakly suppresses osteoclasts leading to the overgeneration of osteoclasts. These two approaches together contribute to osteoporosis.
Conclusion
The present data have revealed that TSHR gene D727E polymorphism is associated with osteoporosis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Favus MJ. Bisphosphonates for osteoporosis. N Engl J Med. 2010;363:2027–35. doi: 10.1056/NEJMct1004903. [DOI] [PubMed] [Google Scholar]
- 2.Cheung CL, Xiao SM, Kung AW. Genetic epidemiology of age-related osteoporosis and its clinical applications. Nat Rev Rheumatol. 2010;6:507–17. doi: 10.1038/nrrheum.2010.106. [DOI] [PubMed] [Google Scholar]
- 3.Ralston SH. Genetics of osteoporosis. Ann N Y Acad Sci. 2010;1192:181–9. doi: 10.1111/j.1749-6632.2009.05317.x. [DOI] [PubMed] [Google Scholar]
- 4.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American college of rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010;62:1515–26. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 5.Clarke BL, Khosla S. Female reproductive system and bone. Arch Biochem Biophys. 2010;503:118–28. doi: 10.1016/j.abb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Vukicevic S, Baliram R, Yang G, Sendak R, McPherson J, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci U S A. 2008;105:4289–94. doi: 10.1073/pnas.0712395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidi M, Davies T, Zallone A, Blair HC, Iqbal J, Moonga SS, et al. Thyroid-stimulating hormone, thyroid hormones, and bone loss. Cur Osteoporos Rep. 2009;7:47–52. doi: 10.1007/s11914-009-0009-0. [DOI] [PubMed] [Google Scholar]
- 9.Gilb V, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 10.Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor -associated diseases: From adenomata to Grave's disease. J Clin Invest. 2005;115:1972–83. doi: 10.1172/JCI26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmentier M, Libert F, Maenhaut C, Lefort A, Gérard C, Perret J, et al. Molecular cloning of the thyrotropin receptor. Science. 1989;246:1620–2. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- 12.Gabriel EM, Bergert ER, Grant CS, van Heerden JA, Thompson GB, Morris JC. Germline polymorphism of codon 727 of human thyroid-stimulating hormone receptor is associated with toxic multinodular goiter. J Clin Endocrinol Metab. 1999;84:3328–35. doi: 10.1210/jcem.84.9.5966. [DOI] [PubMed] [Google Scholar]
- 13.Hase H, Ando T, Eldeiry L, Brebene A, Peng Y, Liu L, et al. TNF-a mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci USA. 2006;103:12849–54. doi: 10.1073/pnas.0600427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novack DV. TSH, the bone suppressing hormone. Cell. 2003;115:129–30. doi: 10.1016/s0092-8674(03)00812-2. [DOI] [PubMed] [Google Scholar]
- 15.Lahiri DK, Numberger JI. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbo RM, Ulizzi L, Piombo L, Martinez-Labarga C, De Stefano GF, Scacchi R. Estrogen receptor alpha polymorphisms and fertility in populations with different reproductive patterns. Mol Hum Reprod. 2007;13:537–40. doi: 10.1093/molehr/gam041. [DOI] [PubMed] [Google Scholar]
- 17.Gustavsson B, Eklof C, Westermark K, Westermark B, Heldin NE. Functional analysis of a variant of the thyrotropin receptor gene in a family with Graves’ disease. Mol Cell Endocrinol. 1995;111:167–73. doi: 10.1016/0303-7207(95)03562-l. [DOI] [PubMed] [Google Scholar]
- 18.Sykiotis GP, Neumann S, Georgopoulos NA, Sgourou A, Papachatzopoulou A, Markou KB, et al. Functional significance of the thyrotropin receptor germline polymorphism D727E. Biochem Biophys Res Commun. 2003;301:1051–6. doi: 10.1016/s0006-291x(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 19.Swarthout JT, D’Alonzo RC, Selvamurugan N, Partridge NC. Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene. 2002;282:1–17. doi: 10.1016/s0378-1119(01)00798-3. [DOI] [PubMed] [Google Scholar]
- 20.Morimura T, Tsunekawa K, Kasahara T, Seki K, Ogiwara T, Mori M, et al. Expression of type 2 iodothyronine deiodinase in human osteoblast is stimulated by thyrotropin. Endocrinology. 2005;146:2077–84. doi: 10.1210/en.2004-1432. [DOI] [PubMed] [Google Scholar]
- 21.Bassett JH, Williams AJ, Murphy E, Boyde A, Howell PG, Swinhoe R, et al. A lack of thyroid hormones rather than excess thyrotropin causes abnormal skeletal development in hypothyroidism. Mol Endocrinol. 2008;22:501–12. doi: 10.1210/me.2007-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–62. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 23.Lee WY, Oh KW, Rhee EJ, Jung CH, Kim SW, Yun EJ, et al. Relationship between subclinical thyroid dysfunction and femoral neck bone mineral density in women. Arch Med Res. 2006;37:511–6. doi: 10.1016/j.arcmed.2005.09.009. [DOI] [PubMed] [Google Scholar]


