Abstract
Vocal indicators of welfare have proven their use for many farmed and zoo animals and may be applied to farmed silver foxes as these animals display high vocal activity toward humans. Farmed silver foxes were selected mainly for fur, size, and litter sizes, but not for attitudes to people, so they are fearful of humans and have short-term welfare problems in their proximity. With a human approach test, we designed here the steady increase and decrease of fox–human distance and registered vocal responses of 25 farmed silver foxes. We analyzed the features of vocalizations produced by the foxes at different fox–human distances, assuming that changes in vocal responses reflect the degrees of human-related discomfort. For revealing the discomfort-related vocal traits in farmed silver foxes, we proposed and tested the algorithm of “joint calls,” equally applicable for analysis of all calls independently on their structure, either tonal or noisy. We discuss that the increase in proportion of time spent vocalizing and the shift of call energy toward higher frequencies may be integral vocal characteristics of short-term welfare problems in farmed silver foxes and probably in other captive mammals.
Keywords: Welfare, Human–animal interaction, Vocalization, Acoustic analysis, Fur farm animals, Vulpes vulpes
Introduction
As many animals respond vocally to discomfort (e.g., Jürgens 1976a, b, 1979; Grandin 1998; Fichtel et al. 2001; Marchant et al. 2001; Blumstein et al. 2006), vocal features may be useful indicators of short-term welfare problems, alongside other behavioral parameters (reviews in Weary and Frazer 1995a; Watts and Stookey 2000; Manteuffel et al. 2004). Moreover, advanced vocal-based automatic systems are already available for welfare monitoring in some farmed animals, such as sows Sus scrofa (Schön et al. 2001, 2004; Moura et al. 2008) and cows Bos taurus (Jahns 2008) and may be actual for other animals, showing high levels of vocalizing in captivity. Theoretical grounds for using the vocal traits as reliable indicators of short-term welfare problems are coming (1) from the findings that most mammalian calls are related to emotional states on the level of brain (Jürgens and Ploog 1970, 1981; Jürgens 1979, 2009), (2) from the empirically stated “motivation–structural rules” linking the call structure and aggressiveness–fearfulness (Morton 1977), and (3) from the concept of the “honest signal,” determining conditions under which the call structure should reflect the degree of psychological comfort (Zahavi 1977, 1982).
On fur farms where keepers do not establish personal relationships with animals, the animals can have short-term welfare problems arising from human intervention by close approach (Broom and Johnson 1993), and the animal–human relations can be defined through the distance between the animal and the human. The human approach test, varying in design and the approach distance, is widely used in welfare-related research with farmed animals (review in Waiblinger et al. 2006) and is applied to silver foxes Vulpes vulpes (e.g. Pedersen and Jeppesen 1990; Pedersen 1993; Bakken 1998; Bakken et al. 1999; Trut 1999; Kukekova et al. 2008). Farmed silver foxes are mainly afraid of humans and try to increase their distance from an approaching human (e.g., Pedersen and Jeppesen 1990; Pedersen 1991, 1993, 1994; Nimon and Broom 2001; Trut 1999; Kukekova et al. 2008). The tendency to keep the maximum distance to humans reflects the elevated physiological stress in silver foxes (expressed in hyperthermia, adrenal response, and changes in blood parameters) in proximity of humans (Pedersen 1994; Moe and Bakken 1997; Bakken et al. 1999, Oskina et al. 2008). These responses were found by Moe and Bakken (1998) to be comparable to human fear responses. The human-related stress can impose harmful effects on animals as the industrial populations of silver foxes were selected mainly for fur quality, body size, and litter size, with little attention to their attitudes toward humans (The Welfare of Animals Kept for Fur Production 2001).
Since the vocal indicators for assessing welfare in silver foxes are poorly developed to date, a majority of welfare-related studies apply the nonvocal behavioral indicators, e.g., the latencies to touch novel object, to move, to defecate (Pedersen and Jeppesen 1990), restraining from eating in the presence of humans (Rekilä et al. 1997), time spent in the front of the cage door (Bakken 1998; Moe et al. 2006), erected ears (Moe et al. 2006), extent of abnormal behaviors (such as tail biting) and reproductive failure (Braastad 1987; Nimon and Broom 2001), infanticide (Bakken 1998), synchrony of activity of family members, use of space available and aggressive acts (Ahola and Mononen 2002), activity levels (Braastad 1996; Bakken et al. 1999), and stereotypies (Moe et al. 2006). At the same time, our earlier findings indicate that farmed silver foxes are extremely active callers, producing up to a few tenses calls per minute in response to human approach (Gogoleva et al. 2008). These calls deserve to be treated as potentially valuable indicators of welfare. For silver foxes, vocal features are rarely considered as indicators of welfare, although Pedersen (1993) and Kukekova et al. (2008) scored vocalizing alongside with other responses to human approach and handling, Pedersen and Jeppesen (1990) registered hissing and screaming as aggressive responses toward people, and Ahola and Mononen (2002) registered growls among other aggressive responses toward conspecifics.
Vocal indicators for assessing hunger, pain, or discomfort have been developed for cows and sows (e.g., Lay et al. 1992a, b; Weary and Frazer 1995b; Grandin 1998; Weary et al. 1998; Watts and Stookey 1999; Jahns 2008; Moura et al. 2008) and for captive rodents (Monticelli et al. 2004), tree shrews (Kirchhof et al. 2001; Schehka et al. 2007), primates (Newman and Goedeking 1992; Schrader and Todt 1993; Fichtel et al. 2001), and carnivores (Pongrácz et al. 2005). However, the results of different studies are not immediately comparable. It is coming from differences in the acoustic structure of calls and therefore in acoustic variables that can be analyzed. Thus, the search for the integral acoustic variables, reflecting welfare in all calls independently on their acoustic structure, tonal or noisy, is actual for the research focused on vocal indicators of welfare.
In a previous study (Gogoleva et al. 2008), we described five call types produced by farmed silver foxes toward people: three voiced or tonal (whine, moo, growl) and two unvoiced or noisy (snort and cough). Tonal calls showed signs of production with vocal source (larynx with vocal folds) reflected in tonal spectrum with the fundamental frequency and its harmonics (e.g., Titze 1994). Noisy calls showed an explosive wideband spectrum without traces of the fundamental frequency, which reflected their production not with vocal folds but with another source, most probably turbulence (vortices), arising during passage of air through a narrowest vocal tract (e.g., Nakagawa 1987; Bachorowski et al. 2001).
In this study, we apply a human approach test modeling the stepwise increase and decrease of the animal–human distance to farmed silver foxes and register their vocal responses. We analyze acoustically the fox calls produced at different animal–human distances, assuming that the changes in vocal traits at each distance reflect the degree of human-related discomfort of the approachable fox. Also, we propose a simple algorithm for revealing the discomfort-related parameters in calls of any acoustic structure and discuss its applicability to the estimation of welfare in other mammals.
Materials and methods
Subjects, site, and dates of work
Experiments and acoustic recordings had been conducted in July–August 2006 at the experimental fur farm of the Institute of Cytology and Genetics, Novosibirsk, Russia. Our subjects were 25 adult (1–3 years old) female farmed silver foxes unselected for any behavioral or vocal trait and not handled in any age. As early exposure to humans can effect the further reactions of foxes to people, it is forbidden to pet any particular fox on this experimental farm. Fox pups socialize with conspecifics when they live together with their mothers until weaning and then live together with their littermates up to separation into individual cages at the age of 2 months. After separation, they remain in visual, olfactory, and auditory contact with foxes from neighboring and opposing cages. This holding regime has been standardized since 1960 and is uniform for all foxes on the farm, thereby excluding the influence of new factors on the behavior of these animals. The study foxes were kept and tested in individual outdoor cages 70×85×90 cm with wire mesh floor. The cages were arranged in batteries of 50 cages per row, with two rows opposite each other and 1.7-m-wide passageway between them. The cages were covered with a slate roof with two sloping surfaces providing protection from wind, rain, and hot sun. Foxes were fed twice a day (beef, meat by-products, minced chicken, cereals, vitamins, and minerals). Water was available ad libitum.
Evidence from enzyme-linked immunosorbent assay analysis obtained with independent set of 15 unselected for behavior foxes living on this experimental farm show that farmed foxes have well-expressed peaks of stress-related hormones in response to human handling. After 10-min restrain in human hands, the level of cortisol in foxes increased from basal mean ± SE 13.97±1.87 to 66.67±6.19 ng/ml, and the level of AKTG increased from 6.39±0.7 to 84.82±10.15 pg/ml (Oskina et al. 2008). These hormonal responses suggest that farmed foxes experience discomfort in response to human interventions, so we could reasonably interpret their vocal responses to human proximity as discomfort-related.
Experimental procedure and acoustic recordings
Human approach tests (one per fox) with parallel acoustic recordings have been done by the same unfamiliar to the foxes researcher (S.S.G). Each test lasted 10 min and included five successive steps, each lasting 2 min. Transitions between the successive test steps were checked with watches and labeled by voice. A test started at the moment of the researcher’s approach to a focal fox cage to a distance of 50 cm. At step 1, the researcher was motionless; at step 2 performed smooth body and hand movements left and right, keeping the distance 50 cm; and at step 3 she shortened a human–fox distance with a one step forward and performed body and hand movements forward and back, touching the cage door by her fingers. Step 4 matched step 2, and step 5 matched step 1. Thus, the human impact to an animal increased between steps 1 and 3 and decreased between steps 3 and 5. The distance between the microphone and a focal fox varied by 25–100 cm; the orientation of an animal to the microphone was mostly frontal or lateral. If a non-focal fox called simultaneously with the focal one, the calls of the focal fox were labeled by voice. The labeling of calls by voice is a traditional practice, inevitable when a few animals call simultaneously. It allows distinguishing between calls of focal and other animals during the following analysis.
We used a Marantz PMD-222 (D&M Professional, Kanagawa, Japan) cassette recorder with an AKG-C1000S (AKG-Acoustics Gmbh, Vienna, Austria) cardioid electret condenser microphone and type II chrome audiocassettes EMTEC-CS II (EMTEC Consumer Media, Ludwigshafen, Germany). The system had a frequency response of 0.04–14 kHz at a tape speed of 4.75 mm/s.
Call analysis
Successive call digitizing (with each test step taken as a separate file) at a 22.05-kHz sampling rate, 16-bit precision, high-pass filtration at 0.1 kHz and measurements were made with Avisoft-SASLab Pro v. 4.33 (Avisoft Bioacoustics, Berlin, Germany). Spectrograms for analysis were created using Hamming window, FFT-length 1024 points, frame 50%, and overlap 87.5%. Figure spectrograms were created with calls downsampled to 11.025 kHz, Hamming window, FFT-length 512 points, frame 50%, and overlap 87.5%.
By spectrogram, one researcher (S.S.G) classified each call visually to one of five types (Fig. 1) according to the vocal traits described in Gogoleva et al. (2008), blindly to the number of the test step during which the calls were recorded. The sound utterances were considered as separate calls if they were separated by a silence space longer than 20 ms. In total, 5,838 calls have been examined.
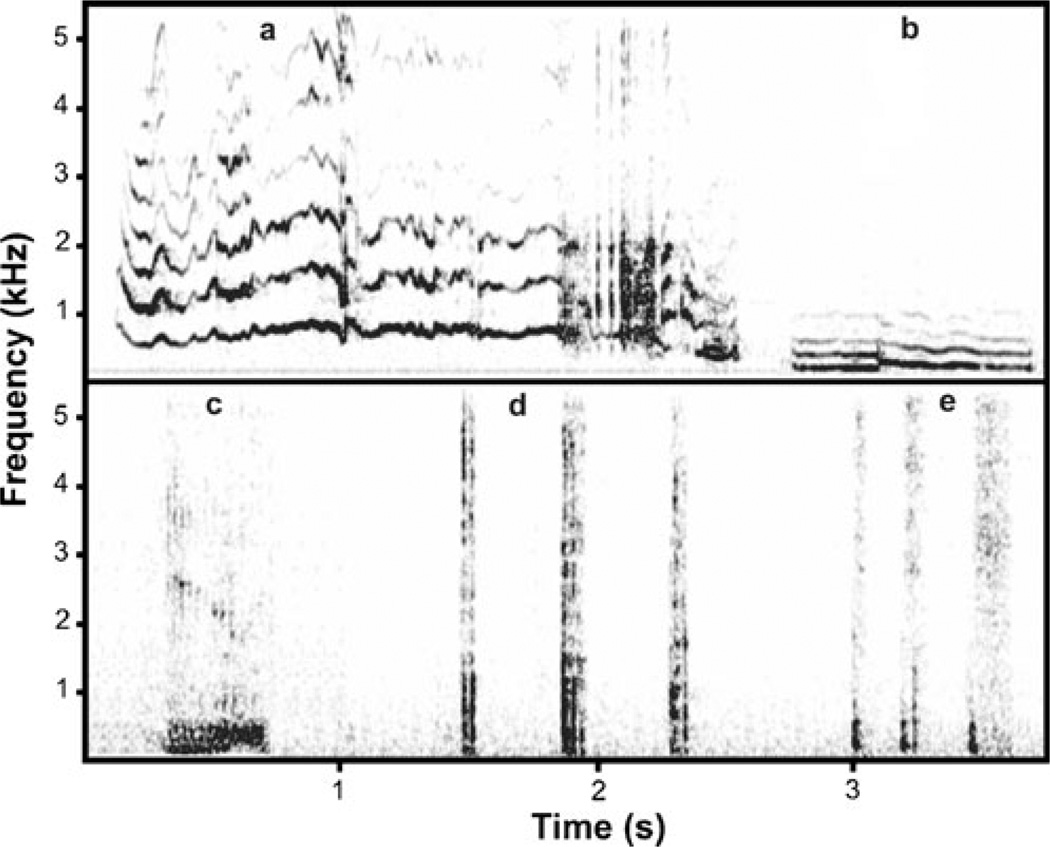
Fig. 1.
Spectrogram illustrating call types produced by farmed silver foxes toward people: a whine, b moo, c growl, d sequence of coughs, e sequence of snorts
To calculate the proportion of time spent vocalizing per fox per test step, i.e., the total duration of calls within a step divided by the duration of the step in minutes (taken in percent), we measured the duration of a given test step and the duration of each call produced during this test step with the standard marker cursor in the main window of Avisoft. The measurements were exported automatically to Excel (Microsoft Corp., Redmond, WA, USA). To calculate the calling rate (calls/min) for each call type, we divided the number of calls of the given type by the duration of the test step (in minutes).
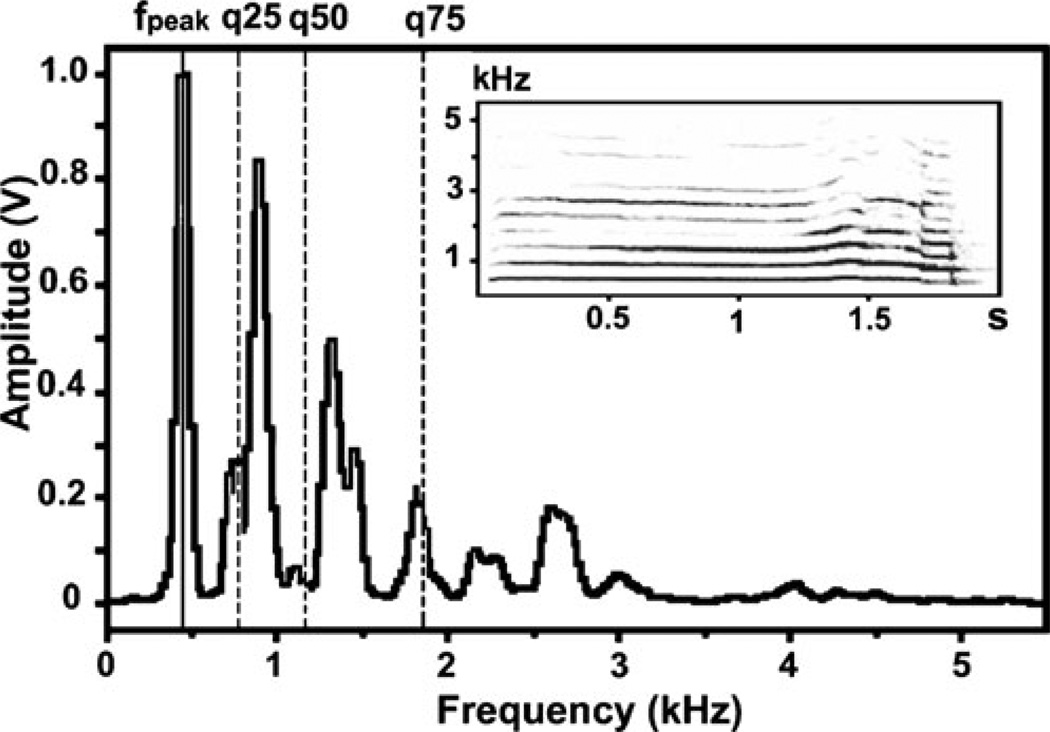
From power spectrum, using automatic parameter measurement option of Avisoft, we measured the maximum amplitude frequency (fpeak) and three quartiles (q25, q50, and q75), covering respectively 25%, 50%, and 75% of call energy (hereafter the lower, medium, and upper quartiles; Fig. 2). These call variables describe the relative distribution of energy over a call spectrum. The low values of the maximum amplitude frequency and of the quartiles reflect the shift of energy toward lower frequencies, while the high values of these variables reflect the energy shift toward the higher frequencies. From a call power spectrum, using automatic parameter measurement option of Avisoft, we measured also the entropy, reflecting the ratio of noisy and harmonic energy. The entropy represents the ratio of the geometric mean to arithmetic mean of the spectrum. This variable is theoretically 0 for pure-tone signals and 1 for random (white) noise (Specht 2006).
Fig. 2.
Power spectrum and the installed spectrogram of a female silver fox’ whine and the measured vocal variables: fpeak maximum amplitude frequency, q25 lower quartile, q50 medium quartile, q75 upper quartile
We measured the duration, maximum amplitude frequency, three quartiles, and entropy for all calls classified as whines, moos, coughs, and snorts (Fig. 1), but not for growls, occurring too rarely to be included into analysis. Then we calculated the average values for all measured variables for each individual at each test step for further statistical analyses.
Also, we prepared “joint calls,” cutting off the silent spaces between calls of a focal fox produced within a test step (Fig. 3). In total, we prepared 125 joint calls for the 25 study foxes (one joint call per fox per test step). As the power characteristics of the joint calls could be affected by strikes, superimposed calls of non-focal foxes, or with fragments of too loud calls of a focal fox, overloading the established level of recording, we cut off such pieces of recordings from 357 (6.11%) of the total number of 5,838 calls (see also Fig. 3). For each joint call, we measured the maximum amplitude frequency, three quartiles and entropy, by similar way as in single calls.
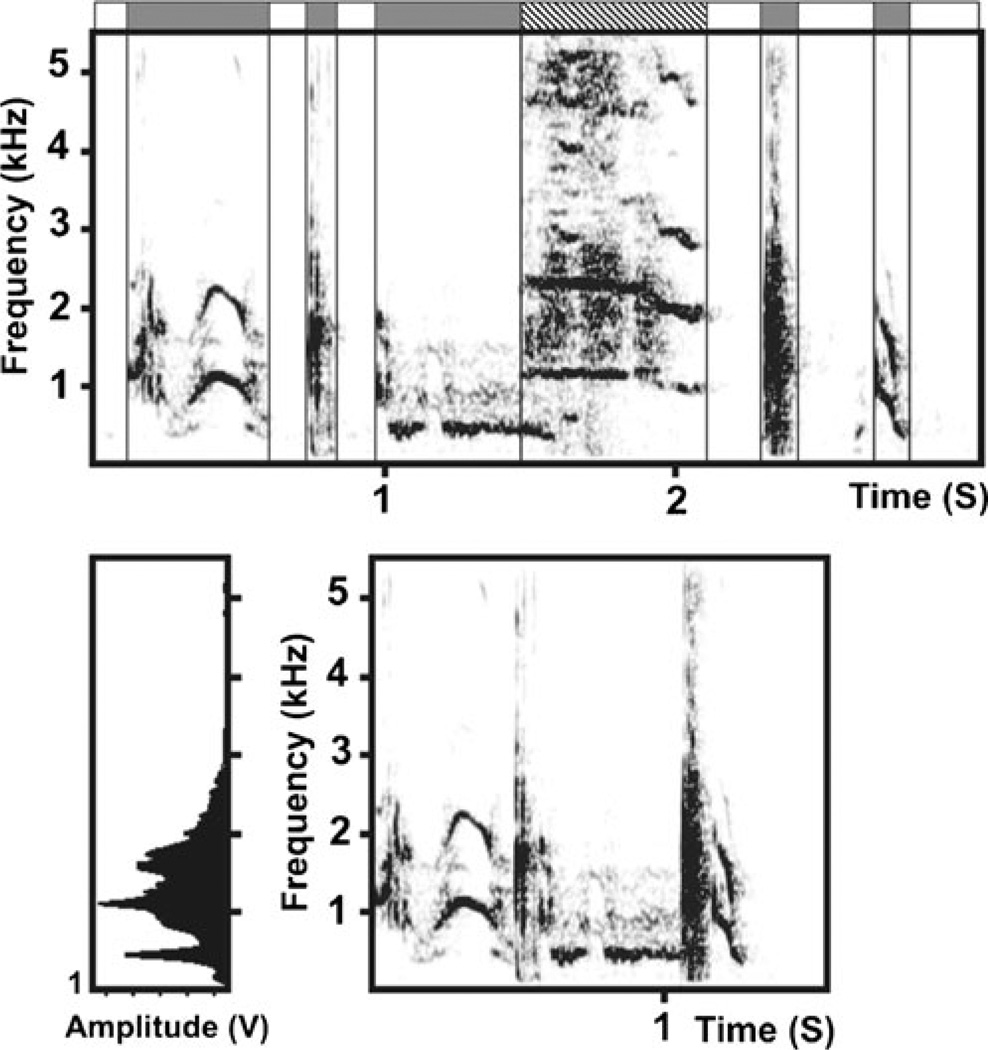
Fig. 3.
Procedure for preparation of a joint call. Above Spectrogram of an intact natural 3-s-long sequence of calls, produced by a focal silver fox during a human approach test. The sequence contains tonal and noisy calls (labeled with dark bars above the spectrogram), separated with silence spaces (labeled with light bars above the spectrogram). The striped bar labels the superimposed call of a non-focal neighboring fox, produced simultaneously with the call of the focal fox. Below Spectrogram and power spectrum of a part of the future joint call made from the call sequence shown above. Only dark-labeled fragments are reserved, while the light-labeled and strip-labeled are cut off
Statistics
All statistical analyses were carried out with STATISTICA, v. 6.0 (StatSoft, Inc., Tulsa, OK, USA). All tests were two-tailed, all means are given as mean ± SE, and differences were considered significant where p<0.05. We used GLM for repeated measures with Newman–Keuls post hoc test to compare the vocal traits between the test steps. The values of acoustic variables, not satisfying the criteria of normality with Kolmogorov–Smirnov test (calling rates of whines, calling rates of coughs, proportions of the time spent vocalizing, and maximum amplitude frequencies of joint calls) were root-square-transformed to be introduced into GLM. Also, we used GLMM (mixed model design with test step as fixed factor and animal as random factor) with Newman–Keuls post hoc test to compare the values of acoustic variables within call types as the numbers of individuals calling certain call types differed among steps. We used χ2 test with Fisher exact test as post hoc to compare the proportions of calls of different types between the successive steps of the human approach test.
Results
The subject foxes produced calls of five types: whines, moos, growls, coughs, and snorts (Fig. 1). The numbers of study foxes (n=25 individuals), produced calls of each type, varied between the successive steps of the human approach test (Table 1). None call type occurred in all the 25 individuals at all test steps. We did not find any single fox silent at all test steps. Moos, snorts, and coughs were the most often produced call types.
Table 1.
Numbers of study foxes (n=25 individuals) produced calls of each type at five successive steps of a human approach test and the numbers of calls at each test step
| Call type | Step 1 | Step 2 | Step 3 | Step 4 | Step 5 |
|---|---|---|---|---|---|
| Whine | 8 | 8 | 15 | 7 | 7 |
| Moo | 20 | 19 | 22 | 24 | 18 |
| Growl | 2 | 3 | 6 | 7 | 6 |
| Cough | 14 | 20 | 23 | 15 | 13 |
| Snort | 23 | 23 | 24 | 21 | 19 |
| All call types | 25 | 25 | 25 | 25 | 25 |
| Total number of calls | 801 | 1,294 | 2,397 | 871 | 475 |
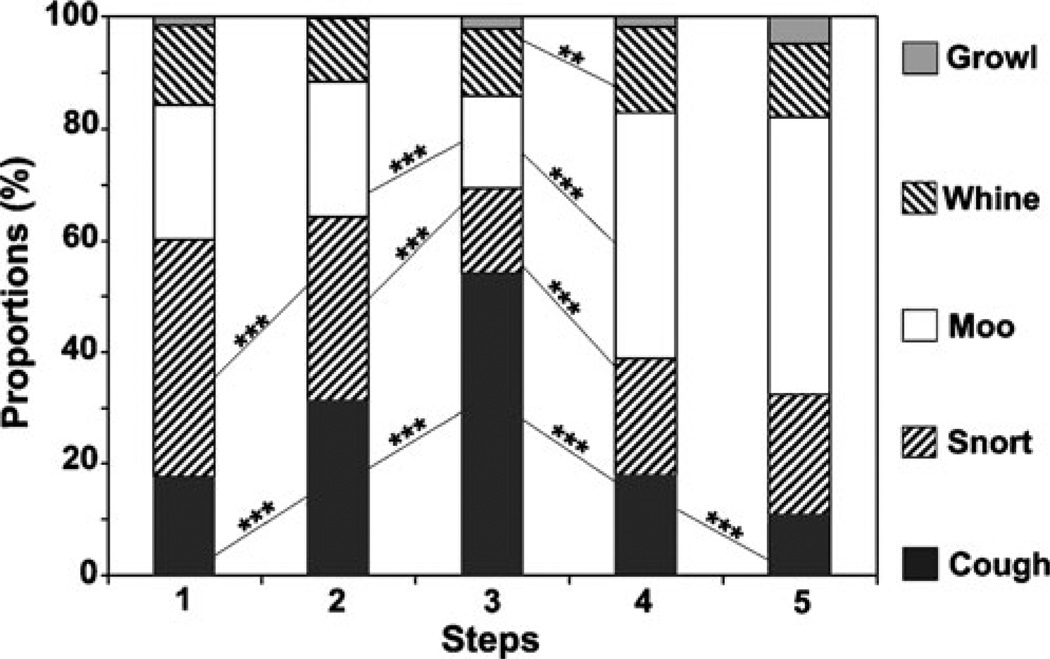
Both total numbers of calls at each test step and the proportions of calls of different types varied strongly between test steps (Table 1 and Fig. 4). The numbers of growls were too small to allow inclusion of this call type into analysis. The χ2 test showed that proportions of calls of different types differed significantly between steps 1 and 2 (χ2=53.2, df=3, p<0.001), steps 2 and 3 (χ2=246.2, df=3, p<0.001), steps 3 and 4 (χ2=401.6, df=3, p<0.001), and steps 4 and 5 (χ2=12.1, df=3, p<0.01). Fisher exact post hoc test showed that the proportion of coughs increased significantly between steps 1 and 2 and between steps 2 and 3 and decreased significantly between steps 3 and 4 and between steps 4 and 5 (Fig. 4). The proportion of snorts decreased significantly between steps 1 and 2 and between steps 2 and 3 and increased significantly between steps 3 and 4. The proportion of moos decreased significantly between steps 2 and 3 and increased significantly between steps 3 and 4. The proportion of whines increased significantly between steps 3 and 4 (Fig. 4).
Fig. 4.
Proportions of calls of different types for five successive steps of a human approach test applied to farmed silver foxes and comparison between adjacent steps with Fisher exact test: ***p<0.001, **p<0.01
GLM for repeated measures revealed significant influence of the test step on the overall calling rate (F4,96=30.55, p<0.001) and on the calling rates of coughs (F4,96=21.36, p<0.001), snorts (F4,96=7.23, p<0.001), whines (F4,96=3.83, p=0.006), and moos (F4,96=4.01, p=0.005). The number of foxes, producing growls, was too small to be included into analysis (Table 1).
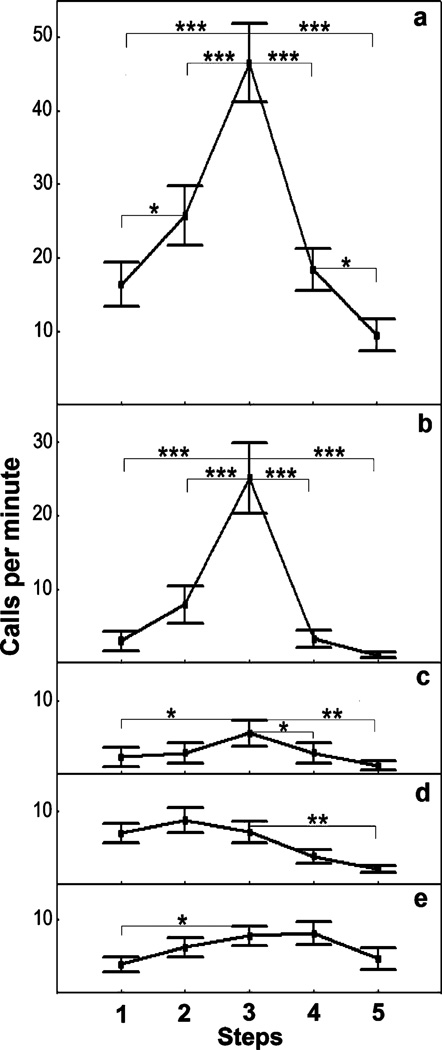
For the overall calling rate, Newman–Keuls post hoc test showed a significant increase between steps 1 and 3, between steps 1 and 2, and between steps 2 and 3 and significant decrease between steps 3 and 5, between steps 3 and 4, and between steps 4 and 5 (Fig. 5a). Similarly to the overall calling rate, the calling rates of coughs increased significantly between steps 1 and 3 and between steps 2 and 3 and decreased significantly between steps 3 and 4 and between steps 3 and 5 (Fig. 5b). The calling rates of whines increased significantly between steps 1 and 3 and decreased significantly between steps 3 and 4 and between steps 3 and 5 (Fig. 5c). The calling rates of snorts decreased significantly between steps 3 and 5 (Fig. 5d), and the calling rates of moos increased significantly between steps 1 and 3 (Fig. 5e).
Fig. 5.
Values (mean ± SE) for the overall calling rate (a) and calling rates of coughs (b), whines (c), snorts (d), and moos (e) for five successive steps of a human approach test applied to farmed silver foxes. Newman–Keuls post hoc test: ***p<0.001, **p<0.01, *p<0.05
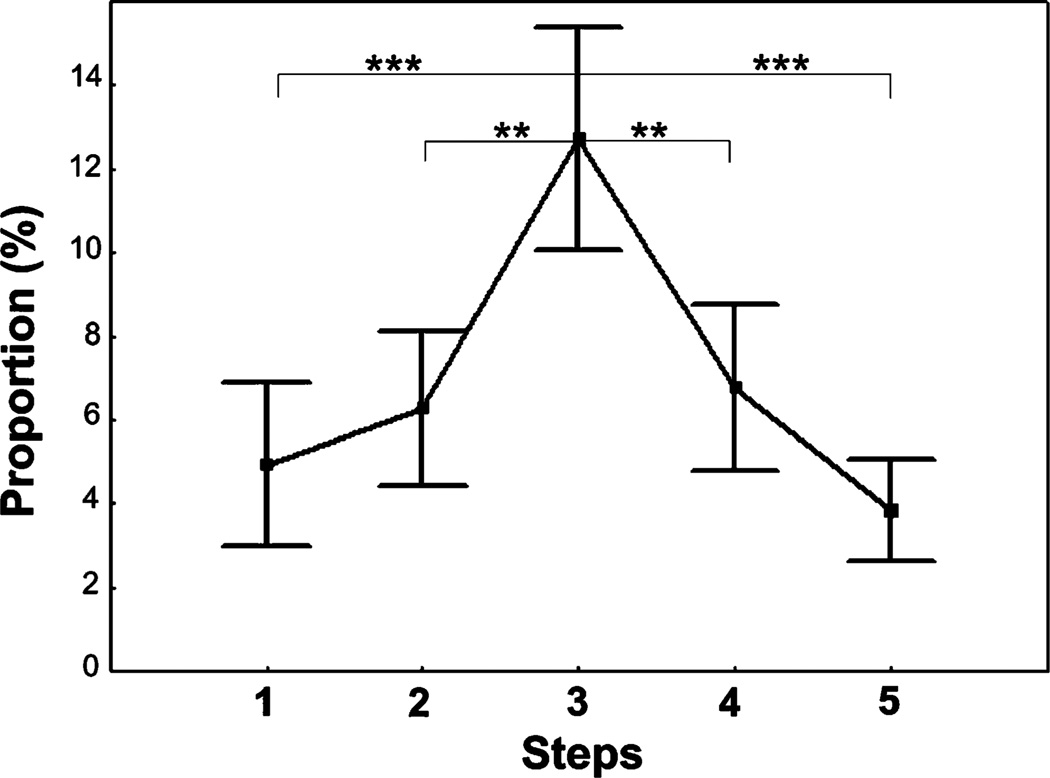
The trend of the proportion of time spent vocalizing matched those of the overall calling rate, increasing between steps 1 and 3 and decreasing between steps 3 and 5 (Fig. 6). GLM for repeated measures revealed a significant influence of the test step on the proportion of time spent vocalizing (F4,96=6.49, p<0.001), and New-man–Keuls post hoc test showed a significant increase in time spent vocalizing between steps 1 and 3 and between the steps 2 and 3 and a significant decrease between steps 3 and 4 and between steps 3 and 5 (Fig. 6).
Fig. 6.
Values (mean ± SE) for the proportion of time spent vocalizing for five successive steps of a human approach test applied to farmed silver foxes. Newman–Keuls post hoc test: ***p<0.001, **p<0.01
The values of acoustic variables measured within whines, moos, coughs, and snorts did not show strong shifts with test step (Table 2). The values of duration did not demonstrate any significant shift with test step for any call type. The maximum amplitude frequency increased significantly between steps 1 and 3 in coughs and snorts (Newman–Keuls post hoc test, p=0.008 and p=0.036, respectively). Lower, medium, and upper quartiles for whines and only lower quartile of coughs increased significantly between steps 1 and 3 and decreased significantly between steps 3 and 5 (Newman–Keuls post hoc test, p<0.05 for all comparisons). Only in whines did the values of entropy increase significantly between steps 2 and 3 and decreased significantly between steps 3 and 5 (Newman–Keuls post hoc test, p=0.028 and p=0.002, respectively; Table 2).
Table 2.
Values (mean ± SE) of the duration (s), maximum amplitude frequency (fpeak, kHz), lower (q25), medium (q50), and upper (q75) quartiles (kHz) and entropy for whines, moos, coughs, and snorts at each step of a human approach test applied to farmed silver foxes and GLMM results for comparison between the successive steps of the test
| Call variable | Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | ANOVA results |
|---|---|---|---|---|---|---|
| Whine | ||||||
| Duration | 0.74±0.07 | 0.62±0.12 | 0.53±0.12 | 0.73±0.14 | 0.53±0.10 | F4,24=1.19, p=0.34 |
| fpeak | 0.43±0.05 | 0.40±0.05 | 0.53±0.07 | 0.39±0.04 | 0.46±0.08 | F4,24=2.50, p=0.07 |
| q25 | 0.40±0.03 | 0.40±0.04 | 0.53±0.04 | 0.38±0.03 | 0.42±0.06 | F4,24=3.10, p=0.03 |
| q50 | 0.63±0.06 | 0.63±0.08 | 0.85±0.09 | 0.58±0.06 | 0.61±0.11 | F4,24=3.42, p=0.02 |
| q75 | 1.12±0.12 | 1.19±0.18 | 1.43±0.16 | 1.01±0.09 | 0.96±0.16 | F4,24=3.53, p=0.02 |
| Entropy | 0.28±0.02 | 0.28±0.02 | 0.33±0.03 | 0.27±0.02 | 0.25±0.02 | F4,24=2.78, p=0.05 |
| Moo | ||||||
| Duration | 0.17±0.03 | 0.20±0.04 | 0.23±0.07 | 0.29±0.05 | 0.27±0.05 | F4,75=2.40, p=0.06 |
| fpeak | 0.27±0.01 | 0.26±0.01 | 0.30±0.01 | 0.29±0.02 | 0.26±0.02 | F4,75=2.46, p=0.06 |
| q25 | 0.30±0.01 | 0.30±0.01 | 0.32±0.01 | 0.30±0.01 | 0.30±0.01 | F4,75=1.80, p=0.14 |
| q50 | 0.49±0.02 | 0.49±0.02 | 0.52±0.03 | 0.49±0.03 | 0.47±0.02 | F4,75=1.40, p=0.24 |
| q75 | 0.98±0.06 | 0.97±0.06 | 0.93±0.05 | 0.96±0.08 | 0.91±0.06 | F4,75=0.12, p=0.98 |
| Entropy | 0.28±0.01 | 0.28±0.01 | 0.26±0.01 | 0.26±0.01 | 0.27±0.01 | F4,75=0.86, p=0.49 |
| Cough | ||||||
| Duration | 0.07±0.01 | 0.07±0.01 | 0.07±0.01 | 0.07±0.01 | 0.07±0.01 | F4,57=0.20, p=0.94 |
| fpeak | 0.70±0.10 | 1.23±0.12 | 1.28±0.08 | 1.18±0.16 | 0.94±0.12 | F4,57=4.10, p=0.005 |
| q25 | 0.75±0.04 | 0.93±0.06 | 0.98±0.05 | 0.93±0.10 | 0.72±0.04 | F4,57=4.76, p=0.002 |
| q50 | 1.53±0.11 | 1.67±0.10 | 1.74±0.08 | 1.71±0.14 | 1.44±0.10 | F4,57=2.51, p=0.06 |
| q75 | 2.74±0.20 | 2.87±0.13 | 2.85±0.12 | 2.77±0.24 | 2.62±0.27 | F4,57=1.14, p=0.35 |
| Entropy | 0.53±0.02 | 0.54±0.01 | 0.54±0.01 | 0.53±0.03 | 0.51±0.02 | F4,57=1.44, p=0.23 |
| Snort | ||||||
| Duration | 0.06±0.01 | 0.05±0.01 | 0.06±0.01 | 0.05±0.01 | 0.06±0.01 | F4,82 = 1.15, p=0.34 |
| fpeak | 0.25±0.01 | 0.28±0.01 | 0.33±0.02 | 0.30±0.02 | 0.27±0.02 | F4,82 = 2.56, p=0.04 |
| q25 | 0.40±0.02 | 0.37±0.01 | 0.40±0.02 | 0.40±0.03 | 0.38±0.03 | F4,82 = 0.47, p=0.76 |
| q50 | 0.95±0.08 | 0.75±0.05 | 0.80±0.07 | 0.78±0.10 | 0.80±0.11 | F4,82 = 1.20, p=0.32 |
| q75 | 2.08±0.17 | 1.66±0.13 | 1.74±0.23 | 1.57±0.18 | 1.67±0.21 | F4,82 = 1.72, p=0.15 |
| Entropy | 0.41±0.02 | 0.37±0.01 | 0.36±0.02 | 0.36±0.02 | 0.36±0.02 | F4,82 = 2.00, p=0.10 |
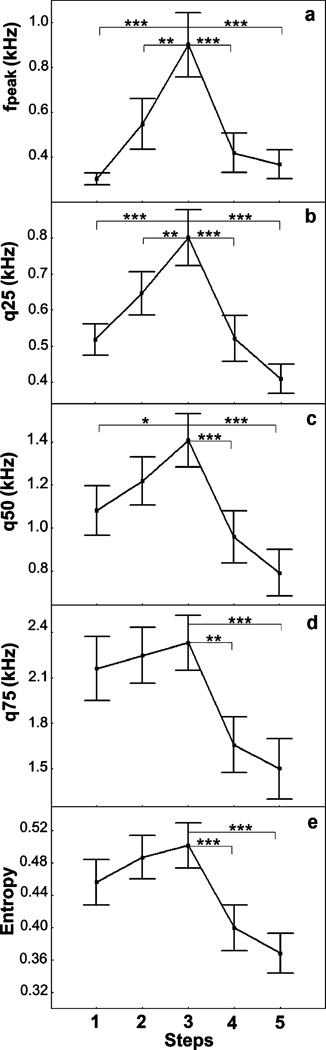
For joint calls, GLM for repeated measures revealed a significant influence of test step on measures of five acoustic variables: the maximum amplitude frequency (F4,96=8.26, p<0.001), lower quartile (F4,96=13.31, p<0.001), medium quartile (F4,96=8.79, p<0.001), upper quartile (F4,96=8.42, p<0.001), and entropy (F4,96=10.67, p<0.001). For all the five measured variables, values increased between steps 1 and 3 and decreased between steps 3 and 5 (Fig. 7). According to Newman–Keuls post hoc test, the maximum amplitude frequency and the lower quartile increased significantly between steps 1 and 3 and between steps 2 and 3 and decreased significantly between steps 3 and 4 and between steps 3 and 5 (Fig. 7a, b). The medium quartile increased significantly between steps 1 and 3 and decreased significantly between steps 3 and 4 and between steps 3 and 5 (Fig. 7c). The upper quartile and the entropy decreased significantly between steps 3 and 4 and between steps 3 and 5 (Fig. 7d, e).
Fig. 7.
Values (mean ± SE) of the maximum amplitude frequency (a), lower (b), medium (c), and upper (d) quartiles, and entropy (e) for joint calls for five successive steps of a human approach test applied to farmed silver foxes. Newman–Keuls post hoc test: ***p<0.001, **p<0.01, *p<0.05
Discussion
We found that farmed silver foxes adjusted their vocal responses according to changes of animal–human distance between successive steps of the human approach test. Changes in discomfort related to the human moving toward and from the animals resulted in shifts in the overall calling rate, calling rate of coughs and whines, proportion of time spent vocalizing, characteristics of joint calls, and proportions of different call types. The vocal traits of joint calls were more sensitive to gradations of discomfort compared to those measured within call types.
Proportions of different call types encoded the changes in a negative emotional arousal of study foxes much better than the changes in values of acoustic variables themselves. It could be due to the foxes using the calls of different types whose structures were very similar within type and very distinctive between types (Gogoleva et al. 2008). Only whines were very variable within type (Gogoleva et al. 2009). Similar findings have been reported for sows: proportions of different call types reflected better the gradations of discomfort compared to shifts in vocal traits (Schrader and Todt 1998; Weary et al. 1998; Taylor and Weary 2000; Taylor et al. 2001). Inconsistent with farmed silver foxes and sows, in other mammals, the shifts in values of acoustic variables reflect gradations of discomfort better compared to proportions of different call types (e.g., Schrader and Todt 1993; Watts and Stookey 1999; Rendall 2003; Monticelli et al. 2004; Pongrácz et al. 2005; Schehka et al. 2007). In this study with farmed silver foxes, the shifts in values of acoustic variables were remarkably noticeable mainly not within call types but in joint calls. The joint calls, enveloping all calls produced at a given test step, allowed us to take into account the acoustic characteristics of all calls independently on their structure, tonal or noisy. The joint calls reflected better the shifts in acoustic characteristics with changes in emotional arousal of animals than the acoustic characteristics measured within call types because they allowed smoothing the abrupt changes in acoustic values between calls of different types.
The proportion of time spent vocalizing, enveloping durations of all calls produced at a test step divided by duration of the test step, allowed us to estimate directly the time which an animal devoted to vocal activity. Potentially, an animal can increase its vocal activity with increasing a duration of its calls (Schrader and Todt 1993; Volodin et al. 1994; Weary and Frazer 1995b; Manser 2001; Rendall 2003), or with shorting inter-call intervals while retaining the duration of calls unchanged (Weary and Frazer 1995b; Blumstein and Armitage 1997; Rendall 2003; Pongrácz et al. 2005; Schehka et al. 2007). In both cases, it results in the increased proportion of time spent vocalizing. Thus, the proportion of time spent vocalizing may represent an integral characteristic of changes enveloping a few temporal variables (call durations, inter-call intervals, and calling rate) according to the changes in discomfort-related emotional arousal.
Consistent with our data for farmed silver foxes, the increased values of maximum amplitude frequency and quartiles (reflecting the shift of sound energy toward higher frequencies of call spectra) with the increase in the level of negative emotional arousal were reported for many mammalian species: common marmosets Callithrix j. jacchus (Newman and Goedeking 1992; Schrader and Todt 1993), great gerbils Rhombomys opimus (Volodin et al. 1994), barbary macaques Macaca sylvanus (Fischer et al. 1995), domestic sows (Weary and Frazer 1995b; Weary et al. 1998; Taylor and Weary 2000), common squirrel monkeys Saimiri sciureus (Fichtel et al. 2001), domestic dogs Canis familiaris (Pongrácz et al. 2005, 2006), and tree shrews Tupaia belangeri (Schehka et al. 2007). This is an advantage of power variables to be measurable both in tonal and in noisy calls in any animal species. We did not measure here the fundamental frequency values because the study foxes produced the tonal whines, moos, and growls where the fundamental frequency could be measured alongside the noisy snorts and coughs, lacking the fundamental frequency. However, it has been reported for many other mammals that the values of the fundamental frequency increased with increased discomfort, e.g., for common marmosets (Newman and Goedeking 1992; Schrader and Todt 1993), tree shrews (Kirchhof et al. 2001; Schehka et al. 2007), baboons Papio hamadrayas ursinus (Rendall 2003), guinea pig Cavia porcellus (Monticelli et al. 2004), and domestic cattle (Watts and Stookey 1999). The increase in values of the fundamental frequency can be considered, however, as particular case of the energy shift toward higher frequencies because in cases where most of the energy is confined within a fundamental frequency, its modulation results in the shift of energy toward higher frequencies. Thus, the shift of acoustic energy toward higher frequencies may represent an integral characteristic of changes enveloping a few frequency variables (frequency of maximum amplitude and fundamental frequency) according to the changes in discomfort-related emotional arousal.
The degree of call noisiness (estimated as entropy measures in this study of farmed silver foxes) is rather rarely applied for the estimation of discomfort levels in mammals. Consistent with the results of our study, the higher levels of noisiness with higher degrees of discomfort were reported for domestic dogs (Riede et al. 2001), common squirrel monkeys (Fichtel et al. 2001), and bonnet macaques Macaca radiata (Coss et al. 2007). On the other side, the noisiness of dog barks did not differ between aggressive and friendly situations (Pongrácz et al. 2005).
Our findings suggest that vocal responses of farmed silver foxes provide reliable indicators of their short-term welfare, similar to the vocal responses of domestic sows and cattle (Weary and Frazer 1995a; Watts and Stookey 2000; Marchant et al. 2001; Manteuffel et al. 2004). While many behavior indicators have been developed for the estimation of long-term welfare problems (stereotypes, enhanced aggressiveness, abnormal behavior, self-damaging), for the estimation of short-term welfare problems, mostly physiological indicators (e.g., levels of stress hormones) are available (Broom and Johnson 1993). The proportion of time spent vocalizing and the shifts of energy toward higher frequencies represent reliable indicators of short-term welfare problems. These variables may be estimated using non-expensive sound recording equipment and free-available software for sound analysis and demand only minimal staff training. These two variables may be considered as good candidates for use as key parameters in advanced automatic systems for welfare monitoring in foxes and other farm animals, similar to sows and cows (Schön et al. 2001, 2004; Jahns 2008; Moura et al. 2008). Vocal responses of animals can be used as good indicators of short-term welfare problems in situations of human approach, handling, transportation, and medical procedures, reflecting current changes in the degrees of discomfort experienced by the animals.
Acknowledgments
We thank the staff of the experimental fur farm of the Institute of Cytology and Genetics RAS, Novosibirsk, Russia for help and support, Dr. Vladimir Lebedev, Nina Vasilieva, and Dr. Andrey Babitsky for consulting in statistics, and Dr. Irina Oskina for consulting in hormonal responses to handling in foxes. Also, we thank two anonymous referees for their constructive and inspiring comments. During our work, we adhered to the Ethical Treatment of Animals in Applied Animal Behaviour Research (Sherwin et al. 2003) and to the laws of Russian Federation, the country where the research was conducted. PHS Approved Animal Welfare Assurance Numbers for Lomonosov Moscow State University is A5751-01 and for Institute of Cytology and Genetics is A5761-01. This study was supported by the Russian Foundation for Basic Research grant 09-04-00416 (for S.S.G, I.A.V and E.V.V), by National Institutes of Health grants R03 TW008098-01 and R01 MH077811, and the Programs of Basic Research of the RAS Presidium “Biodiversity and gene pool dynamics” and “Molecular and Cell Biology” (for A.V.K and L.N.T).
Contributor Information
Svetlana S. Gogoleva, Department of Vertebrate Zoology, Faculty of Biology, Lomonosov Moscow State University, Vorobievy Gory, Moscow 119991, Russia
Elena V. Volodina, Scientific Research Department, Moscow Zoo, B. Gruzinskaya, 1, Moscow 123242, Russia
Ilya A. Volodin, Email: volodinsvoc@gmail.com, Department of Vertebrate Zoology, Faculty of Biology, Lomonosov Moscow State University, Vorobievy Gory, Moscow 119991, Russia; Scientific Research Department, Moscow Zoo, B. Gruzinskaya, 1, Moscow 123242, Russia.
Anastasia V. Kharlamova, Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences, Pr. Lavrentjeva, 10, Novosibirsk 630090, Russia
Lyudmila N. Trut, Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences, Pr. Lavrentjeva, 10, Novosibirsk 630090, Russia
References
- Ahola L, Mononen J. Family break-up in farmed silver foxes (Vulpes vulpes) housed in enlarged cage systems as families. Acta Ethologica. 2002;4:125–127. [Google Scholar]
- Bachorowski J-A, Smoski MJ, Owren MJ. The acoustic features of human laughter. J Acoust Soc Am. 2001;110(3):1581–1597. doi: 10.1121/1.1391244. [DOI] [PubMed] [Google Scholar]
- Bakken M. The effect of an improved man–animal relationship on sex-ratio in litters and on growth and behaviour in cubs among farmed silver foxes (Vulpes vulpes) Appl Anim Behav Sci. 1998;56:309–317. [Google Scholar]
- Bakken M, Moe RO, Smith AJ, Selle G-ME. Effects of environmental stressors on deep body temperature and activity levels in silver fox vixens (Vulpes vulpes) Appl Anim Behav Sci. 1999;64:141–151. [Google Scholar]
- Blumstein DT, Armitage KB. Alarm calling in yellow-bellied marmots: I. The meaning of situationally specific calls. Anim Behav. 1997;53:143–171. [Google Scholar]
- Blumstein DT, Patton ML, Saltzman W. Faecal glucocorticoid metabolites and alarm calling in free-living yellow-bellied marmots. Biol Lett. 2006;2:29–32. doi: 10.1098/rsbl.2005.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braastad BO. Abnormal behaviour in farmed silver fox vixens (Vulpes vulpes L.): tail-biting and infanticide. Appl Anim Behav Sci. 1987;17:376–377. [Google Scholar]
- Braastad BO. Behaviour of silver foxes in traditional breeding boxes and in boxes with an entrance tunnel. Anim Welf. 1996;5:155–166. [Google Scholar]
- Broom DM, Johnson KG. Stress and animal welfare. London: Chapman & Hall; 1993. [Google Scholar]
- Coss RG, McCowan B, Ramakrishnan U. Threat-related acoustical differences in alarm calls by wild bonnet macaques (Macaca radiata) elicited by python and leopard models. Ethology. 2007;113:352–367. [Google Scholar]
- Fichtel C, Hammerschmidt K, Jürgens U. On the vocal expression of emotion, a multi-parametric analysis of different states of aversion in the squirell monkey. Behaviour. 2001;97:97–116. [Google Scholar]
- Fischer J, Hammerschmidt K, Todt D. Factors affecting acoustic variation in Barbary macaque (Macaca sylvanus) disturbance calls. Ethology. 1995;101:51–66. [Google Scholar]
- Gogoleva SS, Volodin IA, Volodina EV, Kharlamova AV, Trut LN. Kind granddaughters of angry grandmothers: the effect of domestication on vocalization in cross-bred silver foxes. Behav Processes. 2009;81:369–375. doi: 10.1016/j.beproc.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogoleva SS, Volodin IA, Volodina EV, Trut LN. To bark or not to bark: Vocalization in red foxes selected for tameness or aggressiveness toward humans. Bioacoustics. 2008;18:99–132. [Google Scholar]
- Grandin T. The feasibility of using vocalization scoring as an indicator of poor welfare during cattle slaughter. Appl Anim Behav Sci. 1998;56:121–128. [Google Scholar]
- Jahns G. Call recognition to identify cow conditions—a call-recogniser translating calls to text. Comput Electron Agric. 2008;62:54–58. [Google Scholar]
- Jürgens U. Reinforcing concomitants of electrically elicited vocalizations. Exp Brain Res. 1976a;26:203–214. doi: 10.1007/BF00238284. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Positive and negative reinforcing properties of electrically elicitable vocalizations in the squirrel monkey. In: Wauquier A, Rolls ET, editors. Brain stimulation reward. Amsterdam: Elsevier; 1976b. pp. 397–402. [Google Scholar]
- Jürgens U. Vocalization as an emotional indicator: a neuroethological study in the squirrel monkey. Behaviour. 1979;69:88–117. doi: 10.1163/156853979x00412. [DOI] [PubMed] [Google Scholar]
- Jürgens U. The neural control of vocalization in mammals: a review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Jürgens U, Ploog D. Cerebral representations of vocalization in squirrel monkey. Exp Brain Res. 1970;10:532–554. doi: 10.1007/BF00234269. [DOI] [PubMed] [Google Scholar]
- Jürgens U, Ploog D. On the neural control of mammalian vocalization. Trends Neurosci. 1981;4:135–137. [Google Scholar]
- Kirchhof J, Hammerschmidt K, Fuchs E. Aggression and dominance in tree shrews (Tupaia belangeri). Agonistic behaviour is reflected in vocal patterns. In: Martinez M, editor. Prevention and control of aggression and the impact on its victims. New York: Kluwer; 2001. pp. 409–414. [Google Scholar]
- Kukekova AV, Trut LN, Chase K, Shepeleva DV, Vladimirova AV, Kharlamova AV, Oskina IN, Stepika A, Klebanov S, Erb HN, Acland GM. Measurement of segregating behaviors in experimental silver fox pedigrees. Behav Genet. 2008;38:185–194. doi: 10.1007/s10519-007-9180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay DC, Friend TH, Bowers CL, Grissom KK, Jenkins OC. A comparative physiological and behavioral study of freeze and hot-iron branding using dairy cows. J Anim Sci. 1992a;70:1121–1125. doi: 10.2527/1992.7041121x. [DOI] [PubMed] [Google Scholar]
- Lay DC, Friend TH, Randell RD, Bowers CL, Grissom KK, Jenkins OC. Behavioral and physiological effects of freeze or hot-iron branding on crossbred cattle. J Anim Sci. 1992b;70:330–336. doi: 10.2527/1992.702330x. [DOI] [PubMed] [Google Scholar]
- Manser MB. The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc R Soc B. 2001;268:2315–2324. doi: 10.1098/rspb.2001.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteuffel G, Puppe B, Schön PC. Vocalization of farm animals as a measure of welfare. Appl Anim Behav Sci. 2004;88:163–182. [Google Scholar]
- Marchant JN, Whittaker X, Broom DM. Vocalisations of the adult female domestic pig during a standard human approach test and their relationships with behavioural and heart rate measures. Appl Anim Behav Sci. 2001;72:23–39. doi: 10.1016/s0168-1591(00)00190-8. [DOI] [PubMed] [Google Scholar]
- Moe RO, Bakken M. Effects of handling and physical restraint on rectal temperature, cortisol, glucose and leucocyte counts in the silver fox (Vulpes vulpes) Acta Vet Scand. 1997;38:29–39. doi: 10.1186/BF03548505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe RO, Bakken M. Anxiolytic drugs inhibit hyperthermia induced by handling in farmed silver foxes (Vulpes vulpes) Anim Welf. 1998;7:97–100. [Google Scholar]
- Moe RO, Bakken M, Kittilsen S, Kingsley-Smith H, Spruijt BM. A note on reward-related behaviour and emotional expressions in farmed silver foxes (Vulpes vulpes)—basis for a novel tool to study animal welfare. Appl Anim Behav Sci. 2006;101:362–368. [Google Scholar]
- Monticelli PF, Tokumaru RS, Ades C. Isolation induced changes in guinea pig Cavia porcellus pup distress whistles. An Brazil Acad Sci. 2004;76:368–372. doi: 10.1590/s0001-37652004000200027. [DOI] [PubMed] [Google Scholar]
- Morton ES. On the occurrence and significance of motivation–structural rules in some bird and mammal sounds. Am Nat. 1977;111(981):855–869. [Google Scholar]
- Moura DJ, Silva WT, Naas IA, Tolón YA, Lima KAO, Vale MM. Real time computer stress monitoring of piglets using vocalization analysis. Comput Electron Agr. 2008;64:11–18. [Google Scholar]
- Nakagawa T. Conversion of vortex energy into acoustic energy. Naturwissenschaften. 1987;74:338–339. [Google Scholar]
- Newman JD, Goedeking P. Noncategorical vocal communication in primates: the example of common marmoset phee calls. In: Papousek H, Jürgens U, Papousek M, editors. Nonverbal vocal communication. Cambridge: Cambridge University Press; 1992. pp. 87–101. [Google Scholar]
- Nimon AJ, Broom DM. The welfare of farmed foxes Vulpes vulpes and Alopex lagopus in relation to housing and management: a review. Anim Welf. 2001;10:223–248. [Google Scholar]
- Oskina IN, Herbeck YE, Shikhevich SG, Plyusnina IZ, Gulevich RG. Alterations in the hypothalamus–pituitary–adrenal and immune systems during selection of animals for tame behavior. VOGiS Herald. 2008;12:39–49. [Google Scholar]
- Pedersen V. Early experience with the farm environment and later effects on behaviour in silver (Vulpes vulpes) and blue (Alopex lagopus) foxes. Behav Processes. 1991;25:163–169. doi: 10.1016/0376-6357(91)90018-U. [DOI] [PubMed] [Google Scholar]
- Pedersen V. Effects of different post-weaning handling procedures on the later behaviour of silver foxes. Appl Anim Behav Sci. 1993;37:239–250. [Google Scholar]
- Pedersen V. Long-term effects of different handling procedures on behavioural, physiological, and production-related parameters in silver foxes. Appl Anim Behav Sci. 1994;40:285–296. [Google Scholar]
- Pedersen V, Jeppesen LL. Effect of early handling on later behaviour and stress responses in the silver fox (Vulpes vulpes) Appl Anim Behav Sci. 1990;26:383–393. [Google Scholar]
- Pongrácz P, Miklosi A, Molnar C, Csanyi V. Human listeners are able to classify dog (Canis familiaris) barks recorded in different situations. J Comp Psychol. 2005;119:136–144. doi: 10.1037/0735-7036.119.2.136. [DOI] [PubMed] [Google Scholar]
- Pongrácz P, Molnar C, Miklosi A. Acoustic parameters of dog barks carry emotional information for humans. Appl Anim Behav Sci. 2006;100:228–240. [Google Scholar]
- Rekilä T, Harri M, Ahola L. Validation of the feeding test as an index of fear in farmed blue (Alopex lagopus) and silver foxes (Vulpes vulpes) Physiol Behav. 1997;62:805–810. doi: 10.1016/s0031-9384(97)00241-2. [DOI] [PubMed] [Google Scholar]
- Rendall D. Acoustic correlates of caller identity and affect intensity in the vowel-like grunt vocalizations of baboons. J Acoust Soc Am. 2003;113:3390–3402. doi: 10.1121/1.1568942. [DOI] [PubMed] [Google Scholar]
- Riede T, Herzel H, Hammerschmidt K, Brunnberg L, Tembrock G. The harmonic-to-noise ratio applied to dog barks. J Acoust Soc Am. 2001;110:2191–2197. doi: 10.1121/1.1398052. [DOI] [PubMed] [Google Scholar]
- Schehka S, Esser K-H, Zimmermann E. Acoustical expression of arousal in conflict situations in tree shrews (Tupaia belangeri) J Comp Physiol A. 2007;93:845–852. doi: 10.1007/s00359-007-0236-8. [DOI] [PubMed] [Google Scholar]
- Schrader L, Todt D. Contact call parameters covary with social context in common marmosets, Callithrix j. jacchus. Anim Behav. 1993;46:1026–1028. [Google Scholar]
- Schrader L, Todt D. Vocal quality is correlated with levels of stress hormones in domestic pigs. Ethology. 1998;104:859–876. [Google Scholar]
- Schön PC, Puppe B, Manteuffel G. Linear prediction coding analysis and self-organizing feature map as tools to classify stress calls of domestic pigs (Sus scrofa) J Acoust Soc Am. 2001;110:1425–1431. doi: 10.1121/1.1388003. [DOI] [PubMed] [Google Scholar]
- Schön PC, Puppe B, Manteuffel G. Automated recording of stress vocalisations as a tool to document impaired welfare in pigs. Anim Welf. 2004;13:105–110. [Google Scholar]
- Sherwin CM, Christiansen SB, Duncan IJ, Erhard H, Lay D, Mench J, O’Connor C, Petherick C. Guidelines for the ethical use of animals in applied ethology studies. Appl Anim Behav Sci. 2003;81:291–305. [Google Scholar]
- Specht R. Advances in Bioacoustics 2. Dissertationes Classis IV: Historia Naturalis. vol 37. Ljubljana: Academy of Sciences and Arts; 2006. Software tools for automatically detecting, measuring and classifying animal sounds; pp. 177–184. [Google Scholar]
- Taylor AA, Weary DM. Vocal responses of piglets to castration: identifying procedural sources of pain. Appl Anim Behav Sci. 2000;70:17–26. doi: 10.1016/s0168-1591(00)00143-x. [DOI] [PubMed] [Google Scholar]
- Taylor AA, Weary DM, Lessard M, Braithwaite L. Behavioural responses of piglets to castration: the effect of piglet age. Appl Anim Behav Sci. 2001;73:35–43. doi: 10.1016/s0168-1591(01)00123-x. [DOI] [PubMed] [Google Scholar]
- The Welfare of Animals Kept for Fur Production. Report of the Scientific Committee on Animal Health and Animal Welfare. EC Health and Consumer Protection Directorate-General. 2001 http://ec.europa.eu/food/animal/welfare/international/out67_en.pdf. [Google Scholar]
- Titze IR. Principles of voice production. Prentice Hall: Englewood Cliffs; 1994. [Google Scholar]
- Trut LN. Early canid domestication: farm-fox experiment. Amer Sci. 1999;87:160–169. [Google Scholar]
- Volodin IA, Goltsman ME, Borisova NG. Situational changes in vocalization of great gerbils (Rhombomys opimus Licht) during defensive behavior. Dokl Biol Sci. 1994;334:65–68. [Google Scholar]
- Waiblinger S, Boivin X, Pedersen V, Tosi M-V, Janczak AM, Visser EK, Jones RB. Assessing the human–animal relationship in farmed species: a critical review. Appl Anim Behav Sci. 2006;101:185–242. [Google Scholar]
- Watts JM, Stookey JM. Effects of restraint and branding on rates and acoustic parameters of vocalization in beef cattle. Appl Anim Behav Sci. 1999;62:125–135. [Google Scholar]
- Watts JM, Stookey JM. Vocal behaviour in cattle: the animal’s commentary on its biological processes and welfare. Appl Anim Behav Sci. 2000;67:15–33. doi: 10.1016/s0168-1591(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Weary DM, Frazer D. Signaling need: costly signals and animal welfare assessment. Appl Anim Behav Sci. 1995a;44:159–169. [Google Scholar]
- Weary DM, Frazer D. Calling by domestic piglets: reliable signals of need? Anim Behav. 1995b;50:1047–1055. [Google Scholar]
- Weary DM, Braithwaite LA, Fraser D. Vocal response to pain in piglets. Appl Anim Behav Sci. 1998;56:161–172. [Google Scholar]
- Zahavi A. Reliability in communication systems and the evolution of altruism. In: Stonehouse B, Perrins C, editors. Evolutionary ecology. London: MacMillan; 1977. pp. 253–260. [Google Scholar]
- Zahavi A. The pattern of vocal signals and the information they convey. Behaviour. 1982;80:1–8. [Google Scholar]