Abstract
Objective
To evaluate and characterize the degree of blood pressure (BP) control in children on chronic dialysis and to identify significant predictors of hypertension and BP control in these patients.
Study design
Linear and logistic regression models were used to examine trends in BP and BP control in a cross-sectional sample of patients on chronic dialysis aged 1–21 years enrolled in the North American Pediatric Renal Trials and Collaborative Studies registry from 1992–2008.
Results
At 6 months after dialysis initiation, 67.9% of patients had uncontrolled or untreated hypertension, and 57.8% were prescribed antihypertensive medications. More recent year of dialysis initiation was associated with a higher use of antihypertensive medication and lower systolic BP and diastolic BP z scores (P < .001) measured over time from 6 months to 3 years post dialysis initiation. Other factors associated with higher BP included black race, glomerular disease, younger age, hemodialysis (systolic BP only), and antihypertensive use. There were significant differences in BP control by dialysis modality and disease etiology, with patients on hemodialysis or those with glomerular diseases having the highest percentage of uncontrolled hypertension.
Conclusions
Despite widespread antihypertensive use, many pediatric patients on dialysis are at risk for untreated or uncontrolled hypertension. Additional efforts are needed to improve management of hypertension in these children.
Despite decades of improvements in care for children with end-stage renal disease (ESRD), recent epidemiological studies have demonstrated a greatly increased risk of cardiovascular mortality as the patients survive into adulthood.1–3 The risk is sufficiently high as to place children with chronic kidney disease (CKD) in the highest American Heart Association cardiovascular risk category, on par with type 1 diabetes and familial hypercholesterolemia.4 Although the specific pathophysiology of cardiovascular disease in patients with CKD is not fully understood, multiple risk factors have been identified. Among these are factors unique to the physiologic milieu of ESRD, such as hyperphosphatemia and chronic inflammation, as well as more traditional risk factors, such as hypertension and left ventricular hypertrophy.
Hypertension is common among adults on dialysis, with a reported prevalence as high as 86%.5 Recent studies have demonstrated that hypertension is similarly common in children, with the majority of patients receiving pediatric hemodialysis (HD) meeting criteria for hypertension (51%–74%), even after 12 months of dialysis.6–8 The pediatric peritoneal dialysis (PD) population is less well-studied, with conflicting reports regarding rates of hypertension in patients on PD relative to patients receiving HD.8,9 Factors contributing to blood pressure (BP) control in the pediatric chronic dialysis population are not well understood, although past studies have reported race, etiology of renal disease, and age to be influential.6–9
The goals of this study were to evaluate and characterize the degree of BP control in children on chronic dialysis and to identify significant predictors of hypertension and BP control in these patients. We also conducted a subanalysis comparing antihypertensive medication use and BP control in periods before and after issuance of revised guidelines for treatment of childhood hypertension in 2004.10,11
Methods
We conducted a cross-sectional study using data from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry, a voluntary collaborative effort comprising 150 pediatric renal disease treatment centers in the United States, Canada, Mexico, and Costa Rica. As of January 1, 2009, there were 6791 patients receiving dialysis from 134 centers in the registry. All pediatric patients with CKD or ESRD are eligible, and written informed consent is obtained locally. Approval for participation is obtained from the institutional review board at each center.
Data in the NAPRTCS registry are collected at 30 days after dialysis initiation and at 6-month intervals thereafter. BP measurements are available from 1992–2008. This analysis includes all patients in the NAPRTCS registry from 1992–2008 who were older than 1 year at the start of chronic dialysis and who remained on dialysis at least 6 months. If a patient had been on dialysis, received a transplant, and then returned to dialysis over the course of the registry, data were only included from the first sequence of dialysis where the patient met study criteria.
The outcomes of interest were systolic BP (SBP) and diastolic BP (DBP) reported at 6 months after dialysis initiation. The 6-month time point was chosen to allow for adjustments in fluid status and medications after initiation of dialysis. A single BP value was recorded for each subject at each study time point. No information is available regarding the timing of BP measurement with respect to dialysis treatment or the method by which it was obtained. Because BP in children is dependent on age, body size (height), and sex, measurements were standardized to a z score using normative values to allow for comparisons.5 Based upon consensus recommendations,10,11 SBP and DBP were also categorized as “controlled” (BP ≤90th percentile for age, sex, and height) or “uncontrolled” (BP >90th percentile). This categorization was made regardless of antihypertensive medication use.
Exposure variables included age in years (grouped as 1, 2–5, 6–12, and ≥13), sex, race (black, nonblack), dialysis modality (PD/HD), grouped calendar year of dialysis initiation (1992–1995, 1996–1999, 2000–2003, 2004–2008), primary disease category (glomerular, structural, other), and antihypertensive use (yes/no). Etiology of ESRD is reported to NAPRTCS as 1 of 30 categories, which includes both “unknown” and “other.” For this analysis, these diagnoses were grouped into the three larger categories just listed. No information is provided in the registry regarding the number, type, or dose of antihypertensive medications—merely whether any have been prescribed for the patient or not. Participants were also categorized at 6 months post-dialysis initiation as normotensive (both SBP and DBP z score <90th percentile and not receiving antihypertensive medications), controlled hypertensive (both SBP and DBP z score <90th percentile and receiving antihypertensive medications), untreated hypertensive (either SBP or DBP z score >90th percentile and not receiving antihypertensive medications), or uncontrolled hypertensive (either SBP or DBP z score >90th percentile and receiving antihypertensive medications).
To examine and test predictors of BP and BP control, 2 regression-based methods were used. The first was a multivariate repeated-measures linear regression analysis to identify factors associated with SBP and DBP z scores. These measurements were collected from 6 months to 3 years post-dialysis initiation. An unstructured covariance matrix was used to model the repeated measures within subjects. The intraclass correlation was calculated using a compound symmetry covariance structure to evaluate the variation between patient BP measurements over time. Second, a logistic regression model was used to generate ORs for the same predictors of “controlled” and “uncontrolled” SBP and DBP at 6 months post-dialysis initiation. Last, a subanalysis was done evaluating whether there were any significant differences in either SBP or DBP or use of antihypertensives in the time periods immediately before (2002–2003) and after (2005–2008) the year 2004, when practice guidelines were revised in the Fourth Report and by The National Kidney Foundation Disease Outcomes Quality Initiative. A Kruskall-Wallis test was used for this portion of the analysis. All analyses were conducted using SAS System for Windows, v 8.2 or higher (SAS Institute, Cary, North Carolina).
Results
A total of 3447 patients were eligible for the main analysis, of whom 2264 (65.7%) were on PD and 1183 (34.3%) were on HD. This distribution is reflective of the overall dialysis population in the NAPRTCS database.12 Patient characteristics are displayed in Table I. The median follow-up time was 12 months and the mean ± SD was 16.7 ± 10.4 months.
Table I.
Patient characteristics
| All n = 3447 |
PD n = 2264 |
HD n = 1183 |
||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age at dialysis initiation (y) | ||||||
| 1 | 159 | 4.6 | 139 | 6.1 | 20 | 1.7 |
| 2–5 | 395 | 11.5 | 318 | 14.0 | 77 | 6.5 |
| 6–12 | 1171 | 34.0 | 799 | 35.3 | 372 | 31.4 |
| 13+ | 1722 | 50.0 | 1008 | 44.5 | 714 | 60.4 |
| Race | ||||||
| White | 1553 | 45.1 | 1138 | 50.3 | 415 | 35.1 |
| Black | 935 | 27.1 | 519 | 22.9 | 416 | 35.2 |
| Hispanic | 710 | 20.6 | 448 | 19.8 | 262 | 22.1 |
| Other | 249 | 7.2 | 159 | 7.0 | 90 | 7.6 |
| Male | 1832 | 53.1 | 1179 | 52.1 | 653 | 55.2 |
| Primary diagnosis | ||||||
| Structural | 926 | 26.9 | 607 | 26.8 | 319 | 27.0 |
| Glomerular | 1546 | 44.9 | 1008 | 44.5 | 538 | 45.5 |
| Other | 716 | 20.8 | 464 | 20.5 | 252 | 21.3 |
| Missing | 259 | 7.5 | 185 | 8.2 | 74 | 6.3 |
| +AH medication use 6 months after dialysis initiation | 1991 | 57.8 | 1245 | 55.0 | 746 | 63.1 |
| Year of dialysis initiation | ||||||
| 1992–1995 | 1045 | 30.3 | 720 | 68.9 | 325 | 31.1 |
| 1996–1999 | 995 | 28.9 | 683 | 68.6 | 312 | 31.4 |
| 2000–2003 | 727 | 21.1 | 456 | 62.7 | 271 | 37.3 |
| 2004–2008 | 680 | 19.7 | 405 | 59.6 | 275 | 40.4 |
AH, antihypertensive.
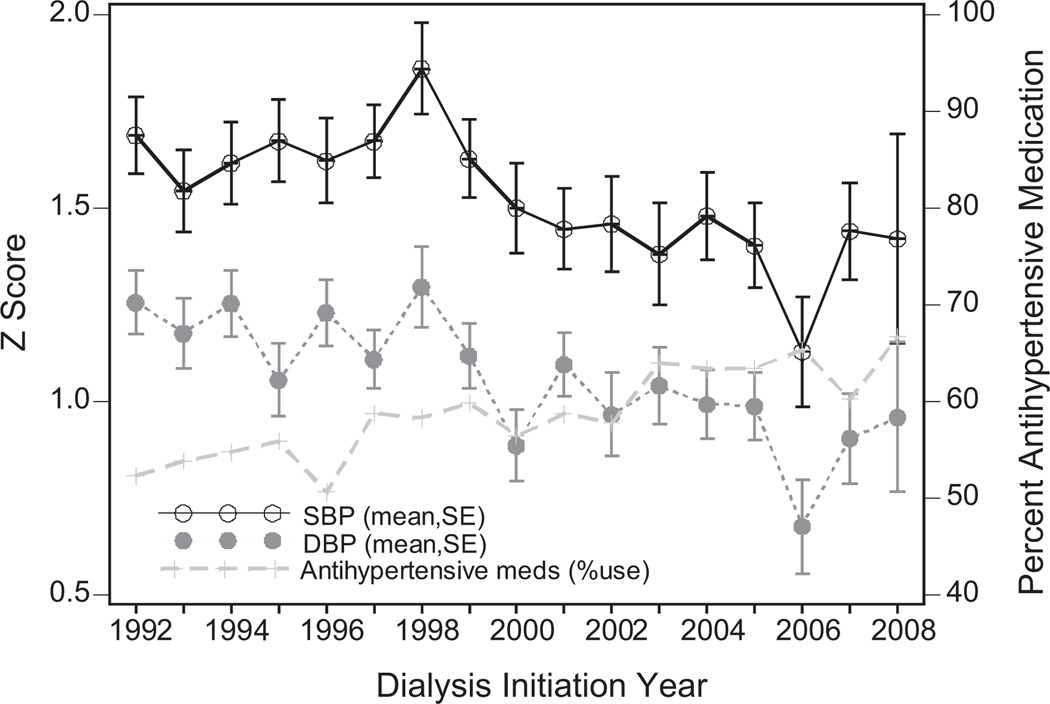
Trends in mean 6-month SBP z score, 6-month DBP z score, and proportion of patients prescribed antihypertensive medications at 6 months, by year of dialysis initiation were shown in Figure 1. Despite substantial variation, an increasing trend of antihypertensive use (52% in 1992 vs 67% in 2008) and decreased mean ± SE SBP z score (1.7 ± 0.1 in 1992 vs 1.4 ± 0.3 in 2008) and DBP z score (1.3 ± 0.1 in 1992 vs 1.0 ± 0.2 in 2008) were observed over the study period. These unadjusted trends were all statistically significant (P < .001 for all, unadjusted linear (BP z scores) and logistic (antihypertensive use) regression). Mean 6-month SBP and DBP z scores in the eras before and after 2004 were also compared. Despite a decrease in mean z scores for both SBP (1.37 vs 1.49, P = .43) and DBP (0.94 vs 1.04, P = .21) in the years after 2004 compared with those before, these differences were not significant.
Figure 1.
Trends in BP z scores and antihypertensive use 30 days after dialysis initiation by year of dialysis initiation, 1992–2008.
Results from the repeated measures linear regression model are shown in Table II. This analysis looks at all BPs from 6 months to 3 years post dialysis initiation and predicts the change in SBP or DBP z score for each factor, while adjusting for the others. Younger age predicted larger increases in SBP and DBP relative to those in the oldest age group (13+ years). Other significant predictors of higher SBP and DBP included black race, glomerular etiology of renal disease, and use of antihypertensive medications at the corresponding follow-up visit. Earlier years of dialysis initiation were predictive of a higher SBP and DBP compared with the reference of 2004–2008. Female sex was predictive of a higher DBP but there was no significant association between sex and SBP. With regard to dialysis modality, PD was predictive of a lower SBP but a higher DBP. BP also decreased during the follow-up period. In terms of magnitude of effect, age, year of dialysis initiation, and antihypertensive use predicted the largest changes in BP z scores. The intraclass correlation coefficient is 0.26 for SBP and 0.29 for DBP z scores.
Table II.
Predictors of SBP and DBP z scores during follow-up (6 months-3 years post dialysis initiation)
| SBP z score n = 3183 |
DBP z score n = 3174 |
|||
|---|---|---|---|---|
| β/Estimate (SE) |
P value |
β/Estimate (SE) |
P value |
|
| Intercept | .412 (.082) | <.0001 | −.040 (.069) | .5596 |
| Age (y) | ||||
| 1 | .406 (.107) | .0002 | .475 (.091) | <.0001 |
| 2–5 | .408 (.072) | <.0001 | .377 (.061) | <.0001 |
| 6–12 | .175 (.048) | .0003 | .217 (.041) | <.0001 |
| 13+ | ref | ref | ||
| Female | .065 (.043) | .14 | .147 (.037) | <.0001 |
| Black | .164 (.049) | .0008 | .109 (.041) | .008 |
| PD | −.148 (.046) | .001 | .093 (.039) | .02 |
| Year of dialysis initiation | ||||
| 1992–1995 | .289 (.063) | <.0001 | .217 (.053) | <.0001 |
| 1996–1999 | .333 (.064) | <.0001 | .234 (.054) | <.0001 |
| 2000–2003 | .163 (.068) | .0161 | .113 (.057) | .0492 |
| 2004–2008 | ref | ref | ||
| +AH | ||||
| at follow up | .625 (.044) | <.0001 | .433 (.037) | <.0001 |
| at day 30 | −.001 (.053) | .99 | −.038 (.044) | .39 |
| Day 30 BP z-score | .208 (.014) | <.0001 | .230 (.014) | <.0001 |
| Follow-up BP (6-months increments) | −.025 (.012) | .0407 | −.033 (.01) | .001 |
| Primary disease | ||||
| Glomerular | .162 (.053) | .002 | .184 (.045) | <.0001 |
| Structural | ref | ref | ||
| Other/unknown | .114 (.061) | .06 | .099 (.052) | .06 |
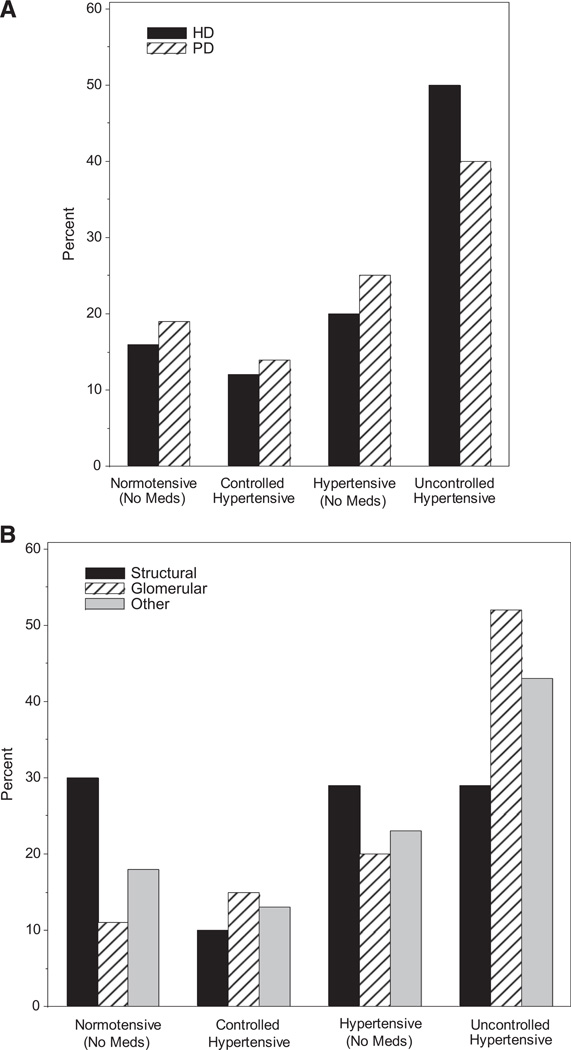
Rates of BP control and antihypertensive use at 6 months post dialysis initiation based on primary disease category and dialysis modality were compared (Figure 2; available at www.jpeds.com). Among patients with glomerular diseases, a smaller percentage are normotensive compared with those with structural diseases (12% vs 31%) and a larger percentage have uncontrolled hypertension (52% vs 29%, P < .001). A greater proportion of patients on HD had uncontrolled hypertension compared with those on PD (51% vs 41%, P < .001).
Figure 2.
Proportion of patients with controlled BP and on AHs by A, primary disease and B, dialysis modality.
Multivariate logistic regression was used as a complementary analysis to evaluate predictors of uncontrolled SBP and DBP (defined as BP >90th percentile, independent of antihypertensive use) at 6 months post-dialysis initiation (Table III). In comparison with patients aged 13 years and older, younger age (<6 years of age) was associated with a higher odds of uncontrolled DBP (OR, 1.47; 95% CI, 1.15–1.88). By contrast, the odds of uncontrolled SBP were lower in the lower age groups, but the findings were only significant for 6- to 12-year-olds. Black race was associated with increased odds of both uncontrolled SBP and DBP (SBP: OR, 1.32; 95% CI, 1.11–1.57; DBP: OR, 1.33; 95% CI, 1.12–1.58). Female sex was associated with increased odds of uncontrolled DBP (OR, 1.19; 95% CI, 1.02–1.38), but there was no significant relationship between sex and uncontrolled SBP. The findings for dialysis modality were similar to those above, with PD associated with a decreased odds of uncontrolled SBP (OR, 0.86; 95% CI, 0.72–1.01) and an increased odds of uncontrolled DBP (OR, 1.06; 95% CI, 0.91–1.25), but neither of these findings were significant. Both antihypertensive medication use at 6 months (SBP: OR, 1.94; 95% CI, 1.65–2.27; DBP: OR, 1.95; 95% CI, 1.66–2.29) and glomerular etiology of disease (SBP: OR, 1.26; 95% CI, 1.05–1.52; DBP: OR, 1.51; 95% CI, 1.26–1.82) were associated with increased odds of uncontrolled SBP and DBP. Again, there was a trend toward increased odds of uncontrolled BP in patients who initiated dialysis earlier in the study period. An interaction model exploring the relationship between dialysis modality and BP based on disease category or age found no significant differences (data not shown).
Table III.
Multivariate logistic regression analysis of characteristics associated with uncontrolled BP at 6 months after dialysis initiation
| SBP >90th percentile |
DBP >90th percentile |
+AH medication use at 6 months |
|
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| SBP at 30 days | 1.33 (1.26–1.40) | 1.37 (1.29–1.45) | |
| SBP at 6 months | 1.28 (1.18–1.40) | ||
| Age at initiation (y) | |||
| 1 | 0.87 (0.60–1.24) | 2.28 (1.57–3.30) | 0.56 (0.35–0.90) |
| 2–5 | 0.92 (0.71–1.18) | 1.47 (1.15–1.88) | 0.53 (0.39–0.73) |
| 6–12 | 0.78 (0.65–0.92) | 1.03 (0.87–1.22) | 1.00 (0.81–1.23) |
| ≥13 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Black race | 1.32 (1.11–1.57) | 1.33 (1.12–1.58) | 1.04 (0.84–1.29) |
| Female | 0.95 (0.82–1.11) | 1.19 (1.02–1.38) | 0.78 (0.65–0.95) |
| Primary disease | |||
| Glomerular | 1.26 (1.05–1.52) | 1.51 (1.26–1.82) | 1.62 (1.28–2.04) |
| Structural | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Other | 1.30 (1.05–1.62) | 1.39 (1.12–1.73) | 1.57 (1.20–2.06) |
| PD | 0.86 (0.72–1.01) | 1.06 (0.91–1.25) | 0.75 (0.61–0.92) |
| +AH medication at 6 months | 1.94 (1.65–2.27) | 1.95 (1.66–2.29) | |
| +AH medication at 30 days | 19.78 (16.10–24.30) | ||
| Year of dialysis initiation | |||
| 1992–1995 | 1.31 (1.06–1.64) | 1.51 (1.22–1.88) | 0.70 (0.53–0.92) |
| 1996–1999 | 1.47 (1.18–1.84) | 1.55 (1.25–1.93) | 0.79 (0.60–1.05) |
| 2000–2003 | 1.12 (0.89–1.42) | 1.06 (0.84–1.34) | 0.87 (0.65–1.17) |
| 2004–2008 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
Results from the model looking at predictors of antihypertensive medication use at 6 months after dialysis initiation are shown in Table III. Predictors associated with increased use of antihypertensive medication included older age at dialysis initiation, male sex, glomerular or other etiology of renal disease, and HD. Baseline variables of antihypertension medication use at 30 days, SBP z score at 6 months, and DBP z score at 6 months were related to antihypertensive medication use at 6 months. Compared with the era 2004–2008, there is a trend toward lower odds of antihypertensive use in earlier eras of dialysis initiation.
Discussion
This study confirms that hypertension remains a significant problem among children on dialysis, with fewer than one-half of the patients studied meeting current criteria for controlled SBP (<90th percentile) after being on dialysis for 6 months, despite the use of antihypertensive medications in more than one-half of the patients.10,11 Among the patients with an SBP >90th percentile, 34% were not prescribed antihypertensive medications. This figure is similar to that reported in a study on children with CKD (39%).13 Prior studies of pediatric patients on dialysis have demonstrated that hypertension is common, but this study also examined trends in BP control over time, particularly as they relate to the use of antihypertensive medication.6–8
The majority of pediatric patients receiving dialysis were prescribed at least one antihypertensive medication at the time of dialysis initiation, and this pattern changed little after 6 months of dialysis. In a prior NAPRTCS study by Mitsnefes et al, the largest changes in BP were seen in the first 6 months after dialysis initiation.7 The correlation of antihypertensive medication use with higher BP and poor BP control cannot answer the question as to whether these medications were or are underutilized in pediatric patients on dialysis or perhaps serve as a marker for a subset of patients in whom BP is more difficult to manage.
Although there are no pediatric studies linking uncontrolled hypertension with mortality, there is mounting evidence that hypertension is associated with multiple intermediate indicators of cardiovascular morbidity. The presence of left ventricular hypertrophy among children with hypertension has been well documented in patients on dialysis, as well as those with CKD, and renal transplants in otherwise healthy children.14–16 Other intermediate markers of cardiovascular morbidity that have been documented in children and young adults with ESRD include increased carotid artery intimal-medial thickness and the presence of coronary artery calcifications.17,18 Although the exact mechanisms and pathophysiology for these findings are not known, hypertension remains a modifiable risk factor that, if treated appropriately, could have significant benefit for the long-term health of children with ESRD.
It is encouraging to see a trend of improvement of BP control in this population over the past 2 decades. Compared with more recent years, earlier year of dialysis initiation was associated with both higher reported BPs and greater odds of uncontrolled BP. There are likely many variables responsible for this change, and the cross-sectional design of the study limits our ability to infer causation between changes in antihypertensive medication use and BP control. Despite this limitation, the association is intriguing and bears further investigation. Many changes in clinical practice have occurred over the study period, including the revision of BP management guidelines, more extensive study on the safety and effectiveness of antihypertensive medications in children, and the advent of the use of blood volume monitoring (BVM).10,11,19 As several pediatric studies have demonstrated an ability to decrease ambulatory BP measures and number of antihypertensive medications prescribed for patients on HD with a BVM protocol, future analyses of BP control in pediatric patients receiving dialysis should examine the role of BVM and other aspects of assessing and addressing volume overload.20,21
Important predictors of BP and BP control in this analysis included age and disease etiology and these relationships were present even when controlling for other confounding factors. Patients with glomerular diseases are typically hypertensive prior to reaching end-stage and often oligo/anuric, making hypertension a common comorbidity on dialysis as well. Our analysis suggests that, despite many patients having been prescribed antihypertensive medications, this is a group for whom BP is poorly controlled. Younger age was also an independent risk factor for uncontrolled hypertension and higher BP. The reason for this is not clear but may be related to undertreatment with antihypertensives, as younger age was also associated with a lower odds of antihypertensive use.
There are few published data offering consensus as to whether PD or HD is a superior modality in achieving BP control in children.7,9 In this study, PD was associated with a lower SBP and lower odds of uncontrolled SBP. The percentage of patients on PD with a controlled SBP was also significantly higher than for patients on HD. Both analyses adjusted for age and etiology of renal disease to address confounding by those two very influential variables on BP, but there may be other, nonmeasured, variables as well that make these two populations different. Factors that make a patient and family suitable candidates for home dialysis may favor closer adherence to taking antihypertensive medications, for example.
The cross-sectional design of this study limits our ability to determine causality. Other important limitations include the lack of information on the method of BP determination, infrequency of BP measurements (every 6 months), and the lack of other potentially important variables, including the timing of BP measurement with respect to dialysis treatment session, patient dry weight, and details on the number and type of antihypertensive medications. Other cardiovascular indicators, such as echocardiographic data, pulse wave velocity, and coronary artery calcification assessment are not standardized aspects of clinical care and thus not collected in the NAPRTCS registry.
Acknowledgments
Supported by the National Institutes of Health (grant T32 DK007662).
Glossary
- BP
Blood pressure
- BVM
Blood volume monitoring
- CKD
Chronic kidney disease
- DBP
Diastolic blood pressure
- ESRD
End-stage renal disease
- HD
Hemodialysis
- NAPRTCS
North American Pediatric Renal Trials and Collaborative Studies
- PD
Peritoneal dialysis
- SBP
Systolic blood pressure
Footnotes
The authors declare no conflicts of interest.
Presented in part at the annual meeting of the American Society of Nephrology, October 29–November 1, 2009, San Diego, CA.
References
- 1.Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141:162–164. doi: 10.1067/mpd.2002.125910. [DOI] [PubMed] [Google Scholar]
- 2.Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, et al. Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int. 2002;61:621–629. doi: 10.1046/j.1523-1755.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 3.McDonald SP, Craig JC. Australian and New Zealand Paediatric Nephrology Association: Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 4.Kavey RW, Allade V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, et al. Cardiovascular risk reduction in high-risk pediatric patients: A scientific statement from the American Heart Association Expert Panel on Population and Prevention Science: The Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment and control of hypertension in chronic hemodialysis patients in the United States. Am J Medicine. 2003;115:291–297. doi: 10.1016/s0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 6.VanDeVoorde RG, Barletta GM, Chand DH, Dresner IG, Lane J, Leiser J, et al. Blood pressure control in pediatric hemodialysis: the Midwest Pediatric Nephrology Consortium Study. Pediatr Nephrol. 2007;22:547–553. doi: 10.1007/s00467-006-0341-x. [DOI] [PubMed] [Google Scholar]
- 7.Mitsnefes M, Stablein D. Hypertension in pediatric patients on long-term dialysis: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Am J Kidney Dis. 2005;45:309–315. doi: 10.1053/j.ajkd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Chavers BM, Solid CA, Daniels FX, Chen SC, Collins AJ, Frankenfield DA, et al. Hypertension in pediatric long-term hemodialysis patients in the United States. Clin J Am Soc Nephrol. 2009;4:1363–1369. doi: 10.2215/CJN.01440209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tkaczyk M, Nowicki M, Balasz-Chmielewska I, Boguszewska-Baczkowska H, Drozdz D, Kollataj B, et al. Hypertension in dialyzed children: the prevalence and therapeutic approach in Poland: a nationwide survey. Nephrol Dial Transplant. 2006;21:736–742. doi: 10.1093/ndt/gfi280. [DOI] [PubMed] [Google Scholar]
- 10.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 11.National Kidney Foundation. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–S290. [PubMed] [Google Scholar]
- 12.North American Pediatric Renal Trials and Collaborative Studies. 2008 Annual Report. Potomac (MD): Emmes Corporation; 2008. [Google Scholar]
- 13.Flynn JF, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Hypertension. 2008;52:631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, et al. Masked hypertension associated with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol. 2010;21:137–144. doi: 10.1681/ASN.2009060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullington N, Kartel J, Khoury P, Mitsnefes M. Left ventricular hypertrophy in pediatric kidney transplant recipients: long term follow up study. Pediatr Transplant. 2006;10:811–815. doi: 10.1111/j.1399-3046.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 16.Sorof JM, Alexandrov AV, Cardwell G, Portman RJ. Carotid artery intimal-medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics. 2003;111:61–66. doi: 10.1542/peds.111.1.61. [DOI] [PubMed] [Google Scholar]
- 17.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106:100–105. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 18.Civilibal M, Caliskan S, Adaletli I, Oflaz H, Sever L, Candan C, et al. Coronary artery calcifications in children with end-stage renal disease. Pediatr Nephrol. 2006;21:1426–1433. doi: 10.1007/s00467-006-0159-6. [DOI] [PubMed] [Google Scholar]
- 19.Flynn JF. Hypertension in the young: epidemiology, sequelae, therapy. Nephrol Dialysis Transplant. 2009;24:370–375. doi: 10.1093/ndt/gfn597. [DOI] [PubMed] [Google Scholar]
- 20.Patel HP, Goldstein SL, Mahan JD, Smith B, Fried CB, Currier H, et al. A standard, noninvasive monitoring of hematocrit algorithm improves blood pressure control in pediatric hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:252–257. doi: 10.2215/CJN.02410706. [DOI] [PubMed] [Google Scholar]
- 21.Civilibal C, Sever L, Civilibal M, Caliskan S, Arisoy N. Blood volume monitoring to adjust dry weight in hypertensive pediatric hemodialysis patients. Pediatr Nephrol. 2009;24:581–587. doi: 10.1007/s00467-008-0985-9. [DOI] [PubMed] [Google Scholar]