The differences in form and texture between a slime mold and a redwood tree originate, in large part, from the structural differences in their extracellular matrices. Indeed, when the slime mold Dictyostelium transforms itself from a creeping blob of amoebae into a cellularized stalk and fruiting body, its cells secret a cellulosic matrix that begins to resemble the plant cell wall and that imparts some mechanical strength to the ascending stalk. As is the case in the slime mold, the rheology of the cell wall is also crucial to plant form and function, albeit in a more complex way. The wall acts as a cellular exoskeleton that encases plant cells, giving them shape and mechanical stability, gluing them together, restraining their growth, and protecting them from assaults by pathogens and the environment. These and other functions of the wall demand varying properties, and hence the wall’s structure and biochemistry are varied and dynamic (1). In growing plant cells, the wall is strong enough to resist the large mechanical forces generated by the high internal pressure (turgor) typical of plants cells yet still pliant enough to permit enlargement by a form of polymer creep. As plant cells begin to mature, their walls typically become inextensible and resistant to degradation, yet certain cell types and tissues remain capable of wall disassembly and dissolution—in some cases transforming a firm tissue into something with a consistency resembling that of a slime mold.
Such is the case for tomatoes and similar fruits, which become softer in the later stages of ripening. This aspect of fruit ripening was long considered to be mediated by pectinases and other wall hydrolases that degrade the major structural polymers of the wall. This idea, however, lost much of its luster in the last decade when experiments with transgenic tomatoes showed that expression of these hydrolytic enzymes could be genetically altered without major effects on fruit softening (2–5). Such experiments have, one by one, downgraded the major candidates from the list of suspected fruit-softening enzymes and clouded the view that fruit softening is the primarily the result of wall hydrolysis. Now, a new and unanticipated candidate appears from the study by Rose et al. (6), published in this issue of the Proceedings. They report that an expansin mRNA is specifically and abundantly expressed in ripening fruit, and they suggest that expansin proteins may contribute to cell wall disassembly during fruit ripening. The involvement of expansins in fruit ripening is surprising because these proteins are not known to possess wall hydrolytic activity and because until now they were known principally as catalysts of plant cell enlargement.
By way of background, growing plant tissues characteristically possess a property known as “acid growth” (7, 8). This term refers to the peculiar ability of growing plant cells to extend rapidly when incubated in acidic buffers (pH <5.5). This pH-dependent growth mechanism is also a property of the walls themselves, as demonstrated when isolated walls from growing cells are clamped in tension in an extensometer. When incubated in a pH 7 buffer, they soon stop extending, but when switched to a pH 4.5 buffer, they extend rapidly and irreversibly by a process of polymer creep. In suitable plant material, the wall may creep for many hours, until thinning of the wall leads to its breakage. Thus, the change in pH transforms the wall from a viscoelastic solid to a viscoelastic liquid. This process of wall creep may be inactivated by treatment with proteases, protein denaturants, and heat and thus is not simply a physico-chemical property of the wall’s polysaccharides (9). In 1992 it was discovered in reconstitution experiments that a crude fraction of wall proteins, when added back to heat-inactivated walls, would restore their ability for acid growth; purification of the active proteins led to the identification of expansins (10). Until now, expansins are the only proteins demonstrated to induce creep of isolated walls.
Although expansins profoundly alter the rheology of growing plant walls, how they accomplish this remains somewhat of a mystery. The plant cell wall is a complex and heterogeneous layer, typically between 0.1 and 1 μm thick, consisting of cellulose microfibrils embedded in a highly hydrated matrix of hemicelluloses and pectins, with smaller quantities of structural protein intercalated in the matrix (Fig. 1). Cellulose is made up of 1 → 4 linked β-d-glucans tightly packed into long, crystalline microfibrils that wind around the cell. Hemicelluloses anchor the microfibril in the matrix by bonding noncovalently to the surface of the microfibril and perhaps by becoming physically entrapped in the microfibril as it is formed by synthase complexes in the plasma membrane. Pectins make up a coextensive hydrophilic phase with gel-like properties; situated in the space between microfibrils, pectins prevent aggregation and collapse of the cellulose network. For years it was hypothesized that enlargement of the growing wall—like the softening of fruit—was primarily based on the activity of wall hydrolases or transglycosylases (11, 12). The concept was that pH-dependent wall-loosening enzymes would cut matrix polysaccharides that hold the microfibrils in place, permitting a kind of chemorheological creep. Thus, it came as a surprise that these enzymatic activities could not be detected in purified expansin preparations (10, 13). Instead, expansins appear to disrupt the noncovalent bonding between cellulose and hemicellulose (14), thereby allowing the wall polymers to yield to the turgor-generated stresses in the cell wall.
Figure 1.
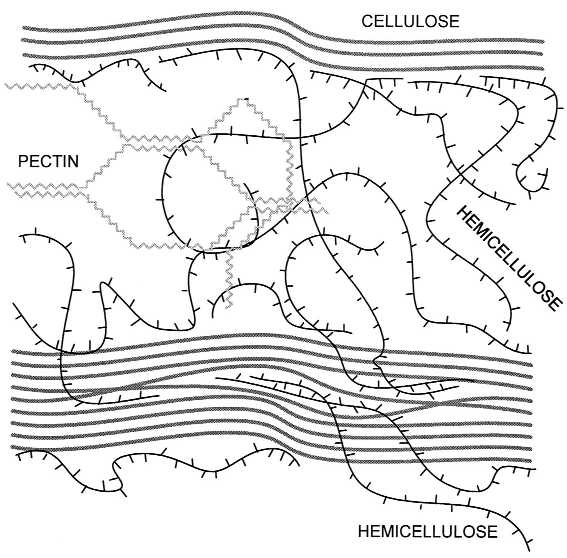
Structure of the primary plant cell wall, showing major structural polymers and their likely arrangement in the wall. Cellulose microfibrils are crystalline aggregates of (1 → 4) β-d-glucans and contain noncrystalline regions that may be formed by entrapment of hemicelluloses. Hemicelluloses can also bond to the surface of cellulose and may link two microfibrils together. Pectins form a hydrophilic gel that surrounds and embeds the microfibrils.
Cloning and sequence analysis showed expansins to be a novel gene family common to the two major branches of angiosperms (monocotyledons and dicotyledons) (15). Expansin cDNAs have also been cloned from the hypocotyls of pine, a gymnosperm (K. Hutchison, personal communication). Despite the 400 million years of evolution separating the angiosperms and gymnosperms, the amino acid sequence of expansins expressed in pine hypocotyls and cucumber hypocotyls is highly conserved, implying strict evolutionary constraints on protein structure and function. In Arabidopsis, 12 different expansin genes have been identified so far (unpublished results), and more will almost certainly be found with further work.
What is the plant doing with so many expansins? One possibility is that different expansin genes are involved in the control of growth in different cell types and by different stimuli. The novel suggestion offered by Rose et al. (6) is that some expansins may have become specialized to serve roles in wall disassembly in nongrowing tissues. This idea is based on their observation that accumulation of the tomato fruit expansin (LeExp1) mRNA is coincident with fruit softening and is regulated by ethylene, a hormone that accelerates tomato ripening. LeExp1 expression is also lacking in tomato mutants (nor and rin) with impaired ripening. Furthermore, Rose et al. (6) report that the sequence of fruit-ripening expansins may define a subclass of expansin genes. If this proves to be generally true, it suggests that the biochemical action and/or substrate specificity of these expansins is different from that of the growth-related expansins. It will thus be of great interest to characterize these expansins with respect to biochemical activity and rheological effects on the wall. Do these expansins mediate acid-induced growth? If so, then why don’t ripening tomatoes grow? Rose et al. (6) suggest that expansins may enhance the accessibility of wall polymers to enzyme action, thereby accelerating wall hydrolysis. The fact that expansins are found in snail digestive juices (16) likewise hints at such a biochemical function. On the other hand, it is also possible that expansins, directly and by themselves, induce fruit softening by weakening the adhesion between wall polymers, just as they directly mediate the creep of walls from growing tissues. Studies with transgenic tomato plants are underway to study these questions further (6).
If some expansins indeed function in wall disassembly, then expansins may also play a role in other developmental changes in the wall, such as in leaf abscission and seed pod dehiscence, during which cell walls separate from one another, or in xylem vessel formation, during which the end walls between adjacent vessel members are dissolved. Perhaps 12 expansins are not enough to accommodate all these functions in Arabidopsis.
In addition to the “classical” expansins described above, a second family of expansins (β-expansins) was recently identified in grass pollen (17). Previously known as group I allergens from grass pollen, these proteins share only 20–25% amino acid sequence identity with expansins, yet they have potent expansin-like activity that can soften the walls of the grass stigma and style. The grass pollen tube grows by tip growth to force its way between the tightly packed cells of the stigma before entering the stylar track, where pollen tube growth involves further intrusion through and between cell walls to reach the ovule. Secretion of cell-wall-loosening agents with expansin-like properties presumably aids invasion of the pollen tube into the maternal tissues. This is yet another example in which control of cell wall rheology by expansins may have an important biological function.
Potential applications of expansins abound and are ripe for testing. Targeted control of plant cell enlargement, i.e., by use of genetic engineering to alter expansin expression in specific organs or tissues, may enhance crop yields and increase food production. In a like manner, genetic engineering of expansins to regulate fruit softening may prove useful in agriculture and the food industry. The plant cell wall is also the source of many man-made products, including paper, cotton, and other natural fibers, wood, and various processed polymers that go into plastics, films, coatings, adhesives, and thickeners in a staggering variety of products (18). Rheology, texture, and polymer accessibility are important physical properties of these materials, so it seems that expansins, with their unique rheological effects on plant cell walls, may also find applications in this arena.
References
- 1.Carpita N C, McCann M, Griffing L R. Plant Cell. 1996;8:1451–1463. doi: 10.1105/tpc.8.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer R L, Bennett A B. Annu Rev Plant Physiol Mol Biol. 1991;42:675–703. [Google Scholar]
- 3.Giovannoni J J, DellaPenna D, Bennett A B, Fischer R L. Plant Cell. 1989;1:53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith C J S, Watson C F, Ray J, Bird C R, Morris P C, Schuch W, Grierson D. Nature (London) 1988;334:724–726. [Google Scholar]
- 5.Hall L M, Tucker G A, Smith C J S, Watson C F, Seymour G B, Bundick Y, Boniwell J M, Fletcher J D, Ray J A, Schuch W, Bird C R, Grierson D. Plant J. 1993;3:121–129. [Google Scholar]
- 6.Rose J K C, Lee H H, Bennett A B. Proc Natl Acad Sci USA. 1997;94:5955–5960. doi: 10.1073/pnas.94.11.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kutschera U. New Phytol. 1994;126:549–569. [Google Scholar]
- 8.Rayle D L, Cleland R E. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosgrove D J. Planta. 1989;177:121–130. [PubMed] [Google Scholar]
- 10.McQueen-Mason S, Durachko D M, Cosgrove D J. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fry S C. Physiol Plant. 1989;75:532–536. [Google Scholar]
- 12.Fry S C. Annu Rev Plant Physiol Mol Biol. 1995;46:497–520. [Google Scholar]
- 13.McQueen-Mason S, Cosgrove D J. Plant Physiol. 1995;107:87–100. doi: 10.1104/pp.107.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McQueen-Mason S, Cosgrove D J. Proc Natl Acad Sci USA. 1994;91:6574–6578. doi: 10.1073/pnas.91.14.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shcherban T Y, Shi J, Durachko D M, Guiltinan M J, McQueen-Mason S, Shieh M, Cosgrove D J. Proc Natl Acad Sci USA. 1995;92:9245–9249. doi: 10.1073/pnas.92.20.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosgrove D J, Durachko D M. J Exp Bot. 1994;45:1711–1719. doi: 10.1093/jxb/45.special_issue.1711. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove, D. J., Bedinger, P. A. & Durachko, D. M. (1997) Proc. Natl. Acad. Sci. USA 94, in press. [DOI] [PMC free article] [PubMed]
- 18.Lapasin R, Pricl S. The Rheology of Industrial Polysaccharides: Theory and Applications. London: Blackie Academic & Professional; 1995. p. 620. [Google Scholar]


