Abstract
Most organisms face the problem of foraging and maintaining growth while avoiding predators. Typical animal responses to predator exposure include reduced feeding, elevated metabolism, and altered development rate, all of which can be beneficial in the presence of predators but detrimental in their absence. How then do animals balance growth and predator avoidance? In a series of field and greenhouse experiments, we document that the tobacco hornworm caterpillar, Manduca sexta, reduced feeding by 30–40% owing to the risk of predation by stink bugs, but developed more rapidly and gained the same mass as unthreatened caterpillars. Assimilation efficiency, extraction of nitrogen from food, and percent body lipid content all increased during the initial phase (1-3 d) of predation risk, indicating that enhanced nutritional physiology allows caterpillars to compensate when threatened. However, we report physiological costs of predation risk, including altered body composition (decreased glycogen) and reductions in assimilation efficiency later in development. Our findings indicate that hornworm caterpillars use temporally dynamic compensatory mechanisms that ameliorate the trade-off between predator avoidance and growth in the short term, deferring costs to a period when they are less vulnerable to predation.
Keywords: antipredator behavior, nonconsumptive effects, predator–prey interactions, phenotypic plasticity
Feeding is dangerous (1, 2). For many animals, reduced feeding is a common response to the watchful eyes of predators that exploit movement as a primary prey location cue (3, 4). In addition, metabolic rate typically increases in response to predation risk (5), potentially exacerbating the costs of reduced food intake. Organisms respond to predation risk and the corresponding food limitation through phenotypically plastic responses, including behavioral, physiological, and developmental changes (6–9). The integration of these factors to ameliorate the impacts of predator exposure has been little studied, however, especially over development during periods of chronic predation risk (10).
After a period of food limitation and associated decreased growth (11, 12), many organisms increase growth through compensatory responses (13–15). Compensation after starvation can be achieved via increased consumption, increased metabolic or digestive efficiency, or altered developmental rate when food becomes available (16). Because predation risk is an important natural phenomenon that constrains foraging and induces physiological stress, compensatory mechanisms may be especially important in this context. Nonetheless, compensatory responses to predation risk, and their behavioral, physiological, and developmental underpinnings, are not well understood (17). Much of the research on responses to predator exposure has focused on behavioral changes in resource selection, where generalist foragers, such as elk and grasshoppers, switch habitat and diet use in the presence of predators (18, 19). Little is known about physiological responses to the stress of predation risk, especially in invertebrates (20). In addition, the bulk of insect herbivores are host plant specialists (21) and cannot readily switch resources to avoid predation. Thus, we predict that specialists will rely on internal compensatory mechanisms (i.e., physiological and developmental), rather than on behavioral mechanisms that are prevalent among polyphagous consumers.
In this study, we used a combination of field and greenhouse experiments to examine how behavioral, physiological, and developmental responses to predation risk are integrated in Manduca sexta (tobacco hornworm), a specialist caterpillar that is relatively immobile early in development. We previously showed that M. sexta reduce foraging and food intake in the presence of stink bug predators (Podisus maculiventris), behaviors that likely protect them against predation (22, 23). In field mesocosms containing tomato plants, caterpillars exposed to “sham predators” that hunt normally but are unable to kill prey consumed 30–40% less leaf material compared with controls, yet surprisingly gained equal mass (24).
Here we examine the physiological mechanisms and costs of maintaining this growth under predator-induced food limitation, and find that the consequences of predation risk for caterpillar growth, development, and physiology are far more complex than previously recognized. We first address how M. sexta caterpillars compensate for reductions in feeding under a short-term risk of predation, and disentangle the physiological mechanisms (i.e., assimilation efficiency and postdigestive allocation of resources) that allow the caterpillars to maintain growth while eating less. Second, we quantify how responses are integrated over longer periods of predation risk and show that substantial delayed costs appear in terms of compensatory feeding and reduced assimilation efficiency. Finally, we show that predator-induced food limitation elicits fundamentally different compensatory responses than non–predator-induced food limitation, and that longer-term responses are a continuing effect of predator exposure (not acclimation to predation risk). Thus, we address how predation risk fundamentally changes the feeding behavior, developmental trajectory, and nutritional physiology of surviving caterpillars in a temporally dynamic manner.
Results
Short-Term Compensation for Predator Exposure.
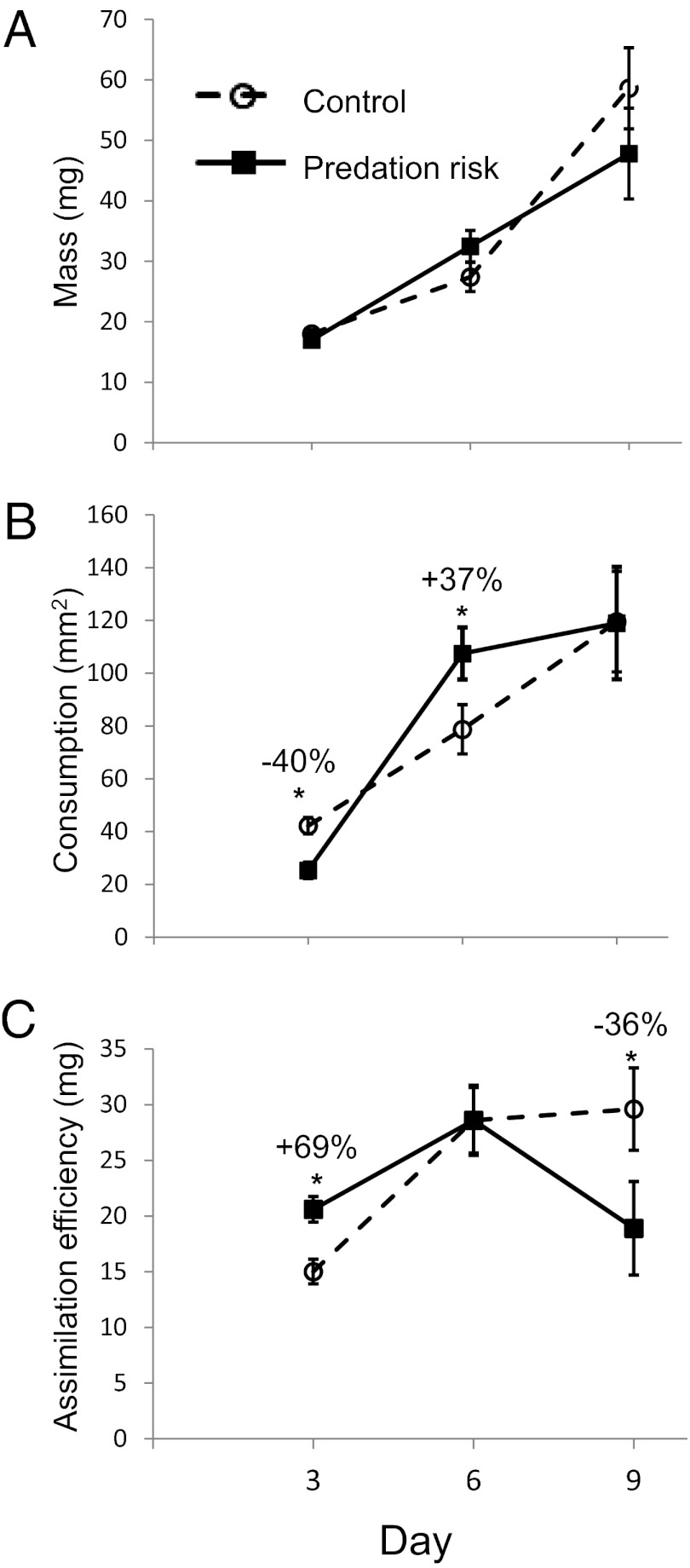
In field mesocosms, caterpillars exposed to predation risk compensated for reduced consumption by increasing the rate of assimilation of ingested food by 28% over a 3-d period, corresponding to ∼15% of the larval development period and to one of the three instars vulnerable to stink bug predation (23). Assimilation efficiency was calculated as mass gain with the amount of ingested food as a covariate in analysis of covariance (25) [least squares (ls) mean assimilation efficiency: control, 16.25 ± 1.43 mg; predation risk, 20.78 ± 1.33 mg; F1,16 = 5.34; P = 0.038]. Similarly, in more controlled greenhouse experiments, we found no difference in mass of second instar caterpillars in the control and predation risk treatments (Fig. 1A, day 3), with a 40% reduction in consumption by the caterpillars exposed to predation risk (Fig. 1B, day 3) compensated for by a 69% increase in assimilation efficiency (Fig. 1C, day 3 and Fig. S1A).
Fig. 1.
Effect of exposure to predaceous stink bugs (P. maculiventris) on M. sexta caterpillar mass, leaf consumption, and assimilation efficiency. Caterpillars in the control treatment are shown with open circles/dashed lines, and those in the predation risk treatment are shown with black squares/solid lines. Measurements are shown for days 3, 6, and 9. Symbols represent least squares mean ± 1 SE. (A) Symbols represent cumulative mass (in mg) of caterpillars. Sample sizes were as follows: day 3: control = 37, predation risk = 41; day 6: control = 29, predation risk = 25; day 9: control = 25, predation risk = 20. (B) Leaf area consumed (in mm2) by M. sexta caterpillars during days 1–3, days 4–6, and days 7–9. Percent differences between control and predation risk treatments are shown for significant effects (P ≤ 0.05; full statistical model given in Table S1). (C) Assimilation efficiency is calculated as the mass gain (in mg), with area consumed as the covariate.
Further evidence of increased assimilation efficiency comes from measurements of nutrients in caterpillar frass. Caterpillars exposed to predation risk excreted 8.5% less nitrogen in their frass compared with controls (ls mean dry weight: control, 3.51 ± 0.11%; predation risk, 3.21 ± 0.11%; F1,121 = 4.08; P = 0.045), demonstrating enhanced extraction of nitrogen from food (percent carbon did not differ; P ≥ 0.3). Predation risk did not change the pattern of feeding damage, measured as the number of damaged leaflets (P ≥ 0.5), and there was no difference in the nitrogen content of leaflets adjacent to damage between predator exposure and control treatments (P ≥ 0.8). The similarity in nitrogen content indicates that caterpillars did not behaviorally select more nutritious food in the presence of predators.
Caterpillars exposed to predation risk had 9% higher lipid content (ls mean: control, 22.08 ± 1.18 μg/mg caterpillar dry weight; predator, 24.11 ± 1.09 μg/mg; F1,133 = 4.44; P = 0.037) and less carbohydrate, driven primarily by a 25% decrease in glycogen (ls mean: control, 37.07 ± 3.10 μg/mg caterpillar dry weight; predation risk, 27.64 ± 2.74 μg/mg; F1,142 = 5.63; P = 0.019). There was a negative correlation between glycogen and lipids among individuals (F1,138 = 9.03; P = 0.032), suggesting that caterpillars exposed to predators may be converting glycogen into lipid. Percent water (P ≥ 0.6) and percent protein (P ≥ 0.1) did not differ between treatments. These findings indicate that two measures of food utilization (assimilation efficiency and nitrogen in frass) and one measure of energy storage (lipid accumulation) demonstrate physiological compensation in response to the initial period of predation risk.
Deferred Costs of Compensating for Predation Risk.
Given the dramatic physiological compensation for short-term predation risk, we assessed the potential breakdown of compensation over a longer period of growth. After the 3-d predation risk exposure reported above, we continued the experiment for another 6 d, to encompass the entire second and third larval instars (∼40% of the larval development period), measuring caterpillar mass, consumption, and assimilation efficiency every 3 d. This period of growth was chosen because it encompasses two of the three instars during which M. sexta are vulnerable to stink bug predation (23). Over the next two developmental assessments, caterpillar mass again did not differ between treatments (Fig. 1A and Table S1), but behavioral and physiological costs of predation risk became apparent.
During the second interval (days 4–6), the caterpillars’ behavioral and physiological responses to predator exposure was reversed compared with that in the first interval, with caterpillars in the predator exposure treatment demonstrating compensatory feeding, eating 37% more than control caterpillars (F1,52= 4.47; P = 0.039) (Fig. 1B, day 6) and with equal assimilation efficiency in the two treatments (F1,52= 0.046; P = 0.83) (Fig. 1C, day 6; Table S1; and Fig. S1B). In the third interval (days 7–9), caterpillars in the two treatments ate the same amount (F1,33 ≤ 0.001; P = 0.99) (Fig. 1B, day 6), but assimilation efficiency declined 36% under continued predation risk compared with controls (F1,33 = 4.16; P = 0.048) (Fig. 1C, day 9; Table S1; and Fig. S1C). Thus, although assimilation efficiency was initially higher in the predation risk treatment (days 1–3), it eventually fell below that of control treatment (days 7–9), indicating a persistent change in assimilation capacity and a delayed cost.
Impact of Predation Risk on Development Rate.
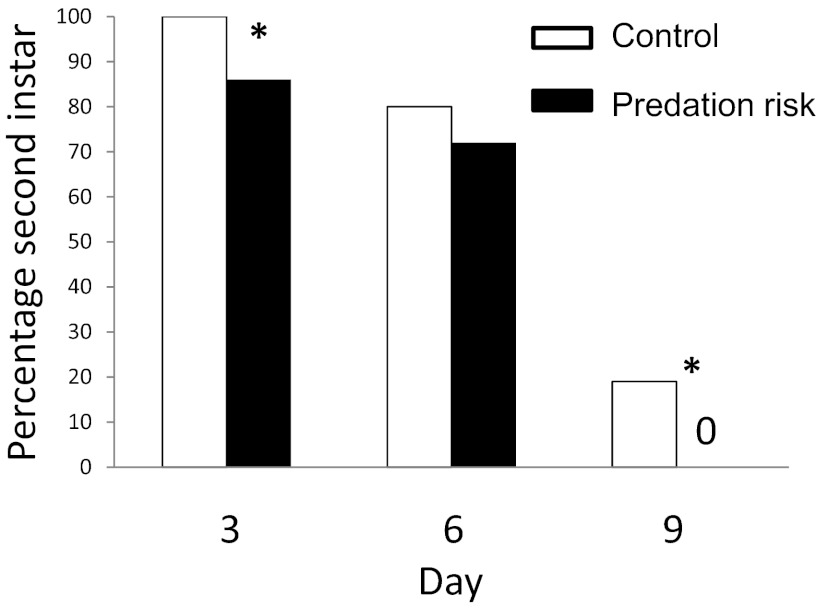
Across the 9-d experiment, we observed a dramatic effect of predation risk on the larval development rate. For example, it took 7.5 d for 25% of the caterpillars to complete the second instar in the control treatment, compared with only 4.5 d in the predation risk treatment (Fig. 2) (Kaplan–Meier χ12 = 4.59; P = 0.032). In addition, more caterpillars in the control treatment remained in the second instar at the end of the experiment (day 9) (Fig. 2). This accelerated development under predator exposure contributed to the increased assimilation efficiency seen during days 1–3, given that third instar caterpillars have higher assimilation efficiency than second instars (P ≤ 0.001) (Fig. S1A). Even if we restrict our analysis to individuals in the second instar on day 3, caterpillars in the predation risk treatment still exhibit higher assimilation efficiency than controls (P = 0.013). In combination with the 3-d greenhouse experiment conducted with third instar caterpillars, our data show that both second and third instar caterpillars increase assimilation efficiency in the predation risk treatment. These results indicate that predation risk enhances assimilation both by physiological mechanisms and by accelerating development.
Fig. 2.
Percentage of M. sexta caterpillars in the second instar in the control and predation risk treatment at 3, 6, and 9 d after the start of the experiment. All caterpillars are either second or third instar; thus, the differences from 100% are third instars. Numbers in each instar on each day are compared across treatments using a G-test (day 3: G = 8.15, P = 0.0046; day 6: G = 0.58, P = 0.45; day 9: G = 5.2, P = 0.024). Asterisks indicate statistical significance. Sample sizes were as follows: day 3: control = 37, predation risk = 41; day 6: control = 29, predation risk = 25; day 9: control = 25, predation risk = 20.
Food Limitation and Predator Acclimation Controls.
To test whether caterpillars acclimate to the presence of the predator during chronic exposure, we exposed caterpillars to “sham predators” for 3 d as before, and measured their continuing daily consumption and mass after removal of the threat of predation. As in our previous experiments, during the 3-d predation risk exposure period, caterpillars in the predator treatment ate 58% less and exhibited a 24% increase in assimilation efficiency compared with controls (Table S2). Nonetheless, caterpillars in the early predation risk treatment did not increase their feeding compared with controls after the predator was removed (1 d after predator removal, leaf area consumed ± SE: control, 68.75 ± 6.75; predator, 55.61 ± 6.43; P = 0.25) (Table S2), and assimilation efficiency returned to levels equal to the control treatment within 1 d (1 d after predator removal, assimilation efficiency ± SE: control, 0.0124 ± 0.0008; predator, 0.0121 ± 0.0007; P = 0.85). This experiment demonstrates that caterpillars had not acclimated to the presence of the predator in the chronic exposure experiment: If the increase in consumption during the second interval (d 4–6) of chronic exposure had occurred due to acclimation, this should have also occurred when the predator was removed.
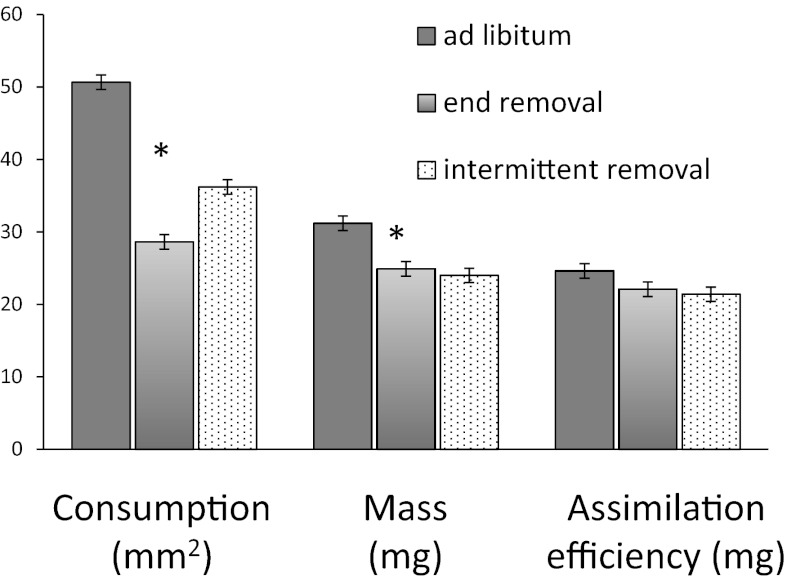
To test whether the increased assimilation efficiency is a predator-specific response or a generalized response to reduced consumption, we experimentally limited food availability in the absence of predators. Caterpillars were divided into three treatments: ad libitum food for 45 h, ad libitum food during the first 30 h with no subsequent food, and interrupted ad libitum food (two periods of 4–6 h of no food interspersed during the trial). Although the interrupted ad libitum design might not mimic the rhythm of predator altered feeding behavior, by having two separate bouts of food limitation, each followed by a feeding period, there were two separate opportunities for the larvae to compensate for reductions in feeding. The latter food limitation treatments resulted in a 51–56% reduction in consumption (P = 0.0022) compared with ad libitum controls (Fig. 3 and Table S3). Unlike caterpillar responses to predation risk, both treatments involving reduced food consumption resulted in 23% less final mass (P = 0.001) and had no effect on assimilation efficiency (P = 0.63). This result indicates that the enhanced assimilation efficiency on exposure to predators is not a general reaction to food deprivation.
Fig. 3.
Effect of food limitation without predation risk on consumption (in mm2), growth (in mg), and assimilation efficiency (mg growth with area consumed as the covariate) of third instar M. sexta caterpillars. Food was offered continuously during the experiment (ad libitum; n = 7), was withheld toward the end (end removal; n = 8), or was withheld three times during the experiment (intermittent removal; n = 8). Bars represent means ± SE. Asterisks indicate significant differences between ad libitum and the two food limitation treatments.
Discussion
To compensate for predator-induced reductions in feeding, animals frequently modify their behavior (e.g., movement to less risky environments) (18) and development (often accelerated) (26). Here we report how, together with such effects, physiological compensatory mechanisms—increased assimilation efficiency and energy storage—also change in the presence of predators. For Manduca caterpillars, although predation risk did not affect growth, it was associated with immediate reductions in feeding, increased assimilation efficiency, and concurrent acceleration in the development rate for more than one-third of the developmental period. In addition, we report that short-term compensation results in long-term physiological costs to prey organisms. The delaying of costs potentially provides an adaptive benefit to prey, allowing the maintenance of growth while minimizing predation during highly susceptible life stages (27, 28).
Insects frequently exhibit plasticity in food utilization efficiency in response to changes in diet quality (29), and predation risk can increase food utilization efficiency directly or through changes in diet. For example, Hawlena et al. (30) found that predation risk indirectly changed morphological traits of grasshoppers associated with feeding ability as a result of changes in the plant species chosen for consumption. McPeek (31) found that three of nine species of damselflies increased their assimilation efficiency under predation risk, despite less growth compared with damselflies in control environments. In the McPeek study, the increased assimilation efficiency could have been related to predation risk or changes in food intake per se, but not to shifts in food use by the damselflies, because only one species of prey was present. Predation risk also has the potential to decrease digestive capacity, as demonstrated in wood frog tadpoles, which have shorter guts when grown with predator cues (32). For Manduca, the increased assimilation efficiency is a plastic response to the predator, not reduced feeding per se, given that food limitation in the absence of predation risk does not increase assimilation efficiency. We hypothesize that in the presence of predators, Manduca may benefit by eating less but extracting more of the recalcitrant nutrients from their food, to maintain growth while remaining inconspicuous.
Prey cannot physiologically compensate for reductions in feeding without costs ad infinitum, but they can alter the timing of physiological processes to maximize performance (16). In nature, predation risk is temporally variable, and thus responses that allow animals to overcome a short-term threat likely will maintain fitness. The costs of compensation appeared to be low early in the predator exposure treatment. The decrease in body glycogen content was expected, given that caterpillars eat less under predation risk and may have increased metabolic demands owing to stress or vigilance. These reserves likely can be replenished when feeding resumes. We also found increased lipid levels in caterpillars exposed to predators, perhaps because of the conversion of glycogen to lipids under predation risk (5). Increasing lipid level has a potential benefit for caterpillars exposed to predators by allowing them to store energy for use after the risk of predation has passed. The increase in lipids also could be a passive response, given that M. sexta caterpillars exposed to predation risk were found to spend less time moving on leaves compared with control caterpillars (24), and decreased activity can result in increased lipid storage (33).
Subsequent to the initial effects of predation risk, several indicators of costs became apparent in the longer-term assay (days 4–9). Feeding rates increased (Fig. 1B, day 6) and assimilation efficiency decreased (Fig. 1C, day 9) under chronic predator exposure. These two factors may interact to pose especially high costs for caterpillars and may lead to reduced performance later in development. Although increased feeding can increase the vulnerability of M. sexta to predators, as reported by Bernays (1), decreased assimilation efficiency may either cause caterpillars to eat even more, exacerbating their exposure to predators, or result in reduced future growth (34). An experiment over the entire larval lifespan and with caterpillars periodically challenged by intact predators would allow us to measure these alternative responses and their impact on fitness.
A faster developmental rate has the potential to benefit caterpillars in the short term by allowing them to advance to a safer stage, but at possible costs later in life. First, molting leads to the production of a larger head capsule (and thus larger mandibles), which facilitates food intake and increased growth (35). Indeed, the caterpillars in our predator exposure treatment ate more in days 3–6 compared with control caterpillars; thus, in addition to physiological adjustments, faster development might have allowed them to maintain equal growth as controls. Second, rapid development likely allows caterpillars to reach a safer developmental stage sooner (e.g., later instars have thicker cuticles that are harder for a predator to pierce compared with early instars) (36). In support of this hypothesis, we previously reported that first and third instar M. sexta caterpillars experienced high predation rates by stink bugs in the field (>60% mortality), but this rate declined to <13% in fourth instar caterpillars (23). The increased developmental rate in the predator exposure treatment has the potential to be costly if it reduces the quality of body tissue (10).
Our findings are consistent with the underlying theory of predator avoidance–growth trade-offs (37), but demonstrate that measuring the costs (and compensatory mechanisms) involves physiological, developmental, and explicitly temporal components. Over a 9-d period, in the M. sexta system, rapid behavioral responses (i.e., reduced feeding) were coupled with physiological responses (i.e, increased assimilation efficiency), but as time progressed and predation risk persisted, physiological responses appeared to fail (i.e, reduced assimilation efficiency) and different behaviors were exhibited (i.e, compensatory feeding). Over the 9-d assay period in which the M. sexta caterpillars remained responsive to predation risk, they did not acclimate to the presence of the predator, but did change the way in which they responded to predators. These temporally dynamic results are consistent with adaptive models of responses to predator exposure that predict combinations of antipredator strategies (38, 39), and indicate that different strategies may be used over an extended period of predator exposure (10).
Although compensation for reduced feeding and associated responses to predation risk are likely quite general, an important next step is the development of testable predictions of the environmental conditions or species traits that favor compensatory ability (31). We advance the hypothesis that immobile specialist prey, such as M. sexta, cannot compensate for predator exposure by changing hosts, and thus will be more likely to experience changes in the physiology and efficiency of host utilization compared with mobile generalist prey, which may be able to maintain feeding by switching to a safer host. Consistent with this hypothesis, a generalist grasshopper did not experience change its efficiency when allowed to forage freely, but did adjust its physiological and morphological traits when constrained to one host plant, such as would occur when exposed to predators (30). If organisms are restricted to feeding on a single resource, especially when constrained by the threat of predation, increased assimilation efficiency may be critical for maintenance of growth.
Methods
Study System.
The tobacco hornworm M. sexta (Lepidoptera: Sphingidae) is a specialist herbivore occurring almost exclusively on solanaceous plants. Caterpillars obtained from a colony at the North Carolina State Insectary were reared on tomato foliage (Solanum lycopersicum cv. Castlemart) until they molted to the second or third instar. The stink bug P. maculiventris (Hemiptera: Pentatomidae), an omnivorous predator, was collected using pheromone traps in Ithaca, New York and maintained in a laboratory colony with tomato plants and caterpillars or mealworms as prey. To manipulate the risk of stink bug predation, we surgically impaired the mouthparts of adult stink bugs to prevent their ability to kill (hereafter referred to as “sham predators”) by removing the terminal 1-mm segment of the stylet using a razor blade. We have previously shown that these surgically altered stink bugs are no longer able to penetrate the cuticle of caterpillars (22–24); however, risk predators survive for 3–4 wk in this altered state, feeding on plants and eating dead prey, and their behavior does not differ from that of lethal predators without stylet alteration (22).
Caterpillar Assimilation Efficiency in Response to Predation Risk in Field Mesocosms.
In the summer of 2008, we performed several field experiments to test the effects of predation risk on the consumption and growth of Manduca hornworm caterpillars. A complete description of that experiment is available elsewhere (24). The experiment used two levels of predator exposure (herbivores alone vs. herbivores exposed to sham predators) on groups of six newly molted third instar M. sexta caterpillars feeding on two tomato plants in 1-m3 mesh field cages. A single sham stink bug was starved for 24 h and added to cages assigned to predator addition. After 3 d, surviving caterpillars were collected, levels of herbivory per plant were quantified using an acetate grid, and caterpillar cohorts were reweighed. The efficiency of conversion of ingested food (i.e, assimilation efficiency) was calculated using one-way ANOVA to test the effects of predator exposure on weight gain, with leaf consumption as a covariate and the interaction between predation treatment and leaf consumed (25). Interactions between main effects are reported if significant. Data were transformed as necessary to meet assumptions of normality and homogeneity of variances.
Behavioral, Digestive, Physiological, and Developmental Responses to Chronic Predation Risk.
To provide further insight into the mechanisms underlying the lack of trade-off between growth and defense found in the field experiments, and to determine how the physiological compensatory mechanisms are coordinated with behavioral compensatory response over periods of prolonged predation risk, we conducted a longer-term predation exposure experiment in the greenhouse. This experiment was conducted for 9 d, encompassing the second and third instars, after which caterpillars are much less vulnerable to predation by hemipterans (23). Control and predation risk treatments were established using single newly molted second instar caterpillars, which were weighed and placed individually on a single potted tomato plant at the three-leaf stage. We started this experiment with second instar caterpillars, who would reach the same instar as the caterpillars in the 3-d field and greenhouse experiment by the end of the experiment. Plants were enclosed in translucent spun-polyester sleeves supported in the center with a bamboo stake and placed in a greenhouse (14-h light/10-h dark, 23–26 °C). On days 3, 6, and 9, caterpillars were weighed, and leaf area eaten was measured. On days 3 and 6, after measurements, caterpillars (and predators if present) were transferred to new plants. If either the predator or the caterpillar died, that replicate was excluded from further analysis. From this experiment, consumption, growth rate, and assimilation efficiency were calculated for each of the three 3-d intervals. The developmental rate and cumulative effect on final mass after 9 d of predator exposure were measured as well. The developmental rate was analyzed by Kaplan–Meier survival analysis.
Carryover Effects of Previous Predation Risk.
To disentangle the responses to continuous predator exposure from the carryover effects of early predator exposure and acclimation to predator exposure, we conducted a greenhouse experiment in which newly molted third instar caterpillars on single plants were either exposed to sham predators for 3 d or left as controls, after which predators were removed. At this time, caterpillars from both treatments were transferred to individual Petri dishes without predators, and their growth and consumption were monitored. Each day until the caterpillars molted to the fourth instar, they were given fresh ad libitum tomato foliage and leaf consumption and mass were measured.
Specificity of the Physiological Compensatory Response.
We tested whether the caterpillars’ increased assimilation efficiency was a specific response to predation risk or was similar to that of food limitation by manipulating food availability in the absence of the predator and measuring subsequent growth and assimilation efficiency. Third instar M. sexta were weighed, placed into Petri dishes, and divided into three treatments: continuous ad libitum food (tomato leaf disks), ad libitum food for the first 30 h and then no further food, and ad libitum food with two periods of no food (at 5–9 h and 19–25 of the experiment). There were seven or eight replicates for each treatment. After 45 h, caterpillars were reweighed, and leaf area eaten was measured. From these data, consumption, growth rate, and assimilation efficiency were calculated.
Nutrient Composition of Caterpillar Body, Frass, and Leaves.
We conducted a companion greenhouse study (to be reported in full elsewhere) with a similar design as the above-described greenhouse experiment to test the effects of predation risk on caterpillar frass production, frass nitrogen, and body nutrient composition. In brief, second instar caterpillars were placed on individual tomato plants and exposed to sham predators or left as controls as described above, but with the soil surface entirely covered with an inverted Petri dish to collect frass dropping down from caterpillars feeding on leaves above. After 3 d of growth in a greenhouse, insects were collected, leaf consumption was measured, and frass was carefully collected from the leaves, stems, and collecting dish at the bottom of the cage. At the same time, leaflets next to where the caterpillar was feeding were collected to assess the nitrogen content of ingested food. Caterpillars, frass, and leaflets were placed in a drying oven (60 °C) for 3 d, after which dry weights were recorded. Dried leaves and frass were then ground with a mortar and pestle and analyzed for percent carbon and nitrogen with a CHN elemental analyzer (Cornell University Stable Isotope Laboratory).
To determine lipid, glycogen, sugar, and protein content, dried caterpillars were prepared following the methods of Van Handel (40). Analysis of samples for glycogen and other sugars, as well as D-glucose standards, were determined using a hot anthrone-based assay. Lipid levels were measured in samples and standards using a vanillin reagent assay. Total protein was determined for a subset of the samples as described previously (41). Optical densities were measured with a Thermo Multiskan Spectrum spectrophotometer (Thermo Scientific) at 625 nm for glycogen and other sugars and at 525 nm for lipids.
Supplementary Material
Acknowledgments
We thank Monica Kersch Becker, Jessica Nix, Guilherme Becker, Ordom Huot, Elena Olsen, and Stephanie Ann Thornton for their assistance with the experiments; Anurag Agrawal and Goggy Davidowitz for useful discussions; and Kailen Mooney, Angela Douglas, Chris Dalton, Os Schmitz, Joel Kingsolver, and an anonymous reviewer for helpful comments on the manuscript. This project was supported by the National Research Initiative of the US Department of Agriculture Grant 2006-35302-17431 (to J.S.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208070109/-/DCSupplemental.
References
- 1.Bernays EA. Feeding by lepidopteran larvae is dangerous. Ecol Entomol. 1997;22:121–123. [Google Scholar]
- 2.Gotthard K. Increased risk of predation as a cost of high growth rate: An experimental test in a butterfly. J Anim Ecol. 2000;69:896–902. doi: 10.1046/j.1365-2656.2000.00432.x. [DOI] [PubMed] [Google Scholar]
- 3.Hassell MP, Southwood TRE. Foraging strategies of insects. Annu Rev Ecol Syst. 1978;9:75–98. [Google Scholar]
- 4.Preisser EL, Bolnick DI, Benard MF. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology. 2005;86:501–509. [Google Scholar]
- 5.Hawlena D, Schmitz OJ. Physiological stress as a fundamental mechanism linking predation to ecosystem functioning. Am Nat. 2010;176:537–556. doi: 10.1086/656495. [DOI] [PubMed] [Google Scholar]
- 6.Bassar RD, et al. Local adaptation in Trinidadian guppies alters ecosystem processes. Proc Natl Acad Sci USA. 2010;107:3616–3621. doi: 10.1073/pnas.0908023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitz OJ, Krivan V, Ovadia O. Trophic cascades: The primacy of trait-mediated indirect interactions. Ecol Lett. 2004;7:153–163. [Google Scholar]
- 8.Sheriff MJ, Krebs CJ, Boonstra R. The sensitive hare: Sublethal effects of predator stress on reproduction in snowshoe hares. J Anim Ecol. 2009;78:1249–1258. doi: 10.1111/j.1365-2656.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- 9.Skelly DK, Werner EE. Behavioral and life-historical responses of larval american toads to an odonate predator. Ecology. 1990;71:2313–2322. [Google Scholar]
- 10.Cressler CE, King AA, Werner EE. Interactions between behavioral and life-history trade-offs in the evolution of integrated predator-defense plasticity. Am Nat. 2010;176:276–288. doi: 10.1086/655425. [DOI] [PubMed] [Google Scholar]
- 11.Stamp NE, Bowers MD. Foraging behavior of specialist and generalist caterpillars on plantain (Plantago lanceolata) altered by predatory stinkbugs. Oecologia. 1992;92:596–602. doi: 10.1007/BF00317854. [DOI] [PubMed] [Google Scholar]
- 12.Relyea RA, Werner EE. Quantifying the relation between predator-induced behavior and growth performance in larval anurans. Ecology. 1999;80:2117–2124. [Google Scholar]
- 13.Metcalfe NB, Monaghan P. Compensation for a bad start: Grow now, pay later? Trends Ecol Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- 14.Tammaru T, Nylin S, Ruohomaki K, Gotthard K. Compensatory responses in lepidopteran larvae: A test of growth rate maximisation. Oikos. 2004;107:352–362. [Google Scholar]
- 15.Auer SK, Arendt JD, Chandramouli R, Reznick DN. Juvenile compensatory growth has negative consequences for reproduction in Trinidadian guppies (Poecilia reticulata) Ecol Lett. 2010;13:998–1007. doi: 10.1111/j.1461-0248.2010.01491.x. [DOI] [PubMed] [Google Scholar]
- 16.Morgan IJ, Metcalfe NB. Deferred costs of compensatory growth after autumnal food shortage in juvenile salmon. Proc Biol Sci. 2001;268:295–301. doi: 10.1098/rspb.2000.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoks R, De Block M, McPeek MA. Physiological costs of compensatory growth in a damselfly. Ecology. 2006;87:1566–1574. doi: 10.1890/0012-9658(2006)87[1566:pcocgi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz OJ. Direct and indirect effects of predation and predation risk in old-field interaction webs. Am Nat. 1998;151:327–342. doi: 10.1086/286122. [DOI] [PubMed] [Google Scholar]
- 19.Christianson D, Creel S. A nutritionally mediated risk effect of wolves on elk. Ecology. 2010;91:1184–1191. doi: 10.1890/09-0221.1. [DOI] [PubMed] [Google Scholar]
- 20.Preisser EL. The physiology of predator stress in free-ranging prey. J Anim Ecol. 2009;78:1103–1105. doi: 10.1111/j.1365-2656.2009.01602.x. [DOI] [PubMed] [Google Scholar]
- 21.Bernays E, Graham M. On the evolution of host specificity in phytophagous arthropods. Ecology. 1988;69:886–892. [Google Scholar]
- 22.Griffin CAM, Thaler JS. Insect predators affect plant resistance via density- and trait-mediated indirect interactions. Ecol Lett. 2006;9:338–346. doi: 10.1111/j.1461-0248.2005.00880.x. [DOI] [PubMed] [Google Scholar]
- 23.Thaler JS, Griffin CAM. Relative importance of consumptive and non-consumptive effects of predators on prey and plant damage: The influence of herbivore ontogeny. Entomol Exp Appl. 2008;128:34–40. [Google Scholar]
- 24.Kaplan I, Thaler JS. Plant resistance attenuates the consumptive and non-consumptive impacts of predators on prey. Oikos. 2010;119:1105–1113. [Google Scholar]
- 25.Raubenheimer D, Simpson SJ. Analysis of covariance: An alternative to nutritional indexes. Entomol Exp Appl. 1992;62:221–231. [Google Scholar]
- 26.Abrams PA, Rowe L. The effects of predation on the age and size of maturity of prey. Evolution. 1996;50:1052–1061. doi: 10.1111/j.1558-5646.1996.tb02346.x. [DOI] [PubMed] [Google Scholar]
- 27.Zanette LY, White AF, Allen MC, Clinchy M. Perceived predation risk reduces the number of offspring songbirds produce per year. Science. 2011;334:1398–1401. doi: 10.1126/science.1210908. [DOI] [PubMed] [Google Scholar]
- 28.Benrey B, Denno RF. The slow-growth–high-mortality hypothesis: A test using the cabbage butterfly. Ecology. 1997;78:987–999. [Google Scholar]
- 29.Yang Y, Joern A. Gut size changes in relation to variable food quality and body size in grasshoppers. Funct Ecol. 1994;8:36–45. [Google Scholar]
- 30.Hawlena D, Hughes KM, Schmitz OJ. Trophic trait plasticity in response to changes in resource availability and predation risk. Funct Ecol. 2011;25:1223–1231. [Google Scholar]
- 31.McPeek MA. The growth/predation risk trade-off: So what is the mechanism? Am Nat. 2004;163:E88–E111. doi: 10.1086/382755. [DOI] [PubMed] [Google Scholar]
- 32.Relyea RA, Auld JR. Having the guts to compete: How intestinal plasticity explains costs of inducible defences. Ecol Lett. 2004;7:869–875. [Google Scholar]
- 33.Johansson F, Andersson J. Scared fish get lazy, and lazy fish get fat. J Anim Ecol. 2009;78:772–777. doi: 10.1111/j.1365-2656.2009.01530.x. [DOI] [PubMed] [Google Scholar]
- 34.De Block M, Stoks R. Short-term larval food stress and associated compensatory growth reduce adult immune function in a damselfly. Ecol Entomol. 2008;33:796–801. [Google Scholar]
- 35.Bernays EA. Diet-induced head allometry among foliage-chewing insects and its importance for graminivores. Science. 1986;231:495–497. doi: 10.1126/science.231.4737.495. [DOI] [PubMed] [Google Scholar]
- 36.Lin HT, Slate DJ, Paetsch CR, Dorfmann AL, Trimmer BA. Scaling of caterpillar body properties and its biomechanical implications for the use of a hydrostatic skeleton. J Exp Biol. 2011;214:1194–1204. doi: 10.1242/jeb.051029. [DOI] [PubMed] [Google Scholar]
- 37.Houston AI, McNamara JM, Hutchinson JMC. General results concerning the trade-off between gaining energy and avoiding predation. Philos Trans R Soc Lond B Biol Sci. 1993;341:375–397. [Google Scholar]
- 38.Boeing WJ, Ramcharan CW, Riessen HP. Multiple predator defence strategies in Daphnia pulex and their relation to native habitat. J Plankton Res. 2006;28:571–584. [Google Scholar]
- 39.Steiner UK, Pfeiffer T. Optimizing time and resource allocation trade-offs for investment into morphological and behavioral defense. Am Nat. 2007;169:118–129. doi: 10.1086/509939. [DOI] [PubMed] [Google Scholar]
- 40.Van Handel E, Day JF. Assay of lipids, glycogen and sugars in individual mosquitoes: Correlations with wing length in field-collected Aedes vexans. J Am Mosq Control Assoc. 1988;4:549–550. [PubMed] [Google Scholar]
- 41.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.