Abstract
NK-lysin is an effector protein of the innate immune system and an important component of host protection. We isolated a SNP in the NK-lysin coding sequence among different chicken breeds. This A to G substitution at the position 271 nucleotide in the ORF results in an Asn (N) to Asp (D) amino acid alteration. We synthesized two 30-aa peptides (N29N and N29D) to compare the biological activity of the helix 2-loop-helix 3 region of NK-lysin resulting from the polymorphic gene. Both peptides were found to be cytotoxic in bacteria and tumor cell cultures at micromolar concentrations. The N29N peptide, however, exhibited greater antibacterial and anticancer activity than the N29D peptide. Circular dichroism spectroscopy of the two peptides in negatively charged single unilamellar vesicles showed spectra typical of α-helical peptides. The helical profile of N29D was reduced substantially compared with that of N29N. However, no structural change was observed in neutral vesicles. ζ-Potential measurements of liposomes incubated with increasing peptide concentrations allowed surface charge neutralization with a negatively charged lipid, but not with a zwitterionic lipid. This result suggests that a difference in electrostatic interaction between lipid membranes and the helical peptides results from the polymorphic gene and is subsequently an important factor in cell lytic activity of variant NK-lysin peptides.
Keywords: antimicrobial peptides, genetic variation, innate immunity
The antimicrobial peptides (AMPs) are important elements of the first line of defense in animals against pathogens, and an important constituent of human innate immunity. AMPs act on a rather broad spectrum of microbial organisms that often belong to the common or natural flora associated with the animal (1), and have been isolated and characterized in organisms ranging from prokaryotes to humans, where they are expressed in a variety of tissues. These peptides generally range from 12 to 50 aa in length, with estimated molecular weights of 2 to ∼6 kDa. Amphipathic secondary structures of AMPs are thought to act by a rapid destruction of the membrane rather than by interaction with specific receptors. However, little is known about the molecular basis for their action, despite their efficacy in animal models of experimentally induced sepsis (2). Cathelicidin and defensin families are the best known AMPs, followed by dermcidin and NK-lysin. NK-lysins are much larger peptides than the classic antimicrobial peptides and have a globular 3D structure. Their primary sequences are rich in positively charged amino acids and contain conserved cysteines that make intradisulfide bonds (3).
NK-lysin is a cationic antibacterial peptide that was originally isolated from porcine intestinal tissue based on its antibacterial activity (4). NK-lysin was also found in the granules of T lymphocytes and natural killer (NK) cells. NK-lysin is a member of saposin-like protein family and is orthologous with human granulysin. The structural and antimicrobial activities of NK-lysin have been investigated for more than a decade (5–7). The microbiocidal and tumor cytolytic activities of NK-lysin, like those of other saposin-like proteins, are believed to be a result of its ability to form pores in the cell membrane because of its α-helical structure (8). Hydrophobicity, cationicity, and relative size of hydrophobic and hydrophilic domains of AMPs generate diverse effects on membranes (9), although their specific contributions to activity are not known. There is little homology among AMPs except for their amphipathic topology, and active antimicrobial peptides can have different types of secondary structure (9).
The genes modulating innate immunity are candidates for providing a relationship between host genomic variation and response to pathogens (10–13). SNPs or other mutations in AMP genes could potentially lead to altered gene function and differential susceptibility to infection (13–16). Granulysin and NK-lysin are polymorphic in nature, with several sequence variations known in pigs (4), cattle (17), and human (18). Although the roles of mammalian NK-lysin and granulysin in the immune response and their polymorphisms have been studied, there has been little study in avian species (19–21). We identified a polymorphism in the chicken NK-lysin gene and discovered functional differences in antimicrobial activity and cancer cell killing in the protein variants encoded by the SNP alleles. Charge difference in the peptide products of the alleles were explored to account for antimicrobial and cytotoxic functional differences.
Results
SNP in the Coding Sequence.
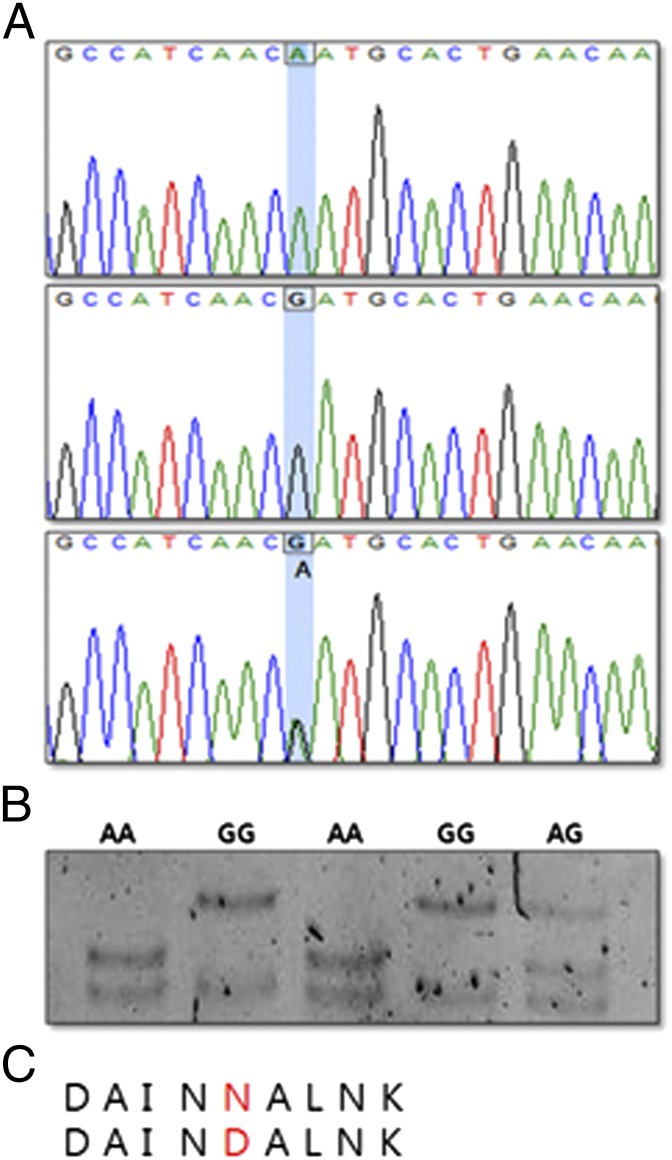
To identify potential polymorphism in different chicken breeds, we screened the coding region of the NK-lysin gene through a PCR-sequencing strategy in a sample of 115 individual chickens. Comparison of the NK-lysin coding sequence of domestic chicken breeds, White Leghorn and Cornish, revealed one SNP, an A to G nucleotide substitution at nucleotide position 271 compared with the nucleotide sequence in GenBank. This is a nonsynonymous SNP, corresponding to position 29 (Asn-29 Asp) in the NK-lysin amino acid sequence (Fig. 1C). Individuals of three genotypes (AA, GG, and AG) were sequenced (Fig. 1A), and three unique single-strand conformation polymorphism (SSCP) banding patterns (AA homozygote, GG homozygote, and AG heterozygote) were observed after PCR-SSCP electrophoresis (Fig. 1B).
Fig. 1.
SNPs of the NK-lysin gene in chicken. (A) The chromatograms of nucleic acid sequence revealed an A-to-G transition. (B) PCR-SSCP showed three different patterns (lanes 1 and 3, AA genotype; lanes 2 and 4, GG genotype; lane 5, AG genotype). (C) Asn (N) to Asp (D) amino acid alteration produced by nonsynonymous SNP.
Allele and genotype frequencies of the NK-lysin gene are in Hardy–Weinberg equilibrium within each of the two breeds, as presented in Table 1. The frequency of the A allele was 0.89 in White Leghorn and 0.52 in Cornish. Not unexpectedly, a homozygous GG genotype was not detected in this small number of 62 White Leghorn individuals.
Table 1.
Genotype and allele frequencies of NK-lysin in two chicken breeds
| Allele frequency |
Genotype frequency |
|||||
| Breed (no.) | G | A | GG | GA | AA | χ2 (P value) |
| White Leghorn (62) | 0.11 | 0.89 | 0 | 0.23 | 0.77 | 0.3163 |
| Cornish (53) | 0.48 | 0.52 | 0.21 | 0.55 | 0.24 | 0.4851 |
Antibacterial Activity of NK-Lysin–Derived Synthetic Peptides.
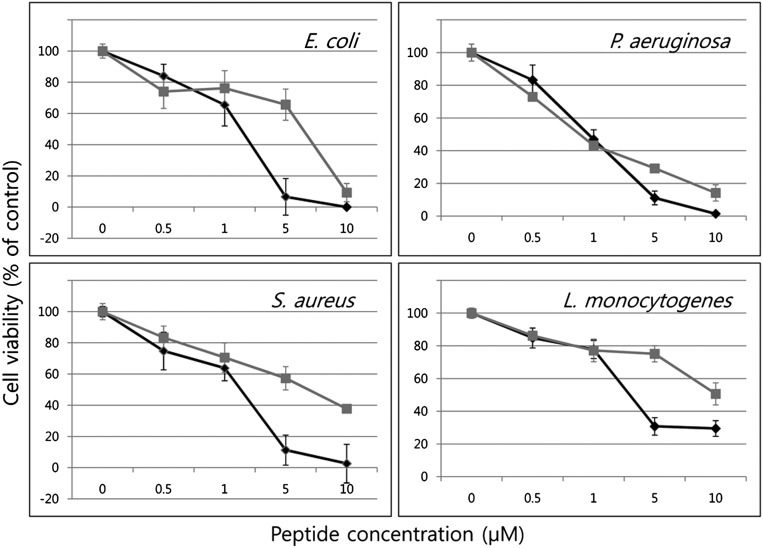
We synthesized two peptides to compare NK-lysin biological activity resulting from the A and G alleles and designated them as N29N (Asn) and N29D (Asp), respectively. The peptide fragments of 30 amino acids, residues 22–51, involving the helix 2-loop-helix 3 region of chicken NK-lysin were synthesized (Table 2). To test antibacterial properties of N29N and N29D synthetic peptides, two Gram-negative and two Gram-positive bacteria strains were used. Both NK-lysin peptides were broadly effective against all tested bacteria, although Gram-negative bacteria were more sensitive to both peptides than were Gram-positive bacteria. N29N and N29D were most active against Gram-negative Pseudomonas aeruginosa and Escherichia coli and moderately active against Staphylococcus aureus. Listeria monocytogenes was more resistant to the peptides than the other bacteria (Fig. 2). All bacteria showed similar viability with up to 1 μM of both peptides, but considerable change was observed with peptide concentrations above 1 μM. Only 10% of E. coli survived 5 μM N29N, whereas 70% survived the same concentration of N29D (Fig. 2). Although the difference in killing of P. aeruginosa was less dramatic than the other three, N29N is clearly more effective in antibacterial activity than N29D against all bacteria at higher concentrations (Fig. 2).
Table 2.
Sequences and properties of NK-lysin peptides
| Peptide | Sequence | Length (aa) | Mw | Net charge | Hydrophobic ratio |
| N29N | PDEDAINNALNKVCSTGRRQRSICKQLLKK | 30 | 3399.9 | 3.9 | 33 |
| N29D | PDEDAINDALNKVCSTGRRQRSICKQLLKK | 30 | 3400.9 | 2.9 | 33 |
Mw, molecular weight. Substituted amino acid is boldface.
Fig. 2.
Antibacterial properties of N29N and N29D peptides against Gram-negative bacteria, E. coli and P. aeruginosa, and Gram-positive bacteria, S. aureus and L. monocytogenes in black and gray color, respectively. Different concentrations of N29N and N29D peptides were incubated with each bacterium and its viability compared with control.
Killing Activity Against Target Cancer Cells.
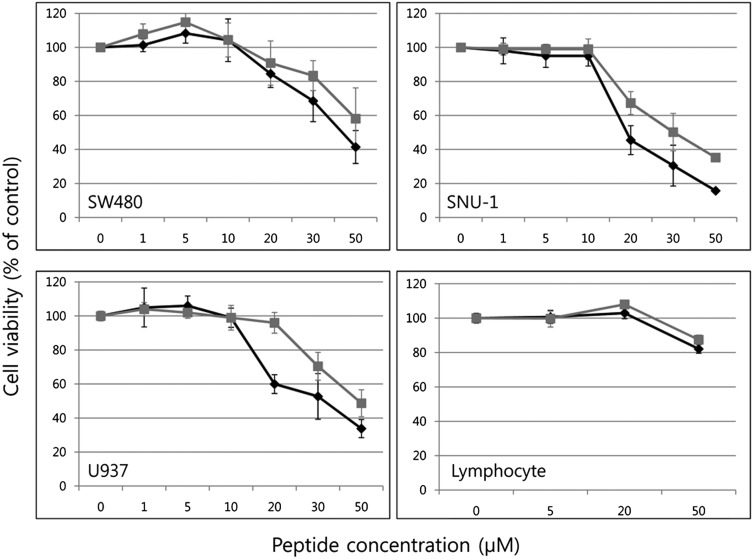
We used a cell viability assay to determine cytotoxicity of N29N and N29D peptides against human coloretal adenocarcinoma cancer cells (SW480), human histolytic lymphoma cancer cells (U937), human gastronic cancer cells (SNU-1), and normal human lymphocytes in complete medium containing 10% (vol/vol) serum. Cells were incubated with peptide concentrations of 1, 5, 10, 20, 30, and 50 μM for 24 h and viability was assayed. Most strikingly, normal lymphocytes were only slightly affected, even at 50-μM concentrations of either peptide (Fig. 3). In contrast, the three cancer cell lines were sensitive to N29N and N29D. Both peptides were broadly effective against all tested cancer cell lines with SNU-1 being more sensitive than the others. There were slightly different effects between two peptides with 1- to 10-μM peptide concentrations, but N29N showed increased activity over N29D at 20 μM on all treated cells, ranging from a 6% (SW480) to a 36% (U937) increase. N29N thus demonstrated greater anticancer activity than N29D against all cancer cells tested in this study.
Fig. 3.
Cytotoxicity of N29N and N29D determined by cell viability assay against SW480, U937, SNU-1 and normal human lymphocytes. Cells were incubated with peptide concentrations of 1, 5, 10, 20, 30, and 50 μM. N29N and N29D treatments are represented in black and gray, respectively. The average was calculated from four independent experiments.
Apoptotic Cell Death.
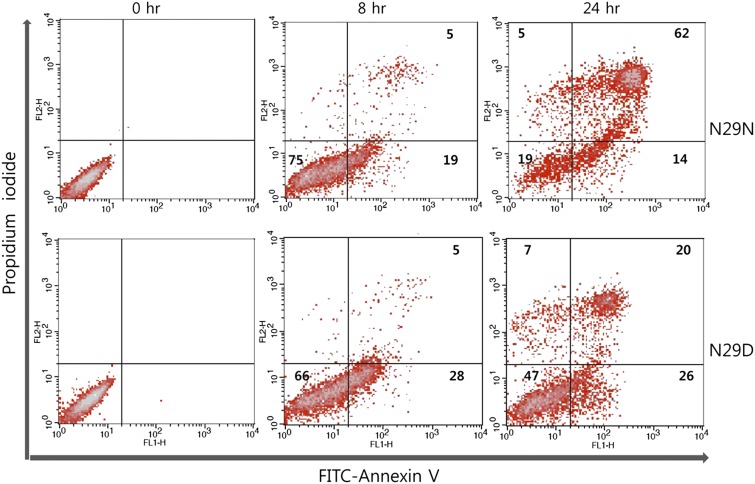
NK-lysin–induced cell death was analyzed by FACS and compared with cell viability assay results. Both N29N- and N29D-treated cells were heavily represented in the annexin V+ sorting, where 76% and 46% of the entire cell population of SNU-1 cells were found after 24 h, respectively (Fig. 4). Interestingly, after 8 h, 28% of the N29D cells were early apoptotic [annexin V/propidium iodide (annexin V+/PI−)] compared with 19% of the N29N-treated cells. Both treatments resulted in 5% late apoptotic cells (annexin V+/PI+). However, by 24 h a total of 76% of the N29N-treated cells were apoptotic, with 62% in the late apoptotic class. N29D-treated cells were only 46% apoptotic with 20% in the late apoptotic class at 24 h. Although we cannot distinguish between cells in late apoptosis and necrotic dead cells in the annexin V+/PI+ category in this assay, it is evident that N29N induces more complete apoptosis in SNU-1 cells than N29D in a 24-h treatment assay.
Fig. 4.
NK-lysin induced apoptosis in SNU-1 cells—FACS flow cytometry. After cell staining with annexin V-FITC and PI, the apoptotic cells (annexin V+/PI− and annexin V+/PI+) were analyzed by a dot-plot using a flow cytometer. The numbers in the quadrants of each plot indicate the percentage of annexin-positive (apoptotic) cells. The figure is representative of three replicates.
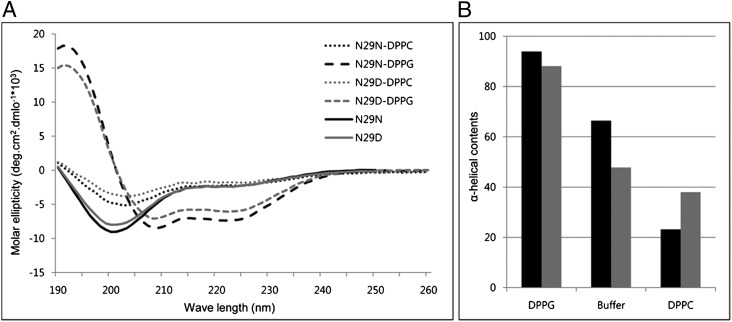
Effects of Lipid Binding on the Secondary Structure of NK-Lysin.
Far-UV circular dichroism (CD) was used to determine the secondary structure of the NK-lysin peptides in lipid-free and lipid-bound states. The CD spectrum of N29N and N29D in aqueous buffer (pH 7.2) displayed a single negative minimum at ∼200 nm, suggesting a less organized form in solution (Fig. 5A). However, upon the addition of dipalmitoylphosphatidyglycerol (DPPG) liposomes the spectra displayed two broad minima at ∼208 and ∼222 nm, indicating the existence a helical structure. This result suggests a conformational transition into an α-helical structure upon binding to anionic vesicles. N29N has a higher degree of helicity than N29D (Fig. 5B). Of interest, addition of dipalmitoylphosphatidylcholine (DPPC) small unilamellar veiscles (SUVs) to N29N and N29D did not induce any observed transition in the conformation of the peptide. This finding might indicate that the peptide does not bind to the zwitterionic DPPC membranes, or it binds too weakly to induce conformational change. This result is consistent with many of the α-helical peptides, which only become helical with anionic phospholipid membranes (22).
Fig. 5.
(A) CD spectra of N29N and N29D peptides in 10 mM phosphate buffer (solid line), DPPC liposomes (dotted line), and DPPG liposomes (dashed line) in black and gray color, respectively. (B) α-Helical contents of N29N and N29D using CDNN deconvolution software.
Surface Charge Compensation by NK-Lysin Peptide.
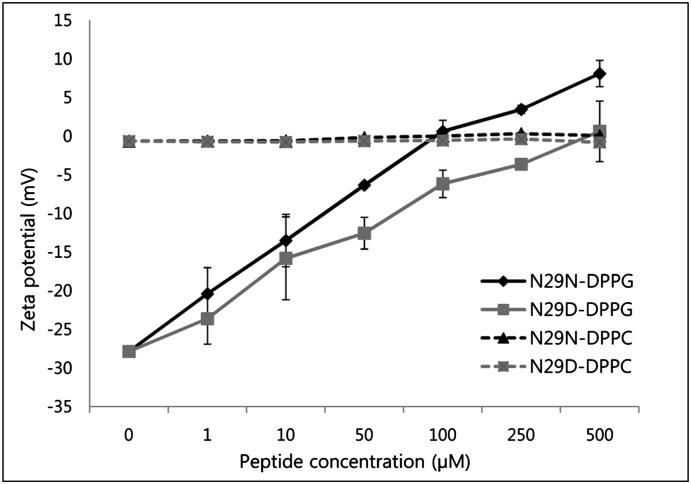
ζ-Potential measurement is a powerful technique to characterize and monitor surface modification and can be used to quantify protein/lipid interactions of a wide variety of charged molecules, such as AMPs (23). Electrostatic interaction between anionic phospholipids, common in bacterial and fungal membranes, and cationic AMPs is a fundamental driving force for adsorption with most AMPs (24). To evaluate whether the peptides interact with liposomes by electrostatic properties, interactions between peptides and SUVs were examined by ζ-potential measurement. As expected, the interaction of the positively charged peptides with negatively charged DPPG vesicles causes reduction in the magnitude of the surface charge of the membrane (Fig. 6). Increasing peptide concentrations lead to the neutralization of the negative charge and even an overcompensation of the liposomes. N29N neutralized DPPG more rapidly than N29D in low concentrations. Only slight ζ-potential changes were observed when DPPC vesicles were incubated with both peptides. This finding indicates a weak or even no interaction of the peptide with neutral lipids. Cationicity is important for the initial electrostatic attraction of antimicrobial peptides to negatively charged phospholipid membranes of bacteria (22, 25, 26). This result with N29N and N29D peptides, with hydrophobicity and amphipathicity kept constant, showed that increasing the charge from +2.9 to +3.9 results in increasing antibacterial activities against Gram-negative and Gram-positive bacteria.
Fig. 6.
Effects of N29N and N29D on the ζ-potential properties of phospholipid liposomes. Peptide concentrations of 0 to 500 μM were tested for negatively charged DPPG and neutral DPPC liposomes to determine ζ-potential. Data shown for ζ-potential represent mean values from at least two independent liposome preparations.
Discussion
NK-lysin is a lymphocytic AMP and a member of the membrane-interacting, saposin-like protein family. Mammalian NK-lysin has been studied intensively over the last two decades since the discovery of porcine NK-lysin for its possible therapeutic application (4, 20, 27–30), but chicken NK-lysin has received relatively little attention. We identified NK-lysin coding sequence variation within two chicken breeds, White Leghorn and Cornish, and have investigated the relationship of genetically distinct NK-lysin alleles to functional variation and immunity.
Several synthetic peptide fragments derived from granulysin and NK-lysin have been investigated to determine the minimal active subunit of antimicrobial activity (6, 31, 32). The peptides corresponding to helix 2 and helix 3 are known to be important for the cytolytic activity of granulysin against bacteria and human cells (32). Allelic variation in the helix 2 region provides an opportunity to examine the effect of naturally occurring amino acid alteration on NK-lysin cell lytic activity. Thus, we designed peptides from the helix 2 and helix 3 regions and compared biological activity of NK-lysin coded by each of the two alleles.
We performed antibacterial and anticancer assays to compare their cytotoxicity, and CD spectra and ζ-potential measurement with SUVs to understand their conformational changes and differences in their electrostatic interactions. The conformational transition from random to α-helix upon lipid binding is thought to provide the energetic source to drive the lipid interaction of amphipathic α-helical AMPs, like apolipoproteins (33), temporins (34), and LL-37 (35). CD analysis showed conformational transition of both peptides to α-helical structure on interaction with anionic membranes but not with zwiterrionic lipids. N29N showed a higher degree of helicity than N29D. The presence of anionic lipids in bacterial membranes enables selective interaction with cationic AMPs (36). Our results demonstrate that different structural transition properties of the two peptides can be one way to differentiate their membrane-modulating activities.
To test possible difference of electrostatic interaction between the two peptides and membranes, ζ-potential measurement was applied after the cationic peptides interacted with anionic SUVs. Cationic NK-lysin peptides N29N and N29D induced reduction of the surface charge of negative SUVs depending on peptide concentration. N29N even showed overcompensation of the liposomes at higher peptide concentration, an entrapment of the peptide beyond electrostatic equivalence, which can be explained by hydrophobic interaction after neutralization of negatively charged liposomes (37). These results corroborate the cytotoxic activity differences in both the antibacterial and anticancer assays and CD-based conformational transition from random to α-helix upon anionic lipid binding. AMPs target negatively charged bacteria by binding to the outmost leaflet of the bacteria by hydrophobic and electrostatic interactions (38).
Difference in electrostatic attraction in the two allelic forms of the peptide is a major factor in modulating NK-lysin activity. Naturally occurring variation in genes responsible for innate immunity can play an important role in host-susceptibility to infectious diseases. Genetic variation in AMPs, such as β-defensins (13), can directly affect susceptibility to diseases. Difference in net charge of the biologically active peptide derived from a nonsynonymous SNP altered NK-lysin activity in chickens and is a potential factor in differential response to pathogens.
In conclusion, our investigation of nonsynonymous SNPs suggests the intriguing possibility that variation in the NK-lysin gene may play a role in inherited differences between individual chickens, and suggests a role of electrostatic charge for antimicrobial and cancer cytotoxicity.
Materials and Methods
Animals.
The care and use of White Leghorn and Cornish chickens were approved by the Institute of Laboratory Animal Resources, Seoul National University (SNU-070823-5) and the Institutional Animal Care and Use Committee at the National Institute of Animal Sciences (No. 2010-000), respectively.
SNP Analysis.
Blood samples were collected from 62 White Leghorn and 53 Cornish chickens. Genomic DNA was extracted from blood using a DNeasy Blood and Tissue kit (Qiagen). The NK-lysin SNP region was synthesized by PCR amplification by using a forward primer of 5′-GGTGCAGAAGATTGTGGGTGA-3′ and a reverse primer of 5′-TTGCAGATACTCCTCTGGCG-3′. The sequences of PCR products of homozygous and heterozygous individuals of different alleles were confirmed by DNA sequencing in both directions. The sequences of chicken NK-lysin were aligned using ClustalW.
PCR-SSCP
Genomic fragments of 105 bp containing the polymorphic sites of the NK-lysin gene were amplified by using primers HRM F1 (5′-AGTTGCAGCCTCACTGACAC-3′) and NK QR1 (5′-TTGCAGA TACTCCTCTGGCG-3′). Two microliters of the PCR products were mixed with 10 μL denaturing solution [98% formamide, 10 mM ethylenediamine tetra acetic acid (EDTA), 0.1% xylene-cyanole and 0.1% bromophenol blue], incubated at 98 °C for 10 min and then chilled on ice. Denatured DNA was loaded onto 15% PAGE gel in 0.5× TBE buffer and electrophoresis was at a constant voltage of 120 V for 6 h. The gel was visualized with silver stain (Bio-Rad).
Peptide Synthesis.
Two 30-aa peptides were synthesized based on coding sequence harboring the nonsynonymous SNP and designated as N29N and N29D, respectively. Peptides were purified to >95% purity grade through reverse-phase HPLCy. Lyophilized peptide was stored in desiccant at −20 °C and dissolved in DMSO and diluted in phosphate buffer (pH 7.2) before use.
Antibacterial Assay.
Gram-negative bacteria (E. coli ATCC25922 and P. aeruginosa ATCC 27853) and Gram-positive bacteria (L. monocytogenes ATCC 19115 and S. aureus subsp. aureus ATCC 25923) were purchased from Korean Collection for Type Culture and tested individually against N29N and N29D. Overnight cultures of bacteria were subcultured for an additional 2–3 h at 37 °C to a midlogarithmic phase, washed once with 10 mM sodium phosphate buffer (pH 7.2), and suspended to a 3 × 105 CFU/mL in the same buffer. Each sample (90 μL) was suspended into 96-well plates, followed by the addition of 10 μL of serial-diluted peptide in triplicate. After 2-h incubation at 37 °C, colonies of surviving bacteria were counted after plating onto agar plates with overnight incubation.
Cytotoxicity Assay.
SW480 (human colorectal adenocarcinoma cancer cells), U937 (human histocytic lymphoma cells), and SNU-1 (human gastronic cancer cells) were obtained from the Korean Cell Line Bank and maintained in RPMI medium 1640 containing 10% (vol/vol) FBS. Normal human lymphocytes, SW480, U937, and SNU-1 were sedimented by centrifugation and washed with RPMI 1640. Ninety microliters of the resulting cell suspension (5 × 104 cells) in RPMI medium 1640 containing 10% FBS were dispensed into each well of a 96-well plate. Ten microliters of each peptide were added to cell suspensions. Cell survival was assayed after 24 h incubation at 37 °C using Ez-CyTox kit (DoGEN). Dye solution was added to each well and the plate was incubated for 2 h at 37 °C and for 5 h in case of lymphocytes. The absorbance was recorded using a 96-well plate reader.
Flow Cytometry Analysis.
Apoptotic cells were measured by the FITC annexin V apoptosis detection kit II (BD Biosciences) according to the manufacturer’s instructions. Briefly, 1 × 105 SNU-1 cells were either untreated or were treated with N29N or N29D peptide in a 50-μM concentration for 24 h and washed twice with cold PBS. After treatment, cells were incubated with FITC annexin V and PI for 15 min at room temperature and analyzed by flow cytometry using a FACS Calibur (BD Biosciences).
Liposome Preparation.
DPPC and DPPG were obtained from Avanti Polar Lipids. The SUV solution was prepared by dissolving the appropriate amounts of lipids in chloroform and methanol (2:1) mixture. The lipid solution was evaporated in a 50-mL round-bottomed flask using a rotary evaporator at 50 °C under vacuum for 10 min. The dried mixture was resuspended in 10 mM sodium phosphate buffer (pH 7.2) and hydrated overnight at 4 °C. The total lipid concentration was 2 mg⋅mL−1. The solution was extruded through a PC membrane with a pore diameter of 100 nm at 50°. The resulting SUVs were collected and stored at 4 °C before use.
CD Spectrum Analysis.
CD spectra were measured in 10 mM sodium phosphate buffer (pH 7.4) at peptide concentrations of 0.3 mg/mL with a JASCO J-715 spectropolarimeter at room temperature. The light path of the cell was 0.2 cm for wavelengths ranging from 190 to 260 nm. All data were expressed as molar ellipticity. The CD spectra results were obtained by averaging three scans and expressed as ellipticity (h) m, (deg⋅cm2⋅mol).
Measurement of ζ-Potential.
ζ-Potentials were determined with a ZetaSizer nano ZS (Malvern Instructure). Measurements were recorded at 25 °C. Peptides were suspended in 10 mM sodium phosphate buffer (pH 7.2) using a phase analysis light scattering mode. The ζ-potential was automatically calculated from electrophoretic mobility based on the Smoluchowski equation. Equal volume of SUV solution of DPPC and DPPG (0.3 mM) were mixed with peptides (0–500 μM) for 5 min and analyzed.
Acknowledgments
We thank Jeong-Yong Seo for advice on liposome synthesis. This research was supported by World Class University Program R31-10056 through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.
Footnotes
The authors declare no conflict of interest.
References
- 1.Boman HG. Antimicrobial peptides. Chairman’s opening remarks. Ciba Found Symp. 1994;186:1–4. [PubMed] [Google Scholar]
- 2.Cirioni O, et al. Single-dose intraperitoneal magainins improve survival in a gram-negative-pathogen septic shock rat model. Antimicrob Agents Chemother. 2002;46:101–104. doi: 10.1128/AAC.46.1.101-104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Wang Y, Xu P, Liu Z. NK-lysin of channel catfish: Gene triplication, sequence variation, and expression analysis. Mol Immunol. 2006;43:1676–1686. doi: 10.1016/j.molimm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Andersson M, et al. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J. 1995;14:1615–1625. doi: 10.1002/j.1460-2075.1995.tb07150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gansert JL, et al. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol. 2003;170:3154–3161. doi: 10.4049/jimmunol.170.6.3154. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs T, Bruhn H, Gaworski I, Fleischer B, Leippe M. NK-lysin and its shortened analog NK-2 exhibit potent activities against Trypanosoma cruzi. Antimicrob Agents Chemother. 2003;47:607–613. doi: 10.1128/AAC.47.2.607-613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenger S, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G, Ross CR, Blecha F. Porcine antimicrobial peptides: New prospects for ancient molecules of host defense. Vet Res. 2000;31:277–296. doi: 10.1051/vetres:2000121. [DOI] [PubMed] [Google Scholar]
- 9.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 10.Barreiro LB, et al. Deciphering the genetic architecture of variation in the immune response to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 2012;109:1204–1209. doi: 10.1073/pnas.1115761109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seabury CM, et al. Diversity and evolution of 11 innate immune genes in Bos taurus taurus and Bos taurus indicus cattle. Proc Natl Acad Sci USA. 2010;107:151–156. doi: 10.1073/pnas.0913006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pankratz VS, Vierkant RA, O’Byrne MM, Ovsyannikova IG, Poland GA. Associations between SNPs in candidate immune-relevant genes and rubella antibody levels: A multigenic assessment. BMC Immunol. 2010;11:48. doi: 10.1186/1471-2172-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasenstein JR, Lamont SJ. Chicken gallinacin gene cluster associated with Salmonella response in advanced intercross line. Avian Dis. 2007;51:561–567. doi: 10.1637/0005-2086(2007)51[561:CGGCAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Rivas-Santiago B, Serrano CJ, Enciso-Moreno JA. Susceptibility to infectious diseases based on antimicrobial peptide production. Infect Immun. 2009;77:4690–4695. doi: 10.1128/IAI.01515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita I, et al. Genetic variants of human beta-defensin-1 and chronic obstructive pulmonary disease. Biochem Biophys Res Commun. 2002;291:17–22. doi: 10.1006/bbrc.2002.6395. [DOI] [PubMed] [Google Scholar]
- 16.Hellgren O, Sheldon BC, Buckling A. In vitro tests of natural allelic variation of innate immune genes (avian β-defensins) reveal functional differences in microbial inhibition. J Evol Biol. 2010;23:2726–2730. doi: 10.1111/j.1420-9101.2010.02115.x. [DOI] [PubMed] [Google Scholar]
- 17.Endsley JJ, et al. Characterization of bovine homologues of granulysin and NK-lysin. J Immunol. 2004;173:2607–2614. doi: 10.4049/jimmunol.173.4.2607. [DOI] [PubMed] [Google Scholar]
- 18.Ericson KG, et al. Sequence analysis of the granulysin and granzyme B genes in familial hemophagocytic lymphohistiocytosis. Hum Genet. 2003;112:98–99. doi: 10.1007/s00439-002-0841-0. [DOI] [PubMed] [Google Scholar]
- 19.Linde CM, et al. Conserved structure and function in the granulysin and NK-lysin peptide family. Infect Immun. 2005;73:6332–6339. doi: 10.1128/IAI.73.10.6332-6339.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong YH, Lillehoj HS, Siragusa GR, Bannerman DD, Lillehoj EP. Antimicrobial activity of chicken NK-lysin against Eimeria sporozoites. Avian Dis. 2008;52:302–305. doi: 10.1637/8083-072307-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 21.Hong YH, et al. Molecular cloning and characterization of chicken NK-lysin. Vet Immunol Immunopathol. 2006;110:339–347. doi: 10.1016/j.vetimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 23.Freire JM, et al. Using zeta-potential measurements to quantify peptide partition to lipid membranes. Eur Biophys J. 2011;40:481–487. doi: 10.1007/s00249-010-0661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strömstedt AA, Pasupuleti M, Schmidtchen A, Malmsten M. Evaluation of strategies for improving proteolytic resistance of antimicrobial peptides by using variants of EFK17, an internal segment of LL-37. Antimicrob Agents Chemother. 2009;53:593–602. doi: 10.1128/AAC.00477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bessalle R, Haas H, Goria A, Shalit I, Fridkin M. Augmentation of the antibacterial activity of magainin by positive-charge chain extension. Antimicrob Agents Chemother. 1992;36:313–317. doi: 10.1128/aac.36.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dathe M, et al. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997;403:208–212. doi: 10.1016/s0014-5793(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 27.Andrä J, Koch MH, Bartels R, Brandenburg K. Biophysical characterization of endotoxin inactivation by NK-2, an antimicrobial peptide derived from mammalian NK-lysin. Antimicrob Agents Chemother. 2004;48:1593–1599. doi: 10.1128/AAC.48.5.1593-1599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson M, Curstedt T, Jörnvall H, Johansson J. An amphipathic helical motif common to tumourolytic polypeptide NK-lysin and pulmonary surfactant polypeptide SP-B. FEBS Lett. 1995;362:328–332. doi: 10.1016/0014-5793(95)00268-e. [DOI] [PubMed] [Google Scholar]
- 29.Davis EG, Sang Y, Rush B, Zhang G, Blecha F. Molecular cloning and characterization of equine NK-lysin. Vet Immunol Immunopathol. 2005;105:163–169. doi: 10.1016/j.vetimm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Gelhaus C, Jacobs T, Andrä J, Leippe M. The antimicrobial peptide NK-2, the core region of mammalian NK-lysin, kills intraerythrocytic Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52:1713–1720. doi: 10.1128/AAC.01342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siano A, et al. Bactericidal and hemolytic activities of synthetic peptides derived from granulysin. Protein Pept Lett. 2010;17:517–521. doi: 10.2174/092986610790963555. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, et al. Hemolysis of erythrocytes by granulysin-derived peptides but not by granulysin. Antimicrob Agents Chemother. 2005;49:388–397. doi: 10.1128/AAC.49.1.388-397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gazzara JA, et al. Interaction of class A amphipathic helical peptides with phospholipid unilamellar vesicles. J Lipid Res. 1997;38:2134–2146. [PubMed] [Google Scholar]
- 34.Mahalka AK, Kinnunen PK. Binding of amphipathic alpha-helical antimicrobial peptides to lipid membranes: Lessons from temporins B and L. Biochim Biophys Acta. 2009;1788:1600–1609. doi: 10.1016/j.bbamem.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Henzler Wildman KA, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 36.Thennarasu S, et al. Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL37. Biophys J. 2010;98:248–257. doi: 10.1016/j.bpj.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domingues MM, Castanho M, Santos NC. rBPI21 promotes lipopolysaccharide aggregation and exerts its antimicrobial effects by (hemi)fusion of PG-containing membranes. PLoS ONE. 2009;4:e8385. doi: 10.1371/journal.pone.0008385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao L, Fang W. Effects of induced tension and electrostatic interactions on the mechanisms of antimicrobial peptide translocation across lipid bilayer. Soft Matter. 2009;5:3312–3318. [Google Scholar]