Abstract
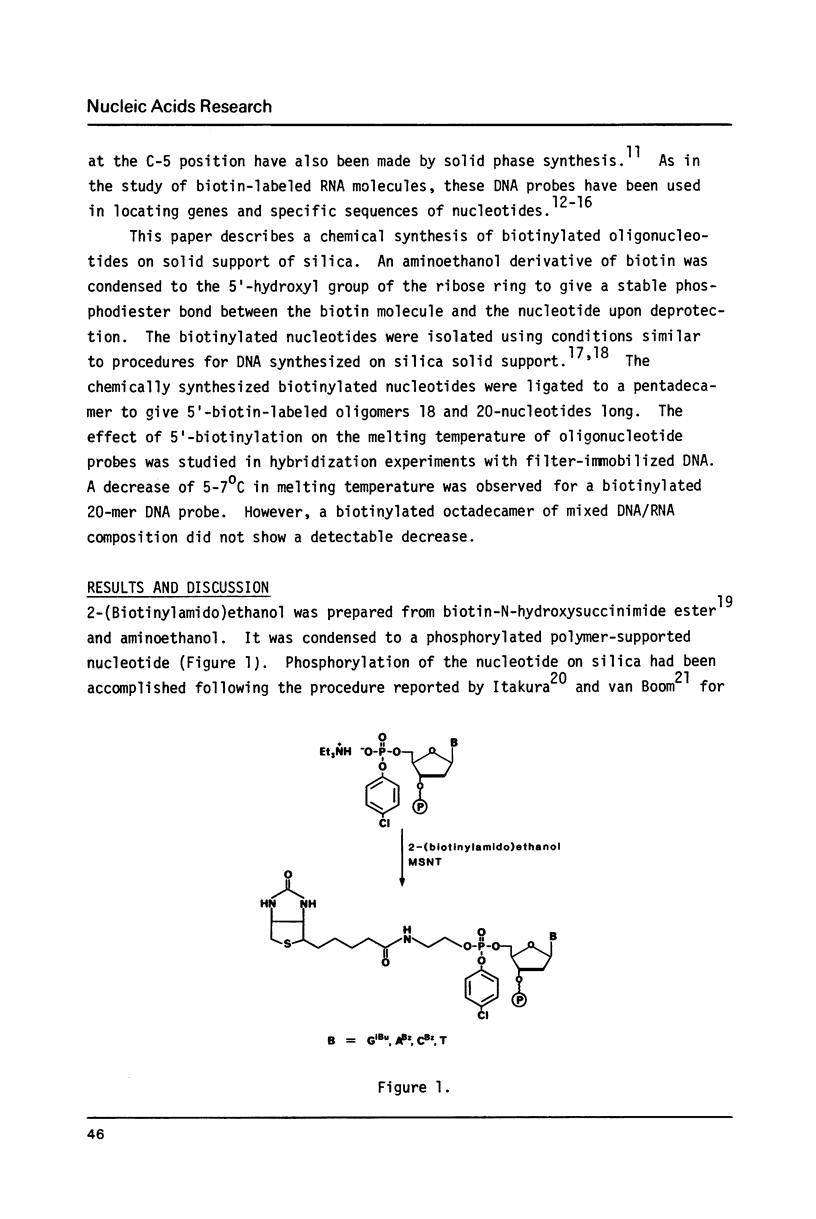
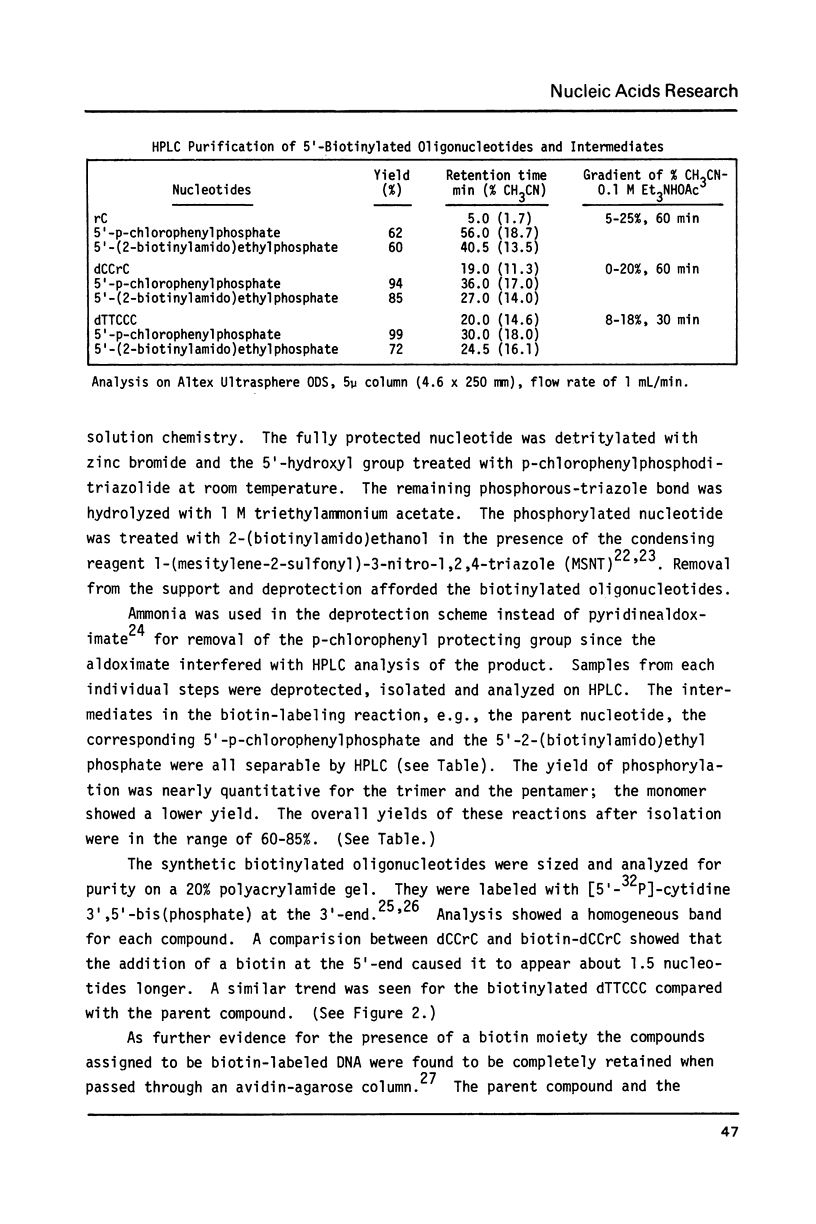
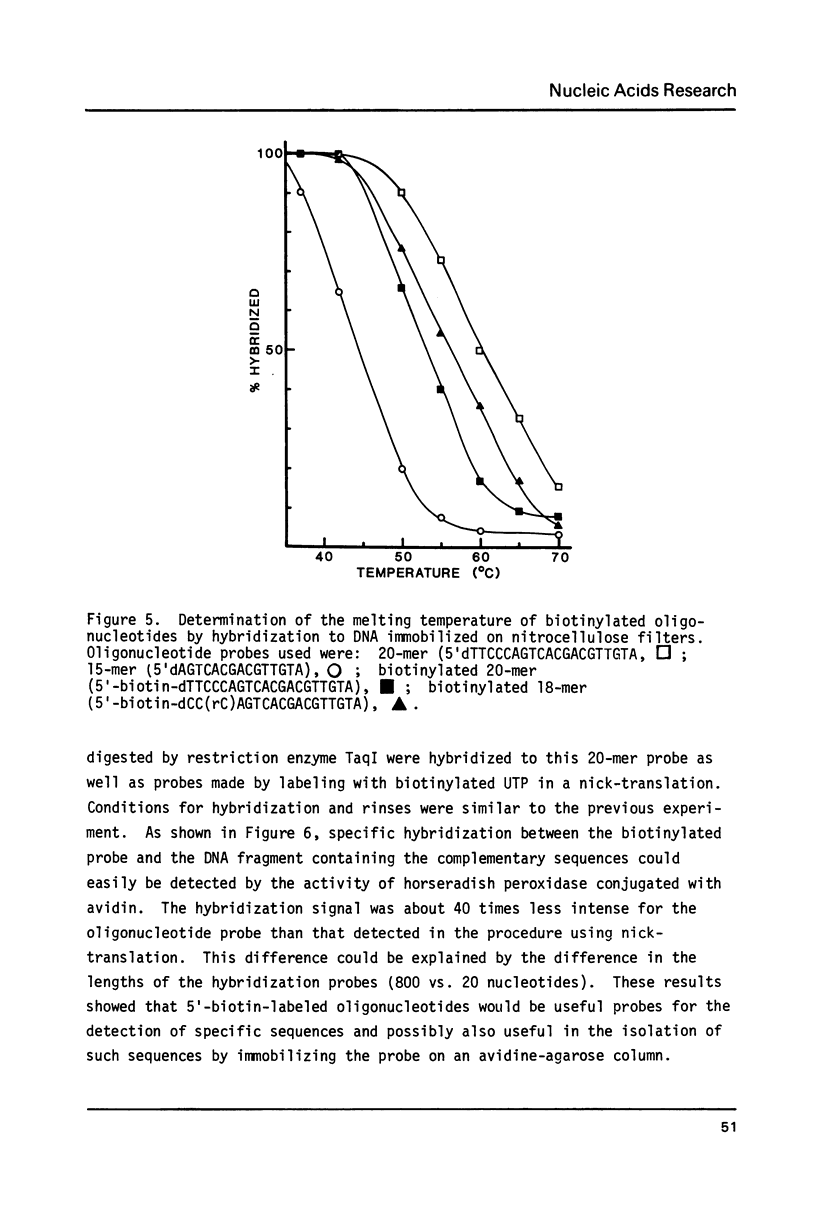
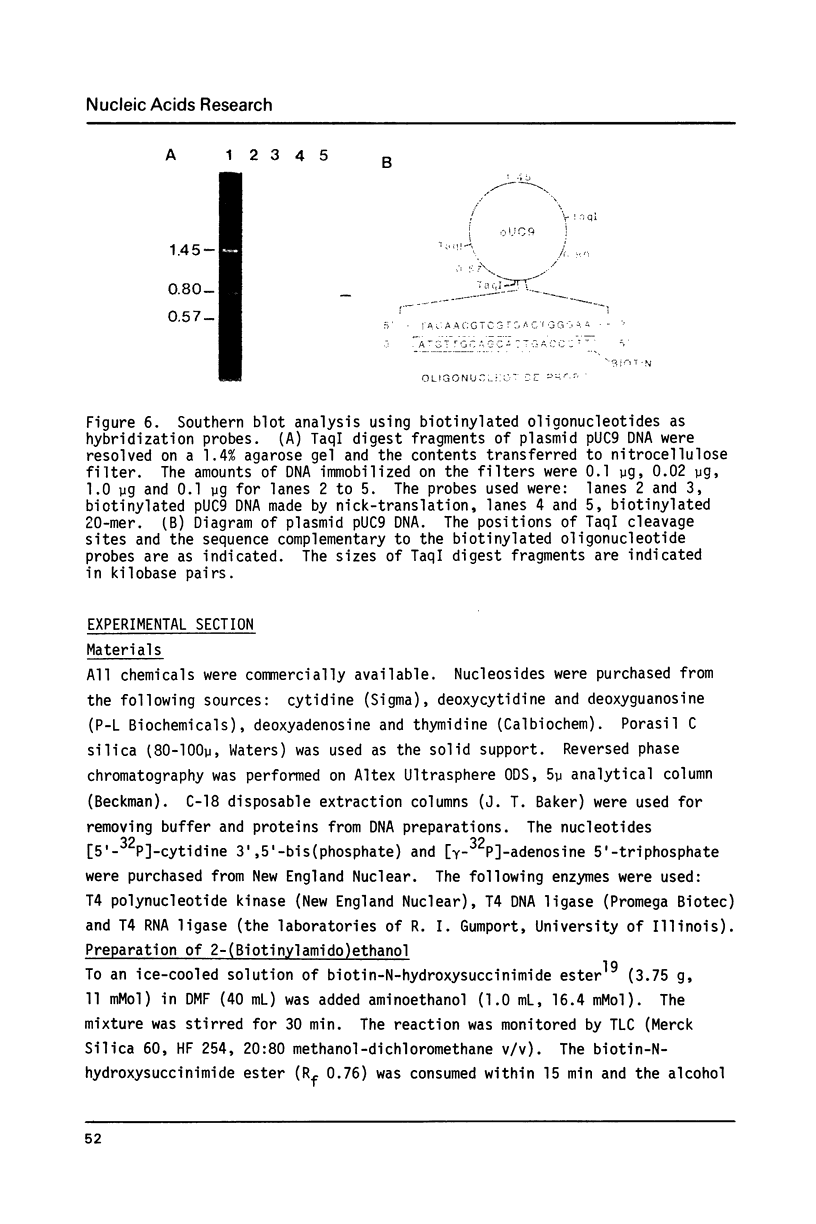
Biotin has been converted to 2-(biotinylamido)ethanol and condensed to phosphorylated oligonucleotides in a solid phase synthesis. The 5'-biotinylated oligonucleotides were enzymatically coupled to other DNA fragments by T4 DNA ligase or T4 RNA ligase. The hybridization properties of such biotin-labeled oligonucleotide probes were studied.
Full text
PDF








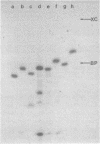
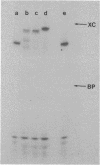
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E., Wilchek M. Insolubilized biotin for the purification of avidin. Methods Enzymol. 1974;34:265–267. doi: 10.1016/s0076-6879(74)34023-2. [DOI] [PubMed] [Google Scholar]
- Brennan C. A., Manthey A. E., Gumport R. I. Using T4 RNA ligase with DNA substrates. Methods Enzymol. 1983;100:38–52. doi: 10.1016/0076-6879(83)00044-0. [DOI] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Angerer L. M., Yen P. H., Hershey N. D., Davidson N. Electron microscopic visualization of tRNA genes with ferritin-avidin: biotin labels. Nucleic Acids Res. 1978 Feb;5(2):363–384. doi: 10.1093/nar/5.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. L., Belagaje R., Ryan M. J., Khorana H. G. Chemical synthesis and cloning of a tyrosine tRNA gene. Methods Enzymol. 1979;68:109–151. doi: 10.1016/0076-6879(79)68010-2. [DOI] [PubMed] [Google Scholar]
- Chow F., Kempe T., Palm G. Synthesis of oligodeoxyribonucleotides on silica gel support. Nucleic Acids Res. 1981 Jun 25;9(12):2807–2817. doi: 10.1093/nar/9.12.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison N. J., Langer-Safer P. R., Ward D. C., Hamkalo B. A. In situ hybridization at the electron microscope level: hybrid detection by autoradiography and colloidal gold. J Cell Biol. 1982 Nov;95(2 Pt 1):609–618. doi: 10.1083/jcb.95.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Ike Y., Ikuta S., Itakura K. Solid phase synthesis of polynucleotides. VI. Further studies on polystyrene copolymers for the solid support. Nucleic Acids Res. 1982 Mar 11;10(5):1755–1769. doi: 10.1093/nar/10.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe T., Chow F., Sundquist W. I., Nardi T. J., Paulson B., Peterson S. M. Selective 2'-benzoylation at the cis 2',3'-diols of protected ribonucleosides. New solid phase synthesis of RNA and DNA-RNA mixtures. Nucleic Acids Res. 1982 Nov 11;10(21):6695–6714. doi: 10.1093/nar/10.21.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehaug J. R., Kleppe K. Effect of salts and polyamines on T4 polynucleotide kinase. Biochemistry. 1975 Mar 25;14(6):1225–1229. doi: 10.1021/bi00677a021. [DOI] [PubMed] [Google Scholar]
- Lillehaug J. R., Kleppe K. Kinetics and specificity of T4 polynucleotide kinase. Biochemistry. 1975 Mar 25;14(6):1221–1225. doi: 10.1021/bi00677a020. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Hershey N. D., Broker T. R., Pellegrini M., Mitchell H. K., Davidson N. A new method of in situ hybridization. Chromosoma. 1975 Nov 24;53(2):107–117. doi: 10.1007/BF00333039. [DOI] [PubMed] [Google Scholar]
- Manning J., Pellegrini M., Davidson N. A method for gene enrichment based on the avidin-biotin interaction. Application to the Drosophila ribosomal RNA genes. Biochemistry. 1977 Apr 5;16(7):1364–1370. doi: 10.1021/bi00626a020. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Langer-Safer P. R., Ward D. C. High-resolution mapping of satellite DNA using biotin-labeled DNA probes. J Cell Biol. 1982 Nov;95(2 Pt 1):619–625. doi: 10.1083/jcb.95.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasugi A., Wallace R. B. Biotin-labeled oligonucleotides: enzymatic synthesis and use as hybridization probes. DNA. 1984 Jun;3(3):269–277. doi: 10.1089/dna.1.1984.3.269. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Richards O. C., Ehrenfeld E., Manning J. Strand-specific attachment of avidin-spheres to double-stranded poliovirus RNA. Proc Natl Acad Sci U S A. 1979 Feb;76(2):676–680. doi: 10.1073/pnas.76.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R. W., Gumport R. I. Biotin and fluorescent labeling of RNA using T4 RNA ligase. Nucleic Acids Res. 1983 Sep 24;11(18):6167–6184. doi: 10.1093/nar/11.18.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., Uhlenbeck O. C. Joining of RNA molecules with RNA ligase. Methods Enzymol. 1983;100:52–59. doi: 10.1016/0076-6879(83)00045-2. [DOI] [PubMed] [Google Scholar]
- Sodja A., Davidson N. Gene mapping and gene enrichment by the avidin-biotin interaction: use of cytochrome-c as a polyamine bridge. Nucleic Acids Res. 1978 Feb;5(2):385–401. doi: 10.1093/nar/5.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swack J. A., Zander G. L., Utter M. F. Use of avidin-sepharose to isolate and idenify biotin polypeptides from crude extracts. Anal Biochem. 1978 Jun 15;87(1):114–126. doi: 10.1016/0003-2697(78)90575-4. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- van der Marel G. A., van Boeckel C. A., Wille G., van Boom J. H. A general method for the synthesis of 5'-monophosphates of DNA fragments via phosphotriester intermediates. Nucleic Acids Res. 1982 Apr 10;10(7):2337–2351. doi: 10.1093/nar/10.7.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]