Abstract
The c-Jun NH2-terminal kinase (JNK) signal transduction pathway is implicated in cancer, but the role of JNK in tumorigenesis is poorly understood. Here, we demonstrate that the JNK signaling pathway reduces the development of invasive adenocarcinoma in the phosphatase and tensin homolog (Pten) conditional deletion model of prostate cancer. Mice with JNK deficiency in the prostate epithelium (ΔJnk ΔPten mice) develop androgen-independent metastatic prostate cancer more rapidly than control (ΔPten) mice. Similarly, prevention of JNK activation in the prostate epithelium (ΔMkk4 ΔMkk7 ΔPten mice) causes rapid development of invasive adenocarcinoma. We found that JNK signaling defects cause an androgen-independent expansion of the immature progenitor cell population in the primary tumor. The JNK-deficient progenitor cells display increased proliferation and tumorigenic potential compared with progenitor cells from control prostate tumors. These data demonstrate that the JNK and PTEN signaling pathways can cooperate to regulate the progression of prostate neoplasia to invasive adenocarcinoma.
The c-Jun NH2-terminal kinase (JNK) signaling pathway can target members of the activating protein 1 (AP1) group of transcription factors, including ATF2, c-Jun, JunB, and JunD (1). These transcription factors represent an important component of the immediate-early gene response to mitogens and inflammatory stimuli (2). AP1 transcription factors are also implicated in dysregulated growth and tumor development (2). Significantly, JNK deficiency suppresses AP1-dependent gene expression and causes defects in cell proliferation, senescence, and apoptosis (3–5). JNK may, therefore, play a role in carcinogenesis.
Studies using mouse models of cancer have confirmed that JNK can play a key role in cancer. Thus, JNK deficiency reduces the development of Bcr/Abl-induced lymphoma (6) and KRas-induced lung tumors (7). Moreover, carcinogen-induced hepatocellular carcinoma (8–10) and skin cancer (11) can be reduced by JNK deficiency. These observations demonstrate that JNK can promote cancer. However, loss of JNK signaling can also promote development of other tumors (2, 9, 12–15). These opposing roles of JNK in tumor development (promotion or repression) may represent differences in JNK function between tumor types (1). Alternatively, these differences may reflect separate functions of JNK in tumor cells and the tumor microenvironment (9).
Mutational inactivation of the tumor suppressor phosphatase and tensin homolog (PTEN) frequently occurs in human prostate cancer (16–18). Mouse models of Pten deficiency in the prostate epithelium demonstrate that loss of PTEN expression is sufficient to cause activation of the AKT signaling pathway, prostatic intraepithelial neoplasia (PIN) lesions, and subsequent development of castration-resistant prostate cancer (19). Loss of PTEN function is, therefore, established to be a key step in the development of prostate cancer. Importantly, PTEN inactivation is associated with increased activity of the JNK signaling pathway in human prostate cancer (20). Indeed, it has been proposed that JNK may be an effector of the PI3K/AKT pathway in prostate cancer with PTEN inactivation (20).
The purpose of this study was to examine the role of the JNK signaling pathway in prostate cancer using a mouse model with selective gene disruption in the prostate epithelium. Previous studies indicate that JNK may be a positive (20) or a negative (21) regulator of prostate cancer development. Here, we report that JNK signaling plays a key role in the development of invasive adenocarcinoma caused by Pten inactivation.
Results
JNK Deficiency in the Prostate Epithelium.
To test the functional role of JNK, we used a model of prostate cancer using mice with conditional (floxed) Pten and selective expression of Cre recombinase in the prostate epithelium (19). We crossed Pten conditional deletion mice to Jnk1−/− and Jnk2−/− mice on the BALB/cJ strain background. The resulting compound mutant mice developed prostatic neoplastic lesions similar to that of Pten single deletion, indicating that JNK1 and JNK2 may be functionally redundant in PTEN-controlled prostate cancer formation. To test this hypothesis, we examined the effect of concomitant deletion of JNK1 plus JNK2 on tumor development. Because Jnk1−/− Jnk2−/− compound mutant mice die during midembryogenesis (1), we used a conditional (floxed) deletion approach. Mice with dual deficiency of Jnk1 plus Jnk2 in the prostate epithelium (ΔJnk mice) were viable and fertile. Histopathological analysis of sections prepared from the prostate gland of wild-type (WT) mice and ΔJnk mice indicated that JNK was not required for prostate gland development (Fig. S1).
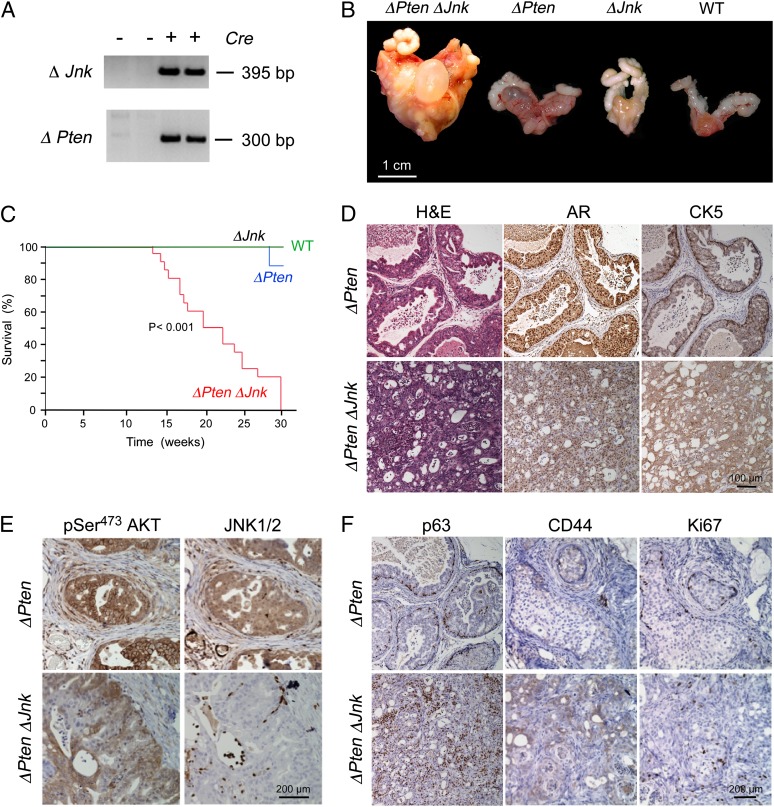
Mice with triple deficiency of Jnk1, Jnk2, plus Pten (ΔJnk ΔPten mice) in the prostate epithelium were also found to be viable and fertile (Fig. 1A). However, the majority of the ΔJnk ΔPten male mice died by age 20 wk with large prostate tumors and urethral obstruction (Fig. 1B). In contrast, the mean lifespan of mice with Pten deficiency alone (ΔPten mice) was greater than 80 wk. Kaplan–Meier analysis demonstrated that the lifespan of ΔJnk ΔPten mice was significantly shorter than WT mice, ΔJnk mice, or ΔPten mice (Fig. 1C). Moreover, the primary tumors present in ΔJnk ΔPten mice were significantly larger than ΔPten mice at age 20 wk (Fig. 1B). Microscopic analysis of sections prepared from these primary tumors demonstrated that the ΔJnk ΔPten mice displayed significant disruption of prostate glandular structure compared with ΔPten tumors (Fig. 1D), consistent with a more advanced tumor phenotype. Indeed, we detected PIN lesions in the primary tumors of ΔPten mice and invasive adenocarcinoma in the primary tumors of ΔJnk ΔPten mice at age 20 wk (Fig. 1D). Immunohistochemical analysis demonstrated the presence of activated AKT in both ΔPten and ΔJnk ΔPten primary tumors, but JNK was detected only in ΔPten tumors (Fig. 1E). Nuclear androgen receptors were detected in both ΔPten and ΔJnk ΔPten tumors (Fig. S2).
Fig. 1.
Loss of JNK cooperates with Pten deficiency to promote prostate cancer. (A) Genomic DNA isolated from the anterior prostate gland of ΔPten ΔJnk mice (Cre+ and Cre−) was examined by PCR using amplimers designed to detect the deleted Pten and Jnk alleles. (B) Representative prostate glands of WT, ΔJnk, ΔPten, and ΔPten ΔJnk mice (age 20 wk) are illustrated. (C) Kaplan–Meier analysis of the survival of WT, ΔJnk, ΔPten, and ΔPten ΔJnk mice. The lifespan of ΔPten ΔJnk mice was significantly shorter than ΔPten mice (P < 0.001). (D) Sections of the anterior prostate of ΔPten and ΔPten ΔJnk mice were stained with H&E or with antibodies to the androgen receptor (AR) or CK5. (E) Sections were stained with antibodies to pSer473 AKT or JNK1/2. (F) Sections were stained with antibodies to p63, CD44, or Ki67.
It is established that JNK can influence proliferation, senescence, and apoptosis (1). The larger prostate tumors in ΔJnk ΔPten mice compared with ΔPten mice (Fig. 1) could, therefore, reflect increased growth and/or decreased apoptosis. The majority of cells detected in ΔPten PIN lesions expressed senescence-associated β-galactosidase and did not stain for the proliferation marker Ki67 (Fig. S3). It is established that cellular senescence in ΔPten PIN lesions limits prostate cancer progression (22, 23). Studies of ΔJnk ΔPten adenocarcinoma cells demonstrated no expression of senescence-associated β-galactosidase and markedly increased Ki67 staining (Fig. S3). No differences in apoptosis were detected between ΔPten and ΔJnk ΔPten primary tumors (Fig. S3). Together, these data indicate that JNK deficiency in ΔPten mice increases prostate cancer development by increasing tumor growth.
Disruption of the JNK Pathway in the Prostate Epithelium.
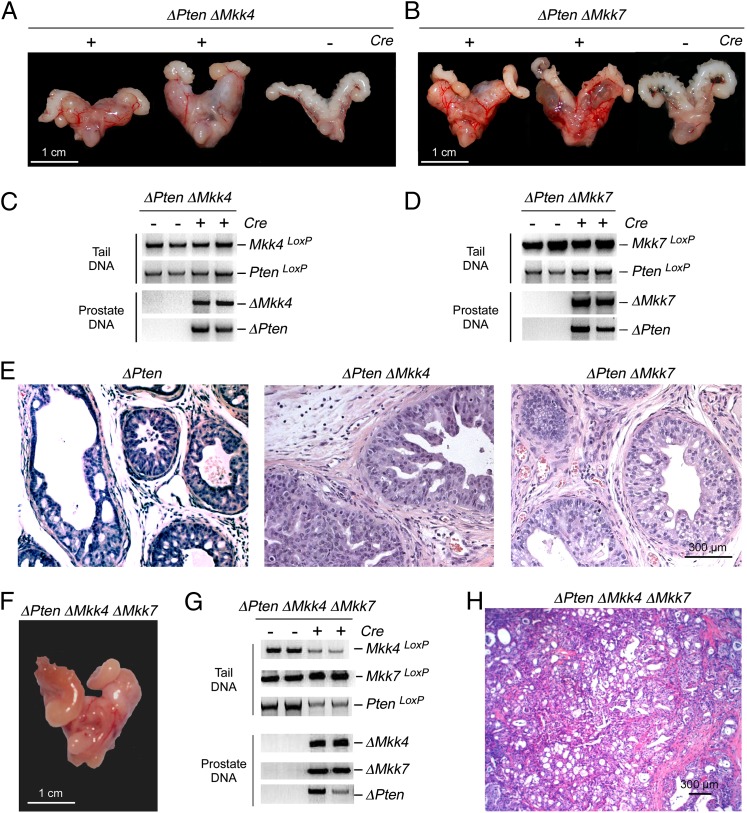
The increased tumor burden in ΔJnk ΔPten mice compared with ΔPten mice (Fig. 1) indicates that JNK may play an important role during prostate cancer development. However, it is unclear whether these data reflect a role of JNK signaling because functions of nonactivated JNK have been reported (24). To test whether JNK signaling contributes to prostate cancer progression, we examined the effect of deficiency of the MAP kinase kinases (MKK4 and MKK7) that phosphorylate and activate JNK (1). It is established that Mkk4−/− and Mkk7−/− mice are not viable (1). We, therefore, used conditional alleles to selectively disrupt Mkk4 and Mkk7 in the prostate epithelium of ΔPten mice (Fig. 2 A–D). This analysis demonstrated similar prostate cancer in ΔMkk4 ΔPten mice and ΔMkk7 ΔPten mice. Analysis of tumor sections indicated that the ΔMkk4 ΔPten and ΔMkk7 ΔPten neoplastic lesions exhibited greater luminal disruption than ΔPten tumors (Fig. 2E), but these tumors did not resemble the invasive carcinoma detected in ΔJnk ΔPten mice (Fig. 1). MKK4 and MKK7 have partially redundant functions, and complete ablation of the JNK pathway requires compound mutation of both Mkk4 and Mkk7 (25). We, therefore, examined prostate tumor development in ΔMkk4 ΔMkk7 ΔPten mice. Compound deficiency of MKK4 plus MKK7 in the prostate epithelium of ΔPten mice caused development of invasive adenocarcinoma that was similar to ΔJnk ΔPten mice (Fig. 2 F–H). The similarity of prostate tumors in ΔJnk ΔPten mice (Fig. 1) and ΔMkk4 ΔMkk7 ΔPten mice (Fig. 2) indicates that JNK signaling plays an important role in ΔPten-dependent prostate carcinogenesis.
Fig. 2.
Effect of Mkk4 and Mkk7 gene ablation on ΔPten-dependent prostate cancer. (A and B) Representative images of prostate glands from ΔMkk4 ΔPten and ΔMkk7 ΔPten mice (age 20 wk) are illustrated. (C and D) Genomic DNA isolated from the prostate gland and tail of ΔMkk4 ΔPten (C) and ΔMkk7 ΔPten mice (D) were genotyped for Mkk4, Mkk7, and Pten alleles. (E) Representative H&E-stained tissue sections of the anterior prostate glands of ΔPten mice, ΔMkk4 ΔPten mice, and ΔMkk7 ΔPten mice (age 20 wk) are presented. (F–H) Representative image of a ΔMkk4 ΔMkk7 ΔPten prostate gland (20-wk-old mouse) is illustrated (F). Genomic DNA isolated from the prostate gland and tail of ΔMkk4 ΔMkk7 ΔPten mice were genotyped for Mkk4, Mkk7, and Pten alleles (G). A representative H&E-stained tissue section prepared from the anterior prostate gland of a ΔMkk4 ΔMkk7 ΔPten mouse (20 wk old) is presented (H).
Androgen Dependence of JNK-Deficient Prostate Tumors.
A hallmark of advanced prostate cancer is the progression to androgen independence (26). Studies of ΔPten mice demonstrate that castration (androgen withdrawal) causes dramatic tumor regression followed by the subsequent development of castration-resistant prostate cancer (19). In contrast, castration-induced tumor regression was suppressed in ΔJnk ΔPten mice compared with ΔPten mice (Fig. S4). The ΔJnk ΔPten tumors from castrated mice exhibited increased glandular disruption, decreased E-cadherin expression, and disorganized expression of α-smooth muscle actin compared with ΔJnk ΔPten tumors (Fig. S5). The aggressive prostate cancers detected in ΔJnk ΔPten mice therefore markedly differ from tumors detected in ΔPten mice in their response to androgen withdrawal. This observation is consistent with a more advanced tumor phenotype in ΔJnk ΔPten mice compared with ΔPten mice.
JNK and Prostate Tumor Metastasis.
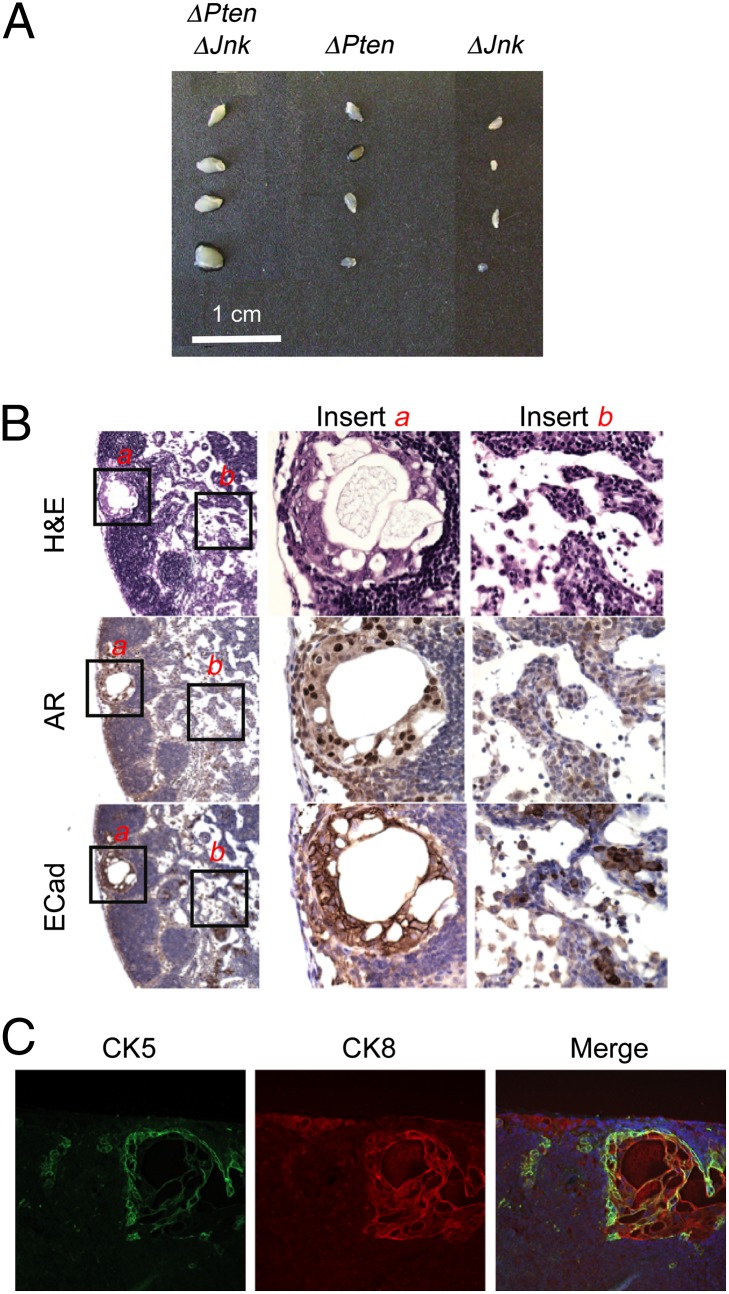
Pathological examination of ΔJnk ΔPten mice indicated the presence of metastatic cells at sites distant from the primary tumor. The lumbar and caudal lymph nodes are established to be preferential sites for metastasis in orthotopic mouse models of prostate cancer (27, 28). Metastasis of ΔPten prostate tumor cells to these lymph nodes has been reported (29). We found that the lumbar lymph nodes of ΔJnk ΔPten mice were enlarged compared with ΔPten mice at age 20 wk (Fig. 3A). Androgen-receptor–positive metastatic cells in the lymph nodes of ΔJnk ΔPten mice were detected (Fig. 3B). These metastatic cells formed organized duct-like structures with basal expression of cytokeratin (CK)5 and luminal expression of CK8 that resemble prostate epithelium (Fig. 3C). In contrast, metastasis was not detected in ΔPten mice (Fig. 3). The failure to detect metastasis in ΔPten mice is consistent with previous observations (23) but differs from the low incidence of metastasis detected in studies of ΔPten mice on a mixed genetic background (19). Together, these observations are consistent with the conclusion that ΔJnk ΔPten mice rapidly develop advanced prostate cancer.
Fig. 3.
Loss of JNK promotes prostate tumor metastasis. (A) Lumbar lymph nodes isolated from ΔJnk, ΔPten, and ΔPten ΔJnk mice (age, 20 wk) are illustrated. (B) Representative sections of ΔPten ΔJnk lumbar lymph nodes were stained with H&E or with antibodies to the androgen receptor (AR) or E-cadherin (ECad). (C) Representative sections of ΔPten ΔJnk lumbar lymph nodes were stained with antibodies to CK5 and CK8. The merged image includes the DNA stain DAPI.
JNK Regulates the Tumorigenic Potential of ΔPten Prostate Tumor Cells.
It is established that ablation of Pten causes an increase in the immature cell compartment within the prostate gland (30). However, the prostate glands of ΔJnk ΔPten mice were found to contain a much larger population of immature prostate cells that stained with antibodies to p63 and cluster of differentiation (CD)44 compared with ΔPten mice at age 20 wk (Fig. 1F). This expansion of immature prostate cells was also detected in castrated mice (Fig. S6) and is, therefore, androgen-independent.
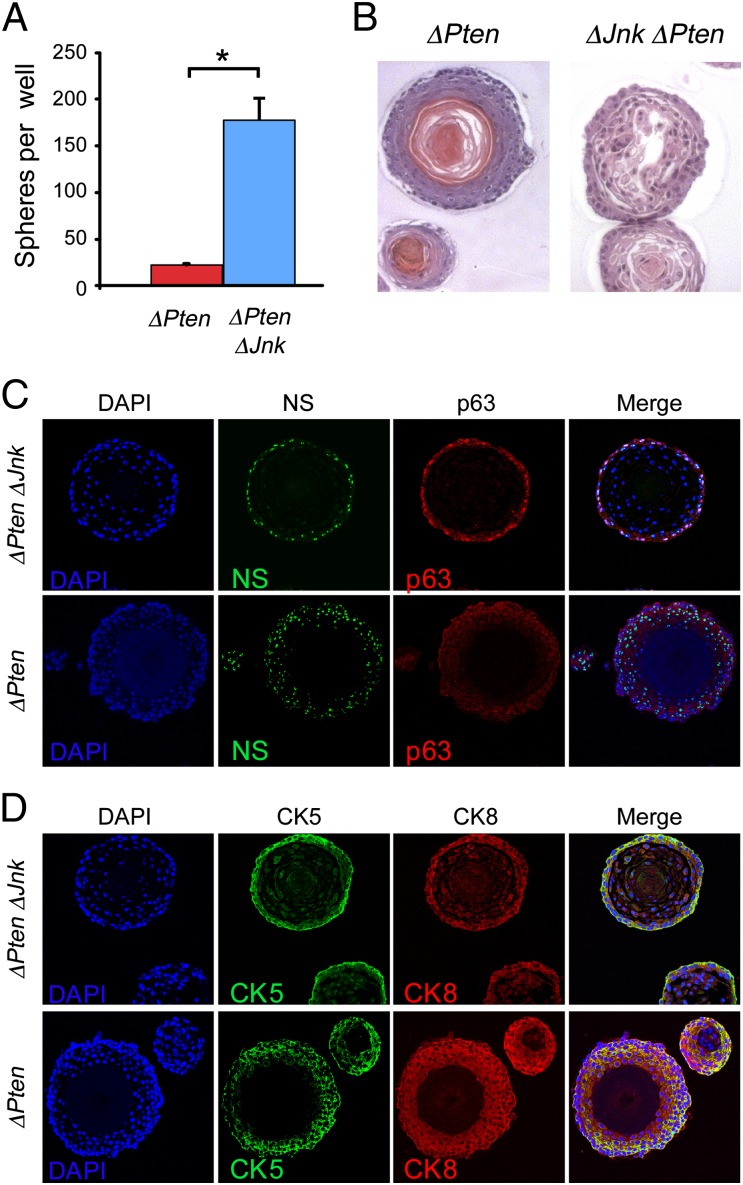
The expansion of the immature cell population in the primary prostate tumors of ΔJnk ΔPten mice compared with ΔPten mice is intriguing. To test whether these cells contribute to the tumor phenotype, we isolated lineage-negative (Lin−) Sca1+ cells from ΔJnk ΔPten and ΔPten primary tumors and cultured these cells in vitro. Previous studies using WT mice have demonstrated that this procedure leads to the formation of spheres (prostaspheres) with a surface location of prostate stem cells and a luminal location of more differentiated cells (31). Luminal prostate stem cells may also be present in these cultures (32, 33). Comparison of ΔJnk ΔPten and ΔPten prostaspheres demonstrated that JNK deficiency caused a significant increase in growth during culture in vitro (Fig. 4A). Microscopic analysis of sections prepared from ΔJnk ΔPten and ΔPten prostaspheres indicated that JNK deficiency caused altered morphology, including reduced luminal cell–cell interactions (Fig. 4B) and increased luminal cell apoptosis (Fig. S7). Immunofluorescence analysis demonstrated strong staining of immature progenitor cell markers (p63, CK5, nucleostemin, and integrin α6) in a distinct surface zone of ΔJnk ΔPten prostaspheres compared with ΔPten prostaspheres (Fig. 4 C and D and Fig. S8).
Fig. 4.
Loss of JNK promotes proliferation of immature ΔPten prostate cells. (A) The cloning efficiency of Sca1+ cells isolated from ΔPten and ΔPten ΔJnk prostate tumors was measured at passage 2 in vitro. Equal numbers of Sca1+ cells were plated and the number of prostaspheres obtained after 14 d in culture was examined (mean ± SD; n = 3). Significant differences are indicated (*P < 0.05). (B) Sections of prostaspheres were stained for DNA (DAPI) and the stem cell markers p63 and nucleostemin (NS). (C and D) Sections of prostaspheres were stained with H&E or with DNA (DAPI) plus basal (CK5) and luminal (CK8) differentiation markers.
The tumorigenic potential of the Lin− Sca1+ cells isolated from ΔJnk ΔPten and ΔPten prostate tumors was examined using renal capsule transplantation assays with immunodeficient host mice (Fig. S9). No growth was detected in studies using Lin− Sca1+ ΔPten cells, a population of cells that includes the tumor-initiating cells of the ΔPten prostate cancer model (34). In contrast, Lin− Sca1+ ΔJnk ΔPten cells efficiently formed kidney capsule tumors (Fig. S9).
Discussion
JNK Is Not Required for the Development of Prostate Neoplasia.
Two protein kinases (MKK4 and MKK7) that phosphorylate and activate JNK (1) are detected in benign human prostate epithelial cells (35). Studies of human prostate cancer demonstrate that the expression of MKK4 and MKK7 (but not JNK) is increased in PIN lesions (35). Signaling by the JNK pathway may, therefore, be increased during the formation of PIN lesions. Indeed, PTEN inactivation in human prostate tumors is associated with increased JNK activity (20). Together, these data indicate that JNK may promote the formation of PIN lesions and may function as an effector of increased PI3K/AKT signaling caused by PTEN inactivation (20). To test this prediction, we used a mouse model with conditional deletion of Pten in the prostate epithelium. This analysis demonstrated that JNK in prostate epithelial cells is not essential for the formation of neoplastic lesions. However, it remains possible that JNK in stromal cells that support epithelial cell morphogenesis and differentiation (36) may play a role in the development of PIN lesions. Further studies will be required to test this hypothesis in the context of prostate cancer, but recent studies have established that JNK in stromal cells can promote carcinogenesis (9). Nevertheless, JNK in prostate epithelial cells is not required for prostate cancer development caused by Pten inactivation in mice.
JNK Regulates Cancer Progression to Invasive Adenocarcinoma.
Although JNK in the prostate epithelium is not essential for the formation of neoplastic lesions in the conditional Pten deletion mouse model, it is possible that JNK may contribute to PIN lesion maintenance by contributing to the cellular senescence program that limits prostate cancer progression (22, 23). Indeed, the senescence of primary ΔPten PIN lesions was not detected in the prostate glands of ΔJnk ΔPten mice. These data indicate that JNK signaling may maintain PIN lesions by reducing growth and inducing senescence and that loss of JNK signaling promotes the development of invasive adenocarcinoma.
The concept that JNK may restrain progression to adenocarcinoma is consistent with the observation that MKK4 is down-regulated in advanced stage human prostate cancer (21). The mechanism of down-regulation is caused, in part, by translation inhibition (37) that may be mediated by microRNA pathways, including miR15b, miR-24, miR-25, and miR-141 (38). Intriguingly, increased MKK4 caused by decreased microRNA expression is associated with senescence (38). Together, these data indicate that decreased JNK signaling in PIN lesions may contribute to cancer progression and the formation of adenocarcinoma.
The MKK4 gene has been identified previously as a putative human metastasis suppressor in prostate cancer (21, 39–41) and other forms of cancer (42–44). Moreover, mutations in JNK pathway genes (MKK4, MKK7, JNK1, and JNK2) have also been identified in human prostate cancer (45–47). A functional role for MKK4 deficiency is consistent with the finding that decreased JNK signaling in the ΔPten mouse model promotes the development of invasive adenocarcinoma. However, the effect of MKK4 or MKK7 deficiency on murine prostate cancer was reduced compared with JNK deficiency (Figs. 1 and 2). This observation is consistent with previous reports that deficiency of MKK4 or MKK7 reduces JNK activity (25). Compound deficiency of MKK4 plus MKK7 is required to ablate JNK signaling (25) and to fully promote adenocarcinoma development (Fig. 2). Additional mechanisms may therefore contribute to the proposed effects of MKK4 deficiency in humans (41). Thus, the dominant-negative activity of catalytically inactive MKK4 proteins (caused by cancer-associated MKK4 gene mutations) may cause greater suppression of JNK activity than MKK4 gene ablation (42, 45). Moreover, MKK4 gene mutation in human prostate cancer may cooperate with other genetic alterations (e.g., increased DUSP1 expression) that suppress JNK activity (48).
JNK and PTEN Cooperate to Regulate the Development of Invasive Adenocarcinoma.
It is established that PTEN inactivation is an important event in the development of human prostate cancer. Loss of PTEN in the prostate epithelium causes activation of the PI3K/AKT pathway (19) and inhibition of androgen receptor signaling (49, 50). Reciprocal regulation of PI3K/AKT and androgen receptor signaling represents a mechanism of signaling cooperation that regulates prostate cancer development. Similarly, dysregulated Smad4 (23), Erg (51, 52), cMyc (53), and Trp53 (22) cooperate with PTEN/AKT to promote prostate cancer. The results of this study identify JNK as another signaling pathway that cooperates with PTEN deficiency to regulate cancer progression in the prostate gland. The effects of JNK deficiency to increase both tumor size and invasive adenocarcinoma/metastasis more closely resembles dysregulated Smad4 than dysregulated cMyc, Erg, or Trp53. This observation indicates that crosstalk between JNK and TGF-β signaling (54) may contribute to tumor progression. PTEN deficiency therefore promotes carcinogenesis by altering the cell signaling network. Perturbation of this signaling network can promote or repress tumorigenesis. In this context, JNK cooperates with PTEN to regulate progression to invasive adenocarcinoma.
Compound JNK deficiency in epithelial cells of the prostate (this study), breast (2), and liver (9) causes an increase in carcinogenesis. This observation suggests that JNK may act to reduce tumor development in these epithelial tissues.
Materials and Methods
Mice.
Mice with ablation of the Jnk1 (55) and Jnk2 (56) genes and also mice with conditional (floxed) alleles of Jnk1 (5), Mkk4 (57), and Pten (58) have been described. BALB/cJ and NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJ mice were obtained from The Jackson Laboratory. Mice with floxed alleles of Mkk7 were created using homologous recombination in embryonic stem cells, blastocyst injection of ES cells to create chimeric mice, and breeding to obtain germ-line transmission of the mutated Mkk7 allele with LoxP sites inserted in intron 3 and intron 7. PB-Cre4 mice were provided by Dr. P. Roy-Burman (59). These mice were back-crossed (ten generations) to the BALB/cJ strain background. These mice were crossed to obtain compound mutants: ΔPten (PB-Cre4+ PtenLoxP/LoxP); ΔJnk (PB-Cre4+ Jnk1LoxP/LoxP Jnk2−/−); ΔJnk ΔPten (PB-Cre4+ PtenLoxP/LoxP Jnk1LoxP/LoxP Jnk2−/−); ΔMkk4 ΔPten (PB-Cre4+ PtenLoxP/LoxP Mkk4LoxP/LoxP); ΔMkk7 ΔPten (PB-Cre4+ PtenLoxP/LoxP Mkk7LoxP/LoxP); and ΔMkk4 ΔMkk7 ΔPten (PB-Cre4+ PtenLoxP/LoxP Mkk7LoxP/LoxP Mkk4LoxP/LoxP). Studies of ΔPten mice demonstrated that tumor development in BALB/cJ mice was similar to previous reports using mixed strain background mice (19), although progression to PIN lesions and invasive adenocarcinoma was slower in the BALB/cJ strain background. Mice were castrated using a surgical procedure (19). The animal studies were approved by the Institutional Animal Care and Use Committees of the University of Massachusetts Medical School and the University of California, Los Angeles. The mice were housed in facilities approved by the American Association of Laboratory Animal Care (AALAC).
Analysis of Tissue Sections.
Histology was performed using tissue fixed in 10% (vol/vol) formalin for 24 h, dehydrated, and embedded in paraffin. Sections (7 μm) were cut and stained using hematoxylin and eosin (H&E) (American Master Tech Scientific). Apoptotic cells were detected using the ApopTag reagent (Millipore). Immunohistochemistry was performed by staining with a primary antibody [androgen receptor (Santa Cruz; sc-816), CK5 (Covance; PRB-160), CD44 (eBioscience; 14–004), E-cadherin (BD Transduction; 610181), P-AKT-Ser473 (Cell Signaling; 3787), JNK1/2 (BD Pharmingen; 554285), smooth muscle actin (Sigma; A5228), and Ki67 (Vector; VP-RM04)], a biotinylated secondary antibody (Biogenex), streptavidin-conjugated horseradish peroxidase (Biogenex), and the substrate 3,3′-diaminobenzidene (Vector Laboratories), followed by brief counter staining with Mayer’s hematoxylin (Sigma). Immunofluorescence analysis was performed by staining with antibodies to CK5 (Covance; PRB-160) and CK8 (Covance; MMS-162) and detection using fluorescence-conjugated secondary antibodies (Invitrogen). Fluorescent images were examined using a fluorescence microscope.
Supplementary Material
Acknowledgments
We thank Dr. P. Roy-Burman for providing PB-Cre4 mice; Dr. D. Garlick for examination of mouse pathology; J. Reilly, L. Paquin, and V. Benoit for assistance with mouse assays; and K. Gemme for administrative assistance. This work was supported by National Institutes of Health Grants CA065861 (to R.J.D.), AI046629 (to D.L.G.), CA112988 (to D.J.M.), CA107166 (to H.W.), and CA121110 (to H.W.), a California Institute for Regenerative Medicine training grant (to D.J.M.), and an award from the Prostate Cancer Foundation (to H.W.). R.J.D. is a member of the National Institute of Diabetes and Digestive and Kidney Diseases Diabetes and Endocrinology Research Center (Grant DK032520) at the University of Massachusetts Medical School. R.A.F., C. L. Sawyers, and R.J.D. are Investigators of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209660109/-/DCSupplemental.
References
- 1.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 2.Cellurale C, et al. Role of JNK in mammary gland development and breast cancer. Cancer Res. 2012;72:472–481. doi: 10.1158/0008-5472.CAN-11-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tournier C, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 4.Lamb JA, Ventura JJ, Hess P, Flavell RA, Davis RJ. JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell. 2003;11:1479–1489. doi: 10.1016/s1097-2765(03)00203-x. [DOI] [PubMed] [Google Scholar]
- 5.Das M, et al. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc Natl Acad Sci USA. 2007;104:15759–15764. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess P, Pihan G, Sawyers CL, Flavell RA, Davis RJ. Survival signaling mediated by c-Jun NH(2)-terminal kinase in transformed B lymphoblasts. Nat Genet. 2002;32:201–205. doi: 10.1038/ng946. [DOI] [PubMed] [Google Scholar]
- 7.Cellurale C, et al. Requirement of c-Jun NH(2)-terminal kinase for Ras-initiated tumor formation. Mol Cell Biol. 2011;31:1565–1576. doi: 10.1128/MCB.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das M, Garlick DS, Greiner DL, Davis RJ. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011;25:634–645. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N, et al. Suppression of skin tumorigenesis in c-Jun NH(2)-terminal kinase-2-deficient mice. Cancer Res. 2001;61:3908–3912. [PubMed] [Google Scholar]
- 12.Schramek D, et al. The stress kinase MKK7 couples oncogenic stress to p53 stability and tumor suppression. Nat Genet. 2011;43:212–219. doi: 10.1038/ng.767. [DOI] [PubMed] [Google Scholar]
- 13.Cellurale C, et al. Role of JNK in a Trp53-dependent mouse model of breast cancer. PLoS ONE. 2010;5:e12469. doi: 10.1371/journal.pone.0012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.She QB, Chen N, Bode AM, Flavell RA, Dong Z. Deficiency of c-Jun-NH(2)-terminal kinase-1 in mice enhances skin tumor development by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 2002;62:1343–1348. [PubMed] [Google Scholar]
- 15.Chen P, et al. Jnk2 effects on tumor development, genetic instability and replicative stress in an oncogene-driven mouse mammary tumor model. PLoS ONE. 2010;5:e10443. doi: 10.1371/journal.pone.0010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 17.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 18.Whang YE, et al. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA. 1998;95:5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 20.Vivanco I, et al. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Kim HL, et al. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res. 2001;61:2833–2837. [PubMed] [Google Scholar]
- 22.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Z, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs SY, et al. JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev. 1998;12:2658–2663. doi: 10.1101/gad.12.17.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tournier C, et al. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craft N, Sawyers CL. Mechanistic concepts in androgen-dependence of prostate cancer. Cancer Metastasis Rev. 1998-1999;17:421–427. doi: 10.1023/a:1006141806801. [DOI] [PubMed] [Google Scholar]
- 27.El Hilali N, Rubio N, Martinez-Villacampa M, Blanco J. Combined noninvasive imaging and luminometric quantification of luciferase-labeled human prostate tumors and metastases. Lab Invest. 2002;82:1563–1571. doi: 10.1097/01.lab.0000036877.36379.1f. [DOI] [PubMed] [Google Scholar]
- 28.Rubio N, Villacampa MM, El Hilali N, Blanco J. Metastatic burden in nude mice organs measured using prostate tumor PC-3 cells expressing the luciferase gene as a quantifiable tumor cell marker. Prostate. 2000;44:133–143. doi: 10.1002/1097-0045(20000701)44:2<133::aid-pros6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Liao CP, et al. Mouse models of prostate adenocarcinoma with the capacity to monitor spontaneous carcinogenesis by bioluminescence or fluorescence. Cancer Res. 2007;67:7525–7533. doi: 10.1158/0008-5472.CAN-07-0668. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, et al. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci USA. 2006;103:1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 2007;25:2760–2769. doi: 10.1634/stemcells.2007-0355. [DOI] [PubMed] [Google Scholar]
- 32.Korsten H, Ziel-van der Made A, Ma X, van der Kwast T, Trapman J. Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model. PLoS ONE. 2009;4:e5662. doi: 10.1371/journal.pone.0005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulholland DJ, et al. Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 2009;69:8555–8562. doi: 10.1158/0008-5472.CAN-08-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotan TL, et al. Up-regulation of MKK4, MKK6 and MKK7 during prostate cancer progression: An important role for SAPK signalling in prostatic neoplasia. J Pathol. 2007;212:386–394. doi: 10.1002/path.2194. [DOI] [PubMed] [Google Scholar]
- 36.Cunha GR. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74(3, Suppl):1030–1044. doi: 10.1002/1097-0142(19940801)74:3+<1030::aid-cncr2820741510>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 37.Robinson VL, et al. Mitogen-activated protein kinase kinase 4/c-Jun NH2-terminal kinase kinase 1 protein expression is subject to translational regulation in prostate cancer cell lines. Mol Cancer Res. 2008;6:501–508. doi: 10.1158/1541-7786.MCR-07-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marasa BS, et al. Increased MKK4 abundance with replicative senescence is linked to the joint reduction of multiple microRNAs. Sci Signal. 2009;2:ra69. doi: 10.1126/scisignal.2000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida BA, et al. Mitogen-activated protein kinase kinase 4/stress-activated protein/Erk kinase 1 (MKK4/SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res. 1999;59:5483–5487. [PubMed] [Google Scholar]
- 40.Taylor JL, et al. New paradigms for the function of JNKK1/MKK4 in controlling growth of disseminated cancer cells. Cancer Lett. 2008;272:12–22. doi: 10.1016/j.canlet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Khamis ZI, Iczkowski KA, Sang QX. Metastasis suppressors in human benign prostate, intraepithelial neoplasia, and invasive cancer: Their prospects as therapeutic agents. Med Res Rev. 2011 doi: 10.1002/med.20232. [DOI] [PubMed] [Google Scholar]
- 42.Teng DH, et al. Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res. 1997;57:4177–4182. [PubMed] [Google Scholar]
- 43.Yamada SD, et al. Mitogen-activated protein kinase kinase 4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Res. 2002;62:6717–6723. [PubMed] [Google Scholar]
- 44.Whitmarsh AJ, Davis RJ. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene. 2007;26:3172–3184. doi: 10.1038/sj.onc.1210410. [DOI] [PubMed] [Google Scholar]
- 45.Kan Z, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 46.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger MF, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magi-Galluzzi C, et al. Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab Invest. 1997;76:37–51. [PubMed] [Google Scholar]
- 49.Carver BS, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulholland DJ, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carver BS, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41:619–624. doi: 10.1038/ng.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.King JC, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009;41:524–526. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clegg NJ, et al. MYC cooperates with AKT in prostate tumorigenesis and alters sensitivity to mTOR inhibitors. PLoS ONE. 2011;6:e17449. doi: 10.1371/journal.pone.0017449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ventura JJ, Kennedy NJ, Flavell RA, Davis RJ. JNK regulates autocrine expression of TGF-beta1. Mol Cell. 2004;15:269–278. doi: 10.1016/j.molcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Dong C, et al. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 56.Yang DD, et al. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, et al. Targeted deletion of the mitogen-activated protein kinase kinase 4 gene in the nervous system causes severe brain developmental defects and premature death. Mol Cell Biol. 2007;27:7935–7946. doi: 10.1128/MCB.00226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lesche R, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 59.Wu X, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.