Abstract
Members of the transforming growth factor-β superfamily play essential roles in various aspects of embryonic development and physiological organ function. Among them, bone morphogenetic protein (BMP) 9 and BMP10 regulate embryonic vascular development by activating their endothelial receptor ALK1 (activin receptor-like kinase 1, also called Acvrl1). ALK1-mediated intracellular signaling is implicated in the etiologies of human diseases, but their downstream functional proteins are largely unknown. In this study, we identified Tmem100, a gene encoding a previously uncharacterized intracellular transmembrane protein, to be an embryonic endothelium-enriched gene activated by BMP9 and BMP10 through the ALK1 receptor. Tmem100 null mice showed embryonic lethality due to impaired differentiation of arterial endothelium and defects of vascular morphogenesis, which phenocopied most of the vascular abnormalities observed with the Acvrl1/Alk1 deficiency. The activity of Notch- and Akt-mediated signaling, which is essential for vascular development, was down-regulated in Tmem100 null mice. Cre-mediated deletion of Tmem100 in endothelial cells was sufficient to recapitulate the null phenotypes. These data indicated that TMEM100 may play indispensable roles downstream of BMP9/BMP10-ALK1 signaling during endothelial differentiation and vascular morphogenesis.
Formation of the cardiovascular network is essential for proper embryonic development, and neovascularization is associated with various adult diseases, such as ischemic heart diseases, retinopathy, and cancer, acting either protectively or deterioratively in those pathological states (1, 2). At early embryonic stages, vasculogenesis occurs as a de novo organization of endothelial cell plexus. A series of processes for vascular remodeling and maturation follows, including an initial phase of endothelial cell migration, proliferation, and tubular reorganization, collectively called angiogenesis, and a maturation phase when the structure of blood vessels is established by the stabilization of endothelial cells, acquirement of arterial or venous identity, and recruitment of mural cells to vascular walls (1, 2).
A variety of cellular signaling pathways controlling vascular development involve cytokines and growth factors, such as vascular endothelial growth factors (VEGFs), angiopoietins, transforming growth factor β (TGF-β), and bone morphogenetic proteins (BMPs) (3, 4). Among them, BMP9 and BMP10 act mainly through an endothelium-specific ALK1 (activin receptor-like kinase 1) receptor and promote arterial endothelial maturation and quiescence (5–7). Targeting disruption of the genes for the ALK1 receptor, a type III coreceptor endoglin, and downstream signaling components such as Tak1/Map3k7 caused embryonic lethality with remarkably similar defects of arterial endothelium differentiation and vascular morphogenesis (8–11). Despite clear demonstration of its critical roles in embryonic development, endothelial functional proteins downstream of the BMP9/BMP10-ALK1 signaling pathway remained unclear.
In a microarray screen to search for endothelial genes downstream of BMP9/BMP10-ALK1 signaling, we found that the expression of Tmem100, a gene encoding an intracellular transmembrane protein of unknown functions, was markedly augmented by BMP9 and BMP10. It was recently reported that Tmem100 mRNA expression significantly decreased in the lung of Acvrl1/Alk1 conditional knockout mice and that Tmem100 drove endothelial-enriched expression of lacZ reporter in mouse embryos (12). In this paper, we demonstrated that Tmem100 null mice and endothelial-specific Tmem100 knockout mice showed fatal defects of arterial endothelium differentiation and vascular morphogenesis, which are virtually identical to the abnormalities due to the Acvrl1/Alk1 deficiency. These results suggest that TMEM100 may play essential roles as a downstream target protein of BMP9/BMP10-ALK1 signaling in embryonic vascular development.
Results
Tmem100: An Arterial Endothelium-Enriched Gene Downstream of BMP9/BMP10-ALK1 Signaling.
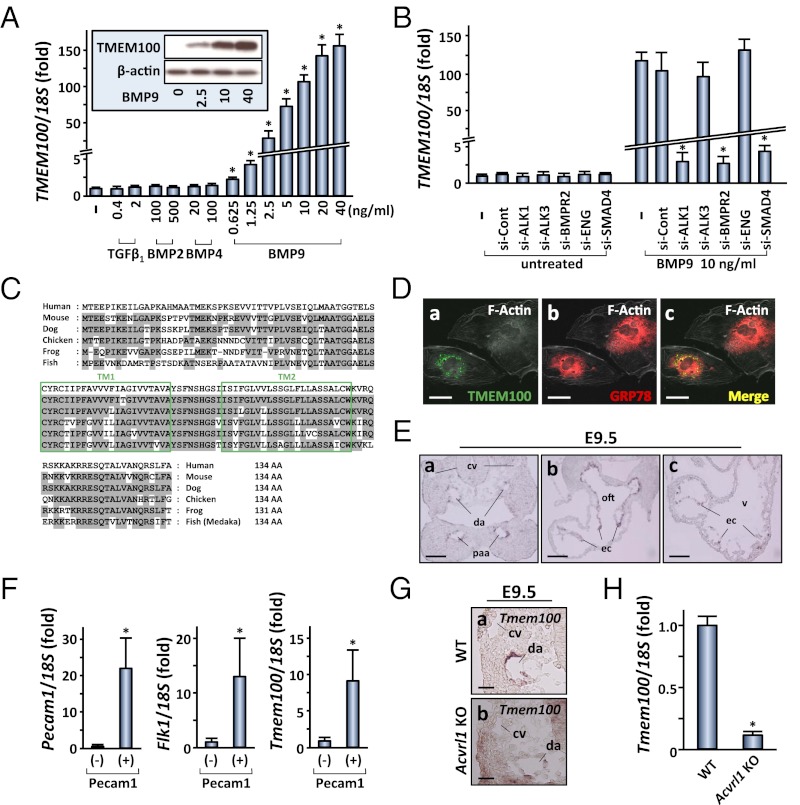
In an attempt to identify endothelial genes downstream of BMP9/BMP10-ALK1 signaling, we first analyzed whether human umbilical artery endothelial cells (HUAEC) responded to the treatment with various TGF-β–related factors. Among several ligands tested, BMP9 potently stimulated the phosphorylation of Smad1/5/8 (Fig. S1 A and B). We then compared gene expression profiles of BMP9-treated HUAEC and control cells by an RNA microarray analysis (Fig. S1C). In addition to known BMP9-target genes such as SMAD6, SMAD7, ID1, and ID2, we found that TMEM100 showed a marked activation of mRNA expression by the BMP9 treatment (Fig. 1A). Quantitative RT-PCR and Western blot analysis confirmed a dose-dependent induction of the TMEM100 expression by BMP9, up to more than 100-fold (Fig. 1A). Similar levels of activation were observed with BMP10 (Fig. S1D), and those effects were significantly inhibited by knockdown of ACVRL1/ALK1, BMPR2, and SMAD4 (Fig. 1B and Fig. S1E). These results indicated that BMP9/BMP10-induced TMEM100 expression occurred through the ALK1/BMPR2 receptor complex and Smad-mediated transcriptional regulation.
Fig. 1.
Tmem100: an arterial endothelium-enriched gene downstream of BMP9/BMP10-ALK1 signaling. (A) BMP9 markedly increases TMEM100 mRNA expression at 24 h in a dose-dependent manner. Quantitative RT-PCR analysis is shown. Fold increase relative to the untreated cells is shown with SD. (Inset) Western blot result shows a dose-dependent increase in TMEM100 protein expression. −, untreated. (B) BMP9-induced TMEM100 mRNA expression is inhibited by pretreatment with ACVRL1/ALK1 siRNA (si-ALK1), BMPR2 siRNA (si-BMPR2), or SMAD4 siRNA (si-SMAD4), but not with BMPR1A/ALK3 siRNA (si-ALK3), Endoglin siRNA (si-ENG), or control siRNA (si-Cont). Quantitative RT-PCR is shown. −, untreated. (C) Amino acid sequences of TMEM100 from various species. Boxes represent two putative transmembrane (TM) domains. Residues conserved with the human sequence are shaded in gray. (D) TMEM100 protein predominantly resides in the perinuclear region overlapping endoplasmic reticulum marked by anti-GRP78/HSPA5 antibody. Immunocytochemistry of HUAEC transfected with a TMEM100-FLAG expression plasmid is shown. (E) Tmem100 mRNA is expressed specifically in arterial endothelial cells of pharyngeal arch artery (paa) and dorsal aorta (da) as well as in endocardium (ec), but not in cardinal vein (cv), at E9.5. In situ hybridization is shown. oft, outflow tract; v, ventricle. (F) Tmem100 expression is enriched in endothelial cells also at earlier developmental stages. Shown is FACS sorting of Pecam1-positive cells from E8.5 embryos followed by quantitative RT-PCR. Pecam1 and Kdr/Flk1 expression was examined to confirm proper selection of the endothelial population. (G) Tmem100 expression in dorsal aorta is reduced in Acvrl1/Alk1 null embryos at E9.5. In situ hybridization is shown. (H) Tmem100 expression significantly decreases in the yolk sac of Acvrl1/Alk1 null mice at E9.5. Quantitative RT-PCR is shown. In A, B, F, and H, asterisks indicate data with statistical significance (*P < 0.05). (Scale bars: D, 10 μm; E, 50 μm; and G, 20 μm.)
TMEM100 is a gene encoding a protein with two putative transmembrane domains, but its physiological significance has not been described. The structure of TMEM100 protein is highly conserved from fish to humans, especially in its putative transmembrane domains (Fig. 1C); however, no structurally related family of proteins was found in any species, indicating that TMEM100 represents a previously uncharacterized entity of functional proteins. Levels of endogenous TMEM100 expression in HUAEC were below detection limits in immunocytochemical analyses, whereas TMEM100 protein expressed using a mammalian expression plasmid resided in the perinuclear region marked by endoplasmic reticulum (ER) proteins such as GRP78/HSPA5 (Fig. 1D and Fig. S2A). Endogenous TMEM100 protein in BMP9-treated HUAEC was recovered to the subcellular membrane fraction and was enriched in the ER microsome (Fig. S2 B and C). In adult mice, Tmem100 was most abundantly expressed in the lung with a lower level of expression in the brain, heart, and muscle (Fig. S2D). In mouse embryos, Tmem100 mRNA was detected from embryonic day (E) 8.5 (Fig. S2E) and was enriched in arterial endothelium and endocardium (Fig. 1E and see Fig. S4A). Quantitative RT-PCR confirmed the enrichment of Tmem100 mRNA in the platelet/endothelial cell adhesion molecule 1 (Pecam1)-positive endothelial cell population sorted from E8.5 embryos (Fig. 1F). Consistent with ALK1-mediated Tmem100 expression in HUAEC (Fig. 1 A and B) and the previous report (12), Tmem100 mRNA expression in arterial endothelium was significantly reduced in Acvrl1/Alk1 null embryos (Fig. 1 G and H).
These results prompted us to examine functional significance of TMEM100 in vascular differentiation and morphogenesis during development.
Embryonic Lethality by Targeted Disruption of Tmem100.
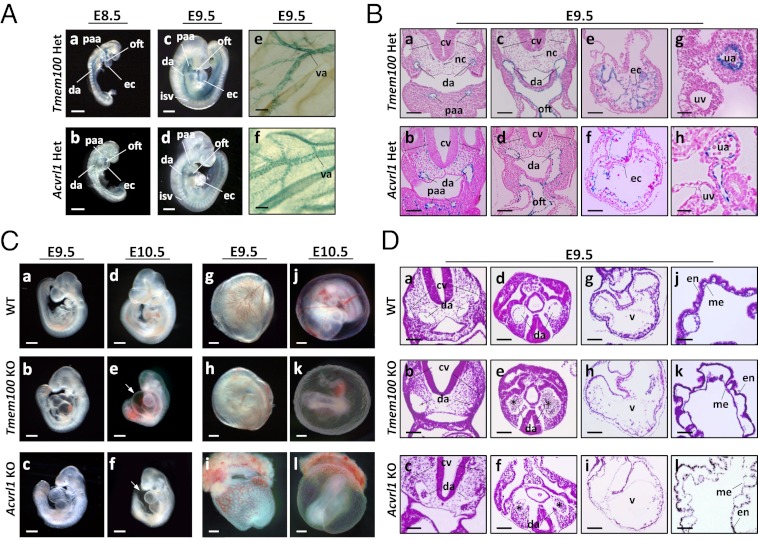
We generated Tmem100-deficient mice in which exon 3 encoding the entire coding region was replaced with a lacZ-neo cassette (Fig. S3 A–C). Mice heterozygous for the Tmem100 mutation survived to adulthood and were fertile. LacZ reporter driven by the Tmem100 locus (Tmem100-lacZ) was predominantly expressed in developing arteries, including dorsal aorta and pharyngeal arch; intersomitic, umbilical, and vitelline arteries; and endocardium (Fig. 2 A and B and Fig. S4 B–D). Vascular expression of Tmem100-lacZ overlapped with that of lacZ reporter knocked into the Acvrl1/Alk1 locus (Fig. 2 A and B) and was observed in the endothelial layer of dorsal aorta but not in smooth muscle cells (Fig. S4 C and D). At later embryonic stages, Tmem100-lacZ expression was also detected in the limb buds, somites, and other tissues (Fig. S4B). In the adult lung, lacZ reporter was expressed in vascular endothelial cells as well as in alveolar cells (Fig. S4E).
Fig. 2.
Tmem100 and Acvrl1/Alk1: arterial expression of knock-in lacZ reporter and angiogenesis defects in null embryos. (A and B) Knock-in lacZ reporter activity is detected in major arteries and endocardium in both Tmem100 and Acvrl1/Alk1 heterozygous mice. Sections counterstained with nuclear fast red are shown in B. (C) Tmem100 null embryos die in utero, showing cardiac dysmorphogenesis and enlargement at E9.5, massive pericardial effusion (arrow) and severe growth retardation at E10.5, and absence of large vitelline vessels in the yolk sac at E10.5. These phenotypes are also observed in Acvrl1/Alk1 null embryos. (D) Tmem100 null and Acvrl1/Alk1 null embryos have remarkably similar defects of cardiovascular morphogenesis. Paired dorsal aortas show marked dilatation and narrowing, and clumps of blood cells are frequently seen in the caudal region (asterisk). Mural layers surrounding the aorta and myocardial wall are thinner and ventricular trabeculation is not formed well. The yolk sac shows detachment of endodermal (en) and mesodermal (me) layers and abnormal vessel dilatation. H&E staining at E9.5 is shown. cv, cardinal vein; da, dorsal aorta; isv, intersomitic vessel; nc, notochord; oft, outflow tract; paa, pharyngeal arch artery; ua, umbilical artery; uv, umbilical vein; v, ventricle; va, vitelline artery. (Scale bars: A, a–d, and C, 200 μm; A, e and f, B, a–f, and D, a–i, 50 μm; and B, g and h, and D, j–l, 20 μm.)
Breeding of heterozygous mice revealed a significant deviation from expected inheritance, and no surviving embryos were recovered at and after E11.0 (Fig. S3D). Tmem100 null embryos at E10.5 were strongly affected, showing abnormal cardiac morphology, massive pericardial effusion, and growth retardation (Fig. 2C), suggesting that cardiovascular failure caused embryonic lethality of Tmem100 null mice.
Vascular Defects in Tmem100 Null Mice.
Tmem100 null embryos showed no detectable phenotypes until E8.5, and the formation of primitive vasculature appeared normal (Fig. S5A). Earliest signs of deficiency were observed in the vasculature at E9.0–9.5. The yolk sac of Tmem100 null embryos showed reduction of vitelline circulation (Fig. 2C). Consistently, Tmem100 null embryos displayed a variety of severe abnormalities in vascular morphology, as shown in Fig. 2D. One of the paired dorsal aortae frequently showed a marked dilatation with a narrowing or closure on the opposite side, and clumps of blood cells were often observed in the caudal region, which is likely to be due to vascular obstruction. Cardiac myocardium was thinner, and the yolk sacs showed detachment of endodermal and mesodermal layers and abnormal vessel dilatation (Fig. 2D).
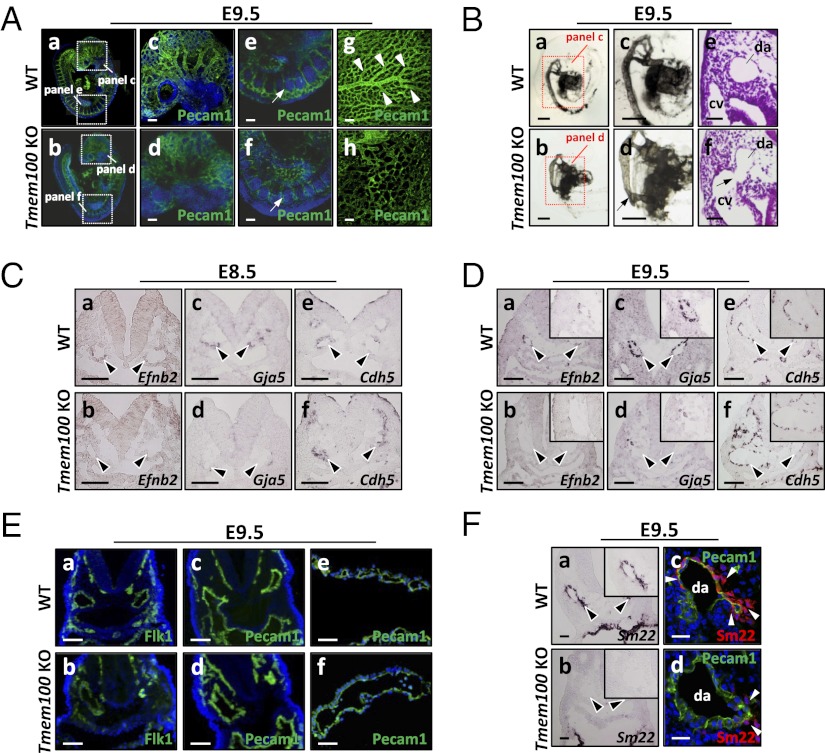
Whole-mount Pecam1 staining revealed abnormally coarse vascular patterns, indicative of impaired vascular remodeling (Fig. 3A), and India ink injection identified arteriovenous malformation from dorsal aorta toward sinus venosus in Tmem100 null embryos (Fig. 3B and Fig. S5B). These phenotypes of Tmem100 null embryos were mostly identical to those observed in Acvrl1/Alk1 null embryos (Fig. 2 C and D and Fig. S6A) and reported in previous studies (8, 9).
Fig. 3.
Impairment of vascular remodeling and arterial endothelium differentiation in Tmem100 null embryos. (A) Impaired vascular remodeling is visualized by whole-mount Pecam1 immunostaining in the Tmem100 null embryos (b, d, and f) and yolk sac (h). Arrows indicate intersomitic vessels. Arrowheads indicate highly organized vascular branches in the wild-type yolk sac. a–f are shown with nuclear DAPI stain. (B) Abnormal vascular connections (arrow) between dorsal aorta and cardinal veins are observed in null embryos. Shown is microangiography by India ink injection and H&E staining. (C and D) Expression of arterial endothelium marker genes Efnb2 and Gja5/Cx40 diminishes in dorsal aorta (arrowheads) of Tmem100 null embryos at E8.5 (C) and E9.5 (D), whereas maintenance of Cdh5 expression indicates existence of endothelial layers. In situ hybridization is shown. (E) Flk1 and Pecam1 expression is preserved in dorsal aorta of Tmem100 null embryo (b and d) and yolk sac vasculature (f). Immunohistochemistry with nuclear DAPI stain is shown. (F) Expression of Sm22/Tagln mRNA and SM22 protein in the mural layers of dorsal aorta is significantly reduced in Tmem100 null embryos (arrowheads). In situ hybridization (a and b) and immunohistochemistry with nuclear DAPI stain (c and d) are shown. Insets in D and F show magnified views of dorsal aorta. (Scale bars: A, C, D, and E, a–d, 50 μm; B, a–d, 200 μm; and B, e and f, E, e and f, and F, 20 μm.)
Defects of Arterial Differentiation in Tmem100 Null Embryos.
ALK1-mediated signaling is implicated in establishing arterial identity of vascular endothelial cells (8, 9). Indeed, the expression of arterial marker genes Efnb2 and Gja5/Cx40 was significantly decreased in dorsal aorta of Tmem100 null embryos as early as E8.5 (Fig. 3 C and D). In contrast, expression of Cdh5 (encoding VE-cadherin), Kdr/Flk1, and Pecam1 was maintained (Fig. 3 C–E), suggesting that arterial specification is compromised by Tmem100 deficiency. Upon normal arterial maturation, mural smooth muscle precursors marked by Sm22 are recruited (1, 2); however, the Sm22/Tagln expression around the dorsal aorta was significantly reduced in Tmem100 null embryos (Fig. 3F). These defects of arterial differentiation and maturation were also observed in Acvrl1/Alk1 null mice (Fig. S6 B–E).
Down-Regulation of Endothelial Signaling Pathways in Tmem100 Null Embryos.
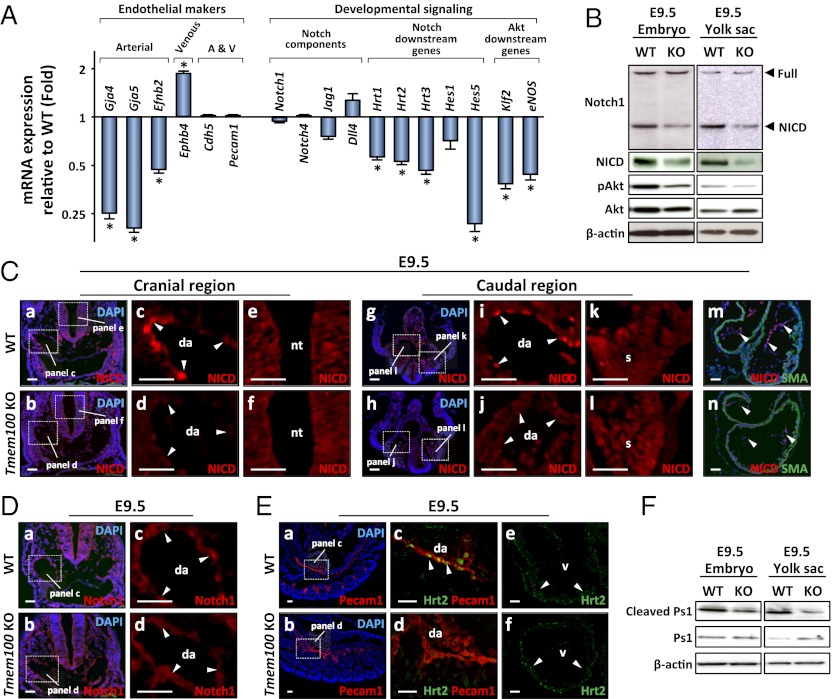
To begin to understand mechanisms of vascular abnormalities caused by the Tmem100 deficiency, we performed a microarray analysis using the yolk sac, which is a vascular-rich tissue with obvious null phenotypes (Fig. S7A). We observed a significant decrease in the expression of arterial markers, such as Efnb2, Gja4/Cx37, and Gja5/Cx40, and an increase in vein-specific Ephb4 expression in the Tmem100 null yolk sac (Fig. 4A and Fig. S7B), which is consistent with our notion that Tmem100 is essential for arterial endothelium differentiation.
Fig. 4.
Notch- and Akt-mediated signaling pathways are down-regulated in Tmem100 null embryos and yolk sac. (A) Expression of arterial endothelium markers, Notch downstream genes, and Akt downstream genes markedly decreases in Tmem100 null yolk sac at E9.5. Expression of a venous endothelium marker, Ephb4, is significantly up-regulated. Quantitative RT-PCR is shown. (B) The amount of the intracellular domain of Notch receptor (NICD) and phosphorylated Akt significantly decreases in Tmem100 null embryos and yolk sac at E9.5, whereas that of full-length Notch1 receptor and total Akt remains unchanged. Western blot analysis is shown. (C) NICD expression markedly decreases in dorsal aorta (da) and endocardium (arrowheads in d, j, and n), but not in the neural tube (nt) and somites (s) (f and l), of E9.5 null embryos. Immunohistochemistry using a NICD-specific antibody is shown. a, b, g, h, m, and n are shown with nuclear DAPI stain. (D) Total amount of Notch receptors does not decrease in vascular endothelium of null embryos. Immunohistochemistry using an antibody recognizing full-length Notch receptors and NICD is shown. a and b are shown with nuclear DAPI stain. (E) Expression of Hrt2, a Notch downstream transcription factor, significantly decreases in the vasculature of Tmem100 null embryos (d), whereas its expression in cardiac muscle was unchanged (f). Immunohistochemistry is shown. a and b are shown with nuclear DAPI stain. (F) Cleaved Presenilin-1 (Ps1) decreases in null embryos and yolk sac at E9.5. Western blot analysis is shown. (Scale bars in C–E, 20 μm.)
We also found that the expression of Notch downstream genes Hrt1/Hey1, Hrt2/Hey2, Hrt3/Heyl, and Hes5 (13, 14) was significantly decreased in Tmem100 null yolk sac (Fig. 4A and Fig. S7C). Because Notch signaling was known to be essential for arterial differentiation (1, 2, 15), we further examined whether the activity of Notch signaling is down-regulated in Tmem100 null embryos. Upon activation by the ligands such as Delta and Jagged, the intracellular domain of Notch receptors, NICD, is enzymatically cleaved and translocated to the nucleus, where it forms a transcriptional activator complex with RBP-J and other cofactors (14). Western blot analysis demonstrated a significant decrement of NICD expression in Tmem100 null embryos and yolk sac (Fig. 4B). Immunohistochemical staining using an anti–NICD-specific antibody indicated that down-regulation of NICD expression was specific to the arterial endothelium and endocardium and was not observed in other Notch-regulated tissues such as somite and neural tube (Fig. 4C). The total amount of Notch receptors, which was examined using an antibody recognizing both full-length Notch receptors and NICD, appeared unchanged (Fig. 4D). Furthermore, the expression of Notch-target transcription factor Hrt2/Hey2 was significantly decreased in the vasculature of Tmem100 null embryos, whereas its expression in the cardiac muscle was unaltered (Fig. 4E).
In addition, we observed a decrease in Akt phosphorylation in Tmem100 null embryos and yolk sac (Fig. 4B). Consistently, the expression of Klf2 and eNOS/Nos3 (Fig. 4A) and the cleavage of Presenilin-1 (Ps1) (Fig. 4F), which can be activated by Akt signaling (16–18), were suppressed in null embryos and yolk sac, suggesting down-regulation of Akt-mediated signaling important for endothelial survival, migration, and homeostasis. Importantly, dysregulation of Notch and Akt signaling was also observed in Acvrl1/Alk1 null mice (Fig. S6 F–K).
In contrast, other signaling pathways implicated in embryonic vascular development did not appear compromised (1, 2, 19–22). The levels of ERK and Smad1/5/8 phosphorylation did not alter in Tmem100 null embryos and yolk sac (Fig. S7 D–F). Components of Angiopoietin, Hedgehog, Semaphorin, and fibroblast growth factor signaling pathways and their downstream target genes did not show aberrant expression in Tmem100 null yolk sac (Fig. S7C). These data suggested that the Tmem100 deficiency caused impairment of arterial differentiation and morphogenesis through, at least in part, down-regulation of Notch- and Akt-mediated signaling.
Embryonic Vascular Defects by Cre-Mediated Deletion of Tmem100 in Endothelial Cells.
We further examined phenotypes of the mice with Tek-Cre–mediated endothelial cell-specific deletion of the Tmem100 gene (Fig. S8 A–D). Endothelial-specific deletion of Tmem100 caused vascular defects remarkably similar to those observed in global Tmem100 null mice and embryonic lethality around E11.0 (Fig. S8 E and F). Expression of arterial endothelium-specific genes and a vascular smooth muscle marker SM22 apparently decreased, and Notch- and Akt-mediated signaling was down-regulated also by the endothelial-specific Tmem100 deletion (Fig. S9 A–D). These results indicated that the endothelial expression of Tmem100 is essential for arterial differentiation and embryonic development.
Discussion
In searching for genes involved in the regulation of endothelial differentiation and vascular development, we identified Tmem100 to be a gene activated downstream of BMP9/BMP10-ALK1 signaling. Tmem100 and Acvrl1/Alk1 were expressed in arterial endothelial cells during vascular development in mouse embryos, and their targeting disruption caused embryonic lethality with remarkably similar abnormalities of arterial endothelium differentiation and vascular morphogenesis. Tmem100 expression significantly decreased in Acvrl1/Alk1 null embryos, which is consistent with down-regulation of Tmem100 expression in the adult lung of Acvrl1/Alk1 conditional knockout mice (12). It has not been studied whether BMP9 and BMP10 are major regulators of Tmem100 expression in vivo. Bmp10 null mice show impaired cardiac growth but not defects of angiogenesis (23), whereas the phenotypes of Gdf2 (encoding BMP9) knockout mice have not been reported. Examining whether the mice null for both Gdf2 and Bmp10 show embryonic vascular defects will provide further insights into in vivo regulatory mechanisms of Tmem100 expression.
Notch-mediated signaling was suppressed in arterial endothelium of Tmem100 null embryos as well as in endothelial-specific Tmem100 knockout embryos. We also found that Notch signaling was down-regulated in Acvrl1/Alk1 null embryos. Notch receptors and ligands are enriched in arterial but not venous endothelium of mouse embryos, and target disruption of Notch signaling components such as Notch1, Notch4, Dll4, Rbpj, Hrt1/Hey1, and Hrt2/Hey2 revealed that Notch activity is crucial for promoting arterial cell fate (1, 2, 15). Impairment of endothelial Notch signaling affects vascular smooth muscle cell recruitment or differentiation during arterial maturation (24–26), which was observed in Tmem100 and Acvrl1/Alk1 knockout mice in the present study. In addition to Notch signaling, the activities of Akt kinase and Ps1 protease appeared repressed in Tmem100 and Acvrl1/Alk1 knockout embryos. It was reported that Akt enhances Ps1-mediated Notch cleavage (16, 27) and that Ps1 provokes Akt activation in turn (28). The signaling relay or mutual interaction involving Akt, Ps1, and Notch might play a role in the pathogenesis of vascular defects by the Tmem100 and Acvrl1/Alk1 deficiency.
A number of biological stimuli including fluid shear stress could influence Akt- and Notch-mediated signaling as a result of insufficient circulation (1, 2, 15). Markers of arterial endothelium differentiation, however, became down-regulated before apparent structural abnormalities in Tmem100 null embryos, suggesting a possibility that TMEM100 is directly involved in acquiring arterial cell fate. Previous studies reported Tmem100 expression in embryonic vasculature and the adult lung, prostate, and kidney in humans and mice (12, 29, 30), but cellular functions of TMEM100 have not been investigated. We found that TMEM100 protein was mainly localized in ER, but not in plasma membrane, of cultured endothelial cells. It is tempting to speculate that TMEM100 works for posttranslational protein modification or intracellular sorting in the membrane of ER and surrounding structures. Elucidating molecular functions of TMEM100 is essential to clarify how TMEM100 conveys the BMP9/BMP10-ALK1 signals toward the downstream signaling cascade for proper endothelial differentiation.
Mutations in ACVRL1/ALK1, BMPR2, ENG, or SMAD4 cause hereditary hemorrhagic telangiectasia as well as pulmonary arterial hypertension in humans (31, 32). TMEM100 might be involved in the mechanisms of these diseases as an additional causative gene or a modifier. At late embryonic and adult stages, Tmem100 expression appears to be not restricted to vascular endothelial cells. Studying the significance of TMEM100 may lead to a better understanding of human diseases in the cardiovascular system and other organs.
Materials and Methods
Cell Culture and Microarray Analysis.
HUAEC (Takara Bio) were precultured in Endothelial Basal Medium 2 medium with 0.2% FBS for 3 h and treated with TGF-β superfamily ligands (R&D Systems) for 24 h. siRNA was electroporated to HUAEC 36 h before the BMP9 or BMP10 treatment. Other cell culture experiments were performed using standard procedures.
Two-color microarray analyses using the human and mouse whole-genome 4x44K v2 oligo microarray systems (Agilent Technologies) were performed to examine mRNA expression profiles of BMP9-treated HUAEC and the yolk sac of Tmem100 null mice, respectively.
Generation of Tmem100 Null Mice.
The Tmem100-lacZ targeting vector was constructed using a BAC clone containing the Tmem100 locus genomic DNA (BACPAC Resources) and was electroporated into C57BL/6 embryonic stem cells for homologous recombination (Fig. S3A). A correctly targeted clone, as identified by Southern blot analysis, was injected into BALB/c blastocysts. Chimeras were bred to obtain heterozygous mice that carry the targeted Tmem100 locus in their germ line. The floxed PGK-neo cassette was removed by breeding with a CAG-Cre transgenic line. All breeding was done with mice under the C57BL/6 genetic background. Procedures to generate mice for conditional Tmem100 deletion are described in SI Materials and Methods. All animal experiments were approved by the institutional committee.
Biochemical, Histological, and Anatomical Analyses.
Quantitative RT-PCR, Northern blot analysis, Western blot analysis, in situ hybridization, immunohistochemistry, and lacZ staining were performed using standard procedures. A rabbit polyclonal antibody generated against a carboxyl-terminal 27-aa fragment of mouse TMEM100 protein was used for Western blot analysis of HUAEC. Details of PCR primers, Northern probe, and primary antibodies are described in SI Materials and Methods. Microangiography was performed by India ink injection to the outflow tract of mouse embryos.
Supplementary Material
Acknowledgments
The authors thank Drs. E. Olson, J. Hill, and P. ten Dijke for profitable advice and support. The authors also thank Drs. A. Wanaka, K. Tatsumi, H. Yamagishi, K. Uchida, H. Bannai, H. Kurihara, R. Asai, S. Itoh, F. Itoh, T. Morioka, and M. Itoh for experimental information and Ms. M. Ikugawa, T. Fujita, Y. Yoshioka, and M. Sakaida for technical assistance. This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology (to O.N., S.S., H.H., M.S., and Y.S.); grants from the Astellas Foundation for Research on Metabolic Disorders, the Mitsubishi Pharma Research Foundation, the Miyata Cardiac Research Promotion Foundation, Novartis Foundation (Japan) for the Promotion of Science, Suzuken Memorial Foundation, the Takeda Science Foundation, The Mother and Child Health Foundation, The Naito Foundation, The Smoking Research Foundation, and the Uehara Memorial Foundation (to O.N.); grants from the Banyu Life Science International Foundation and Takeda Science Foundation (to S.S.); grants from the Takeda Science Foundation, The Ichiro Kanahara Foundation, and The Nakatomi Foundation (to H.H.); a grant from Japan Heart Foundation (to M.S.); and a research grant for Cardiovascular Diseases (20A-3) from the Ministry of Health, Labor and Welfare (to Y.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207210109/-/DCSupplemental.
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 3.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20:203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 5.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 6.Scharpfenecker M, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, et al. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123:1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 8.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26:328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 9.Oh SP, et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li DY, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 11.Jadrich JL, O’Connor MB, Coucouvanis E. The TGF beta activated kinase TAK1 regulates vascular development in vivo. Development. 2006;133:1529–1541. doi: 10.1242/dev.02333. [DOI] [PubMed] [Google Scholar]
- 12.Moon EH, et al. Generation of mice with a conditional and reporter allele for Tmem100. Genesis. 2010;48:673–678. doi: 10.1002/dvg.20674. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa O, et al. Members of the HRT family of basic helix-loop-helix proteins act as transcriptional repressors downstream of Notch signaling. Proc Natl Acad Sci USA. 2000;97:13655–13660. doi: 10.1073/pnas.250485597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopan R, Ilagan MX. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita K, et al. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70–78. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sako K, et al. Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J Biol Chem. 2009;284:5592–5601. doi: 10.1074/jbc.M806928200. [DOI] [PubMed] [Google Scholar]
- 18.SenBanerjee S, et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan R, et al. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS ONE. 2009;4:e8283. doi: 10.1371/journal.pone.0008283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechleider RJ, et al. Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol. 2001;240:157–167. doi: 10.1006/dbio.2001.0469. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, et al. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126:1571–1580. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]
- 22.Chang H, et al. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrow D, et al. Notch and vascular smooth muscle cell phenotype. Circ Res. 2008;103:1370–1382. doi: 10.1161/CIRCRESAHA.108.187534. [DOI] [PubMed] [Google Scholar]
- 25.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.High FA, et al. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci USA. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamizu K, et al. Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. J Cell Biol. 2010;189:325–338. doi: 10.1083/jcb.200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baki L, et al. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: Effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Heul-Nieuwenhuijsen L, Hendriksen PJ, van der Kwast TH, Jenster G. Gene expression profiling of the human prostate zones. BJU Int. 2006;98:886–897. doi: 10.1111/j.1464-410X.2006.06427.x. [DOI] [PubMed] [Google Scholar]
- 30.Georgas K, et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol. 2009;332:273–286. doi: 10.1016/j.ydbio.2009.05.578. [DOI] [PubMed] [Google Scholar]
- 31.Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: A clinical and scientific review. Eur J Hum Genet. 2009;17:860–871. doi: 10.1038/ejhg.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowery JW, de Caestecker MP. BMP signaling in vascular development and disease. Cytokine Growth Factor Rev. 2010;21:287–298. doi: 10.1016/j.cytogfr.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.