Abstract
GABAB receptors mediate slow inhibitory neurotransmission in the brain and feature during excitatory synaptic plasticity, as well as various neurological conditions. These receptors are obligate heterodimers composed of GABABR1 and R2 subunits. The two predominant R1 isoforms differ by the presence of two complement control protein modules or Sushi domains (SDs) in the N terminus of R1a. By using live imaging, with an α-bungarotoxin-binding site (BBS) and fluorophore-linked bungarotoxin, we studied how R2 stabilizes R1b subunits at the cell surface. Heterodimerization with R2 reduced the rate of internalization of R1b, compared with R1b homomers. However, R1aR2 heteromers exhibited increased cell surface stability compared with R1bR2 receptors in hippocampal neurons, suggesting that for receptors containing the R1a subunit, the SDs play an additional role in the surface stability of GABAB receptors. Both SDs were necessary to increase the stability of R1aR2 because single deletions caused the receptors to be internalized at the same rate and extent as R1bR2 receptors. Consistent with these findings, a chimera formed from the metabotropic glutamate receptor (mGluR)2 and the SDs from R1a increased the surface stability of mGluR2. These results suggest a role for SDs in stabilizing cell surface receptors that could impart different pre- and postsynaptic trafficking itineraries on GABAB receptors, thereby contributing to their physiological and pathological roles.
Keywords: G-protein coupled receptor, synapses, hippocampus, bungarotoxin labelling
GABAB receptors are G protein–coupled receptors (GPCRs) underlying slow GABA-mediated inhibitory neurotransmission (1) that regulates neuronal excitability (2–4). These receptors are increasingly considered as potential therapeutic targets for a range of diseases, including epilepsy, schizophrenia, anxiety, depression, and substance abuse (1, 5). Functional GABAB receptors are heteromers formed from GABABR1 subunits containing the agonist binding domain (6) and R2 subunits that link to G protein signaling (7, 8).
To date, just one isoform of the R2 subunit has been reported (9, 10), whereas several isoforms of the R1 subunit exist [R1a, R1b, R1c, R1e, R1j (1)]. Among these, R1a and R1b predominate in the central nervous system (CNS), and their expression arises by the use of different promoters (11) of the GABBR1 gene. R1a subunits contain 143 aa forming two Sushi domains (SDs) in the N terminus, which are also known as complement control proteins or short consensus repeats (12). These SDs are absent in R1b being replaced by 18 unique amino acids.
Heteromers formed from R1aR2 and R1bR2 are thought to play distinct roles in neurotransmission (13–18), with R1aR2 contributing to presynaptic heteroreceptors to inhibit neurotransmitter release, although both R1aR2 and R1bR2 populate postsynaptic membranes to dampen excitability. These heteromeric subtypes exhibit different subcellular compartmentalization with the SDs acting as an axonal-targeting sequence to deliver R1aR2 more efficiently to axons compared with R1bR2 (19). This would, in part, explain the differential pre- and postsynaptic signaling properties of the two subtypes of the GABAB receptor. Although the physiological functions of GABAB receptors are influenced by their differential targeting, it is unknown how the SDs affect the surface stability of these receptors, which is a critical factor in determining the efficacy of GABA-mediated inhibitory signaling. Here, we examine how SDs affect GABAB receptor trafficking by inserting the α-bungarotoxin-binding site (BBS) into R1b to distinguish its mobility from R1a subunits. This approach has been successfully applied to monitor the trafficking of R1a and R2 subunits (20, 21).
Results
Bungarotoxin Tagging of R1bBBS.
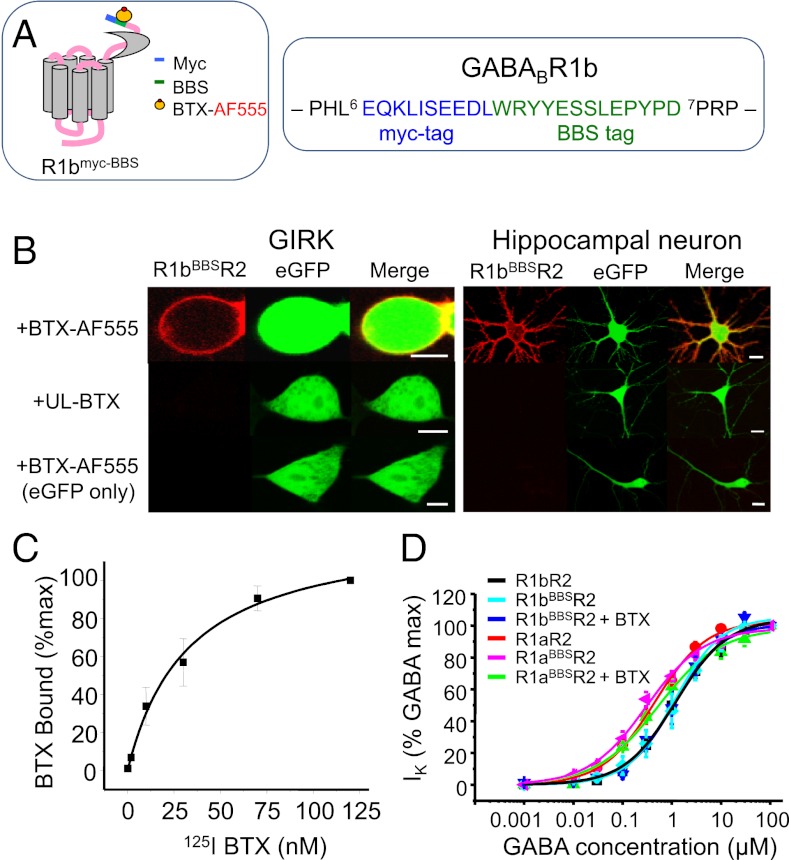
Constitutive internalization of the R1bR2 was followed by inserting a BBS site in the R1b N terminus adjacent to a myc tag near the start of the mature protein (R1bBBS; Fig. 1A). The ability of R1bBBS to bind α-bungarotoxin (BTX) coupled to Alexa Fluor 555 (BTX-AF555) was demonstrated in GIRK cells (HEK293 cells stably expressing inwardly-rectifying potassium channels, Kir 3.1 and Kir 3.2) and cultured hippocampal neurons expressing either R1bBBS, R2 and eGFP, or just eGFP (Fig. 1B). GIRK cells were incubated with BTX-AF555 or unlabeled (UL)-BTX and then fixed and imaged. Hippocampal neurons were treated similarly except for preincubating with 1 mM d-tubocurarine (d-TC) to block BTX binding to α7 nicotinic acetylcholine receptors (nAChRs) (22). Clear surface labeling was detected with BTX-AF555, but not UL-BTX, in cells expressing R1bBBSR2 and eGFP. Moreover, incubating cells expressing R1bBBSR2 and eGFP with UL-BTX followed by BTX-AF555 prevented fluorophore labeling (SI Appendix, Fig. S1), demonstrating the specificity of BTX labeling.
Fig. 1.
BBS in R1b binds to BTX-AF555 and is functionally silent. (A) Schematic diagram for R1b showing the relative locations of the BBS and the myc epitope. The BBS was inserted between Leu6 and Pro7 in the mature R1b protein. (B) Images of GIRK cells (Left) and hippocampal neurons (Right) expressing R1bBBSR2 and/or eGFP incubated with 3 μg/mL BTX-AF555 or UL-BTX for 10 min at RT. Neurons were preincubated with 1 mM d-TC for 5 min. [Scale bars: 5 μm (GIRK cells); 10 μm (neurons)]. (C) Whole-cell radioligand binding experiments with [125I]BTX for the R1bBBSR2 (n = 6). (D) GABA concentration–response curves for R1aR2, R1aBBSR2, R1bR2, R1bBBSR2, and BTX-bound R1aBBSR2 and R1bBBSR2 expressed in GIRK cells (n = 5–6).
Radioligand-binding studies with iodinated BTX, [125I]BTX, was used to assess the saturation binding of BTX to R1bBBS. Increasing concentrations of [125I]BTX were applied to GIRK cells expressing R1bBBSR2 for 1 h at room temperature (RT). [125I]BTX binding was concentration-dependent (Fig. 1C), and Scatchard analysis revealed a Kd of 32.6 ± 5.1 nM (n = 6). This is eightfold lower than that for α7/5HT3a receptors (3.9 ± 2.4 nM; n = 3) (20) and threefold lower than BTX binding to R1aBBS in R1aR2 heteromers (9.8 ± 2.6 nM) (21).
The functional neutrality of the BBS tag on R1bBBS was examined using whole-cell recording and GABA concentration response curves for activating Kir 3.1 and 3.2. Wild-type (R1bR2 or R1aR2) and R1bBBSR2 or R1aBBSR2 receptors were studied in the presence and absence of BTX-AF555 (Fig. 1D). Inserting the BBS into R1b had no effect on GABA potency determined from the concentration–response curve EC50s for R1bBBSR2 in the absence (1.2 ± 0.07 μM; n = 5) or presence (1.14 ± 0.17 μM; n = 6) of BTX-AF555, compared with R1bR2 (1.10 ± 0.07 μM; n = 5; P > 0.05; Fig. 1D). Similarly, the EC50s for R1aBBSR2 in the absence (0.30 ± 0.04 μM; n = 5–11) or presence of BTX-AF555 (0.52 ± 0.10 μM; n = 5) were unaltered compared with R1aR2 (0.45 ± 0.04 μM; n = 5; P > 0.05; Fig. 1D).
These results suggest that as for R1aBBS (21) and R2BBS (20), inserting a BBS site into R1b enables high affinity BTX binding, and when coexpressed with R2, the pharmacological profile of the receptor is unaffected. Thus, BBS tagging and associated imaging strategies can be used to study the trafficking of R1bR2.
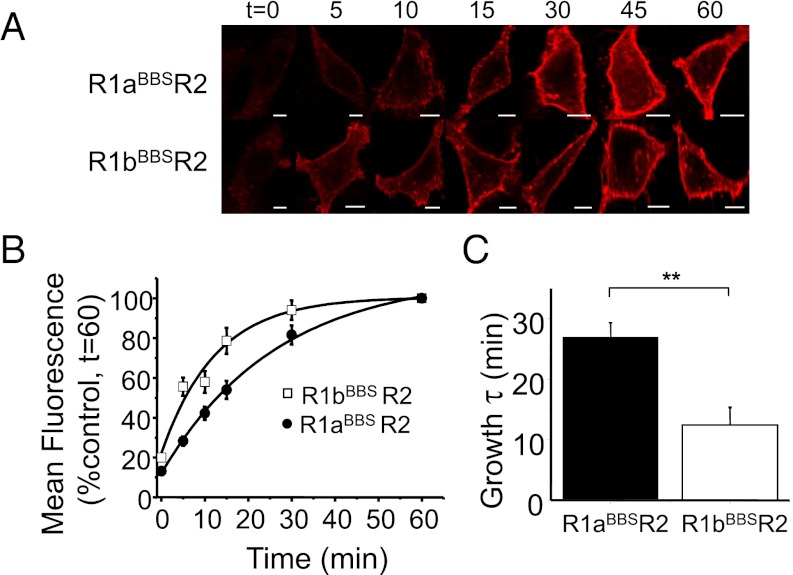
R2 Slows the Internalization of R1b Homomers.
To monitor the cell surface residency of R1b alone, the endoplasmic reticulum (ER) retention motif (-RSR-) in the coiled-coil domain was replaced by single amino acid code -ASA-, enabling R1b to travel to the cell surface. Constitutive internalization of R1bBBS-ASA and R1bBBSR2 was evident at RT from monitoring the decay in surface membrane fluorescence (Fig. 2A). This was faster for R1bBBS-ASA (τ = 8.6 ± 1.1 min; n = 8) compared with R1bBBSR2 (τ = 14.6 ± 1.4 min; n = 9; P < 0.05; Fig. 2B and C), indicating R2 slows the rate of internalization for R1b following heterodimerization (Table S1).
Fig. 2.
R2 stabilizes R1b subunits at the cell surface. (A) GIRK cells expressing either R1bBBSR2 (Top), R1bBBS-ASA (Middle), or R1bBBS-ASA-AA (Bottom) were incubated in 3 μg/mL BTX-AF555 for 10 min at RT to label surface GABAB receptors and imaged over 0–60 min at RT. (B) Rate of internalization of BTX-AF555–tagged R1bBBSR2 heteromers (□) and R1bBBS-ASA ( ) and R1bBBS-ASA-AA (
) and R1bBBS-ASA-AA ( ) homomers (n = 8–9) at RT. Data for R1aBBS-ASA, R1aBBS-ASA-AA, and R1aBBSR2 are shown for comparison as dashed or gray lines (from ref. 20). (C) Exponential decay time constants for membrane fluorescence for R1bBBSR2, R1bBBS-ASA, and R1bBBS-ASA-AA. (D) Extent of internalization for R1bBBSR2 receptors and R1bBBS-ASA or R1bBBS-ASA-AA homomers. NS, not significant; *P < 0.05 (one-way ANOVA). (Scale bar: 5 μm.)
) homomers (n = 8–9) at RT. Data for R1aBBS-ASA, R1aBBS-ASA-AA, and R1aBBSR2 are shown for comparison as dashed or gray lines (from ref. 20). (C) Exponential decay time constants for membrane fluorescence for R1bBBSR2, R1bBBS-ASA, and R1bBBS-ASA-AA. (D) Extent of internalization for R1bBBSR2 receptors and R1bBBS-ASA or R1bBBS-ASA-AA homomers. NS, not significant; *P < 0.05 (one-way ANOVA). (Scale bar: 5 μm.)
We know that R2 subunits prolong the residence of R1a at the cell surface by interacting with a dileucine (LL) motif in the C terminus coiled-coil domain of R1a (20). Because R1a and R1b have identical coiled-coil domains, we expected that R2 would behave similarly with R1b by interacting with its LL motif (L773, L774).
The importance of the LL motif in R1b was determined by substitution with alanines. The rate of internalization for R1bBBS-ASA-AA was significantly slower (τ = 14.1 ± 1.9 min; n = 8; P < 0.05; Fig. 2A) compared with R1bBBS-ASA and similar to that for R1bBBSR2 (P > 0.05; Fig. 2 B and C). However, the extent of constitutive internalization for R1bBBS-ASA (25.9 ± 2%; n = 8), as determined by the steady state of the fluorescence decay curve, was similar to that for R1bBBSR2 (23.9 ± 2.1%; n = 9; P > 0.05) and R1bBBS-ASA-AA (21.8 ± 1.7%; n = 8; P > 0.05). This profile was different for R1a homomers, where the extent of internalization was greater for R1aBBS-ASA compared with those for R1aBBS-ASA-AA and R1aR2 (20).
Thus, although the R2 subunit slows the internalization of both R1aR2 (20) and R1bR2 by interacting with the LL motif in the R1 C terminus, the disparity between the relative extents of internalization implied that the longer residency of R1aR2 at the cell surface may be a consequence of the SDs in R1a. In addition, the observation that there is no difference in extents of internalization between R1a and R1b homomers compared with R1aR2 and R1bR2 suggests that role of the SDs is enhanced in the presence of R2.
To ensure that photobleaching of BTX-AF555 does not affect the decay in surface fluorescence, we scanned expressing cells at low temperatures that prevented internalization (SI Appendix, Fig. S2). Using the same number of scans as for live cell imaging, the fluorescence intensity decreased by only 5%. This photobleaching profile was unaltered by BTX-AF555 binding to R1aBBSR2 and R1bBBSR2, suggesting that any difference in fluorescence decay for these receptor isoforms is attributable to internalization and not photobleaching.
R1aR2 Is More Stable Than R1bR2 on the Cell Surface of Hippocampal Neurons.
To determine whether R1aR2 receptors are more durable on the surface of hippocampal neurons than R1bR2, the constitutive internalization of R1aBBSR2 and R1bBBSR2 was measured in the soma of cultured hippocampal neurons at near physiological temperatures (PTs) (30 °C to 32 °C; Fig. 3A). A rapid decrease in surface fluorescence was evident for each receptor isoform (Fig. 3B), with the rate of internalization for R1bBBSR2 being significantly faster (τ = 9.4 ±1.5 min; n = 12) and proceeding to a greater extent (18.1 ± 1.2%; n = 12) compared with R1aBBSR2 (τ = 16.3 ± 2.5 min, P < 0.05; 30.7 ± 2.2%, P < 0.001; n = 7; Fig. 3 C and D). This is consistent with R1aR2 receptors being retained for longer periods on the somatic cell membrane, further implicating the SDs in GABAB receptor trafficking. To check that these differences did not reflect any dissociation of BTX from the BBS in live cells, internalization of BTX-AF555–tagged R1aBBSR2 and R1bBBSR2 receptors was studied using fixed GIRK cells (SI Appendix, Fig. S3 A–C). This confirmed our results with live cells, with R1bBBSR2 internalizing faster and to a greater extent than R1aBBSR2.
Fig. 3.
R1bR2 internalize faster and to a greater extent than R1aR2. (A) Hippocampal neurons [14–21 days in vitro (DIV)] expressing R1aBBSR2 or R1bBBSR2 with eGFP were incubated in 1 mM d-TC, followed by 3 μg/mL BTX-AF555, at RT for 10 min and imaged at 30–32 °C. (B) Rates of constitutive internalization of BTX-AF555–tagged R1aBBSR2 (●) and R1bBBSR2 (□) receptors (n = 7–12). (C) Exponential decay time constants for membrane fluorescence of R1aBBSR2 and R1bBBSR2 receptors. (D) Extent of constitutive internalization for R1aBBSR2 and R1bBBSR2 receptors. *P < 0.05; ***P < 0.001. (Scale bar = 10 μm.)
Membrane Insertion Is Faster for R1bR2.
We next investigated whether the rates of insertion into the cell surface membrane for R1bBBSR2 and R1aBBSR2 receptors were also different. UL-BTX (20 μg/mL) was applied at RT for 10 min to label all cell surface GABAB receptors. After washing to remove unbound UL-BTX, BTX-AF555 was applied at 37 °C for different periods before fixation (Fig. 4A). By measuring membrane fluorescence, a significant difference was observed in the rates of receptor insertion, with R1bBBSR2 appearing at a faster rate (τ = 12.4 ± 2.9 min; n = 9) compared with R1aBBSR2 (τ = 26.5 ± 2.4 min; n = 9; P < 0.01; Fig. 4 B and C). These results suggested that the two subtypes of GABAB receptors have distinct mobility dynamics at the cell surface, with R1bR2 constitutively inserted at the cell surface at a faster rate than R1aR2 receptors, which is possibly also a consequence of the SDs.
Fig. 4.
R1bBBSR2 is inserted faster than R1aBBSR2 into the cell membrane. (A) Images of GIRK cells transfected with cDNAs encoding for R1aBBSR2 or R1bBBSR2 with eGFP incubated in 20 μg/mL UL-BTX for 10 min at RT, washed, and then incubated at 37 °C for up to 60 min in 3 μg/mL BTX-AF555 before being fixed. (B) Rates of constitutive insertion of R1aBBSR2 (●) and R1bBBSR2 (□) receptors (n = 9). (C) Exponential growth time constants for the increased surface fluorescence representing R1aBBSR2 and R1bBBSR2 receptors. **P < 0.01. (Scale bar: 5 μm.)
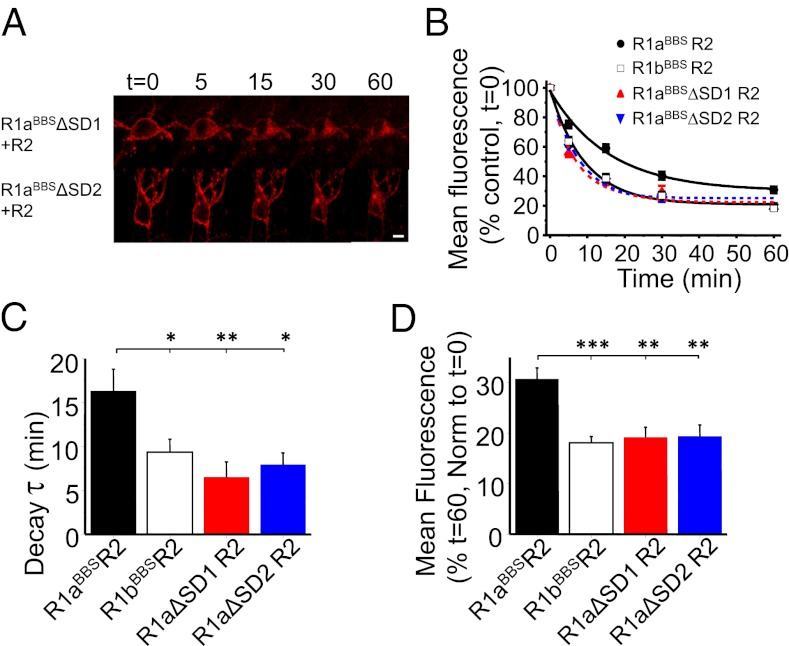
SDs Increase the Cell Surface Residence Time for R1aR2.
Interpretation of NMR structures for the SDs have revealed that the N-terminal SD (SD1) is less compact than its downstream “C-terminal” counterpart (23), implying that the SDs could have different roles. To investigate whether R1aR2 cell surface stability was differentially regulated by the SDs, we deleted either SD1 (R1aBBSΔSD1) or SD2 (R1aBBSΔSD2) in R1aBBS and studied constitutive internalization of the R1aBBSΔSD1R2 and R1aBBSΔSD2R2 heteromers. Both R1aBBSΔSD1R2 (τ = 6.5 ± 1.8 min; n = 9; Fig. 5 A–C) and R1aBBSΔSD2R2 (τ = 7.9 ± 1.3 min; n = 12; Fig. 5 A–C) internalized at similar rates, indistinguishable from R1bBBSR2 (P > 0.05; Fig. 5C) but faster than R1aBBSR2 (P < 0.01 and P < 0.05, respectively; Fig. 5C).
Fig. 5.
SDs in R1a increase the surface stability of R1aR2. (A) Hippocampal neurons expressing either R1aBBSΔSD1R2 or R1aBBSΔSD2R2 with eGFP were incubated in 1 mM d-TC, followed by 3 μg/mL BTX-AF555, for 10 min at RT and imaged at different time points at 30–32 °C. (B) Rates of internalization for BTX-AF555 tagged R1aBBSR2 (●), R1bBBSR2 (□), R1aBBSΔSD1R2 ( ), and R1aBBSΔSD2R2 (
), and R1aBBSΔSD2R2 ( ) receptors (n = 7–12). (C and D) Exponential decay time constants for membrane fluorescence (C) and extent of constitutive internalization (D) of R1aBBSR2, R1bBBSR2, R1aBBSΔSD1R2, and R1aBBSΔSD2R2. ***P < 0.001; **P < 0.01, *P < 0.05 (one-way ANOVA). (Scale bar: 10 μm.)
) receptors (n = 7–12). (C and D) Exponential decay time constants for membrane fluorescence (C) and extent of constitutive internalization (D) of R1aBBSR2, R1bBBSR2, R1aBBSΔSD1R2, and R1aBBSΔSD2R2. ***P < 0.001; **P < 0.01, *P < 0.05 (one-way ANOVA). (Scale bar: 10 μm.)
The extent of internalization for R1aBBSΔSD1R2 (19.2 ± 2%; n = 9) and R1aBBSΔSD2R2 (19.4 ± 2.3%; n = 12; Fig. 5D) was also similar to R1bBBSR2 but significantly greater compared with R1aBBSR2 (P < 0.01). These results suggest that the increased stability conferred by the SDs on R1aR2 requires the presence of both SD1 and SD2 in the N-terminal domain.
By deleting either one of the SDs in R1a, the functional integrity of the expressed GABAB receptors in GIRK cells was unaltered, as determined from the GABA concentration–response curve profiles and the GABA EC50s (R1aBBSΔSD1R2: 0.29 ± 0.03 μM, n = 4–9; R1aBBSΔSD2R2: 0.47 ± 0.05 μM, n = 5; SI Appendix, Fig. S4), which remained comparable to R1aR2 (0.45 ± 0.04 μM) and R1aBBSR2 (0.30 ± 0.04 μM).
Sushi Domains Stabilize R1aR2 Receptors at Dendrites and Spines.
In neurons, R1aR2 and R1bR2 are located postsynaptically near glutamatergic terminals (13), typically opposite dendritic spines. To explore GABAB receptor mobility in native cell membranes, the internalization of R1aR2 and R1bR2 was studied in the dendrites and spines of transfected hippocampal neurons. In dendritic spines, the rate of internalization for R1aBBSR2 (τ = 11.4 ± 3.1 min; n = 78 spines; Fig. 6 A and C) was slower compared with R1bBBSR2 (τ = 6.6 ± 1 min; n = 75 spines; P < 0.001; Fig. 6 A, C, and E), whereas the extents of internalization for R1aBBSR2 (32.8 ± 1%; Fig. 6 A and C) and R1bBBSR2 (30.6 ± 1%; P > 0.05; Fig. 6 A, C, and F) were similar after 60 min.
Fig. 6.
SDs increase surface stability of R1aR2 in dendrites and spines. (A and B) Hippocampal neurons expressing either R1aBBSR2 or R1bBBSR2 with eGFP were incubated in 1 mM d-TC, followed by 3 μg/mL BTX-AF555, for 10 min at RT and spines (A) or dendrites (B) imaged up to 60 min at 30–32 °C. (C and D) Rates of constitutive internalization of R1aBBSR2 (●) and R1bBBSR2 (□) on dendritic spines (n = 75–78) (C) and membranes (n = 7–10) (D). (E) Exponential decay time constants for membrane fluorescence of R1aBBSR2 and R1bBBSR2 in dendrites (Left) and spines (Right). (F) Extent of constitutive internalization of R1aBBSR2 and R1bBBSR2 in dendrites (Left) and spines (Right). NS, not significant; ***P < 0.001; **P < 0.01. (Scale bar: 2 μm.)
In accord with GABAB receptor trafficking in hippocampal somatic membranes and dendritic spines, internalization of R1aBBSR2 was again slower in dendritic shafts of spiny neurons (τ = 19.4 ± 3.3 min; n = 7; Fig. 6 B and D) compared with R1bBBSR2 (τ = 9.2 ± 0.9 min; n = 10; P < 0.001; Fig. 6 B, D, and E), but now the extent of internalization was greater for R1bBBSR2 (22.8 ± 2.5%; Fig. 6 B and D) compared with R1aBBSR2 (36.3 ± 2.7%; P < 0.01; Fig. 6 B, D, and F). This is indicative of different trafficking itineraries for the two major GABAB receptor isoforms in hippocampal postsynaptic membranes.
BBS Tag on mGluR2 Receptors.
To establish the importance of the SDs for regulating receptor trafficking, we inserted them into another GPCR, the metabotropic glutamate receptor (mGluR)2, which lacks an equivalent structure. We inserted a BBS site 6 aa from the N terminus of mGluR2 (mGluR2BBS; Fig. 7A) and then created a chimera from the mGluR2BBS receptor and the SDs from R1a (mGluR2BBS-SD). This involved inserting amino acids G16 to N159, comprising the two SDs in R1a, adjacent to the BBS in mGluR2 (Fig. 7A). This insertion of the SDs and the BBS did not affect the functional properties of mGluR2 (SI Appendix, Fig. S5).
Fig. 7.
SDs stabilize cell surface mGluR2 receptors. (A) Schematic diagram showing the location of the BBS in mGluR2. The BBS was inserted between Lys6 and Val7. (B) Hippocampal neurons expressing either mGluR2BBS or mGluR2BBS-SD were incubated in 1 mM d-TC, followed by 3 μg/mL BTX-AF555, for 10 min at RT and imaged up to 60 min at 30–32 °C. (C) Rates of constitutive internalization for BTX-AF555–tagged mGluR2BBS (▽ ), mGluR2BBS-SD (▲), R1aBBSR2 (●), and R1bBBSR2 (□) (n = 7–12). (D and E) Exponential decay time constants for membrane fluorescence (D) and extents of constitutive internalization (E) of mGluR2BBS, mGluR2BBS-SD, R1aBBSR2, and R1bBBSR2. ***P < 0.001; **P < 0.01; *P < 0.05 (one-way ANOVA). (Scale bar: 10 μm.)
Neurons expressing either mGluR2BBS or mGluR2BBS-SD bound BTX-AF555 with high specificity (SI Appendix, Fig. S6). Live-cell imaging of BTX-AF555–tagged mGluR2BBS revealed that compared with both R1aR2 and R1bR2, the mGluR2BBS receptors exhibited increased stability at the cell surface (P < 0.001; Fig. 7 B and C), with slower rates (τ = 29.8 ± 4.5 min; n = 8; Fig. 7D) and lower extents (41.2 ± 3; n = 8; Fig. 7E) of internalization. Indeed, over a 1-h imaging period, only a relatively small amount of internal BTX-AF555 fluorescence was observed, in comparison with GABAB receptors. Significantly, incorporating the two SDs in the chimera, mGluR2BBS-SD, further increased receptor cell surface stability, reducing the rate (τ = 42.3 ± 8 min; n = 7; P < 0.001; Fig. 7D) and lowering the extent of internalization (64.5 ± 2.2%; n = 7; P < 0.001; Fig. 7E) compared with that for mGluR2BBS. These data confirm that SDs can confer increased cell surface stability on at least two major types of GPCRs.
Discussion
By using the BTX-tagging method, we have identified separate trafficking itineraries for the major GABAB receptor subtypes in the CNS, R1aR2 and R1bR2. Placing the BBS in the N terminus of R1b allowed heteromers containing this subunit to be followed in real-time using fluorophore-labeled BTX. The BBS did not alter the functional electrophysiological properties of recombinant R1bBBSR2 receptors, and high-affinity binding of BTX to R1bBBS allowed us to monitor the cell surface stability of R1bR2 receptors in somatic and dendritic membranes, as well as dendritic spines.
R2 and Stabilization of GABAB Receptors.
Functional GABAB receptors are heterodimers (9, 24, 25) with R1 and R2 subunits playing distinct roles in signaling and trafficking. Recently, we proposed a role for R2, which is based on the stabilization of R1a subunits at the cell surface after formation of the heterodimer (20). This is achieved by an interaction between the two subunit C-terminal tails, involving an LL motif in the coiled-coil domain of R1a. In the present study, R2 performs a similar role with R1b. This was evident from the replacement of the ER retention motif on R1b and comparing the fast rate of internalization for R1bBBS-ASA with the slower rate for R1bBBSR2. The significance of the LL motif on R1b for fast internalization was emphasized by its substitution in R1bBBS-ASA-AA, which slowed the rate of internalization comparable to that of R1bBBSR2. However, there is an important distinction between the trafficking behaviors for R1a and R1b: the extent of internalization of R1bBBS-ASA was similar to R1bBBSR2, differing from R1aBBS-ASA, which internalized to a greater extent than R1aBBSR2. Given that the major structural difference between the R1 isoforms are the two SDs in R1a, this implied they may critically impart additional stability upon R1aR2 receptors compared with R1bR2.
Sushi Domains Regulate GABAB Trafficking.
The different cell surface stabilities of R1aR2 and R1bR2 receptors in hippocampal neurons required the presence of both SDs in the R1a subunit because R1aR2 receptors that contained deletions of either SD (R1aBBSΔSD1R2 or R1aBBSΔSD2R2) exhibited internalization profiles indistinguishable from R1bR2 receptors.
This important role of the SDs in trafficking extends beyond GABAB receptors. This was revealed by inserting a BBS into the N terminus of another class C GPCR, the mGluR2 receptor, which does not contain innate SDs but otherwise closely resembles GABAB receptors (26, 27). Notably, mGluR2BBS receptors were more stable on the cell surface compared with either subtype of GABAB receptor, suggesting the existence of diverse trafficking mechanisms within the class C GPCR family. Inserting the SDs into mGluR2BBS increased their cell surface stability, concurring with a role for these structures in stabilizing surface R1aR2 receptors. The mechanism by which SDs stabilize R1aR2 and the chimeric mGluR2 receptors is unknown. Given the N-terminal location of the SDs, it is likely that protein–lipid interactions in the extracellular matrix are important (1).
The SDs in R1a are noted for their importance in the differential subcellular targeting of R1aR2 over R1bR2 receptors (13, 15). The SDs form an axonal targeting signal for the preferential transport of R1aR2 receptors, although R1aR2 and R1bR2 receptors can also be found in the cell soma and dendrites (19). The interaction partners that are important for axonal targeting have not yet been identified. However, one important distinction is that either SD can fulfill the targeting role, which differs from their receptor stabilizing role, where both are an absolute requirement. It is possible that the SDs may interact across the subunits in the R1aR2 heteromer enabling SD1-SD2 to stabilize larger oligomeric complexes (28, 29) on the cell surface.
Generally, SDs are known to engage in specific protein–protein interactions, and their role in the immune system has been relatively well characterized (30). The N-terminal SD (SD1) of R1a is less compact compared with its C-terminal partner SD (SD2) (23), and it is also atypical in structure to the rest of the complement control protein family. SD1 interacts with the extracellular matrix protein fibulin-2 in vitro (23), and there is evidence that CCAAT/enhancer-binding protein homologous protein (CHOP) differentially interacts with R1a (31). In addition, a soluble excreted isoform of part of R1a, defined as R1j, is mostly composed from the two SDs (32, 33), and this inhibits the function of GABAB heteroreceptors and recognizes binding partners on neuronal membranes. However, the sites of attachment have not been identified (33). It is, therefore, likely that the SDs interact with other proteins that could modulate the signaling and trafficking of GABAB R1aR2 receptors.
Physiological Consequences.
Presynaptic GABAB receptors are classified into autoreceptors or heteroreceptors, depending on whether they inhibit the release of GABA or other transmitters, respectively. This is achieved mostly by the inhibition of voltage-dependent Ca2+ channels (see ref. 1 for a review). Because of preferential targeting, R1aR2 receptors are considered more abundant at axon terminals, specifically glutamatergic terminals, where R1aR2 is more effective at inhibiting Ca2+ influx (13, 17). By contrast, at postsynaptic sites, both R1aR2 and R1bR2 appear similarly effective at activating inwardly rectifying K+ currents (17). Significantly, R1aR2 presynaptic heteroreceptors are selectively activated by low concentrations of baclofen to reduce glutamate release. The role of the SDs in stabilizing the R1aR2 subtype at the cell surface would ensure this population of presynaptic GABAB receptors are not easily internalized, acting as a brake against uncontrolled release of glutamate during periods of excitotoxicity. Indeed, presynaptic GABAB receptors reduce multivesicular glutamate release (34), specifically reducing synaptic cleft levels of glutamate that will affect the amplitude of excitatory postsynaptic potentials (EPSPs).
By contrast, at postsynaptic sites, the R1bR2 subtype, at least in layer 5 neocortical neurons, is mostly responsible for Ca2+ spike inhibition (14). Given the propensity for the R1bR2 subtype to internalize to a greater extent compared with R1aR2 receptors, this would allow the development of NMDA-receptor–mediated Ca2+-dependent postsynaptic plasticity (35–37) under physiological conditions. However, under extreme pathophysiological conditions, such internalization of R1bR2 may also contribute to neurotoxicity.
Clearly R1aR2 receptors will also be present at postsynaptic (extrasynaptic) sites, and these will be capable of ameliorating excessive glutamate-mediated excitation. However, in the neck of the dendritic spines, very near the crucial locus of glutamatergic afferents, R1aR2 receptors are prone to internalize to a greater extent, compared with R1aR2 receptors elsewhere. This tendency would also facilitate glutamate-mediated synaptic plasticity. It would increase Ca2+ influx via NMDA channels by limiting the GABAB-receptor–mediated inhibition of protein kinase A, presumably promoting phosphorylation of NMDA receptors (34, 38). This important caveat, in terms of R1a stability, implies that whatever protein(s) the R1a subunit SDs are interacting with at the cell surface, these must be absent around dendritic spines.
Thus, by exercising control of GABAB receptor trafficking through the R1a subunit SDs, fine tuning of local glutamate-mediated synaptic plasticity is potentially enabled without the need to alter the trafficking dynamics of GABAB receptors at other locations on the cell surface.
Materials and Methods
Additional experimental procedures are described in SI Materials and Methods.
cDNA and Plasmids.
myc-R1b was kindly donated by Andreas Couve (University of Chile, Santiago, Chile). A BBS was inserted into the human mGluR2 N terminus [Missouri S&T cDNA Resource Centre (www.cdna.org)], 6 aa from the start of the mature protein to create mGluR2BBS.
Cell Culture and Transfection.
HEK293 cells stably expressing Kir 3.1 and Kir 3.2 potassium channels (GIRK cells) and embryonic day (E)18 rat hippocampal neurons were grown and transfected as described previously (20, 39).
BTX Radioligand-Binding Assay.
The apparent affinity of BTX for its binding site on the GABAB R1bBBSR2 receptor was determined as described previously using [125I]BTX (20, 21).
Whole-Cell Patch Clamp Electrophysiology.
GABA-activated membrane potassium currents were recorded from single transfected GIRK cells using whole-cell patch-clamp recording as indicated previously (21).
Membrane Insertion Assays and Imaging.
Membrane insertion of receptors was studied as described previously (21).
Imaging and Image Analysis.
GIRK cells and hippocampal neurons (14-21 DIV) were imaged and analyzed as described previously (20) using ImageJ (version 1.40g) and Origin (version 6; OriginLab).
Supplementary Material
Acknowledgments
We thank Dr. Stuart Lansdell for assistance with the radioligand binding experiments. This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC), GlaxoSmithKline (GSK), and the Medical Research Council (MRC).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201660109/-/DCSupplemental.
References
- 1.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 2.Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- 3.Patenaude C, Chapman CA, Bertrand S, Congar P, Lacaille JC. GABAB receptor- and metabotropic glutamate receptor-dependent cooperative long-term potentiation of rat hippocampal GABAA synaptic transmission. J Physiol. 2003;553:155–167. doi: 10.1113/jphysiol.2003.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogt KE, Nicoll RA. Glutamate and γ-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci USA. 1999;96:1118–1122. doi: 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettler B, Kaupmann K, Bowery N. GABAB receptors: Drugs meet clones. Curr Opin Neurobiol. 1998;8:345–350. doi: 10.1016/s0959-4388(98)80059-7. [DOI] [PubMed] [Google Scholar]
- 6.Malitschek B, et al. The N-terminal domain of γ-aminobutyric Acid(B) receptors is sufficient to specify agonist and antagonist binding. Mol Pharmacol. 1999;56:448–454. doi: 10.1124/mol.56.2.448. [DOI] [PubMed] [Google Scholar]
- 7.Margeta-Mitrovic M, Jan YN, Jan LY. Function of GB1 and GB2 subunits in G protein coupling of GABA(B) receptors. Proc Natl Acad Sci USA. 2001;98:14649–14654. doi: 10.1073/pnas.251554498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvez T, et al. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones KA, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 10.Martin SC, Russek SJ, Farb DH. Human GABA(B)R genomic structure: Evidence for splice variants in GABA(B)R1 but not GABA(B)R2. Gene. 2001;278:63–79. doi: 10.1016/s0378-1119(01)00678-3. [DOI] [PubMed] [Google Scholar]
- 11.Kaupmann K, et al. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 12.Hawrot E, et al. Demonstration of a tandem pair of complement protein modules in GABA(B) receptor 1a. FEBS Lett. 1998;432:103–108. doi: 10.1016/s0014-5793(98)00794-7. [DOI] [PubMed] [Google Scholar]
- 13.Vigot R, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Gassmann M, et al. Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J Neurosci. 2004;24:6086–6097. doi: 10.1523/JNEUROSCI.5635-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaban H, et al. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- 17.Guetg N, et al. The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses. J Neurosci. 2009;29:1414–1423. doi: 10.1523/JNEUROSCI.3697-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulrich D, Bettler B. GABA(B) receptors: Synaptic functions and mechanisms of diversity. Curr Opin Neurobiol. 2007;17:298–303. doi: 10.1016/j.conb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Biermann B, et al. The Sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci. 2010;30:1385–1394. doi: 10.1523/JNEUROSCI.3172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannan S, et al. γ-aminobutyric acid type B (GABA(B)) receptor internalization is regulated by the R2 subunit. J Biol Chem. 2011;286:24324–24335. doi: 10.1074/jbc.M110.220814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkins ME, Li X, Smart TG. Tracking cell surface GABAB receptors using an α-bungarotoxin tag. J Biol Chem. 2008;283:34745–34752. doi: 10.1074/jbc.M803197200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekine-Aizawa Y, Huganir RL. Imaging of receptor trafficking by using α-bungarotoxin-binding-site-tagged receptors. Proc Natl Acad Sci USA. 2004;101:17114–17119. doi: 10.1073/pnas.0407563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blein S, et al. Structural analysis of the complement control protein (CCP) modules of GABA(B) receptor 1a: Only one of the two CCP modules is compactly folded. J Biol Chem. 2004;279:48292–48306. doi: 10.1074/jbc.M406540200. [DOI] [PubMed] [Google Scholar]
- 24.White JH, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 25.Kaupmann K, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 26.Pin JP, Galvez T, Prézeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 27.Pin JP, et al. G-protein-coupled receptor oligomers: Two or more for what? Lessons from mGlu and GABAB receptors. J Physiol. 2009;587:5337–5344. doi: 10.1113/jphysiol.2009.179978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurel D, et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: Application to GPCR oligomerization. Nat Methods. 2008;5:561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villemure JF, et al. Subcellular distribution of GABA(B) receptor homo- and hetero-dimers. Biochem J. 2005;388:47–55. doi: 10.1042/BJ20041435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkitadze MD, Barlow PN. Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol Rev. 2001;180:146–161. doi: 10.1034/j.1600-065x.2001.1800113.x. [DOI] [PubMed] [Google Scholar]
- 31.Sauter K, et al. Subtype-selective interaction with the transcription factor CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) regulates cell surface expression of GABA(B) receptors. J Biol Chem. 2005;280:33566–33572. doi: 10.1074/jbc.M503482200. [DOI] [PubMed] [Google Scholar]
- 32.Lee C, Mayfield RD, Harris RA. Intron 4 containing novel GABAB1 isoforms impair GABAB receptor function. PLoS ONE. 2010;5:e14044. doi: 10.1371/journal.pone.0014044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiao JY, et al. The sushi domains of secreted GABA(B1) isoforms selectively impair GABA(B) heteroreceptor function. J Biol Chem. 2008;283:31005–31011. doi: 10.1074/jbc.M804464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalifoux JR, Carter AG. GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron. 2010;66:101–113. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terunuma M, et al. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc Natl Acad Sci USA. 2010;107:13918–13923. doi: 10.1073/pnas.1000853107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guetg N, et al. NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proc Natl Acad Sci USA. 2010;107:13924–13929. doi: 10.1073/pnas.1000909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier PJ, Marin I, Grampp T, Sommer A, Benke D. Sustained glutamate receptor activation down-regulates GABAB receptors by shifting the balance from recycling to lysosomal degradation. J Biol Chem. 2010;285:35606–35614. doi: 10.1074/jbc.M110.142406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalifoux JR, Carter AG. GABAB receptor modulation of voltage-sensitive calcium channels in spines and dendrites. J Neurosci. 2011;31:4221–4232. doi: 10.1523/JNEUROSCI.4561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.