Abstract
Carbonaceous chondrites are meteoritic fragments of asteroids that avoided the geological reprocessing of larger planets and allow laboratory probing of early solar-nebula materials. Among these, Renazzo-type (CR) chondrites found in Antarctica appear remarkably pristine and are distinguished by abundant organic materials and water-soluble molecules such as amino acids and ammonia. We present a comprehensive analysis of the organic composition of selected CR meteorites of different petrographic classification and compare compounds’ abundance and distribution as they may relate to asteroidal aqueous processing and concomitant evolution of the mineral phases. We found that several CR compounds such as amino acids and sugar alcohols are fully represented in stones with no or minimal water exposure indicating a formation that, if solar, preceded parent body processes. The most pristine CRs also revealed natal enantiomeric excesses (ee) of up to 60%, much larger than ever recorded. However, aqueous alteration appears to affect CR soluble organic composition and abundances, in particular some diastereomeric amino acids may gauge its extent by the consequent racemization of their ee.
Keywords: primitive asteroids, abiotic molecular evolution, solar ices, clays
CR meteorites are characterized by several petrologic, geochemical, and isotopic features, such as containing large chondrules, high Fe-Ni metal abundances, and distinct whole-rock O-isotopic distributions (1). However, only recent comprehensive analyses of the soluble and insoluble organic materials in two pristine* CR2† meteorites, GRA 95229 (GRA1) and LAP 02342 (LAP) (2, 3) revealed what is possibly their most distinguishing characteristic vis-à-vis other carbonaceous chondrites (CC) types (i.e., soluble organic suites that are overall unique for having a preponderance of water- over solvent-soluble compounds). Ammonia is the most abundant single molecule detected in CR2 water extracts, and amino acids are their largest component, unlike, for example, in the Murchison meteorite where hydrocarbons are the dominant molecular species.
Amino acids have been the most captivating findings in CC meteorites since the fall of Murchison (4) because several of the compounds have exact molecular correspondence with biomolecules and possible pre-biotic significance. In intervening studies, however, the detection of a diverse and apparently stochastic suite of compounds in many meteorites made it difficult to propose how this random mix could have found effective chemical pathways toward the origin of life (5). CR2s have significantly changed that perspective with their main compositional abundances of water-soluble and N-containing molecular species.
CC materials relied on a long cosmic history for their formation, which spanned solar as well as presolar environments. In particular, the high D and 13C isotopic enrichments of some CC organic compounds have revealed their lineage from cold cosmic regimes (6). Among CRs, GRA1 amino acids display δD values up to +7,200‰ (2), the largest ever determined for an extraterrestrial molecule by direct analysis and falls within δD values established spectroscopically for molecules of the interstellar medium (+5,800 to +45,000) (7). Although available knowledge for the cosmic distribution of nitrogen isotopes is less detailed than for D/H or 13C/12C ratios, δ15N values of +77 to +139‰ for GRA1 amino acids (3), +137‰ for GRA1 ammonia (3), and +223‰ for its insoluble organic material-bound ammonia (8) speak to a long cosmic lineage for these compounds as well (9, 10).
Most CCs known are also at least in part aqueously altered. The provenance of asteroidal water has been questioned and the focus of recent literature (11, 12); the apparent consensus as yet suggests a continuum (13–15) of water accretion between anhydrous asteroids and comets and, based in part from analysis of Stardust cometary grains (16), a possible common origin for their water. Water may affect asteroidal petrology post-accretionally (17) with a conversion of anhydrous to hydrous silicates that is sometimes pervasive, as found represented in petrographic grade 1 (C1) CC; in these samples primary silicate minerals and metals are nearly completely replaced by alteration products (18). For the CR2s analyzed so far, the finding of the more reactive hydroxy-amino acids serine, threonine, and tyrosine never identified in other meteorites, plus abundant suites of aldehydes and ketones (3), also suggested that their asteroidal aqueous chemistry was only mildly oxidizing and somewhat limited by time, temperature, water/rock ratio, or combination of the above factors to keep many organic compounds from further reactions or decomposition. Two stones classified as CR2 upon collection (MET 00426, MET, and QUE 99177, QUE) were recently assigned petrologic type 3 (C3), on the basis of their mineralogical analyses showing minimal thermal and/or aqueous alteration (19).
Therefore, CR chondrites seem to offer ideal samples to study a selected, primitive distribution of organic compounds in the early Solar System and their evolution in small planetesimals as it may relate to the extent of aqueous processing and the concomitant evolution of hydrated mineral phases. In this study, we have extracted and analyzed the soluble organic composition of the following meteorites: the CR1 GRO 95577 (GRO1); CR2s MIL 07525 (MIL), GRO 03116 (GRO2), EET 92042 (EET), GRA 06100 (GRA2), PCA 91082 (PCA); CR3s MET and QUE. We then assessed and further characterized the mineralogy and petrology of the samples with the aim of discovering any possible association between the evolutionary processes of the organic and inorganic meteoritic components. GRA2 and GRO2 were thoroughly contaminated, and results of our analyses are mentioned only when pertinent (Table S1). Terrestrial contamination levels for all meteorites were evaluated based on the presence of proteinogenic amino acids’ L-excesses and accounted for when needed (Table 1). The weathering conditions of the stones, which could alter the organic compounds’ yield, are listed in Table S2. The small amount of available EET material prevented analyses beyond its amino acids, hydroxyacids, polyols, ammonia, and amines.
Table 1.
Amino acids identified in CR meteorites, in nanomol/g
| GRO1 |
MIL |
PCA |
EET |
QUE |
MET |
||
| Linear α-amino acids R*-CH(NH2)-COOH | |||||||
| 1 | glycine | 19.8 | 534.3 | 932.3 | 431.0 | 234.8 | 394.0 |
| 2 | DL-alanine | 2.4† | 353.7 | 634.2 | 400.6 | 99.2 | 452.9 |
| 3 | DL 2aba‡ | nf§ | 67.3 | 106.2 | 39.1 | 21.7 | 105.0 |
| 4 | DL 2ava | nf | 8.5 | 6.1 | 3.8 | 20.7 | 11.3 |
| 5 | DL 2ahex | nf | 1.9 | 1.4 | ≤ 1 | ≤ 1 | 1.7 |
| Branched R-C(CH3)(NH2)-COOH | |||||||
| 6 | aib | 1.2 | 445.5 | 352.8 | 390.1 | 323.3 | 181.9 |
| 7 | DL isovaline | nf | 80.8 | 109.3 | 161.6 | 125.6 | 138.7 |
| 8 | DL 2meva | “ | 3.9 | 7.2 | 7.0 | 10.3 | 18.8 |
| 9 | DL val | “ | 31.7 | 50.4 | 18.4 | 24.3 | 50.8 |
| 10 | DL ile | “ | 5.4† | 5.1 | 2.1 | 5.8 | 8.2 |
| 11 | DL alloile | “ | 5.4 | 7.3 | 3.9 | 6.5 | 21.4 |
| 12 | DL leucine | “ | 3.0† | 3.4 | 3.0 | 1.7† | 18† |
| 13 | DL 2mehex | “ | 1.8 | nf | 1.4 | nf | 1.7 |
| Non-α-amino acids R-CH2(NH2)-R-COOH | |||||||
| 14 | β-alanine | 3.6 | 19.5 | 10.0 | 8.3 | 9.3 | 44.3 |
| 15 | N-meβ-ala | nf | 6.0 | 1.6 | ≤ 1 | 5.1 | 1.3 |
| 16 | DL β-aba | “ | 15.9 | 2.5 | 4.6 | 23.0 | 10.0 |
| 17 | DL β-aib | “ | 8.6 | 1.3 | 1.0 | ≤ 1 | 4.0 |
| 18 | γ-aba | 3.3 | 18.6 | ≤ 1 | 2.0 | 29.5 | 10.4 |
| 19 | δ-ava | 4.5 | 2.3 | 5.4 | ≤ 1 | 2.2 | 1.5 |
| Di-amino & Di-carboxyl amino acids | |||||||
| 20 | ornithine | nf | 20.4 | nf | nf | 2.5 | 5.0 |
| 21 | DL aspartic | “ | 5.1† | 1.2† | ≤ 1 | 4.5† | 3.8† |
| 22 | DL glutamic | “ | 8.2† | 16.4† | 1.6 | 9.4† | 16.7† |
| 23 | DL N-meglu | “ | 11.5 | 1.9 | nf | nf | 2.9 |
| 24 | DL 2-aadi | “ | 3.0 | ≤ 1 | ≤ 1 | ≤ 1 | ≤ 1 |
| 25 | DL 2-meglu | “ | nf | ≤ 1 | nf | nf | ≤ 1 |
| Hydroxy α-amino acids R-C(OH)-CH(NH2)-COOH | |||||||
| 26 | DL serine | 1.0† | 3.5† | 11.5† | ≤ 1* | 3.5† | 18.7† |
| 27 | isoserine | nf | 4.0 | nf | nf | ≤ 1 | ≤ 1 |
| 28 | DL threo | “ | 16.4 | ≤ 1 | nf | 15.7 | 4.3 |
| 29 | DL allothreo | “ | 2.5 | ≤ 1 | nf | nf | ≤ 1 |
| 30 | homoserine | “ | 3.0 | nf | nf | nf | 4.2 |
| N-alkyl α –amino acids R-CH(NHR)-COOH | |||||||
| 31 | sarcosine | nf | 115.5 | 38.1 | 27.7 | 38.8 | 97.2 |
| 32 | N-etgly | “ | 14.4 | 4.5 | ≤ 1 | 3.3 | 8.5 |
| 33 | DL N-meala | “ | 8.8 | 17.1 | 3.8 | 4.8 | 38.8 |
| 34 | N-meaib | “ | 9.1 | 10.7 | 4.4 | 9.2 | 12.2 |
| 35 | DL N-meaba | “ | 3.5 | 11.1 | ≤ 1 | 2.4 | 9.8 |
| N-cyclic and aromatic amino acids | |||||||
| 36 | DL proline | ≤ 1 | 4.2† | 9.2† | 4.4 | 3.8† | 6.7 |
| 37 | DL pipecolic | “ | 20.7 | 7.4 | 7.8 | 7.4 | 7.1 |
| 38 | DL phe-ala | “ | nf | nf | 8.5 | nf | 17.8 |
| 39 | DL tyrosine | “ | 12.8 | ≤1 | 3.0 | ≤ 1 | 8.0 |
| Totals | 37 | 1878 | 2496 | 1546 | 1053 | 1742 | |
Results and Discussion
CR2 and CR3 amino acids were found to be abundant and characterized by the general preponderance of lower molecular weight species (Table 1) [e.g., α-amino isobutyric acid, glycine, and alanine, seen also in LAP and GRA1 (2, 3)]. In a Strecker-type synthetic scenario of meteoritic amino acids forming from aldehydes, HCN, water, and ammonia (20, 21) (Scheme 1), the data would indicate a large abundance of formaldehyde, acetaldehyde, and acetone in their precursors’ pool. The suite of N-substituted amino acids is also distinctive and includes different subgroups of acids, such as N-methyl β-alanine and N-methyl glutamic never described before; via a Strecker syntheses of α-amino acids, amines would have substituted ammonia as reactants.
Scheme 1.
Outline of the Strecker-type synthetic pathways for the formation of amino acids and hydroxyacids from aldehydes and ketones, hydrogen cyanide, ammonia, and water.
Of the CR2 chiral‡ amino acids analyzed that were considered indigenous, only MET and QUE isovaline (#7 in Table 1) showed a small but significant L-ee of 2% comparable to the one observed for GRA1 (2). This represents a departure from CM§ and CI4 compositions, where several 2-methyl amino acids display ee (22, 23). Ee in meteorites offer the only example of chiral asymmetry outside the biosphere and have raised expectations of a possible link between cosmochemical evolution and the origin of life, which is characterized by chiral homogeneity. Their origin is not known, but one question often asked is whether other amino acid species, such as the 2-H-2-amino acids found in terrestrial proteins, had also been affected by asymmetry at some stage in their synthetic history, but their ee record was lost to racemization during parent body aqueous alteration.
Racemization is the conversion of a chiral compound from one configuration to the other and can lead ee-containing compounds to become racemic with time (i.e., a mixture having equal amounts of D- and L-enantiomers). Protein amino acids all have a hydrogen attached to the second carbon in the alkyl chain and are prone to racemization in water because this carbon, slightly acidic for its proximity to an electron-withdrawing carboxyl group, will lose and reacquire its H through attainment of equilibrium. If an alkyl group replaces the H, as is the case for the 2-methyl-2-amino acids, racemization is not possible.
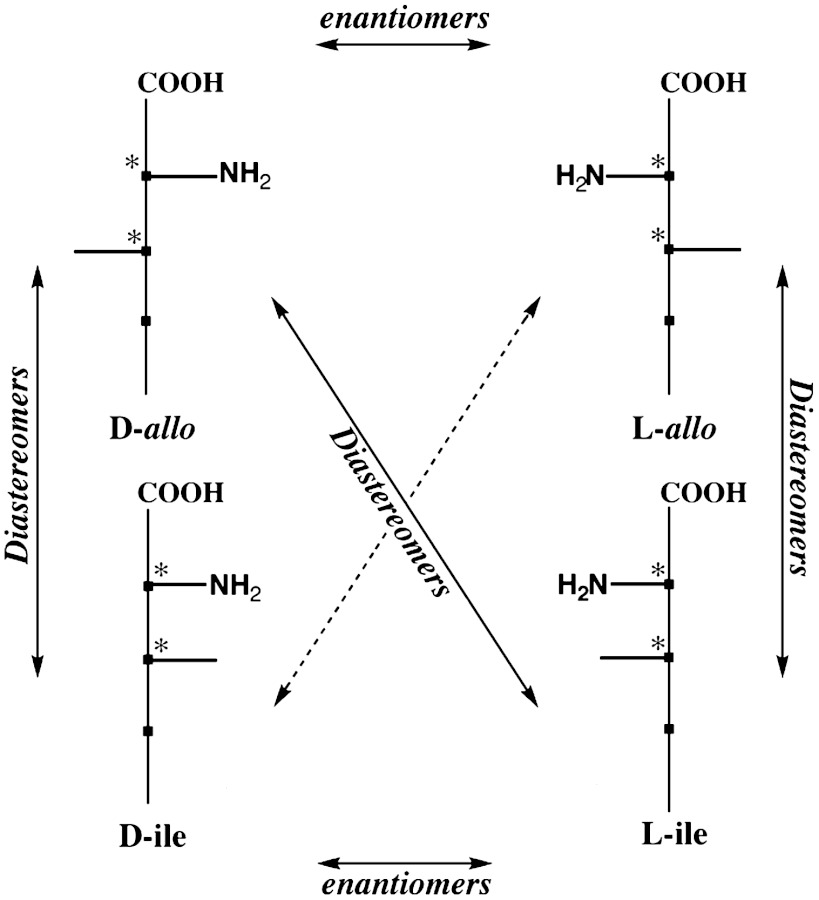
These properties would lead to the conclusion that, if the conditions of the parent body were such that they allowed complete racemization, no amino acid with an α-H would show ee in meteorites after aqueous alteration. However, this is not necessarily true for the particular case of racemization that involves molecules with two chiral centers and are present as four stereoisomers (i.e., two sets of enantiomers called diastereomers). In this case, it is known that racemization of any diastereomer at C2 (the C3-chiral carbon removed from the carboxyl is not acidic and subject to racemization) will not lead from one enantiomer to the other but instead to its conversion to a diastereomer of opposite α-configuration. This process, called epimerization, is shown in the diagram of Scheme 2 for the isoleucine (ile) and alloisoleucine (allo) diastereomers; L-ile is a constituent of proteins while the other three diastereomers are unknown in the biosphere.
Scheme 2.
The enantiomeric and diastereomeric relation of the four 2-amino-3-methylpentanoic acid isomers and their interconversion upon racemization at C2; allo: alloisoleucine, ile: isoleucine.
Epimerization entails that any initial ee of a meteoritic 2-H amino acid diastereomer could be revealed not just by the ee of that amino acid but also by ee of its epimerization product and in spite of racemization. Therefore, if we assume that initial ee of given diastereomers were equal in meteorites of the same family (e.g., caused by comparable ee of their aldehyde precursors), differences in their ee between stones would signify variations in the extent of their racemization in water, (i.e., their chiral distribution could become a reliable indicator of parent bodies’ aqueous conditions).
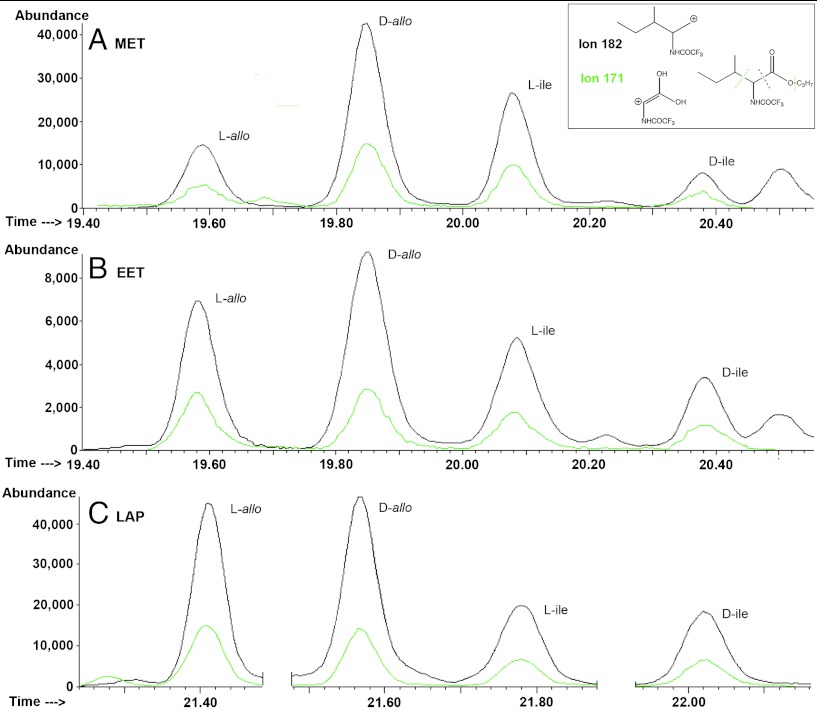
The CR2 meteorites we have analyzed for isoleucine diastereomers show their ee to vary considerably from a maximum of about 60% for MET to less than 5% for LAP (3) (Table 2 and Fig. 1; GRO1, a CR1, did not preserve these amino acids). Based on the expectation of ee depending upon water exposure, therefore, data would show an alteration sequence between CR2s that was most prolonged for LAP, sequentially less so for GRA1 (2), MIL, PCA, EET, QUE and least extensive for MET. Terrestrial contamination does not appear to void these estimates because, although contaminant ile could racemize with long terrestrial exposure and produce some allo, we can consider racemization not to be efficient under the mean subfreezing temperatures and very dry climate of Antarctica. However, there is an approximation in our working assumption and it resides in the possible variability of precursor aldeydes’ ee (e.g., due to its early racemization, and/or the known molecular and chiral heterogeneity of CC) (e.g., ref. 24; see also SI Text).
Table 2.
Enantiomeric distributions of the Allo isoleucine and isoleucine diastereomers in selected CR2 meteorites
| LAP* |
EET |
MET |
PCA |
QUE |
MIL |
GRA1* |
|
| Ile L-ee | 3.6 | 26† | 50 | 19 | 50† | 46† | 14.0 |
| Alloile D-ee | 2.2 | 21 | 60 | 19 | 34.5 | 18 | 12.1 |
| Allo/ile | 1.4 | 1.8† | 2.2 | 2.0 | 1.1† | 1.4† | 2.3 |
*From ref. 3.
†Shows L-proteinogenic amino acid excesses in the extracts, could be in part contaminant and ile/allo ratios were estimated from L-allo/D-ile.
Fig. 1.
Gas chromatography–mass spectrometric analyses of the isoleucine (ile) and allo-isoleucine (allo) diastereomers in the CR meteorites, single ion: (A) MET 00426, (B) EET 92042, and (C) LAP 02342. Inset: Fragmentation pattern and characteristic ions of the two isomeric compounds used to estimate enantiomeric excesses.
Do these findings match petrographic analyses? CR chondrites are known to be highly heterogeneous, due also to large chondrules varying unpredictably the estimate of matrix abundances. For example, the matrix abundances of the CRs in this study ranged from 23.5 to 65.8 vol.%. However, their abundance of metal, sulfide, and Ca-carbonates, while also varying among samples, had GRO1 containing the highest abundance of Ca-carbonate (1.3 ± 0.3) and GRO2, MET, PCA, and QUE the lowest (0.1 ± 0.1) (Table S3). Normalizing data to matrix abundances (where carbonates are mostly found), the samples with lowest to highest Ca-carbonate abundance were: MET; QUE; LAP/GRA1/PCA/EET; and GRO1 (Table S3); i.e., mineralogical traits match well with the above predictions of the least altered CR2s but roughly group those more altered, with the stereochemical evaluation seemingly refining the estimate for their extent of water exposure. Furthermore, secondary alteration minerals such as phyllosilicates and magnetite are rare in MET and QUE and also suggest that these meteorites are among the least aqueously altered CR chondrites (19). The above relative degrees of aqueous alteration also agree well with mineralogical trends inferred from their whole-rock O-isotope composition (25).
Two significant conclusions can be drawn from the overall amino acid data. The first is that because ile/allo five-carbon precursor aldehyde is chiral (CH3CH2C∗H(CH3)CHO) and racemizes readily (in fact, much more rapidly than amino acids), for CR amino acids to show ee at all, their synthetic window must have been short and icy, i.e., in early parent body, proto-planetesimal aggregates (26), or protoplanetary disc (27) environments, with the ammonia abundance that characterizes these type of stones providing a lower freezing point of water (8). Alternative formation pathways to α-amino acids besides Strecker-type syntheses might also allow molecular asymmetry but are not known or easy to propose based on current data.
Abundant amino acids in MET and QUE, combined with observations of their minimal parent body aqueous alteration, also argue against the commonly held paradigm (e.g., ref. 28 and references therein) that an asteroidal water-alteration phase was determinant in meteoritic organics’ formation and support instead their syntheses prior to asteroidal alteration processes. A protracted aqueous phase leading to chondrule and matrix alteration such as in CR2s may foster further syntheses of amino acids and other molecular species but was not essential to their formation. Some amino acid gains could also be challenged by careful comparison between compound abundances and weathering grades of the stones (Table S2), which was not allowed in this study due to limited available samples.
As debated below, this conclusion excludes neither an active role of hydrous silicates in the adsorption, preservation, and further buildup of organic materials in parent bodies [e.g., as seen by the formation of hydrolysable compounds in CMs (29)] nor a determinate participation of minerals in syntheses of amino acids, such as catalysis by FeNi, magnetite, and meteoritic matrixes that has been demonstrated experimentally (30).
The stereochemical data establish the second surprising finding that ee in extraterrestrial environments can be much larger than previously recorded. So far, these were found to be up to 20% for isovaline (23, 24) and 14% for ile/allo (2) amino acids. That 2-H amino acids’ ee were easily racemizeable and reduced during the aqueous phase validates the ee of CR3 stones as primitive and further supports their formation prior to parent body processes. The possible symmetry-breaking mechanism(s) leading to these ee have been debated but are not known (31); by implying that natal ee might have resided with aldehydes, however, these new data shift the focus of inquiry to amino acid precursors. Because ketones leading to isovaline and other ee-carrying 2-me amino acids are not chiral, other synthetic steps leading to amino acids such as imine formation during syntheses could have alternatively been affected asymmetrically [e.g., through mineral interactions as shown for the reverse de-amination reaction (32)].
CR ammonia and amines distributions are less easy to interpret (Table S5) because their abundances do not follow a common or distinguishable trend among stones. For example, highly altered GRO1 contains only one amine (methylamine), consistent with its amino acids depletion, but also displays a significant ammonia amount, while less aqueously altered LAP has the largest observed abundance of both ammonia and amines (3). Given that CR2 insoluble organic material (IOM) was shown to thermolytically release ammonia and traces of methylamine (8, 33), these results could be reasonably explained by assuming that extended water phases had a similar effect on CR IOMs at lower T, increased free ammonia, and, possibly, preserved it on clays during their concomitant formation (vide infra).
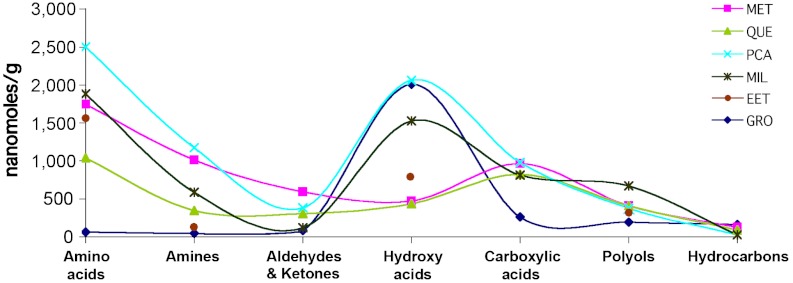
Results for CR alkyl compounds containing only oxygen (Tables S6–S9) can be best overviewed in Fig. 2. Hydroxyacids are compounds whose abundance and distribution in meteorites are most compared to amino acids’ because of their possible synthetic correlation through common precursors via Strecker syntheses (Scheme 1), where their relative yields would be modulated by ammonia’s abundance (34). We found molecular correspondence in linear and α-branched hydroxyacids and amino acids (Table S6), with abundance ratios clearly increasing in water-altered stones. These data also support the hypothesis of their common synthetic pathways but at chronologically distinct stages, after and before water alteration, respectively (35).
Fig. 2.
Abundance distribution of the soluble organic compounds in selected CR meteorites, in nanomole/g of meteorite. Inset: From top to bottom: GRO 95577; MET 00426; QUE 99177; PCA 91082; MIL 07525 and EET 92042 meteorites.
Non-α hydroxyacids are proportionally less abundant than the corresponding amino acids, and only GRO1 contains them at CR2 amino acids levels. Because only α-amino acids can form via Strecker syntheses, the data leave open the question of what alternative syntheses led to these compounds. As for their possible endurance in the meteorite, were they simply more stable and better survivors of alteration phases (e.g., they lack acidic carbons and do not enolize) (36), some would be also found in more pristine meteorites but were not.
Another question raised by the comparative data in Fig. 2 is why hydroxyacid abundances did not decrease in extensively water-altered C1 environments (e.g., GRO1; ref. 18) as most amino acids did. The effect of hydrous silicates may have been essential here, as the seminal work by Garvie and Buseck (37) indicates. The authors carefully isolated small bentonite clay flakes from the Tagish Lake meteorite and demonstrated retention within their layers of soluble organic compounds by electron microscopy. Because the TL fragment they investigated had been found depleted of several compounds [e.g., amino acids (38)], the findings suggested that clays intervened as sinks for the retention of some organic molecules. Hydroxyacids are suited for clay binding via interactions of their carboxyl groups, mostly anionic at the pH of the extracts (6.5–7.8), with clays’ cations. The above study (37) would confirm this possibility recognizing aliphatic and carboxylic groups within clay interlayers by their distinct electron energy-loss spectral features.
Amino acids, zwitterions at these pH values, may have failed to be incorporated into clay minerals, remained adsorbed on their surfaces and decomposed upon oxidation; in GRO1, a high T alteration phase could have been further cause for their degradation. Ammonia abundance in GRO1, either primitive or extracted from the IOM during alteration, could have been preserved in matrix’ clays as well. In fact, clays of altered meteorites have shown high nitrogen content (37) and cation substitutions in the silicate frame-work with ammonium ions has been documented experimentally (39).
Aldehydes and ketones, carboxylic acids, and sugar alcohols (Tables S7–S9) could also have benefited from the appearance of clays because their abundances do not change significantly with CR2s’ alteration degrees; however, literature on the subject is absent. GRO1 has an unusual aldehyde composition that is suggestive of terrestrial contamination; if contaminated, this meteorite’s indigenous aldehydes would have been lost as amino acids and amines. However, by their constant distribution through variously altered meteorites, CR carboxylic acids and polyols are as likely as the amino acids to have been synthesized ahead of parent body aqueous processes.
Hydrocarbons (Table S10) are the least represented compounds in all CR meteorites analyzed, with total amounts below 200 nanomoles. Abundances are the highest for GRO1 but not sufficiently so for results to prove or disprove the probability that this meteorite IOM leached material, including ammonia, during the alteration phase.
Conclusions
Analyses of C3 to C1-type CR chondrites revealed that minimally altered stones carry abundant water-soluble organic compounds and demonstrate that an extended asteroidal aqueous alteration phase was not essential to their formation.
CR2 diastereomeric amino acids show that aqueous processes induced racemization of chiral α-H amino acids, which is dependent on these processes’ extent and validates the ee of more pristine CR3 stones as primitive. Ee values are as high 60% for MET and the largest ever recorded.
Asteroidal aqueous processes changed the organic composition of CR stones, leading mostly to more abundant hydroxyacids, whose molecular makeup reflects in part that of amino acids. This result confirms common, albeit chronologically distinct, Strecker-type syntheses for the two compound groups.
Aqueous processes foster modest increases of amino acid, ammonia, carboxylic acid, and polyol amounts. Expandable clays formed during these processes may have been important in preserving hydroxyacids and ammonia abundances in more aqueously altered stones.
Materials and Methods
Curatorial parent numbers/weights (in mg) of the meteorite fragments analyzed were, respectively, GRO1: .47 and .59/58 + 160; GRO2: .22 and .27/57 + 290; MET: .48/300; EET: .59/120; QUE: .52/270; GRA2: .43/250; PCA: .40/250; MIL: .5/240. All were interior fragments obtained from the Johnson Space Center.
Samples were powdered in agate mortar and extracted in evacuated vials with triple distilled water at 100 °C for 24 h. For analyses of amino acids, hydroxyacids, and polyols the extracts were applied in sequence to cation- and anion-exchange columns (BIORAD AG-50, x4; BIORAD AG-4, x8), for separate collection of cationic (amino acid), and neutral and acidic compounds (37). Dry eluates were derivatized in sequence with acidified isopropanol/isobutanol and trifluoroacetic anhydride for gas chromatography–mass spectrometric (GC–MS) analyses. Amines and monocarboxylic acids were analyzed from dedicated portions of the extracts by collecting their volatile forms by cryogenic transfer in vacuum (37); aldehydes and ketones were derivatized directly from water-extract aliquots by adding a 1∶1 (v∶v) 2.5 mM solution of 2,3,4,5,6-pentafluorobenzyl hydroxylamine (3). Hydrocarbons were obtained by dichloromethane:methanol (9∶1) extraction of water-extracted powders and analyzed by GC–MS. GC–MS methodology is described in SI Text.
For elemental and mineralogical distributions in the samples, we used an optical microscope to characterize each thin section (except MIL and EET, for which a paired sample, EET 87770, was characterized instead) with backscattered electron (BSE) images and full thin section X-ray element maps (Cameca SX-50 electron microprobe analyzer; operating conditions: 15.0 kV and 40.0 nA). Modal abundances of CR chondrite components and select mineral phases (i.e., matrix, chondrules, metal, sulfides, oxides, Ca-carbonates) for entire thin sections were obtained from full BSE images or composite X-ray element maps using the IQmaterials® program and the Adobe Photoshop® software package (26). Meteorites’ weathering grades and modal abundances are given in Table S2.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Cecilia Satterwhite, Kevin Righter, and the Meteorite Working Group for providing the CR meteorite fragments used in this study. S.P. is grateful to John Brucato, Laurence Garvie, Everett Shock, Lynda Williams, and Mike Zolensky for helpful discussions. D.L.S. thanks Ken Domanik for assistance with the electron microprobe and Harold C. Connolly Jr. for helpful discussions. Funding was provided by NASA Exobiology and Cosmochemistry Programs (S.P., A.M.) and the NASA Astrobiology Director’s Fund (S.P., D.L.S., D.S.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204865109/-/DCSupplemental.
*The term pristine is reserved for meteorites having no or minor L-amino acid contaminants while primitive refers to non-, or minimally altered stones (see ref. 2).
†The number represents a classification of petrographic type and estimates asteroidal secondary water processes and varies from 1 to 3; 1 indicates most processed (vide infra and SI Text).
‡Chirality is a property of objects that cannot be brought into congruence with their mirror image by translation or rotation and is intrinsic to the chemistry of carbon due to tetrahedral distribution of its four bonds; a C having four different substituents is asymmetric (C*), the molecules that contain at least one such carbon are chiral and exist in two “handed” forms called enantiomers.
§CC are classified in subgroups by general compositional traits, chondrules’ distribution and named after the first meteorite of the type recognized. CM indicates Mighei-like, CI Ivuna-like.
References
- 1.Weisberg MK, Prinz M, Clayton RN, Mayeda TK. The CR (Renazzo-like) carbonaceous chondrite group and its implications. Geochim Cosmochim Acta. 1993;57:1567–1586. [Google Scholar]
- 2.Pizzarello S, Huang Y, Alexandre MDR. Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc Natl Acad Sci USA. 2008;105:7300–7304. doi: 10.1073/pnas.0709909105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzarello S, Holmes W. Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim Cosmochim Acta. 2009;73:2150–2162. [Google Scholar]
- 4.Kvenvolden K, et al. Evidence of extraterrestrial amino acids and hydrocarbons in the Murchison meteorite. Nature. 1970;228:923–926. doi: 10.1038/228923a0. [DOI] [PubMed] [Google Scholar]
- 5.Cronin JR, Chang S. Organic matter in meteorites: Molecular and isotopic analyses of the Murchison meteorite. In: Greenberg JM, et al., editors. The Chemistry of Life’s Origins. Dordrecht, The Netherlands: Kluwer; 1993. pp. 209–258. [Google Scholar]
- 6.Epstein S, Krishnamurthy RV, Cronin JR, Pizzarello S, Yuen GU. Unusual stable isotope ratios in amino acid and carboxylic acid extracts from the Murchison meteorite. Nature. 1993;326:477–479. doi: 10.1038/326477a0. [DOI] [PubMed] [Google Scholar]
- 7.Roueff E, Gerin M. Deuterium in molecules of the interstellar medium. Space Sci Rev. 2003;106:61–72. [Google Scholar]
- 8.Pizzarello S, Williams LB, Lehman J, Holland GP, Yarger JL. Abundant ammonia in primitive asteroids and the case for a possible exobiology. Proc Natl Acad Sci USA. 2011;108:4303–4306. doi: 10.1073/pnas.1014961108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terzieva R, Herbst E. The possibility of nitrogen fractionation in interstellar clouds. Mon Not R Astron Soc. 2000;317:563–568. [Google Scholar]
- 10.Rodgers SB, Charnley SB. Nitrogen superfractionation in dense cloud cores. Mon Not R Astron Soc. 2008;569:L48–L52. [Google Scholar]
- 11.Jing D, He J, Brucato J, De Sio A, Tozzetti L, Vidali G. On water formation in the interstellar medium: Laboratory study of the O + D reaction on surfaces. Astrophys J Lett. 2011;741:L9. [Google Scholar]
- 12.King HE, et al. Computer simulations of water interactions with low coordinated forsterite surface sites: Implications for the origin of water in the early solar system. Earth Planet Sci Lett. 2010;300:11–18. [Google Scholar]
- 13.Jewitt DC, Luu J. Crystalline water ice on the Kuiper belt object 50000. Nature. 2004;432:731–733. doi: 10.1038/nature03111. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh HH, Jewitt DC. A population of comets in the main asteroid belt. Science. 2006;312:561–563. doi: 10.1126/science.1125150. [DOI] [PubMed] [Google Scholar]
- 15.Briani G, Morbidelli A, Gounelle M, Nesvorny D. Evidence of asteroid-comet continuum from simulations of carbonaceous microxenolith dynamical evolution. Meteorit Planet Sci. 2011;46:1863–1877. [Google Scholar]
- 16.Sanford SA, et al. Organics captured from comet 81P/Wild 3 by Stardust spacecraft. Science. 2006;314:1720–1724. doi: 10.1126/science.1135841. [DOI] [PubMed] [Google Scholar]
- 17.Zolensky M, McSween HY., Jr . Aqueous alteration. In: Kerridge JF, Matthews MS, editors. Meteorites and the Early Solar System. Tucson, AZ: University of Arizona Press; 1988. pp. 114–143. [Google Scholar]
- 18.Weisberg MK, Huber H. The GRO 95577 CR1 chondrite and hydration of the CR parent body. Meteorit Planet Sci. 2007;42:1495–1503. [Google Scholar]
- 19.Abreu NM, Brearley AJ. Early solar system processes recorded in matrices of two highly pristine CR3 carbonaceous chondrites, MET00426 and QUE 99177. Geochim Cosmochim Acta. 2010;74:1146–1171. [Google Scholar]
- 20.Peltzer ET, Bada JL. α-Hydroxycarboxylic acids in the Murchison meteorite. Nature. 1978;272:443–444. [Google Scholar]
- 21.Lerner NR, Cooper GW. Iminodicarboxylic acids in the Murchison meteorite: Evidence of Strecker reactions. Geochim Cosmochim Acta. 2005;69:2901–2906. [Google Scholar]
- 22.Cronin JR, Pizzarello S. Enantiomeric excesses in meteoritic amino acids. Science. 1997;275:951–955. doi: 10.1126/science.275.5302.951. [DOI] [PubMed] [Google Scholar]
- 23.Glavin DP, Dworkin JP. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc Natl Acad Sci USA. 2009;106:5487–5492. doi: 10.1073/pnas.0811618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizzarello S, Zolensky M, Turk KA. Non racemic isovaline in the Murchison meteorite: Chiral distribution and mineral association. Geochim Cosmochim Acta. 2003;67:1589–1595. [Google Scholar]
- 25.Schrader DL, et al. The formation and alteration of the Renazzo-like carbonaceous chondrites I: Implications of bulk-oxygen isotopic composition. Geochim Cosmochim Acta. 2011;75:308–325. [Google Scholar]
- 26.Cuzzi NJ, Hogan RC, Shariff K. Toward planetesimals: Dense chondrule clumps in the protoplanetary nebula. Astrophys J. 2008;687:1432–1447. [Google Scholar]
- 27.Throop H. UV photolysis, organic molecules in young disks, and the origin of meteoritic amino acids. Icarus. 2011;211:885–895. [Google Scholar]
- 28.Pizzarello S, Cooper GW, Flynn GJ. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In: Lauretta D, McSween HY Jr, editors. Meteorites and the Early Solar System II. Tucson, AZ: University of Arizona Press; 2006. pp. 625–651. [Google Scholar]
- 29.Cronin JR. Acid-labile amino acid precursors in the Murchison meteorite: Chromatographic fractionation. Orig Life. 1976;7:337–342. doi: 10.1007/BF00927928. [DOI] [PubMed] [Google Scholar]
- 30.Pizzarello S. Catalytic syntheses of amino acids: Significance for nebular and planetary chemistry; 33rd Annual Lunar and Planetary Science Conference; Houston, TX: 2002. Abstract # 1236. [Google Scholar]
- 31.Pizzarello S, Groy TL. Molecular asymmetry in extraterrestrial organic chemistry: An analytical perspective. Geochim Cosmochim Acta. 2011;75:645–656. [Google Scholar]
- 32.Pizzarello S. Evolution of Solar System Materials: A New Perspective from Antarctic Meteorites. Tokyo: National Institute of Polar Research; 2003. The effect of meteorite powders in model amino acid syntheses and reactions; p. 121. [Google Scholar]
- 33.Pizzarello S, Williams LB. Ammonia in the early solar system: An account from carbonaceous meteorites. Astrophys J. 2012;749:161–166. [Google Scholar]
- 34.Peltzer ET, Bada JL, Schlesinger G, Miller SL. The chemical conditions on the parent body of the Murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Adv Space Res. 1984;4:69–74. doi: 10.1016/0273-1177(84)90546-5. [DOI] [PubMed] [Google Scholar]
- 35.Pizzarello S, Wang Y, Chaban GM. A comparative study of the hydroxy acids fromthe Murchison GRA 95229 and LAP 02342 meteorites. Geochim Cosmochim Acta. 2010;74:6206–6217. [Google Scholar]
- 36.Monroe AA, Pizzarello S. The soluble organic compounds of the Bells meteorite: Not a unique or unusual composition. Geochim Cosmochim Acta. 2011;75:7585–7595. [Google Scholar]
- 37.Garvie LAJ, Buseck PR. Prebiotic carbon in clays from Orgueil and Ivuna (CI), and Tagish Lake (C2 ungrouped) meteorites. Meteorit Planet Sci. 2007;42:2111–2117. [Google Scholar]
- 38.Pizzarello S, et al. The organic content of the Tagish Lake meteorite. Science. 2001;293:2236–2239. doi: 10.1126/science.1062614. [DOI] [PubMed] [Google Scholar]
- 39.Gautier M, Muller F, Le Forestier L, Beny J-M, Guegan R. NH4-smectite: Characterization, hydration properties and hydro mechanical behaviour. Appl Clay Sci. 2010;49:247–254. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.