Abstract
Cilia and flagella are highly conserved motile and sensory organelles in eukaryotes, and defects in ciliary assembly and motility cause many ciliopathies. The two-headed I1 inner arm dynein is a critical regulator of ciliary and flagellar beating. To understand I1 architecture and function better, we analyzed the 3D structure and composition of the I1 dynein in Chlamydomonas axonemes by cryoelectron tomography and subtomogram averaging. Our data revealed several connections from the I1 dynein to neighboring structures that are likely to be important for assembly and/or regulation, including a tether linking one I1 motor domain to the doublet microtubule and doublet-specific differences potentially contributing to the asymmetrical distribution of dynein activity required for ciliary beating. We also imaged three I1 mutants and analyzed their polypeptide composition using 2D gel-based proteomics. Structural and biochemical comparisons revealed the likely location of the regulatory IC138 phosphoprotein and its associated subcomplex. Overall, our studies demonstrate that I1 dynein is connected to multiple structures within the axoneme, and therefore ideally positioned to integrate signals that regulate ciliary motility.
Keywords: dynein f, flagella
Cilia and flagella are highly conserved organelles with roles in cellular movement and signal transduction. In humans, defects in cilia can lead to a number of diseases, such as polycystic kidney disease and primary ciliary dyskinesia (1, 2). The axoneme core of most motile cilia and flagella consists of nine doublet microtubules (DMTs) surrounding two single microtubules (MTs) known as the central pair complex (CPC) (Fig. 1). DMTs are highly periodic with a 96-nm-long unit that repeats along the MT length. They are decorated with two rows of dynein motors, the inner dynein arms (IDAs) and the outer dynein arms (ODAs), which drive MT sliding and axoneme bending (3). Generating the diverse ciliary and flagellar waveforms requires the precise coordination of the activity of thousands of dynein motors within a single organelle (4).
Fig. 1.
Organization of the Chlamydomonas flagellum. (A) Differential interference contrast light microscopy (LM) image shows a Chlamydomonas cell with its two flagella. (B–D) The ultrastructure of the axoneme is visible in EM images; slices from a cryotomogram show longitudinal (B), 3D (C), and cross-sectional (D) views. Key features are highlighted, such as the cylindrical arrangement of nine DMTs (red box in D) and the organization of 96-nm repeats (red box in B) along the length of the axoneme. (E and F) Schematic representations of a 96-nm repeat modified from Nicastro et al. (27) are shown in longitudinal front (E) and cross-sectional (F) views observed from proximal (flagellar base) toward distal (flagellar tip). These orientations are maintained in all figures. The IDAs are numbered in E, and the A- and B-tubule (At and Bt) protofilament numbers are indicated in F. [E and F from ref. 27. Adapted with permission from AAAS.] (Scale bar: 50 nm, valid for B and D.)
The primary signaling pathway known to regulate dynein function in axonemes involves signals traveling from the CPC through radial spokes (RSs) to the IDAs and ODAs (reviewed in 5, 6). Many CPC and RS mutants are paralyzed (7). However, MT sliding can be restored to WT levels in isolated CPC/RS mutant axonemes using protein kinase inhibitors (8, 9). These observations, together with biochemical evidence of phosphorylation of dynein subunits, have implicated the dyneins as a major signaling target of the CPC/RS signaling pathway (10, 11). However, the regulatory mechanisms and physical interactions that participate in signal transduction to the dynein targets are not well understood.
Isolated axonemes require only ATP for reactivation, suggesting that direct interactions between the dyneins and their regulators are physically built into the axoneme (4). Therefore, studies that visualize axonemal structures and their connections at high resolution can provide new insights into interactions that might be important for assembly and/or regulation. For example, a recent cryoelectron tomography (cryo-ET) study of Chlamydomonas axonemes has shed new light on the function of the nexin–dynein regulatory complex (N-DRC) and further defined its role as a highly connected regulatory hub in the distal part of the axonemal repeat (12).
The I1 dynein (dynein f) is the only two-headed IDA in axonemes. Defects in the I1 dynein reduce the swimming velocity of Chlamydomonas and paralyze the flagellum of Trypanosoma brucei (13, 14). The I1 dynein shares some unusual features with the N-DRC. Like N-DRC mutants, I1 mutants were also isolated as suppressors of paralyzed flagella in CPC/RS mutants (15, 16), suggesting that the I1 dynein is not just a motor but has a regulatory function. Biochemical studies and MT sliding assays have demonstrated that both casein kinase 1 (CK1) and type 2 protein phosphatase regulate dynein activity and, either directly or indirectly, phosphorylate I1 intermediate chain IC138, a subunit of the I1 intermediate chain and light chain complex (ICLC) (9, 17, 18). MT sliding assays showed that the phosphorylation status of IC138 correlates with dynein activity, whereby the phosphorylated IC138 appears to be the inactive form (19, 20). Despite continuing studies, the locations of the proteins involved in I1-dependent dynein regulation, and which components and direct interactions participate in signal transduction, remain unknown.
Classic 2D EM of chemically fixed specimens (11, 13, 21–26) and averaged 3D cryo-ET data (27) have demonstrated that the I1 dynein is located at the proximal end of the axonemal repeat between RS1 and the ODA row. The I1 dynein has a “trilobed” structure composed of the two dynein heavy chains (HCs), 1α HC and 1β HC, and the ICLC (Fig. 1). A number of I1 components have been identified biochemically, including three intermediate chains (IC140, IC138, and IC97), several light chains (LC8, LC7a, LC7b, Tctex1, and Tctex2b), and the accessory protein FAP120 (11, 13, 16, 23–25, 28–34). A recent study of an IC138 null mutant (bop5-2) revealed that IC138 is required for assembly of an “IC138 subcomplex” consisting of IC138, IC97, LC7b, and FAP120 (21). However, the precise location of this subcomplex within the I1 ICLC is unknown.
To obtain more detailed information about the specific positions and interactions of the I1 subunits, we analyzed the 3D structure and protein abundance in Chlamydomonas axonemes from WT and three I1 mutants (bop5-1, bop5-2, and pf9-3) using state-of-the art cryo-ET complemented by gel-based proteomics. Consistent with previous studies that implicated I1’s importance for proper motility of cilia and flagella, our averages reveal the I1 dynein as a highly connected regulatory node on the proximal end of the axonemal repeat. We also discovered unique and previously undescribed features of the I1 complex in WT axonemes, such as doublet-specific differences and a large complex called the tether and tether head, which links the 1α HC motor domain directly to the A-tubule. Through correlation of biochemical and 3D structural data, we localized both IC138 and the IC138 subcomplex within the ICLC. These results provide a high-resolution view of the I1 dynein as well as unique insights into the distribution of CPC/RS signals to axonemal dyneins and the mechanism of I1-dependent regulation of axoneme motility.
Results
Using cryo-ET combined with subtomogram averaging, we analyzed the 3D structure of the I1 complex in five Chlamydomonas strains with different degrees of I1 defects. For each strain, well-preserved frozen-hydrated axonemes were reconstructed (Fig. S1) and hundreds of repeat units were averaged to increase the signal-to-noise ratio, and thus the image resolution (more details are provided in Table S1). The pseudo-WT (pWT) strain was generated by transforming the N-DRC mutant pf2 with a GFP-tagged WT PF2 construct; the presence of the PF2-GFP transgene rescues the motility, as well as biochemical and structural defects in the pf2 mutant, and yields averages of the axonemal repeat that are basically identical to WT (side-by-side comparison in Fig. S2) (12). Because of higher resolution in the pWT average (Table S1), some structural details are clearer in the pWT than in the WT averages; therefore, both averages were used to determine the boundaries, 3D structure, and connections of the I1 complex. To determine the location of I1 subunits, we also correlated our structural data with the relative abundance of I1 subunits in the same five stains, as established by gel-based proteomics.
Cryo-ET Reveals the 3D Ultrastructure of the I1 Dynein and a Tether of a Dynein Motor Domain to the DMT.
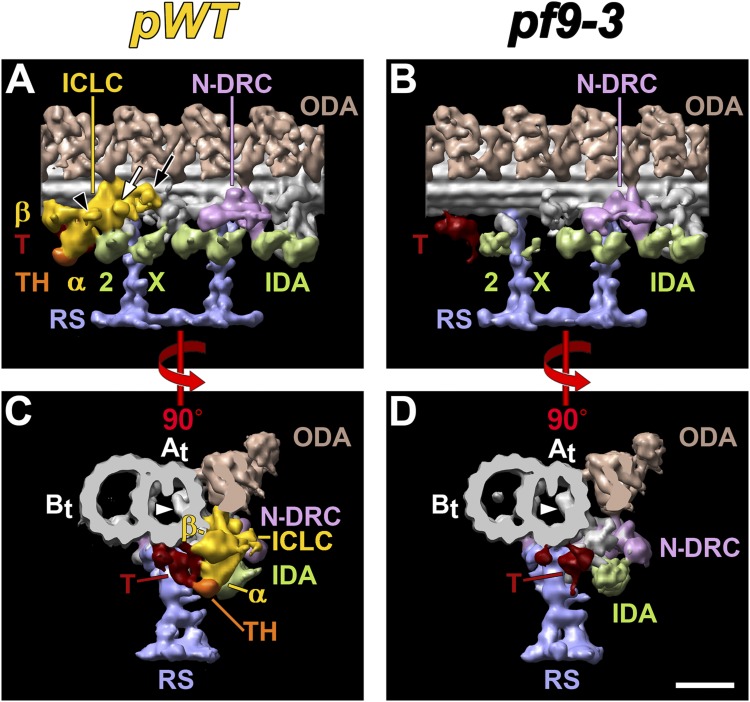
The boundaries of the I1 complex (i.e., which densities form the I1 dynein) were determined by comparing the structures present in the WT/pWT axonemal repeat relative to those missing in the 96-nm repeat from the I1 mutant strain pf9-3 (Fig. 2, Table 1, and Movies S1 and S2). This I1 mutant is missing all the known I1 components (13, 23, 35); therefore, we defined all structures missing in pf9-3 as part of the I1 complex. The overall “trilobed” shape of the I1 dynein (22) is confirmed by our results. However, the increased resolution of our 3D averages provides previously undescribed details and identifies unique features of this two-headed dynein complex (Figs. 2–4 and Figs. S2–S6). The ICLC is associated with the tail domains of the I1 dynein HCs and forms the ATP-insensitive binding site to the A-tubule of the DMTs (36). Given I1’s large mass of ∼2.5 MDa, we expected that the ICLC would be robustly attached to the A-tubule. Surprisingly, the I1 ICLC is a sheet-like structure that appears to be suspended at a distance of ∼5 nm from the A-tubule, with only a single narrow connection (connection 1 in Fig. 4 and Fig. S5) spanning the distance between the ICLC and the A-tubule (Fig. 4, Figs. S3 and S5, and Movie S1). Interestingly, the same protofilament that the ICLC connects to also links to the density of a MT inner protein, MIP1 (27, 37) (Fig. 2 and Figs. S2 and S3). The protein composition and biological function of MIP1 are unknown. The ICLC has three protrusions toward the neighboring DMT, termed the proximal, central, and distal protrusions (Fig. 2A), as well as a small hole near the distal protrusion (red arrow in Fig. S5H).
Fig. 2.
Defining the WT structure of I1 dynein. Isosurface renderings show averaged axonemal repeats in longitudinal (A and B) and cross-sectional (C and D) views. The pWT strain (A and C) is indistinguishable from WT (Fig. S2). The pf9-3 (B and D) mutant lacks the entire I1 dynein. A comparison of both strains is used to define the boundaries of the I1 complex. Both dynein HCs (α and β), the ICLC, and the tether head (TH) are part of the I1 complex because they are present in pWT but missing in pf9-3. The tether (T) is present in both strains, and is thus not considered part of the I1 complex. The three protrusions of the ICLC were named proximal (black arrowhead), central (white arrow), and distal (black arrow) protrusions in A. Note the location of MIP1 (white arrowheads) (C and D). Dynein IAX (X) is only present in some doublets and thus appears weaker and variable in density (A and B). The A- and B-tubules (At and Bt) are also labeled. (Scale bar: D, 20 nm.)
Table 1.
Structural phenotypes of I1 mutants determined by cryo-ET
| Strain |
||||
| Component | WT/pWT | bop5-1 | bop5-2 | pf9-3 |
| α HC | + (500 kDa) | + | + | − |
| β HC | + (500 kDa) | + | + | − |
| ICLC | + (1.3 MDa) | ± (750 kDa) | ± (650 kDa) | − |
| Tether head | + (100–150 kDa) | + | + | − |
| Tether | + (700 kDa) | + | + | + |
Mass estimates were calculated from the electron densities according to Quillin and Matthews (68). +, present; −, absent; ±, reduced.
Fig. 4.
Connections between the I1 complex and neighboring structures. Isosurface renderings of averaged axonemal repeats from pWT show seven connections (magenta-colored densities and numbers) between the I1 complex and adjacent structures in longitudinal (A–B′) and cross-sectional (C–D′) views. Note that B and B′, as well as D and D′, are pairs of stereo images. A summary of connections is indicated in magenta: 1, ICLC attachment to the A-tubule (At); 2, proximal outer-inner dynein (OID) linker between the ICLC and ODAs; 3, bridge (magenta arrowhead in A) between the distal part of the I1 complex and the N-DRC; 4, ICLC to the IA2 dynein; 5, I1 α motor domain to IA2; 6, I1 α motor domain to tether; 7, tether head (TH) to tether. Other axonemal structures are also labeled: β, I1 β motor domain; Bt, B-tubule. See also Fig. S5.
Consistent with previous studies, the 1α and 1β HC motor domains are located at the proximal end of I1 (13, 24, 27, 38) (Fig. 2A and Fig. S2A). The two I1 dynein heads are not only connected to the ICLC but to each other (Fig. 2A and Fig. S2 A and B) by interactions similar to those seen between the stacked heads of the ODAs (27, 38). A previously undescribed density, named the tether head in this study (colored orange in Fig. 2), is attached to the proximal side of the 1α HC motor domain. The tether head is connected to the A-tubule through another large, previously undescribed density we call the tether (Fig. 2 A and C and Fig. S3). The tether is not considered part of the I1 complex because it is present in pf9-3 (Fig. 2 B and D).
Doublet-Specific Differences of the I1 ICLC Structure in WT Axonemes.
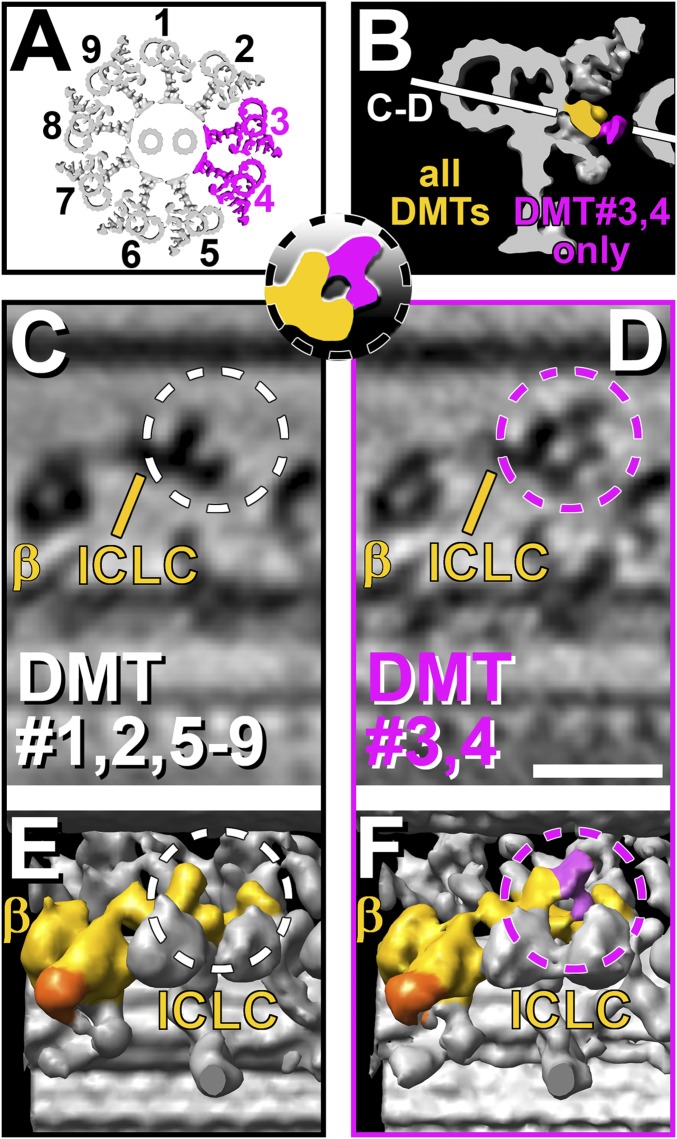
Previous studies have documented some structures that are present only on subsets of doublets in Chlamydomonas axonemes, such as the B-tubule projections (beaks or beak-MIPs) in DMTs 1/5/6, the ODAs missing from DMT 1, and the inner arm (IA) dynein IAX missing from some DMTs (37, 39–43). To investigate if the I1 complex has doublet-specific structures, we generated 3D averages of each individual DMT from two different strains with wild-type phenotypes, pWT and pWT2 (Table S1). The doublet-specific averages of both strains confirmed the same overall 3D structure of the I1 complex on all nine DMTs (Fig. S4). The ICLC central protrusions of DMTs 1, 2, and 5–9 are short; have a mass of ∼150 kDa; and end ∼5 nm from the neighboring B-tubule (Fig. 3 C and E and Fig. S4). The central protrusions of DMTs 3 and 4, however, have an additional density of ∼100 kDa that projects from the central protrusion toward the distal protrusion, forming a ring-shaped structure that extends to within ∼3 nm of the neighboring doublet (Fig. 3 D and F and Fig. S4).
Fig. 3.
Doublet-specific differences of the I1 ICLC structure in pWT axonemes. (A) Simplified schematic model of an axoneme in cross-section highlighting DMTs 3 and 4, which show a doublet-specific feature in the I1 complex, whereas all other DMTs lack this feature. (B and Inset) Isosurface rendering of a DMT in cross-section. The I1 dynein structure present in all DMTs is colored yellow, and the doublet-specific structure present only in DMTs 3 and 4 is highlighted in magenta. Orientation of views shown in C and D is indicated by a white line. (C–F) Tomographic slices (C and D) and isosurface renderings (E and F) of averaged axonemal repeats from pWT viewed from the axoneme center looking outward. Averaged repeats from DMTs 1, 2, and 5–9 (C and E) are compared with averages from DMTs 3 and 4 (D and F). Note the doublet-specific difference: In the average of DMTs 3 and 4, an additional density is present (magenta-colored density and dashed circles in D and F) at the central protrusion, forming a larger ring-shaped structure extending further toward the adjacent B-tubule and the distal protrusion. This additional density is absent from DMTs 1, 2, and 5–9 (white dashed circles in C and E) (Scale bar: D, 20 nm.). Averages of each individual doublet and a second strain, pWT2, are presented in Fig. S4.
Dynein Heads and ICLC of the I1 Complex Are Highly Connected to Neighboring Axonemal Structures.
As described above, we used a comparison between WT/pWT and pf9-3 to establish the boundaries of the I1 complex. We identified several connections based on continuous electron density between the I1 complex and neighboring axonemal structures. At least seven connections to neighboring structures can be identified, which is a unique feature of the I1 dynein in comparison to all the other axonemal dyneins. The first four connections originate from the ICLC, and the remaining three originate from the 1α HC motor domain. Connection 1 (described above) is the attachment of the ICLC to the A-tubule (Fig. 4 and Fig. S5 B, C, and G). Connection 2 is the proximal outer-inner dynein (OID) linker that connects the ICLC to the row of ODAs (27) (Fig. 4 and Fig. S5 A, D, and H). Connection 3 is the linkage of the distal end of the ICLC to a neighboring, unidentified density that ultimately links to the N-DRC (12) (Fig. 4, and Figs. S3N and S5 A and H). This unidentified density also connects to the doublet-specific dynein IAX (Figs. 2A and 4 A and B). The last ICLC connection 4 is large and links between the ICLC and the dynein head of IA2 (dynein a/d) (Fig. 4 and Fig. S5 A and D).
Connections 5–7 are unusual because they originate from the 1α HC motor domain. Connection 5 links the dynein head domains of I1α and IA2 (Fig. 4 and Fig. S5 A and I), similar to interactions seen between the stacked heads of the ODAs (27, 38). This connection, however, is smaller than connection 4 between IA2 and the ICLC. Connections 6 and 7 both link the 1α dynein head to the newly described tether density, and ultimately to the A-tubule. The tether consists of two filamentous parts: One links to the 1α motor domain at connection 6 (Fig. 4 and Fig. S5 B and E), whereas the second links to the tether head at connection 7 (Fig. 4 and Fig. S5 B and F). Both parts of the tether are interconnected and attach to multiple protofilaments of the A-tubule (Fig. 4 and Fig. S5 B, E, and F).
Comparison Between WT and bop5 Mutants Reveals the Location of the IC138 Subcomplex.
The bop5-1 mutant has a point mutation in the IC138 gene that results in a truncated IC138 polypeptide and defective assembly of the ICLC, lacking FAP120 and LC7b (31, 32). According to our cryo-ET structure, the mass of the bop5-1 ICLC is ∼40% reduced compared with WT (Fig. 5, Fig. S6, and Movies S1 and S3). The missing density includes parts of the central and distal protrusions and the lower portion of the ICLC, such that the ICLC no longer connects to the IA2 dynein (Fig. 5B). The IA2 density appears reduced compared with the other dynein heads (except for the doublet-specific IAX). This suggests that IA2 may be more flexible in the absence of connection 4, leading to a blurred density in the averages and an apparent reduction in mass. The 1α and 1β HCs (including the tether head), as well as most other axonemal structures, appear similar between bop5-1 and WT (Fig. 5 and Fig. S6).
Fig. 5.
Comparisons of I1 structure between WT and IC138 mutants. Three-dimensional structure of the averaged axonemal repeat from WT (A and D) is compared with the averages from bop5-1 (B and E) and bop5-2 (C and F). The IC138 subcomplex, which is part of the ICLC, is reduced in bop5-1 and missing in bop5-2. Isosurface renderings show close-ups of the I1 complex in longitudinal (A–C) and cross-sectional (D–F) views. Note that in comparison to WT, the size of the ICLC is smaller in bop5-1 and even more reduced in bop5-2. Other I1 components, such as the α and β HCs and the tether head (TH), and other axonemal structures, including the tether (T), IDA (including IA2), ODA, RS, and A-tubule (At), look similar in all three strains. (Scale bars: 10 nm.) See also Fig. S6.
Bop5-2 is an IC138 null mutation that disrupts assembly of IC138, IC97, FAP120, and presumably LC7b into the axoneme (21). This is a substantially larger defect than that published for bop5-1. However, our structural data show that the bop5-2 ICLC is ∼50% reduced compared with WT (Fig. 5, Fig. S6, and Movies S1 and S4), which is only slightly more than the ∼40% reduction in bop5-1. Bop5-2 lacks the same density that was missing in bop5-1, plus a more central region of the ICLC, including the entire central protrusion (compare Fig. 5 B and C). In addition, the ICLC–A-tubule attachment (connection 1) appears reduced. The structural components present or absent in each strain are summarized in Table 1.
I1 Intermediate Chain Assembly into the Axoneme of WT and I1 Mutants.
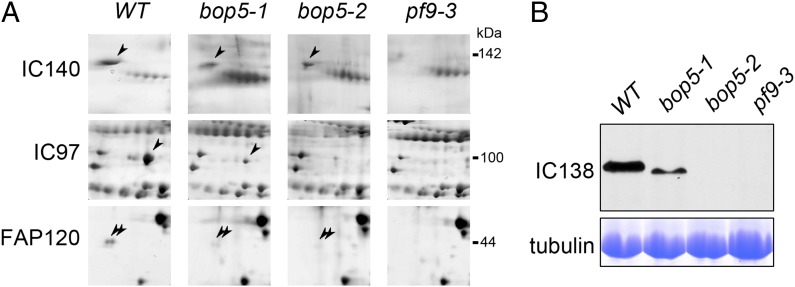
The observed mass differences between the I1 complex in bop5-1 and WT were three- to fivefold larger than expected based on previous biochemical studies (31, 32), whereas the differences between bop5-1 and bop5-2 were surprisingly small. This suggested that the proteomic deficiencies in the bop5 mutants had not been fully characterized, making the interpretation of our structural data difficult. To figure out which subunits correspond to the observed or reduced structures, we used 2D gel electrophoresis (2DE) to reanalyze the I1 subunit composition in WT and I1 mutant axonemes. Thirty-seven silver nitrate-stained 2DE gels (4–17 gels for each strain; Fig. S7) were analyzed for statistically significant differences in protein abundance among strains. Spots of interest were cut from colloidal Coomassie blue-stained gels and analyzed by MS for protein identification. Table 2 summarizes the relative abundance of I1 proteins in the analyzed strains.
Table 2.
Abundance of I1 complex proteins in WT and mutant axonemes determined by 2DE analysis
To characterize the protein composition of the entire I1 complex, we compared 2DE gels from WT and pf9-3 (Fig. 6A and Fig. S7). Our 2DE analysis did not identify additional I1 components, but polypeptides larger than ∼150 kDa are difficult to resolve by this technique. Consistent with previous studies, we identified only a single isoform of IC97 (20, 34) (Fig. 6 and Fig. S7). FAP120 was previously detected as a single, relatively unfocused spot (32). Here, we resolved FAP120 into two spots, indicating a previously unknown posttranslational modification (Fig. 6A). Previous 2DE gel studies did not identify an IC140 spot (20, 44). However, improved proteomic techniques allowed us to localize IC140 on our 2DE gels (Fig. 6A and Fig. S7) in a spot comigrating with FAP61. This comigration is likely the reason why the IC140 spot was not identified previously. Although reductions can be seen in the size of the IC140/FAP61 spot in pf9-3, the FAP61 background makes a more detailed analysis difficult.
Fig. 6.
Assembly of I1 dynein subunits in WT and mutant axonemes. (A) Close-up images of 2DE gels show the changes in the abundance of I1 dynein subunits in different strains (examples of full-sized 2DE gels are shown in Fig. S7). Arrowheads highlight the position(s) of the protein of interest. (B, Upper) One-dimensional Western blot analysis shows that IC138 is present in WT, reduced in bop5-1, and completely missing in bop5-2 and pf9-3. (B, Lower) Coomassie-stained gel of the same samples shows the tubulin bands demonstrating equal protein loading for all samples.
Having characterized the WT I1 protein components, we turned to analysis of the biochemical defects in the bop5 mutants. In addition to the previously reported bop5-1 deficiencies, our 2DE gels and Western blots of bop5-1 suggest that IC138 and IC97 are significantly reduced compared with WT (Fig. 6). These observations suggest that the C-terminal domain of IC138, which is missing in bop5-1, influences the assembly of IC138 and IC97 into the axoneme. In bop5-2, the levels of I1 ICs detected by 2DE and Western blot (Fig. 6) largely confirmed previous reports (21): IC97 and IC138 are missing, and IC140 appears slightly reduced. However, trace amounts of FAP120 (previously believed to be absent in the bop5 mutants) were detected in both bop5 mutants (Fig. 6A). Table 2 and Fig. 7 summarize the subunit composition of the I1 complex in different strains. These proteomics results agree well with our structural data, increasing confidence in our data interpretation.
Fig. 7.
I1 subunit composition, localization. and model of I1 interactions. (A–D) Simplified models of the I1 subunit composition in WT (A), bop5-2 (B), bop5-1 (C), and pf9-3 (D) based on biochemical data. Strong, bright colors represent subunits present at WT levels, whereas weak, faded colors show reduced components and missing proteins are outlined in gray. (C) Truncated IC138 in bop5-1 is denoted by an ellipsoid with a missing part. The I1 α and β HCs are shown in gray, and light chains are not depicted because they were not resolved by our 2DE gels. (E–H) Isosurface renderings from averaged axonemal repeats show the 3D structure of the I1 complex in WT (E), bop5-2 (F), bop5-1 (G), and pf9-3 (H). The dashed white line outlines the WT I1 complex (E) and is superimposed on the mutant structures as a reference (F–H). Rose-colored lines in F and G outline the difference between WT and bop5-2 (density present in WT but missing in bop5-2). The black arrow in E points to the central protrusion, and the red arrow in G highlights the difference between the two bop5 mutants (density present in bop5-1 but missing in bop5-2). (I–K) Subunit localization model based on a biochemical and structural WT-mutant comparison. Isosurface renderings of axonemal repeats show the I1 complex in a longitudinal overview (J), close-up (I), and cross-section (K); the red box in J indicates the region shown in I. The “core I1 complex,” including IC140 and the α and β HCs, is colored yellow. The rose color highlights the proposed location of the IC138 subcomplex (IC138 sub-c), and the suggested position of IC138 itself is indicated in red. The tether head (TH) is colored orange, and other axonemal structures are shown in gray: IDAs, ODAs, RS, and A- and B-tubules (At and Bt). (L) This model summarizes the connections between the I1 complex and neighboring structures, highlighting possible regulatory interactions. This model includes the N-DRC as a regulatory hub [adapted from (12) © Heuser et al., 2009. Originally published in J Cell Biol, 187:921–933]. (Scale bars: I, 10 nm; J and K, 20 nm.)
Discussion
Dynein Regulatory Network and Major Regulatory Hubs in the Axoneme: I1 Dynein and N-DRC.
Cryo-ET allowed us to reconstruct the 3D structure of the I1 dynein in WT and mutant axonemes to the highest resolution currently available. The tomographic averages revealed unique details about the I1 dynein, including its large overall size and shape, its high connectivity to other axonemal structures, and also a surprisingly small attachment of the ICLC to the A-tubule. The large number of connections of the I1 dynein with neighboring structures, as well as its demonstrated role in regulating flagellar motility (reviewed in 45), suggests that the I1 complex is a major regulatory hub in the axoneme and is the proximal counterpart of the distal regulatory node, the N-DRC (12). Both structures form links to the ODAs and other IDAs, and they are also connected to one another through an unidentified density (12) (Fig. 4A and Fig. S3N), which may allow coordination/integration of regulatory signals. The major differences are that the I1 complex is itself a dynein and the I1 complex does not connect directly to a RS.
CPC/RS Signaling and Phosphorylation of IC138.
Both I1 and N-DRC mutants have been identified as suppressors of paralyzed CPC/RS mutants, indicating that the I1 complex and the N-DRC regulate dynein activity in response to CPC/RS signaling (15, 16, 46). Although the N-DRC makes a small, direct connection to RS2 (connection 8 in figures 3 F and G of ref. 12), we found no evidence of a direct connection between RS1 and the I1 complex. The only obvious connections between the I1 ICLC and RSs are indirect, either through the ICLC-to-IA2 connection (connection 4), because IA2’s tail is attached to the base of RS1 (47) (Fig. S3J), or through ICLC’s distal connection (connection 3) to the N-DRC that links to RS2. Therefore, these connections may be critical for phosphorylation of IC138 and regulation of I1 dynein activity.
Many protein kinases have been implicated in the regulation of I1; however, their locations and targets remain unclear (4, 9, 17, 18, 48, 49). Of these kinases, CK1 is thought to be the most likely to phosphorylate IC138 directly (18, 19). Here, we show that IC138 is located in the center of the ICLC. This is surprising, because previous studies have shown that CK1 is present in all known I1 mutants (18), suggesting it is not part of the ICLC and thus not in a position to directly interact with IC138. Similar to previous RNA interference studies (50–52), future CK1 knockdowns may provide insights into the axonemal location of this kinase.
1α HC Tether.
Only a few cases have been reported where proteins are thought to interact directly with the motor domain of a dynein (53–55). Based on biochemical evidence, Patel-King and King (55) have reported that the 22-kDa LC1 likely forms a bridge between the ODA γ HC motor domain and the DMT in axonemes, possibly providing limiting doublet sliding in the axonemal bend. Our study identified a unique structure tethering the I1α HC motor domain to the A-tubule. The identities of the proteins that form the tether and tether head are unknown. Because the tether is present in all strains we have analyzed by cryo-ET thus far, it is not identifiable by comparative proteomics of I1 mutants. The tether head, with a predicted size of 100–150 kDa, appears to be missing in pf9-3 averages (Fig. 2), but comparison of WT and pf9-3 2DE gels did not reveal a polypeptide that could correspond to the tether head. This could be attributable to inherent limitations of the gel-based approach (e.g., protein size). Alternatively, but less likely, the tether head (or parts of it) may remain in pf9-3 but is not visible in the averages because of increased flexibility in the absence of the I1 dynein.
Although the function of the I1α tether in the axoneme is unknown, it would be in an ideal position to work as a tension sensor detecting relative movement between the I1 dynein and the A-tubule during dynein stepping or axoneme bending. Thus, tethering may serve as part of a mechanical feedback mechanism for dynein coordination. This is consistent with previous studies of I1 HC mutants reporting distinct roles for the I1α and I1β HCs: The I1β HC seems to be mostly responsible for generating motor force, whereas the I1α HC is suggested to have a regulatory and/or restraining function (13, 24, 25, 35). Alternatively, the substantial connection between the 1α motor domain and the A-tubule may serve to stabilize the I1 complex.
Asymmetry Provided by Doublet-Specific Differences of the I1 ICLC Could Be Important for Generating the Effective Stroke.
Based on sliding disintegration assays, the activity of dyneins on doublets 2–4 is believed to be crucial for generating interdoublet sliding during the effective stroke in Chlamydomonas flagella, whereas the activity of dyneins on doublets 6–8 drives the recovery stroke (56). It has been suggested that the generation and direction of the effective and recovery stroke are regulated by structural components or signals that provide inherent asymmetry to the axonemes (40, 56–59). Our doublet-specific averages identified just such a unique structure of unknown protein composition associated with the ICLC on DMTs 3 and 4. Here, the central ICLC protrusion is part of a larger ring structure that extends closer to the adjacent B-tubule compared with the ICLC seen on all other DMTs (Fig. 3 and Fig. S4). Although we did not observe a connection from the ICLC protrusions on DMTs 3 and 4 to the neighboring B-tubule, we cannot exclude a small linkage beyond our resolution limit. This doublet-specific structure could play a role in generating the asymmetrical distribution of dynein activity required for effective motility, which is supported by recent studies of bop5 mutants. These studies revealed defects in both MT sliding and the flagellar waveform of bop5 mutants that are consistent with a role for the IC138 subcomplex in the selective activation or inactivation of dyneins during flagellar bending (21, 60).
Internal Architecture of the I1 Complex and Localization of I1 Subunits.
By combining our 3D structural data with both previously published biochemical results and those described in this study (summarized in Fig. 7 A–H) to compare the WT and mutant I1 dyneins, we can propose a likely location of both IC138 and the IC138 subcomplex within the I1 ICLC. Fig. 7 I–K and Movie S5 depict our summary model by showing color-coded regions for proposed subunit locations projected onto the WT structure.
Bop5-2 axonemes lack the entire IC138 subcomplex: IC138, IC97, LC7b, and FAP120 (21) (Fig. 6). Bop5-1 was previously thought to be missing only the C-terminal 8% of IC138, LC7b, and FAP120 (2–3 copies per I1), that is, roughly a difference of 110–150 kDa compared with WT (31, 32). It was therefore surprising that our bop5-1 averages suggested a loss of ∼500 kDa compared with WT (Fig. 5 A and B). Such a large discrepancy made interpretation of the structures difficult. However, our biochemical studies here revealed that IC138 and IC97 are also significantly reduced in bop5-1 axonemes (Fig. 6 and Table 2), which is consistent with the structural defect seen in the ICLC of bop5-1. Additional possibilities that could explain part of the missing mass include the following: (i) some of the structure is present but blurred out as a result of increased flexibility, and thus appears smaller in our averages; (ii) some of the ICLC subunits are present in multiple copies, resulting in an underestimation of the total missing mass; or (iii) there are additional subunits in the IC138 subcomplex that have not yet been identified.
Most of the structural difference between bop5-1 and bop5-2, which consists of a central region of the ICLC, is likely caused by different amounts of IC138 and IC97 in the two mutants. IC97 is known to bind tubulin (34), and is therefore probably located near connection 1. If IC97 is indeed part of connection 1, this could explain why connection 1 appeared weaker in bop5-2 axonemes. In previous analyses, IC97 and IC140 were both thought to be potential ICLC-to-MT anchors because IC97 interacts directly with tubulin (34) and purified IC140 cross-links to I1-depleted axonemes (36). Both IC140 and IC97 could form extensions into connection 1; however, IC140 was reported to be present at near-WT levels in all the bop5 mutants (21, 31, 32, 34, 60). This suggests that IC140 is located in the upper ICLC density present in both bop5 mutants and that IC97 is likely located more centrally, at or below the ICLC-to-MT connection (connection 1).
In both bop5 mutants, the ICLC-to-IA2 connection 4 is clearly missing. Meanwhile, the largest protein mass missing in both bop5 mutants should be FAP120 (estimated mass of ∼40 kDa), which is believed to be present in two to three copies per I1 complex (32). Genetic evidence supports an association between FAP120 and the ICLC because it is reduced in I1 mutants (32). However, instead of copurifying with the I1 dynein (dynein f) during FPLC ion exchange chromatography, FAP120 coelutes with dyneins d and e (32). Dynein e is located at the IA4 position next to the N-DRC (12, 61), making an association of FAP120 with dynein e unlikely. The precise position of dynein d is not known, but IA2 and IA6 are possible locations (38, 61). One interesting interpretation of these data is that FAP120 associates with dynein d at the ICLC-to-IA2 connection 4, but additional work is needed to test this hypothesis. FAP120 is not reduced in ida5, which is missing dyneins a, c, d, and e (32). However, this is not necessarily inconsistent with the proposed localization for FAP120, because the I1 ICLC could be sufficient for proper FAP120 localization even when FAP120 cannot connect to IA2. The ICLC-to-IA2 connection 4 is one possible pathway for RS signals to reach the I1 dynein, but further study of FAP120 isoforms in different mutant backgrounds will be required to understand FAP120’s role in the pathway(s) that regulate I1 activity.
The suspension of the I1 complex above the A-tubule through connections on all sides, like a spider web, does not seem ideal for providing resistance to force production by the two I1 dynein heads. However, its high connectivity and flexibility in combination with the tethering of the 1α motor domain to the MT suggest important roles in signal transduction, and possibly tension sensing, in a regulatory system that can generate and modify complex waveforms during motility.
Our results provide a unique, high-resolution view into the 3D structure and subunit organization of the I1 dynein, revealing probable pathways for regulatory signals. Overall, we demonstrate that I1 dynein is a highly connected structure within the axoneme, ideally positioned to integrate signals that regulate axonemal dynein activity. A deeper insight into the structure and protein composition of the I1 dynein is an important step toward understanding how axonemal movement is regulated and how it fails in disease.
Materials and Methods
Axonemal Sample Preparation.
Chlamydomonas reinhardtii strains used in this study are listed in Table S1. Axonemes were isolated and purified from WT and mutant C. reinhardtii cells as previously described (12, 21) with minimal modifications. Briefly, cells were grown in Tris acetate⋅phosphate medium (62), collected by centrifugation, washed twice with minimal medium, and resuspended in 10 mM Hepes (pH 7.4), 1 mM SrCl2, 4% (wt/wt) sucrose, and 1 mM DTT. Flagella were detached using pH-shock (43), followed by the addition of 5 mM MgSO4; 1 mM EGTA; 0.1 mM EDTA; and 100 μg/mL aprotinin, pepstatin, and leupeptin or, for proteomics, 0.4% protease inhibitor mixture (P9599; Sigma–Aldrich). Cell bodies were collected at 1,800 × g at 4 °C for 10 min, and flagella were purified by two additional centrifugation steps over 20% (wt/wt) sucrose cushions. Purified flagella were demembranated with 0.1% Nonidet-P-40 or 1% (vol/vol) IGEPAL CA-630 (Sigma–Aldrich) for 30 min at 4 °C, and axonemes were collected by centrifugation at 35,000 × g for 1 h. The isolation procedure was performed in the absence of ATP. Axonemes for cryo-ET were prepared by resuspension in 10 mM Hepes (pH 7.4), 25 mM NaCl, 4 mM MgSO4, 1 mM EGTA, and 0.1 mM EDTA and processed within 24 h.
For 1D and 2D gel analysis, proteins were dissolved directly from axoneme pellets with 2DE lysis buffer [7 M urea, 2 M thiourea, 4% (wt/wt) CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate), 65 mM DTT, and 2% (vol/vol) IPG (immobilized pH gradient) buffer (pH 3–10NL; GE Healthcare)] with vigorous stirring for 30 min. Cell debris and insoluble substances were removed by centrifugation at 45,000 × g for 1 h. Protein concentrations were determined using a 2-D Quant Kit (GE Healthcare). The supernatant was aliquoted and stored at −70 °C until further use.
Proteomic Analysis.
Protein samples (70 μg for analytical gels or 300 μg for semipreparative gels) were separated with 13-cm, immobilized, pH 3–10 nonlinear gradient dry strips (GE Healthcare) for the first dimension and 10% SDS/PAGE gels for the second dimension. All samples (2–7 axoneme preparations for each strain) were run at least in duplicate to confirm reproducibility. Analytical gels were stained with silver nitrate, and semipreparative gels were stained with colloidal Coomassie blue. The 2DE images were analyzed using ImageMaster 2D Platinum software (version 5.0; GE Healthcare). Two-sample t tests were used to analyze differences in protein abundance between WT and mutant samples. P values of less than 0.05 were considered statistically significant. Spots that showed significant changes in abundance between WT and I1 mutants (greater than ±1.5-fold) were identified as affected I1 dynein components.
Spots of interest were excised from the semipreparative gels, trypsin-digested as described by Lin et al. (63), and analyzed using a Bruker Daltonics Microflex mass spectrometer. Peptide mass fingerprinting was interpreted by the Mascot sequence database search engine configured with a mass tolerance of 50 ppm and the variable modifications “carbamidomethylation of cysteine and oxidation of methionine” against the National Center for Biotechnology Information database. Probability-based Mascot scores were used to evaluate MS results: peptide mixtures that yielded statistically significant search scores (>95% confidence interval, which is equivalent to Mascot expected values <0.05) and accounted for the majority of ions present in the mass spectra were defined as positive identifications.
For 1D immunoblotting of IC138, a total of 15 μg of axonemal protein for each cell line was separated on 10% SDS/PAGE minigels under standard denaturing conditions. A polyclonal antibody against IC138 was used in a 1:10,000 dilution (31). Signals were visualized using the ECL detection system (Pierce).
Preparation of Cryosamples and EM.
Cryosamples were prepared, examined by EM, and processed as previously described (12, 42). Briefly, Quantifoil holey carbon grids (Quantifoil Micro Tools GmbH) were glow-discharged and coated with 10 nm of colloidal gold (Sigma–Aldrich). Grids were loaded in a homemade plunge freezer; 3 μL of axoneme sample and 1 μL of 10-fold concentrated 10-nm colloidal gold were added to the grid, blotted with a filter paper from the front side, and immediately plunge-frozen in liquid ethane. Grids were stored in liquid nitrogen until examined by EM.
Cryosamples were transferred into a transmission electron microscope (Tecnai F30; FEI) with a cryoholder (Gatan, Inc.). Tilt series were recorded by rotating the specimen from −65° to +65° with 1.5° to 2.5° angular increments. The accumulative electron dose was limited to ∼100 e/Å2. Using the microscope control software SerialEM (64), samples were imaged at 300 keV, with −6 μm or −8 μm defocus, under low-dose conditions and in zero loss mode of the energy filter (Gatan, Inc.) (slit width of 20 eV). All images were digitally recorded with a 2,000 × 2,000 pixel CCD camera (Gatan, Inc.) at a magnification of 13,500×, resulting in a pixel size of ∼1 nm.
Image Processing.
Cryoelectron tomograms were reconstructed using fiducial alignment and the IMOD software package (65). Only tomograms of intact and noncompressed axonemes were used for further data analysis (Fig. S1). The software PEET (Particle Estimation for Electron Tomography) (27) was used for subtomogram averaging of the 96-nm repeats. The extracted subvolumes were aligned and averaged with missing wedge compensation to increase the signal-to-noise ratio, and thus the resolution (27).
Two different alignment strategies were applied and compared during subtomogram averaging: The “global alignment” approach used the entire 96-nm repeat unit (volume size: 100 nm × 100 nm × 80 nm) for 3D alignment, whereas the “local alignment” approach used only the region surrounding the I1 complex for alignment (volume size: 60 nm × 36 nm × 40 nm). Both strategies resulted in comparable but slightly different averages. The strongest differences between alignment strategies were seen in bop5-1 (Fig. S6E and Movie S3) and bop5-2 (Fig. S6F and Movie S4). The local alignment yielded the best I1 averages for both bop-5 strains and pWT, whereas the global alignment was slightly better for WT. Because of the absence of the entire I1 complex in the pf9-3 mutant, only global alignment was used for this strain. The I1 structures described in this study refer to the best averages obtained for each strain (i.e., global alignment for pf9-3 and WT, local alignment for all other strains).
The number of tomograms and repeat units included in the final averages, as well as the estimated resolution, is summarized in Table S1. Resolution was measured at the base of RS1 in close proximity to the I1 complex. This density, which is present in all strains, permits a direct comparison among all averages. The resolution was estimated using the Fourier shell correlation method with a criterion of 0.5 (66). For 3D visualization by isosurface rendering of axonemal averages, analysis, animation, and volume measurements, we used the University of California, San Francisco Chimera software package (67). For mass estimations, an average protein density of 1.43 g/cm3 was assumed (68). The 3D structure of the WT I1 dynein from this publication has been submitted to the Electron Microscopy Data Bank (EMDB; http://www.emdatabank.org) and assigned the EMDB accession code EMD-5330.
Supplementary Material
Acknowledgments
We thank Raqual Bower and Douglas Tritschler (University of Minnesota) for providing the pf2-4::PF2-GFP (pWT) and ida6::IDA6-GFP (pWT2) strains, as well as the Brandeis University MS facility directed by Dr. Jeff Agar for access to instruments; Dr. Win Sale for critical reading of the manuscript; Dr. Chen Xu for outstanding management of the Brandeis EM facility; and Alan Tso and Avraham Tukachinsky for their help in tomogram reconstruction. This work was supported by National Institutes of Health Grants GM083122 (to D.N.) and GM55667 (to M.E.P.), the W. M. Keck Foundation, and National Science Foundation Grant DMR-MRSEC-0820492 (to T.H.), as well as a Pew Biomedical Scholars Award (to D.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.N. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited in the Electron Microscopy Data Bank (EMDB), http://www.emdatabank.org (accession no. EMD-5330).
See Author Summary on page 11912 (volume 109, number 30).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120690109/-/DCSupplemental.
References
- 1.Afzelius BA. Cilia-related diseases. J Pathol. 2004;204:470–477. doi: 10.1002/path.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fliegauf M, Benzing T, Omran H. When cilia go bad: Cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 3.Summers KE, Gibbons IR. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci USA. 1971;68:3092–3096. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151:F37–F42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith EF, Lefebvre PA. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil Cytoskeleton. 1997;38:1–8. doi: 10.1002/(SICI)1097-0169(1997)38:1<1::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Wirschell M, et al. Regulation of ciliary motility: Conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme. Arch Biochem Biophys. 2011;510:93–100. doi: 10.1016/j.abb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witman GB, Plummer J, Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol. 1978;76:729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard DR, Habermacher G, Glass DB, Smith EF, Sale WS. Regulation of Chlamydomonas flagellar dynein by an axonemal protein kinase. J Cell Biol. 1994;127:1683–1692. doi: 10.1083/jcb.127.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith EF. Regulation of flagellar dynein by the axonemal central apparatus. Cell Motil Cytoskeleton. 2002;52:33–42. doi: 10.1002/cm.10031. [DOI] [PubMed] [Google Scholar]
- 10.Habermacher G, Sale WS. Regulation of flagellar dynein by an axonemal type-1 phosphatase in Chlamydomonas. J Cell Sci. 1996;109:1899–1907. doi: 10.1242/jcs.109.7.1899. [DOI] [PubMed] [Google Scholar]
- 11.Piperno G, Ramanis Z, Smith EF, Sale WS. Three distinct inner dynein arms in Chlamydomonas flagella: Molecular composition and location in the axoneme. J Cell Biol. 1990;110:379–389. doi: 10.1083/jcb.110.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–933. doi: 10.1083/jcb.200908067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myster SH, Knott JA, Wysocki KM, O’Toole E, Porter ME. Domains in the 1alpha dynein heavy chain required for inner arm assembly and flagellar motility in Chlamydomonas. J Cell Biol. 1999;146:801–818. doi: 10.1083/jcb.146.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springer AL, et al. Silencing of a putative inner arm dynein heavy chain results in flagellar immotility in Trypanosoma brucei. Mol Biochem Parasitol. 2011;175:68–75. doi: 10.1016/j.molbiopara.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutcher SK, Gibbons W, Inwood WB. A genetic analysis of suppressors of the PF10 mutation in Chlamydomonas reinhardtii. Genetics. 1988;120:965–976. doi: 10.1093/genetics/120.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porter ME, Power J, Dutcher SK. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J Cell Biol. 1992;118:1163–1176. doi: 10.1083/jcb.118.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gokhale A, Wirschell M, Sale WS. Regulation of dynein-driven microtubule sliding by the axonemal protein kinase CK1 in Chlamydomonas flagella. J Cell Biol. 2009;186:817–824. doi: 10.1083/jcb.200906168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang P, Sale WS. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J Biol Chem. 2000;275:18905–18912. doi: 10.1074/jbc.M002134200. [DOI] [PubMed] [Google Scholar]
- 19.Habermacher G, Sale WS. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain. J Cell Biol. 1997;136:167–176. doi: 10.1083/jcb.136.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King SJ, Dutcher SK. Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains. J Cell Biol. 1997;136:177–191. doi: 10.1083/jcb.136.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bower R, et al. IC138 defines a subdomain at the base of the I1 dynein that regulates microtubule sliding and flagellar motility. Mol Biol Cell. 2009;20:3055–3063. doi: 10.1091/mbc.E09-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastronarde DN, O’Toole ET, McDonald KL, McIntosh JR, Porter ME. Arrangement of inner dynein arms in wild-type and mutant flagella of Chlamydomonas. J Cell Biol. 1992;118:1145–1162. doi: 10.1083/jcb.118.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myster SH, Knott JA, O’Toole E, Porter ME. The Chlamydomonas Dhc1 gene encodes a dynein heavy chain subunit required for assembly of the I1 inner arm complex. Mol Biol Cell. 1997;8:607–620. doi: 10.1091/mbc.8.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrone CA, Myster SH, Bower R, O’Toole ET, Porter ME. Insights into the structural organization of the I1 inner arm dynein from a domain analysis of the 1beta dynein heavy chain. Mol Biol Cell. 2000;11:2297–2313. doi: 10.1091/mbc.11.7.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrone CA, Yang PF, O’Toole E, Sale WS, Porter ME. The Chlamydomonas IDA7 locus encodes a 140-kDa dynein intermediate chain required to assemble the I1 inner arm complex. Mol Biol Cell. 1998;9:3351–3365. doi: 10.1091/mbc.9.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith EF, Sale WS. Structural and functional reconstitution of inner dynein arms in Chlamydomonas flagellar axonemes. J Cell Biol. 1992;117:573–581. doi: 10.1083/jcb.117.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicastro D, et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science. 2006;313:944–948. doi: 10.1126/science.1128618. [DOI] [PubMed] [Google Scholar]
- 28.DiBella LM, Sakato M, Patel-King RS, Pazour GJ, King SM. The LC7 light chains of Chlamydomonas flagellar dyneins interact with components required for both motor assembly and regulation. Mol Biol Cell. 2004;15:4633–4646. doi: 10.1091/mbc.E04-06-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiBella LM, Smith EF, Patel-King RS, Wakabayashi K, King SM. A novel Tctex2-related light chain is required for stability of inner dynein arm I1 and motor function in the Chlamydomonas flagellum. J Biol Chem. 2004;279:21666–21676. doi: 10.1074/jbc.M313540200. [DOI] [PubMed] [Google Scholar]
- 30.Harrison A, Olds-Clarke P, King SM. Identification of the t complex-encoded cytoplasmic dynein light chain tctex1 in inner arm I1 supports the involvement of flagellar dyneins in meiotic drive. J Cell Biol. 1998;140:1137–1147. doi: 10.1083/jcb.140.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendrickson TW, et al. IC138 is a WD-repeat dynein intermediate chain required for light chain assembly and regulation of flagellar bending. Mol Biol Cell. 2004;15:5431–5442. doi: 10.1091/mbc.E04-08-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda K, et al. A novel ankyrin-repeat protein interacts with the regulatory proteins of inner arm dynein f (I1) of Chlamydomonas reinhardtii. Cell Motil Cytoskeleton. 2009;66:448–456. doi: 10.1002/cm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith EF, Sale WS. Microtubule binding and translocation by inner dynein arm subtype I1. Cell Motil Cytoskeleton. 1991;18:258–268. doi: 10.1002/cm.970180403. [DOI] [PubMed] [Google Scholar]
- 34.Wirschell M, et al. IC97 is a novel intermediate chain of I1 dynein that interacts with tubulin and regulates interdoublet sliding. Mol Biol Cell. 2009;20:3044–3054. doi: 10.1091/mbc.E09-04-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toba S, et al. Distinct roles of 1alpha and 1beta heavy chains of the inner arm dynein I1 of Chlamydomonas flagella. Mol Biol Cell. 2011;22:342–353. doi: 10.1091/mbc.E10-10-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang PF, Sale WS. The Mr 140,000 intermediate chain of Chlamydomonas flagellar inner arm dynein is a WD-repeat protein implicated in dynein arm anchoring. Mol Biol Cell. 1998;9:3335–3349. doi: 10.1091/mbc.9.12.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicastro D, et al. Cryo-electron tomography reveals conserved features of doublet microtubules in flagella. Proc Natl Acad Sci USA. 2011;108:E845–E853. doi: 10.1073/pnas.1106178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bui KH, Sakakibara H, Movassagh T, Oiwa K, Ishikawa T. Molecular architecture of inner dynein arms in situ in Chlamydomonas reinhardtii flagella. J Cell Biol. 2008;183:923–932. doi: 10.1083/jcb.200808050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bui KH, Sakakibara H, Movassagh T, Oiwa K, Ishikawa T. Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella. J Cell Biol. 2009;186:437–446. doi: 10.1083/jcb.200903082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoops HJ, Witman GB. Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J Cell Biol. 1983;97:902–908. doi: 10.1083/jcb.97.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King SJ, Inwood WB, O’Toole ET, Power J, Dutcher SK. The bop2-1 mutation reveals radial asymmetry in the inner dynein arm region of Chlamydomonas reinhardtii. J Cell Biol. 1994;126:1255–1266. doi: 10.1083/jcb.126.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicastro D. Cryo-electron microscope tomography to study axonemal organization. Methods Cell Biol. 2009;91:1–39. doi: 10.1016/S0091-679X(08)91001-3. [DOI] [PubMed] [Google Scholar]
- 43.Witman GB, Carlson K, Berliner J, Rosenbaum JL. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972;54:507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piperno G, Luck DJ. Inner arm dyneins from flagella of Chlamydomonas reinhardtii. Cell. 1981;27:331–340. doi: 10.1016/0092-8674(81)90416-5. [DOI] [PubMed] [Google Scholar]
- 45.Wirschell M, Hendrickson T, Sale WS. Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation. Cell Motil Cytoskeleton. 2007;64:569–579. doi: 10.1002/cm.20211. [DOI] [PubMed] [Google Scholar]
- 46.Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for Flagellar function. Cell. 1982;28:115–124. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- 47.Barber CF, Heuser T, Carbajal-González BI, Botchkarev VV, Jr, Nicastro D. Three-dimensional structure of the radial spokes reveals heterogeneity and interactions with dyneins in Chlamydomonas flagella. Mol Biol Cell. 2012;23:111–120. doi: 10.1091/mbc.E11-08-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaillard AR, Fox LA, Rhea JM, Craige B, Sale WS. Disruption of the A-kinase anchoring domain in flagellar radial spoke protein 3 results in unregulated axonemal cAMP-dependent protein kinase activity and abnormal flagellar motility. Mol Biol Cell. 2006;17:2626–2635. doi: 10.1091/mbc.E06-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White D, de Lamirande E, Gagnon C. Protein kinase C is an important signaling mediator associated with motility of intact sea urchin spermatozoa. J Exp Biol. 2007;210:4053–4064. doi: 10.1242/jeb.007013. [DOI] [PubMed] [Google Scholar]
- 50.Boesger J, Wagner V, Weisheit W, Mittag M. Analysis of flagellar phosphoproteins from Chlamydomonas reinhardtii. Eukaryot Cell. 2009;8:922–932. doi: 10.1128/EC.00067-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dymek EE, Heuser T, Nicastro D, Smith EF. The CSC is required for complete radial spoke assembly and wild-type ciliary motility. Mol Biol Cell. 2011;22:2520–2531. doi: 10.1091/mbc.E11-03-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt M, et al. Proteomic analysis of the eyespot of Chlamydomonas reinhardtii provides novel insights into its components and tactic movements. Plant Cell. 2006;18:1908–1930. doi: 10.1105/tpc.106.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King SM. Integrated control of axonemal dynein AAA(+) motors. J Struct Biol. 2012 doi: 10.1016/j.jsb.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKenney RJ, Vershinin M, Kunwar A, Vallee RB, Gross SP. LIS1 and NudE induce a persistent dynein force-producing state. Cell. 2010;141:304–314. doi: 10.1016/j.cell.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel-King RS, King SM. An outer arm dynein light chain acts in a conformational switch for flagellar motility. J Cell Biol. 2009;186:283–295. doi: 10.1083/jcb.200905083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wargo MJ, McPeek MA, Smith EF. Analysis of microtubule sliding patterns in Chlamydomonas flagellar axonemes reveals dynein activity on specific doublet microtubules. J Cell Sci. 2004;117:2533–2544. doi: 10.1242/jcs.01082. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell DR. Orientation of the central pair complex during flagellar bend formation in Chlamydomonas. Cell Motil Cytoskeleton. 2003;56:120–129. doi: 10.1002/cm.10142. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell DR, Nakatsugawa M. Bend propagation drives central pair rotation in Chlamydomonas reinhardtii flagella. J Cell Biol. 2004;166:709–715. doi: 10.1083/jcb.200406148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wargo MJ, Smith EF. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. Proc Natl Acad Sci USA. 2003;100:137–142. doi: 10.1073/pnas.0135800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.VanderWaal KE, et al. bop5 Mutations reveal new roles for the IC138 phosphoprotein in the regulation of flagellar motility and asymmetric waveforms. Mol Biol Cell. 2011;22:2862–2874. doi: 10.1091/mbc.E11-03-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardner LC, O’Toole E, Perrone CA, Giddings T, Porter ME. Components of a “dynein regulatory complex” are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J Cell Biol. 1994;127:1311–1325. doi: 10.1083/jcb.127.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorman DS, Levine RP. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin JF, et al. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int J Cancer. 2007;121:2596–2605. doi: 10.1002/ijc.23016. [DOI] [PubMed] [Google Scholar]
- 64.Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 66.Harauz G, Van Heel M. Exact filters for general geometry three dimensional reconstruction. Optik (Stuttg) 1986;73:146–156. [Google Scholar]
- 67.Pettersen EF, et al. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 68.Quillin ML, Matthews BW. Accurate calculation of the density of proteins. Acta Crystallogr D Biol Crystallogr. 2000;56:791–794. doi: 10.1107/s090744490000679x. [DOI] [PubMed] [Google Scholar]