Abstract
The debate surrounding the safety of shale gas development in the Appalachian Basin has generated increased awareness of drinking water quality in rural communities. Concerns include the potential for migration of stray gas, metal-rich formation brines, and hydraulic fracturing and/or flowback fluids to drinking water aquifers. A critical question common to these environmental risks is the hydraulic connectivity between the shale gas formations and the overlying shallow drinking water aquifers. We present geochemical evidence from northeastern Pennsylvania showing that pathways, unrelated to recent drilling activities, exist in some locations between deep underlying formations and shallow drinking water aquifers. Integration of chemical data (Br, Cl, Na, Ba, Sr, and Li) and isotopic ratios (87Sr/86Sr, 2H/H, 18O/16O, and 228Ra/226Ra) from this and previous studies in 426 shallow groundwater samples and 83 northern Appalachian brine samples suggest that mixing relationships between shallow ground water and a deep formation brine causes groundwater salinization in some locations. The strong geochemical fingerprint in the salinized (Cl > 20 mg/L) groundwater sampled from the Alluvium, Catskill, and Lock Haven aquifers suggests possible migration of Marcellus brine through naturally occurring pathways. The occurrences of saline water do not correlate with the location of shale-gas wells and are consistent with reported data before rapid shale-gas development in the region; however, the presence of these fluids suggests conductive pathways and specific geostructural and/or hydrodynamic regimes in northeastern Pennsylvania that are at increased risk for contamination of shallow drinking water resources, particularly by fugitive gases, because of natural hydraulic connections to deeper formations.
Keywords: formation water, isotopes, Marcellus Shale, water chemistry
The extraction of natural gas resources from the Marcellus Shale in the Appalachian Basin of the northeastern United States (1, 2) has increased awareness of potential contamination in shallow aquifers routinely used for drinking water. The current debate surrounding the safety of shale gas extraction (3) has focused on stray gas migration to shallow groundwater (4) and the atmosphere (5) as well as the potential for contamination from toxic substances in hydraulic fracturing fluid and/or produced brines during drilling, transport, and disposal (6–9).
The potential for shallow groundwater contamination caused by natural gas drilling is often dismissed because of the large vertical separation between the shallow drinking water wells and shale gas formations and the relatively narrow zone (up to 300 m) of seismic activity reported during the deep hydraulic fracturing of shale gas wells (10, 11). Recent findings in northeastern Pennsylvania (NE PA) demonstrated that shallow water wells in close proximity to natural gas wells (i.e., < 1 km) yielded, on average, higher concentrations of methane, ethane, and propane with thermogenic isotopic signature. By comparison, water wells farther away from natural gas development had lower combustible gas concentrations and an isotopic signature consistent with a mixture between thermogenic and biogenic components (4). In contrast, when inorganic water geochemistry from active drilling areas was compared to nonactive areas and historical background values, no statistically significant differences were observed (4). Increasing reports of changes in drinking water quality have nevertheless been blamed on the accelerated rate of shale gas development.
The study area in NE PA consists of six counties (Fig. 1) that lie within the Appalachian Plateaus physiographic province in the structurally and tectonically complex transition between the highly deformed Valley and Ridge Province and the less deformed Appalachian Plateau (12, 13). The geologic setting and shallow aquifer characteristics are described and mapped in greater detail in multiple sources (4, 14–19) and in SI Methods. The study area contains a surficial cover composed of a mix of unconsolidated glacial till, outwash, alluvium and deltaic sediments, and postglacial deposits (the Alluvium aquifer) that are thicker in the valleys (17–19) (Fig. S1). These sediments are underlain by Upper Devonian through Pennsylvanian age sedimentary sequences that are gently folded and dip shallowly (1–3°) to the east and south (Fig. S2). The gentle folding creates alternating exposure of synclines and anticlines at the surface that are offset surface expressions of deeper deformation (12, 20). The two major bedrock aquifers are the Upper Devonian Catskill and the underlying Lock Haven Formations (14, 15, 18, 19). The average depth of drinking water wells in the study area is between 60 and 90 m (Table S1). The underlying geological formations, including the Marcellus Shale (at a depth of 1,200–2,500 m below the surface) are presented in Fig. 2, Fig. S2 A and B, and SI Methods.
Fig. 1.
Digital elevation model (DEM) map of northeastern PA. Shaded brown areas indicate higher elevations and blue-green shaded areas indicate lower elevations (valleys). The distribution of shallow (< 90 m) groundwater samples from this study and previous studies (18, 19) are labeled based on water type. Two low salinity (Cl < 20 mg/L) water types dominated by Ca-HCO3 (type A = green circles) or Na-HCO3 (type B = blue triangles) were the most common, and two higher salinity (Cl > 20 mg/L) water types were also observed: Br/Cl < 0.001 (type C = pink squares) and brine-type groundwater Br/Cl > 0.001 (type D = red diamonds). Type D groundwater samples appear associated with valleys (Table S1) and are sourced from conservative mixing between a brine and fresh meteoric water. The DEM data were obtained from NASA’ Shuttle Radar Topography Mission http://srtm.usgs.gov/.
Fig. 2.
Generalized stratigraphic section in the subsurface of western and eastern PA plateau adapted from (14, 15, 18, 19) and Sr isotope data of Appalachian brines and type D saline groundwater. Variations of 87Sr/86Sr ratios in Appalachian Brine and type-D groundwater samples show enrichment compared to the Paleozoic secular seawater curve (dashed grey line) (49). Note the overlap in values of type-D shallow ground water with 87Sr/86Sr values in Marcellus brines or older formations (21, 22, 24) but no overlap with the Upper Devonian brines in stratigraphically equivalent formations (Table S2) (21, 24).
In this study, we analyze the geochemistry of 109 newly-collected water samples and 49 wells from our previous study (4) from the three principal aquifers, Alluvium (n = 11), Catskill (n = 102), and Lock Haven (n = 45), categorizing these waters into four types based on their salinity and chemical constituents (Figs. 1 and 2, and S1 Text). We combine these data with 268 previously-published data for wells in the Alluvium (n = 57), Catskill (n = 147), and Lock Haven (n = 64) aquifers (18, 19) for a total of 426 shallow groundwater samples. We analyzed major and trace element geochemistry and a broad spectrum of isotopic tracers (δ18O, δ2H, 87Sr/86Sr, 228Ra/226Ra) in shallow ground water and compared these to published (6, 21, 22) and new data of 83 samples from underlying Appalachian brines in deeper formations from the region (Table S2) to examine the possibility of fluid migration between the hydrocarbon producing Marcellus Formation and shallow aquifers in NE PA. We hypothesize that integration of these geochemical tracers could delineate possible mixing between the Appalachian brines and shallow groundwater.
Results and Discussion
The water chemistry data from the Alluvial, Catskill, and Lock Haven shallow aquifers (Table S1) reveal a wide range of solute concentrations from dilute groundwater with total dissolved solids (TDS) < 500 mg/L and Cl < 20 mg/L to highly saline water (e.g., a salt spring with TDS of 7,800 mg/L and Cl approximately 4,000). Based on these characteristics, we divide the water samples into four types of ground water (Fig. 1). Two groundwater types (A and B; n = 118 of 158 samples from this and our previous study (4) are characterized by low salinity and high Na/Cl and Br/Cl (all ratios reported as molar) ratios (Table S1). The two elevated salinity (Cl > 20 mg/L) water types (C and D) were divided based on their Br/Cl ratios. Type (C) (n = 13 of 158) has a distinctive low (< 0.001) Br/Cl ratio (Fig. 3) and higher  concentrations that we attribute to salinization from domestic sources such as wastewater and/or road salt that have typically low Br/Cl ratios. The fourth subset of shallow groundwater (type D) (n = 27 of 158) was identified with a relatively high Br/Cl ratio (> 0.001) and low Na/Cl ratio (Na/Cl < 5) with a statistically significant difference in water chemistry from types A–C (Table S3).
concentrations that we attribute to salinization from domestic sources such as wastewater and/or road salt that have typically low Br/Cl ratios. The fourth subset of shallow groundwater (type D) (n = 27 of 158) was identified with a relatively high Br/Cl ratio (> 0.001) and low Na/Cl ratio (Na/Cl < 5) with a statistically significant difference in water chemistry from types A–C (Table S3).
Fig. 3.
Bromide vs. chloride concentrations (log-log scale) in shallow groundwater in NE PA and Appalachian brines from this and previous studies (18, 19). The linear relationship (type D: r2 = 0.99, p < 1 × 10-5; sample types A–C: r2 = 0.14) between the conservative elements Br and Cl demonstrates that the majority of the higher salinity samples of type D are derived from dilution of Appalachian brines that originated from evaporated seawater. Even with a large dilution of the original brine, the geochemical signature of type-D waters are still discernable in shallow groundwater from other high salinity (Cl > 20 mg/L) groundwater with low Br/Cl ratios (type C). Type C water likely originated from shallow sources such as septic systems or road deicing. Seawater evaporation line is from (25).
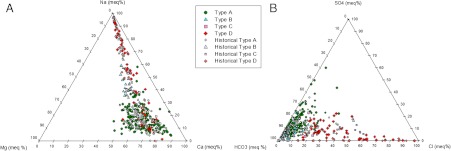
A geochemical analysis of published data collected in the 1980s (18, 19) revealed similar shallow salinized groundwater with a distinctive higher Cl (> 20 mg/L) and low Na/Cl ratio. The saline groundwater mimics type D water with statistically indistinguishable (Table S3) concentrations of major cations and anions (Fig. 4 A and B); however, bromide concentrations were not available in the historical data set. Nonetheless, we designated historical samples with high Cl (> 20 mg/L) and low Na/Cl ratio (Na/Cl < 5) as possible type D (n = 56 of 268). The remaining historical samples with Cl concentrations (> 20 mg/L) were designated as type C. All water types (A–D) were statistically indistinguishable from their respective historical types (A–D) (Table S3).
Fig. 4.
Ternary diagrams that display the relative percent of the major cations (A) and anions (B) in shallow groundwater samples from this and previous studies (18, 19). The overlap indicates that Na-Ca-Cl type saline water was present prior to the recent shale-gas development in the region and could be from natural mixing.
Type D saline waters are characterized by a Na-Ca-Cl composition with Na/Cl, Sr/Cl, Ba/Cl, Li/Cl, and Br/Cl ratios similar to brines found in deeper Appalachian formations (e.g., the Marcellus brine) (4, 6, 21, 22) (Table S2). This suggests mixing of shallow modern water with deep formation brines. Furthermore, the linear correlations observed for Br, Na, Sr, Li, and Ba with chloride (Fig. 3 and Fig. S3 A–F) demonstrate the relatively conservative and nonreactive behavior of these constituents and that the salinity in these shallow aquifers is most likely derived from mixing of deeper formation brines.
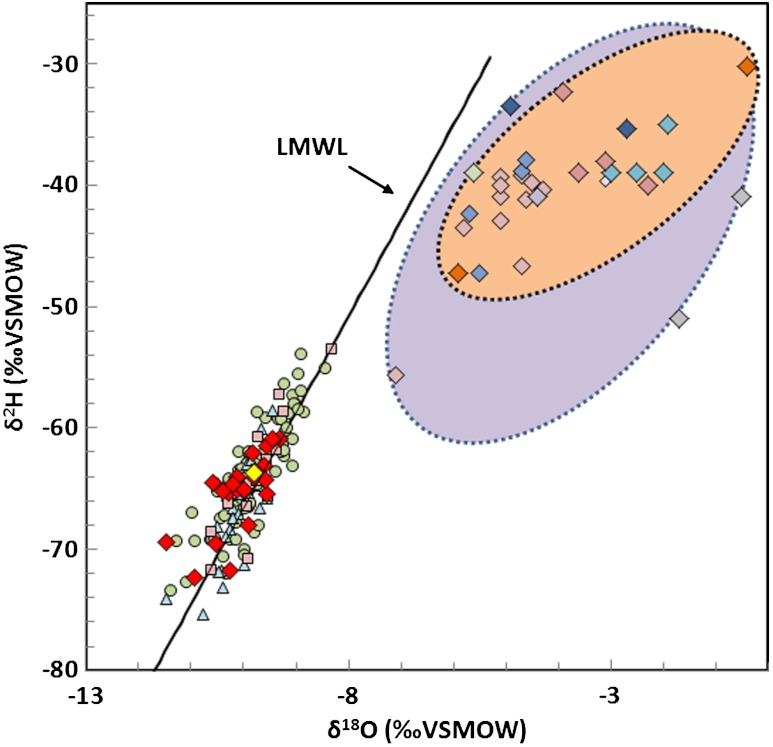
The stable isotopes (δ18O = -8 to -11‰; δ2H = -53 to -74‰) of all shallow groundwater types (A–D) are indistinguishable (p > 0.231) and fall along the local meteoric water line (LMWL) (23) (Fig. 5). The similarity of the stable isotopic compositions to the modern LMWL likely indicate dilution with modern (post-glacial) meteoric water. Shallow groundwater isotopic compositions do not show any positive δ18O shifts towards the seawater evaporation isotopic signature (i.e., higher δ18O relative to δ2H) as observed in the Appalachian brines (Fig. 5 and Table S2). Because of the large difference in concentrations between the brines and fresh water, very small contributions of brine have a large and measureable effect on the geochemistry and isotopes of dissolved salts (Fig. 3) but limited effect on δ18O and δ2H. Mass-balance calculations indicate that only a brine fraction of higher than approximately 20% would change the δ18O and δ2H of salinized groundwater measurably. Oxygen and hydrogen isotopes are, therefore, not sensitive tracers for the mixing of the Appalachian brines and shallow groundwater because of the large percentage of the fresh water component in the mixing blend. For example, the salt spring at Salt Springs State Park with the highest salinity among shallow groundwater samples is calculated to contain < 7% brine.
Fig. 5.
δ2H vs. δ18O in shallow groundwater from this study and Appalachian brines. The water isotope composition of the shallow groundwater samples including the Salt Spring appear indistinguishable from each other and the local meteoric water line (LMWL) (23) and do not show any apparent trends toward the stable isotope ratios of the Appalachian brines (6, 22). The data indicate that dilution of the type-D waters likely occurred on modern (post-glacial) time scales. Symbol legend is provided in Fig. 3.
The discrete areas of type D water have lower average elevations and closer distances to valley centers but do not correlate with distance to the nearest shale gas wells (Fig. 1 and Fig. S1 and Table S1). The lack of geospatial association with shale-gas wells and the occurrence of this type of saline water prior to shale gas development in the study area (14, 15, 18, 19) (see distribution in Fig. 4 A and B) suggests that it is unlikely that hydraulic fracturing for shale gas caused this salinization and that it is instead a naturally occurring phenomenon that occurs over longer timescales.
Distinguishing the ultimate source of the salinized water in NE PA requires an evaluation of the geochemical signatures of underlying brines in the Appalachian Basin. The data presented in this study (Figs. 2 and 3, and Fig. S3 A–F and Table S2) and previous studies (4, 6, 22, 24), suggest that the Appalachian brines evolved by evaporation from a common seawater origin but underwent varying stages of alteration. The first stage of evolution common to all of the brines is the evaporation of seawater beyond halite saturation resulting in brines with high Br/Cl and low Na/Cl ratios relative to seawater (6). The degree of evaporation that is computed based on the Br/Cl ratio in the Appalachian brines (4–7·10-3)(Fig. 3) as compared to the evaporated sea water curve (25) is equivalent to 20–40-fold, though mixing between brines of different evaporation stages cannot be excluded. The brines then likely underwent dolomitization with carbonate rocks that enriched Ca and depleted Mg in the brine relative to the seawater evaporation curve (6) (Fig. S3 B and C) and sulfate reduction that removed all sulfate. In addition, the composition of each respective hypersaline Ca-Cl Appalachian brine (i.e., Salina and/or Marcellus) was differentially altered by interactions with the host aquifer rocks presumably under tectonically-induced thermal conditions (26) that resulted in resolvable variations in Sr/Ca, Ba/Sr, and 87Sr/86Sr ratios. The final stage of brine alteration that accounts for the observed brine compositions is dilution (6).
The net results of these processes generated large variations in brine salinity (TDS of 10–343 g/L), relatively homogeneous elevated Br/Cl ratios (range of 2.4·10-3 to 7.6·10-3) and enriched δ18O (0‰ to -7‰) and δ2H (-33‰ to -45‰) in all Appalachian brines. The remnant geochemical signatures (i.e., Sr/Ca, Ba/Sr, and 87Sr/86Sr) of formation specific brine-rock interactions provide the most suitable basis for differentiating the Appalachian brines. The Sr/Ca ratios (0.03–0.17) of the produced waters from Marcellus wells are significantly higher than brines evolved through calcite (0.4–1.6·10-3) or aragonite (1.5–2.2·10-2) dolomitization but are consistent with equilibrium with other minerals such as gypsum or celestite (27). Similarly, the Ba/Sr (0.01–1.78) ratios range up to values observed for typical upper continental crust (Ba/Sr = 1.3–1.7) (28).
New and compiled data presented in Table S2 show distinctive geochemical fingerprints (Sr/Ca, Ba/Sr, Sr/Cl, Ba/Cl, Li/Cl, and 87Sr/86Sr) among the Appalachian brines in the different formations. We, therefore, used these variables as independent tracers to differentiate possible brine sources for the shallow type D groundwater. Brines from the Marcellus Formation show systematically low (less radiogenic) 87Sr/86Sr (0.71000–0.71212; n = 50) and high Sr/Ca (0.03–0.17) ratios compared to the more radiogenic Upper Devonian brines (87Sr/86Sr ratio = 0.71580–0.72200; n = 12; Fig. 6) and low Sr/Ca (0.002–0.08)(Fig. S4). Because of the relatively high Sr concentration and diagnostic Sr/Ca, Ba/Sr, and 87Sr/86Sr ratios, this geochemical proxy has the potential to elucidate regional flow paths, salinity sources, and the specific source of the Appalachian brines (21, 24) (Fig. 6). The 87Sr/86Sr ratios (0.71030–0.71725 ± 0.000003 SE) of low-saline groundwater (type A and B) vary widely in the shallow aquifers, but the overwhelming majority are distinctly different from values of produced water brines from Upper Devonian (0.71580–0.72200) (24) (Table S2) and Middle Devonian Marcellus Formation (0.71000–0.71212) (21) (Fig. 6). Conversely, the type D shallow groundwater data show a linear correlation between Sr and Cl (i.e., conservative behavior of Sr) (Fig. S3D) and a decrease of 87Sr/86Sr from 0.71453–0.70960 with increasing Sr concentrations and salinity confirming that the resulting salinity is likely derived from mixing with Marcellus Formation brine (Fig. 6). Our data also display a strong association between 87Sr/86Sr and Sr/Ca ratios (Fig. S4), a relationship suggested as a sensitive indicator of Marcellus brines because of the unique combination of low 87Sr/86Sr ratios and high Sr/Ca ratios reported for brines from the Marcellus Formation (21).
Fig. 6.
87Sr/86Sr vs. Sr concentrations (log scale) of Appalachian Brines (21, 24) and shallow groundwater samples in the study area. The shallow groundwater samples are divided in the figure based on water types. Increased concentrations of Sr in the shallow aquifers are likely derived from two component mixing: (i) A low salinity, radiogenic 87Sr/86Sr groundwater sourced from local aquifer reactions; and (ii) A high salinity, less radiogenic 87Sr/86Sr water consistent with Marcellus Formation brine. The Marcellus Formation 87Sr/86Sr appears lower in western Bradford than in Susquehanna and Wayne counties. Other brine sources such as the Upper Devonian formations have a more radiogenic 87Sr/86Sr ratio that does not appear to show any relationship to the salinized shallow groundwater. Symbol legend is provided in Fig. 3.
The saline waters in the eastern portion of the study area follow the expected Sr-isotope mixing trend hypothesized from new and published data on produced water from the Marcellus Formation (Fig. 6). In contrast, the saline waters from the western portion of our study area show systematic mixing with an end member of a slightly lower 87Sr/86Sr ratio (0.70960). This lower ratio could reflect provenance variations within the formation (e.g., lower siliclastic detrital component away from the Acadian clastic source) in the region (21). In sum, whereas the high Br/Cl ratio in type D saline groundwater reflects mixing with underlying Appalachian brines from a common evaporated seawater origin, the 87Sr/86Sr ratios indicate mixing with brines with lower 87Sr/86Sr fingerprints of approximately 0.7096–0.7110 that cannot be accounted for by Upper Devonian formations but are similar to the underlying Marcellus Formation brines.
Other features that characterize the produced waters from the Marcellus Formation are the high activities of naturally occurring nuclides of 226Ra and 228Ra and low 228Ra/226Ra ratios (7). 226Ra and 228Ra are the disintegration products of 238U and 232Th, respectively, and are generated in groundwater from alpha recoil, desorption from sediments, and dissolution of aquifer material (7, 29). In most of the shallow groundwater we sampled (Table S1), combined Ra activities were low (< 5 pCi/L). In contrast, reported activities of Ra in Marcellus brines from the study area were high (1,500–3,100 pCi/L) (Fig. S5) with low 228Ra/226Ra ratios (0.12–0.73) (7). The highest Ra activities that we measured were in type D waters, and the range (0.4 to 28 pCi/L) is consistent with our calculated mixing range of approximately 0.01–7% based on chloride and bromide mass-balance calculations (Fig. 3), though some interaction such as adsorption with the aquifer rocks (29) is likely. In addition, the 228Ra/226Ra ratio in the salinized groundwater (mean = 0.56) is higher than that of the majority of the Marcellus produced waters from the study area (mean = 0.33) (7) (Table S2) indicating that the dissolved Ra in the shallow groundwater is likely derived from a combination of local water-rock interactions and conservative mixing.
Methane data from our previous studies (4, 30) can be examined based on the four water types (A–D) we found in this study. The highest average methane concentrations were observed in type D waters throughout the dataset, followed by type B and A. In locations > 1 km away from shale gas drilling sites only one sample, a type B water, out of total of 41 samples contained elevated methane concentrations (> 10 mg/L). One newly sampled type D water from the spring at Salt Springs State Park (30) also had concentrations > 10 mg/L. Within 1 km of a natural gas well, three type A, three type B, and five type D samples had methane concentrations > 10 mg/L. In three type D groundwater samples that were located in the lowland valleys > 1 km from shale gas drilling sites, methane concentrations were 2–4 mg/L for the two previously sampled shallow ground waters and 26 mg/L for the newly sampled salt spring. In contrast, type A groundwater > 1 km away from drilling sites had methane concentrations < 0.01 mg/L in all samples (n = 14). This could suggest that methane in type D water > 1 km away from drilling sites could be derived from natural seepage (31) but at concentrations much lower than those observed near drilling (4).
Cross-formational pathways allowing deeper saline water to migrate into shallower, fresher aquifers have been documented in numerous study areas including western Texas (32, 33), Michigan Basin (34, 35), Jordan Rift Valley (36), Appalachian Basin (26), and Alberta, Canada (37). In the Michigan Basin, upward migration of saline fluid into the overlying glacial sediments (34, 35) was interpreted to reflect isostatic rebound following the retreat of glaciers, leading to fracture intensification and increased permeability (34). Alternatively, vertical migration of over-pressured hydrocarbons has been proposed for the Appalachian Basin in response to tectonic deformation and catagensis (i.e., natural gas induced fracturing) during the Alleghenian Orogeny (38–40). This deformation resulted in joints that cut across formations (J2) in Middle and Upper Devonian formations (39). In addition, the lithostatic and isostatic rebound following glacial retreat significantly increased fracture intensification and permeability in the Upper Devonian aquifers within our study area.
We hypothesize that regions with the combination of deep high hydrodynamic pressure and enhanced natural flow paths (i.e., fracture zones) (39, 41, 42) could induce steep hydraulic gradients and allow the flow of deeper fluids to zones of lower hydrodynamic pressure (43, 44). The higher frequency of the saline type D water occurrence in valleys (Table S1) is consistent with hydrogeological modeling of regional discharge to lower hydrodynamic pressure in the valleys with greater connectivity to the deep subsurface (43–45).
The possibility of drilling and hydraulic fracturing causing rapid flow of brine to shallow groundwater in lower hydrodynamic pressure zones is unlikely but still unknown. By contrast, the time scale for fugitive gas contamination of shallow aquifers can be decoupled from natural brine movement specifically when gas concentrations exceed solubility (approximately 30 cc/kg) and forms mobile free phase gases (i.e., bubbles). In western PA, on the Appalachian Plateau, contamination of shallow aquifers has been described as leakage of highly pressurized gas through the over-pressurized annulus of gas wells and into the overlying freshwater aquifers via fractures and faults (43, 44). The faults are often connected to local and regional discharge areas (i.e., valleys) where the methane contamination is observed (43). Buoyant flow of methane gas bubbles through these fractures is far more rapid than head-driven flow of dense brine, occurring on time scales of less than a year (46).
This study shows that some areas of elevated salinity with type D composition in NE PA were present prior to shale-gas development and most likely are unrelated to the most recent shale gas drilling; however, the coincidence of elevated salinity in shallow groundwater with a geochemical signature similar to produced water from the Marcellus Formation suggests that these areas could be at greater risk of contamination from shale gas development because of a preexisting network of cross-formational pathways that has enhanced hydraulic connectivity to deeper geological formations (43). Future research should focus on systematically monitoring these areas to test potential mechanisms of enhanced hydraulic connectivity to deeper formations, confirm the brine source, and determine the timescales for possible brine migration.
Methods
Drinking water wells were purged until pH, electrical conductance, and temperature were stabilized. Samples were collected prior to any treatment systems and filtered/preserved following USGS protocols (47). All major element and isotopic chemistry analyses were conducted at Duke University. Major anions were determined by ion chromatography, major cations by direct current plasma optical emission spectrometry, and trace metals by VG PlasmaQuad-3 inductively coupled plasma mass-spectrometry. Alkalinity was determined by titration with HCl to pH 4.5. Stable isotopes were determined by continuous flow isotope ratio mass spectrometry using a ThermoFinnigan TCEA and Delta + XL mass spectrometer at the Duke Environmental Isotope Laboratory (DEVIL). Analytical precisions for δ18O and δ2H were estimated as ± 0.1‰ and ± 1.5‰, respectively. Radium isotope analyses (226Ra and 228Ra) were measured at the Laboratory for Environmental Analysis of RadioNuclides (LEARN) using a Durridge RAD7 radon-in-air monitor (226Ra) and Canberra DSA2000BEGe gamma detector (228Ra) following methods described in (29) and (48). Strontium isotopes were analyzed by a thermal ionization mass spectrometer on a ThermoFisher Triton. The mean 87Sr/86Sr of the Standard Reference Material-987 standard was 0.710266 ± 0.000005 (SD).
Supplementary Material
ACKNOWLEDGMENTS.
Gary Dwyer performed trace element analysis and provided valuable guidance on sample preparation and analysis throughout the research. Jon Karr performed analyses of δ18O and δ2H. Discussions with Bob Poreda helped refine this manuscript. Tom Bullen, Gary Dwyer, Flip Froelich, Terry Engelder, Karl Turekian, and two anonymous reviewers provided valuable and critical comments that greatly improved the manuscript. We thank William Chameides, the Dean of the Nicholas School of Environment, for supporting this research. We gratefully acknowledge financial support from Fred and Alice Stanback.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121181109/-/DCSupplemental.
References
- 1.Kerr RA. Natural gas from shale bursts onto the scene. Science. 2010;328:1624–1626. doi: 10.1126/science.328.5986.1624. [DOI] [PubMed] [Google Scholar]
- 2.Kargbo DM, Wilhelm RG, Campbell DJ. Natural gas plays in the Marcellus Shale: Challenges and potential opportunities. Environ Sci Technol. 2010;44:5679–5684. doi: 10.1021/es903811p. [DOI] [PubMed] [Google Scholar]
- 3.Howarth RW, Ingraffea A, Engelder T. Natural gas: Should fracking stop? Nature. 2011;477:271–275. doi: 10.1038/477271a. [DOI] [PubMed] [Google Scholar]
- 4.Osborn SG, Vengosh A, Warner NR, Jackson RB. Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing. Proc Natl Acad Sci USA. 2011;108:8172–8176. doi: 10.1073/pnas.1100682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang M, et al. Life cycle greenhouse gas emissions of Marcellus shale gas. Environ Res Lett. 2011;6:034014. [Google Scholar]
- 6.Dresel P, Rose A. Chemistry and origin of oil and gas well brines in Western Pennsylvania: Pennsylvania Geological Survey; 4th series Open-File Report OFOG 10-01.0; 2010. p. 48. (Pennsylvania Department of Conservation and Natural Resources) [Google Scholar]
- 7.Rowan E, Engle M, Kirby C, Kraemer T. Radium content of oil- and gas-field produced waters in the Northern Appalachian basin (USA)—Summary and discussion of data; U.S. Geological Survey Scientific Investigations Report 2011–5135; 2011. p. 31. (U.S. Geological Survey) [Google Scholar]
- 8.Gregory KB, Vidic RD, Dzombak DA. Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements. 2011;7:181–186. [Google Scholar]
- 9.Hayes T. Sampling and analysis of water streams associated with the development of Marcellus shale gas; Marcellus Shale Initiative Publications Database; 2009. [Google Scholar]
- 10.Veil J. Society of Petroleum Engineers. Houston, TX: Society of Petroleum Engineers; 2011. White Paper on SPE Summit on Hydraulic Fracturing. [Google Scholar]
- 11.Fisher K. Data confirm safety of well fracturing. Reporter. 2010 http://www.fidelityepco.com/Documents/OilGasRept_072010.pdf. [Google Scholar]
- 12.Frey MG. Influence of salina salt on structure in New York-Pennsylvania part of Appalachian plateau. AAPG Bull. 1973;57:1027–1037. [Google Scholar]
- 13.Faill R. The Acadian orogeny and the Catskill Delta. Geol S Am S. 1985;201:15–38. [Google Scholar]
- 14.Lohman SW. Ground Water in North-Central Pennsylvania. Pennsylvania: Department of Conservation and Natural Resources; 1973. p. 219. [Google Scholar]
- 15.Lohman SW. Ground Water in Northeastern Pennsylvania. Pennsylvania: Department of Conservation and Natural Resources; 1957. p. 312. [Google Scholar]
- 16.Alexander S, Cakir R, Doden AG, Gold DP, Root SI. Basementdepth and related geospatial database for Pennsylvania; Pennsylvania Geological Survey; 2005. Open-File General Geology) Report 05-01.00. [Google Scholar]
- 17.Geyer A, Wilshusen JP. Engineering characteristics of the rocks of Pennsylvania; environmental geology supplement to the state geologic map; Pennsylvania Geological Survey; 1982. p. 300. [Google Scholar]
- 18.Taylor L. Groundwater Resources of the Upper Susquehanna River Basin, Pennsylvania: Water Resources Report 58. 1984. p. 136. (Pennsylvania Department of Environmental Resources, Office of Parks and Forestry, Bureau of Topographic and Geologic Survey)
- 19.Williams J, Taylor L, Low D. Hydrogeology and Groundwater Quality of the Glaciated Valleys of Bradford, Tioga, and Potter Counties, Pennsylvania: Water Resources Report 68. 1998. p. 89. (Commonwealth of Pennsylvania Department of Conservation and Natural Resources)
- 20.Trapp H, Jr, Horn MA. Ground Water Atlas of the United States: Delaware, Maryland, New Jersey, North Carolina, Pennsylvania, Virginia, West Virginia HA 730-L. 1997. (U.S. Geological Survey)
- 21.Chapman EC, et al. Geochemical and strontium isotope characterization of produced waters from Marcellus Shale natural gas extraction. Environ Sci Technol. 2012;46:3545–3553. doi: 10.1021/es204005g. [DOI] [PubMed] [Google Scholar]
- 22.Osborn SG, McIntosh JC. Chemical and isotopic tracers of the contribution of microbial gas in Devonian organic-rich shales and reservoir sandstones, northern Appalachian Basin. Appl Geochem. 2010;25:456–471. [Google Scholar]
- 23.Kendall C, Coplen T. Distribution of oxygen-18 and deuterium in river waters across the United States. Hydrol Process. 2001;15:1363–1393. [Google Scholar]
- 24.Osborn SG, Mcintosh J, Hanor J, Biddulph D. Iodine-129, 87Sr/86Sr, and trace elemental geochemistry of northern Appalachian Basin brines: Evidence for basinal-scale fluid migration and clay mineral diagenesis. Am J Sci. 2012 in press. [Google Scholar]
- 25.McCaffrey M, Lazar B, Holland H. The evaporation path of seawater and the coprecipitation of Br and K with halite. J Sediment Petrol. 1987;57:928–937. doi: 10.1306/212f8cab-2b24-11d7-8648000102c1865d. [DOI] [PubMed] [Google Scholar]
- 26.Schedl A, McCabe C, Montanez I, Fullagar P, Valley J. Alleghenian regional diagenesis: A response to the migration of modified metamorphic fluids derived from beneath the Blue Ridge-Piedmont thrust sheet. J Geol. 1992;100:339–352. [Google Scholar]
- 27.Sass E, Starinsky A. Behaviour of strontium in subsurface calcium chloride brines: Southern Israel and Dead Sea rift valley. Geochim Cosmochim Acta. 1979;43:885–895. [Google Scholar]
- 28.Rudnick R, Gao S. The Composition of the Continental Crust, in the Crust. Oxford: Elsevier-Pergamon; 2003. [Google Scholar]
- 29.Vinson DS, Vengosh A, Hirschfeld D, Dwyer GS. Relationships between radium and radon occurrence and hydrochemistry in fresh groundwater from fractured crystalline rocks, North Carolina. Chem Geol. 2009;260:159–171. [Google Scholar]
- 30.Osborn SG, Vengosh A, Warner NR, Jackson RB. Reply to Saba and Orzechowski and Schon: Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing. Proc Natl Acad Sci USA. 2011;108:E665–E666. doi: 10.1073/pnas.1100682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molofsky LJ, Connor JA, Farhat SK, Wylie AS, Jr, Wagner T. Methane in Pennsylvania water wells unrelated to Marcellus shale fracturing. Oil Gas J. 2011;109:54–67. [Google Scholar]
- 32.Mehta S, Fryar AE, Banner JL. Controls on the regional-scale salinization of the Ogallala aquifer, Southern High Plains, Texas, USA. Appl Geochem. 2000;15:849–864. [Google Scholar]
- 33.Hogan JF, et al. Geologic origins of salinization in a semi-arid river: The role of sedimentary basin brines. Geology. 2007;35:1063–1066. [Google Scholar]
- 34.Weaver TR, Frape SK, Cherry JA. Recent cross-formational fluid flow and mixing in the shallow Michigan Basin. Geol Soc Am Bull. 1995;107:697–707. [Google Scholar]
- 35.Long DT, Wilson TP, Takacs MJ, Rezabek DH. Stable-isotope geochemistry of saline near-surface ground water: East-central Michigan basin. Geol Soc Am Bull. 1988;100:1568–1577. [Google Scholar]
- 36.Farber E, et al. The origin and mechanisms of salinization of the lower Jordan river. Geochim Cosmochim Acta. 2004;68:1989–2006. [Google Scholar]
- 37.Tilley B, et al. Gas isotope reversals in fractured gas reservoirs of the western Canadian Foothills: Mature shale gases in disguise. AAPG Bull. 2011;95:1399–1422. [Google Scholar]
- 38.Lash GG, Engelder T. Tracking the burial and tectonic history of Devonian shale of the Appalachian Basin by analysis of joint intersection style. Geol Soc Am Bull. 2009;121:265–277. [Google Scholar]
- 39.Engelder T, Lash GG, Uzcategui RS. Joint sets that enhance production from Middle and Upper Devonian gas shales of the Appalachian Basin. AAPG Bull. 2009;93:857–889. [Google Scholar]
- 40.Lash G, Blood DR. Origin of early overpressure in the Upper Devonian Catskill Delta Complex, western New York State. Basin Res. 2007;19:51–66. [Google Scholar]
- 41.Llewellyn G. Structural and topographic assessment of shallow bedrock permeability variations throughout Susquehanna County PA: A focus area of Marcellus Shale Gas Development. Abstr Programs Geol Soc Am. 2011;43:567. [Google Scholar]
- 42.Jacobi RD. Basement faults and seismicity in the Appalachian Basin of New York State. Tectonophysics. 2002;353:75–113. [Google Scholar]
- 43.Harrison SS. Evaluating system for ground-water contamination hazards due to gas-well drilling on the glaciated Appalachian plateau. Ground Water. 1983;21:689–700. [Google Scholar]
- 44.Harrison SS. Contamination of aquifers by overpressuring the annulus of oil and gas wells. Ground Water. 1985;23:317–324. [Google Scholar]
- 45.Tóth J. A conceptual model of the groundwater regime and the hydrogeologic environment. J Hydrol. 1970;10:164–176. [Google Scholar]
- 46.Etiope G, Klusman RW. Geologic emissions of methane to the atmosphere. Chemosphere. 2002;49:777–789. doi: 10.1016/s0045-6535(02)00380-6. [DOI] [PubMed] [Google Scholar]
- 47.USGS. Washington, DC: US Geological Survey; 2011. National Field Manual for the Collection of Water-Quality Data. [Google Scholar]
- 48.Kim G, Burnett WC, Dulaiova H, Swarzenski PW, Moore WS. Measurement of 224Ra and 226Ra activities in natural waters using a radon-in-air monitor. Environ Sci Technol. 2001;35:4680–4683. doi: 10.1021/es010804u. [DOI] [PubMed] [Google Scholar]
- 49.Denison RE, Kirkland DW, Evans R. Using strontium isotopes to determine the age and origin of gypsum and anhydrite beds. J Geol. 1998;106:1–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.