Abstract
Two so-called “secretory Rabs,” Rab3 and Rab27, regulate late steps during dense-core vesicle exocytosis in neuroendocrine cells. Sperm contain a single large dense-core granule that is released by regulated exocytosis (termed the acrosome reaction) during fertilization or on exposure to inducers in vitro. Sperm exocytosis uses the same fusion machinery as neurons and neuroendocrine cells, with an additional requirement for active Rab3. Here we show that Rab27 is also required for the acrosome reaction, as demonstrated by the inability of inducers to elicit exocytosis when streptolysin O-permeabilized human sperm were loaded with inhibitory anti-Rab27 antibodies or the Rab27–GTP binding domain of the effector Slac2-b. The levels of GTP-bound Rab27 increased on initiation of exocytosis, as did the proportion of GTP-bound Rab3A. We have developed a fluorescence microscopy-based method for detecting endogenous Rab3A-GTP and Rab27-GTP in the acrosomal region of human sperm. Challenge with an inducer increased the population of cells exhibiting GTP-bound Rabs in this subcellular domain. Interestingly, introducing recombinant Rab27A loaded with GTP-γ-S into sperm elicited a remarkable increase in the number of cells evincing GTP-bound Rab3A. In the converse condition, recombinant Rab3A did not modify the percentage of Rab27-GTP–containing cells. Furthermore, Rab27A-GTP recruited a Rab3 GDP/GTP exchange factor (GEF) activity. Our findings suggest that Rab27/Rab3A constitutes a Rab-GEF cascade in dense-core vesicle exocytosis.
Keywords: Rab cascade, GTP-binding protein, Rab pull down, calcium, cyclic AMP/Epac
Evolution has endowed secretory cells with sophisticated and highly regulated exocytosis mechanisms that are recruited by the intracellular calcium fluctuations elicited by extracellular signals. These cells contain vesicles that store and release secreted products. The machinery of regulated exocytosis is highly conserved among conventional Golgi-derived granules in exocrine and endocrine cells, lysosome-related organelles in hematopoietic cells and melanocytes, and synaptic or synaptic-like vesicles in neurons and neuroendocrine cells (1–5). To reach the fusion competent state, vesicles must undergo a series of maturation steps that include their tethering and docking to the plasma membrane and priming of the fusion machinery on both membranes. One of the best-known functions of the Rab GTPases is to govern the initial attachment, or tethering, of the membranes that are fusing (6). Current knowledge suggests that Rabs function as molecular switches that alternate between a GTP and membrane-bound “on” state and a GDP-bound, cytosolic, “off” state. During the activation phase of the Rab cycle, a GDP/GTP exchange factor (GEF) releases GDP bound in the nucleotide pocket. Rab immediately binds GTP, which has a much higher cytosolic concentration than GDP.
During the inactivation phase, hydrolysis converts GTP to GDP, a reaction catalyzed by Rab’s intrinsic GTPase activity assisted by GTPase-activating proteins (GAPs). Guanine nucleotide dissociation inhibitors recycle GDP-bound Rabs back to the cytosol. GTP-bound Rabs recruit other proteins called effectors, forming protein complexes that tether the transport vesicle to the acceptor membrane (7–11), and participate in the priming of synaptic vesicles to a readily releasable state (12).
Rab3 (A, B, C, and D) and Rab27 (A and B) constitute the two main Rab subfamilies directly implicated in regulated exocytosis. These “secretory Rabs” localize to vesicles and secretory granules in a variety of secretory cell types (1, 8, 13–15). They control the recruitment, tethering, and/or docking of secretory vesicles to the plasma membrane through interaction with effectors (16). The coexistence of Rab3 and Rab27 on the same membrane in several secretory cells raises the questions of functional redundancy, and if and how their roles are coordinated to regulate exocytosis. A handful of laboratories have addressed these issues by investigating the behavior of both Rabs in parallel. In one study, for instance, in PC12 cells, both overexpressed and endogenous Rab3A and Rab27A localized to dense-core vesicles (17). Silencing each Rab individually reduced the number of vesicles docked at the plasma membrane and exocytotic events. Both phenomena were further reduced by silencing Rab3A and Rab27A simultaneously, which led to the conclusion that these small GTPases cooperate to regulate the docking of dense-core vesicles to the plasma membrane (17). Another study conducted in the same neuroendocrine cell line and published a year later found that overexpressed Rab3A cycles continuously between the cytosol and membranes, and that this cycling is not coupled to exocytosis (18). Unlike Rab3A, Rab27A does not exchange between granules and cytosol (18). In the pancreatic β-cell line MIN6, Rab27A localizes primarily to membranes, whereas Rab3A distributes between membranes and cytosol. A study found that overexpression of WT or GTPase-deficient Rab27A enhanced K+ depolarization-induced insulin release by 50–60%, whereas overexpression of the equivalent Rab3A proteins had no effect (19). The authors concluded that Rab27A and Rab3A have different roles in insulin secretion. Likewise, a kinetic study demonstrated that β-cells from Rab27A−/− (ashen) mice, but not from Rab3A null mice, exhibit defects in refilling certain pools of insulin granules in response to depolarization (20). Ashen islets exhibit decreased insulin secretion from undocked granules (21). In the most recent study analyzing Rab3 and Rab27 simultaneously, both proteomic and quantitative Western blot analyses of subcellular fractions prepared from rat brain homogenates demonstrated the marked enrichment of three isoforms of Rab3 (A, B, and C), as well as Rab27B, on synaptic vesicles. Subpopulations of Rab3A molecules also localize to brain cytosol and plasma membrane, whereas Rab27B is not found in the cytosol (15). In a recent review, the authors hypothesized that the roles of Rab3A and Rab27B in the synaptic vesicle cycle are complementary, and that these isoforms act in successive steps (22).
The acrosome is a dense-core secretory vesicle that overlies the nucleus of the mature spermatozoon. In response to physiological or pharmacologic stimuli, the acrosome undergoes a special type of calcium-dependent exocytosis termed the acrosome reaction (AR), which is a prerequisite for fertilization (23). We and others have shown that the AR relies on the same highly conserved molecules and goes through the same stages as exocytosis in neuronal, endocrine, and all other types of cells studied to date (reviewed in refs 24–26), so much so that some authors refer to the anterior region of the acrosomal cap as the “acrosomal synapse” (27). Rab3A is present in the acrosomal region of human (28), rat, and mouse sperm (29); in the latter it is predominantly membrane-bound and disappears from cells that have undergone the AR (30). Rab3A is required for the AR triggered by calcium (31, 32), cAMP (33), and sphingosine 1-phosphate (34) in human sperm. Treatment with AR inducers increases the levels of GTP-bound Rab3A (35).
Here we combine biochemical with functional and microscopy-based methods to show that, like Rab3A, Rab27 is present in, localizes to the acrosomal region of, and is required for exocytosis in human sperm. Whereas Rab27 is predominantly membrane-bound, Rab3A binding to membranes increases when sperm are challenged with AR inducers. Both Rabs exchange GDP for GTP in response to exocytosis initiators. By means of a protocol developed in our laboratory, we are able to show that Rab3A-GTP and Rab27-GTP localize to the acrosomal region of the sperm head. More importantly, we present direct evidence that active Rab27 increases the exchange of GDP for GTP on Rab3A. The molecular mechanism for this activation appears to be through a Rab-GEF cascade, given that active Rab27 recruits a Rab3 GEF from human sperm extracts. Therefore, we propose that Rab27 and Rab3A work sequentially during sperm dense-core granule exocytosis, with Rab27 acting first.
Results
Presence and Subcellular Localization of Rab27 in Human Sperm.
Rabs from the Rab3 and Rab27 families usually coexist in secretory cells. A few years ago, we and others reported that Rab3A is present in mammalian sperm and exhibits a role in the AR, as discussed in the Introduction. In the present study, we investigated the presence of Rab27 in human sperm using a polyclonal antibody raised against purified recombinant full-length rat Rab27A. According to the manufacturer, this antibody recognizes Rab27A and B, but does not cross-react with other Rab proteins. Nevertheless, we ran specificity controls for this antibody and the anti-Rab3A antibody and confirmed a lack of cross-reactivity between their target proteins. In brief, we electrophoresed recombinant GST-Rab3A and GST-Rab27A and probed them on Western blots. The anti-Rab3A antibody detected Rab3A, but not Rab27A, and vice-versa; an anti-GST antibody recognized both (Fig. S1A).
Probing extracts of whole human sperm (Fig. 1A) and mouse testis (Fig. 1C) with the anti-Rab27 antibody on Western blots revealed a single protein band with apparent molecular mass of ∼28 kDa corresponding to Rab27. We inferred that Rab27 is geranylgeranylated in sperm because it partitioned entirely into the detergent phase after Triton X-114 phase separation (Fig. 1A). To determine its subcellular localization, we fractionated human sperm into membrane and cytosolic fractions and probed them on Western blots. Rab27 was predominantly membrane-bound, with a negligible amount detected in the cytosol (Fig. 1B). All membrane-bound Rab27 partitioned into the detergent fraction after Triton X-114 phase separation (Fig. 1C).
Fig. 1.

Rab27 is membrane-associated in human sperm. (A and B) Whole sperm homogenates were partitioned in Triton X-114 (A) or subjected to subcellular fractionation (B) as described in SI Materials and Methods. Proteins from unfractionated (wc extract) sperm extract, from the aqueous (ap) and detergent phases (dp), and from the particulate (membranes) and soluble (cytosol) fractions were electrophoresed on 10% Tris-tricine-SDS-polyacrylamide gels, transferred to nitrocellulose, and immunoblotted with the anti-Rab27 antibody. Mr standards (× 103) are indicated on the left. Data are from experiments representative of three repetitions. Quantification (expressed as percent Rab27 in each fraction, carried out with ImageJ) is depicted to the right of each immunoblot as mean ± SEM from all replicates. (C) Human sperm samples were subjected to subcellular fractionation into membranes and cytosol, followed by Triton X-114 partition. Proteins from the aqueous and detergent phases were precipitated with organic solvents, resolved on 10% Tris-tricine-SDS-polyacrylamide gels, transferred to nitrocellulose, and probed with the anti-Rab27 antibody. Mr standards (× 103) are shown on the left. Data are from an experiment representative of two repetitions.
Rab27 Is Required for Human Sperm AR.
Streptolysin O (SLO) permeabilization is a widespread methodology used in analysis of dense-core granule exocytosis that we and others have applied to studying the AR (28, 36–39). This method permits the introduction of exogenous factors—which are not otherwise able to cross membranes—into the cytosol through pores in the plasma membrane generated by SLO. Given the important role of Rab3 in the AR and its coexistence with Rab27 in human sperm, we predicted that Rab27 also would be required for sperm exocytosis. We tested this hypothesis and characterized Rab27’s site of action during human sperm AR by means of a plasma membrane permeabilization technique. To do so, we first introduced anti-Rab27 antibodies into SLO-permeabilized human sperm and monitored their effects on the calcium-triggered AR. The antibodies inhibited the AR in a dose–response fashion (Fig. 2A). To determine whether active Rab27 is required for the AR, we conducted similar experiments, but substituting the anti-Rab27 antibodies with the Rab27-GTP binding domain of the effector Slac2-b. This protein blocked calcium-triggered exocytosis in a concentration-dependent manner (Fig. 2B). Finally, we tested the inhibitory effect of the Rab27 blockers when initiating the signaling pathway leading to the AR with a different exocytosis inducer. When we challenged sperm with the Epac- selective cAMP analogue 8-(p-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (8-pCPT-2′-O-Me-cAMP) instead of calcium, we observed that the anti-Rab27 antibodies and Slac2-b blocked exocytosis (Fig. 2C, black bars). Taken together, these data indicate that active Rab27 is necessary for the human sperm AR.
Fig. 2.
Active Rab27 is required for human sperm AR. (A and B) SLO-permeabilized human sperm were treated for 15 min at 37 °C with increasing concentrations of the anti-Rab27 antibody (A) or the Rab27-GTP binding domain of Slac2-b (B). The AR was then initiated by adding 0.5 mM CaCl2 and incubating for 15 min at 37 °C. In B, ▪ indicates that Slac2-b alone has no effect on exocytosis. (C) Permeabilized spermatozoa were incubated with 7 nM anti-Rab27 or 140 nM Slac2-b for 15 min at 37 °C. Acrosomal exocytosis was then initiated by adding 0.5 mM CaCl2 (calcium) or 50 μM 8-pCPT-2′-O-Me-cAMP (8pCPT) and another 15-min incubation at 37 °C. (D) Permeabilized spermatozoa were loaded with 10 μM NP-EGTA-AM (NP) for 10 min at 37 °C to chelate intra-acrosomal calcium. The AR was then initiated by adding 0.5 mM CaCl2. After another 15-min incubation at 37 °C to allow exocytosis to proceed up to the intra-acrosomal calcium-sensitive step, sperm were treated for 15 min at 37 °C with 7 nM anti-Rab27 antibodies or 140 nM Slac2-b. All of these procedures were carried out in the dark. UV flash photolysis of the chelator was induced at the end of the incubation period (hν), and the samples were incubated for 5 min (NP→calcium→inhibitor→hν, black bars). Controls (gray bars) included background AR in the absence of any stimulation (control), AR stimulated by 0.5 mM CaCl2 (calcium), the inhibitory effect of NP-EGTA-AM in the dark (NP→calcium→dark) and recovery on illumination (NP→calcium→hν), and the effect of the inhibitors when present throughout the experiment (NP→inhibitor→calcium→hν). Exocytosis was evaluated by FITC-PSA binding, and the data were normalized as described in Materials and Methods. Plotted data represent the mean ± SEM of at least three independent experiments.
Because Rab27 exhibits homology with members of the Rab3 subfamily (40% similarity at the amino acid level) (19, 40), and because we derive strong conclusions from experiments conducted with antibodies, we performed specificity controls for the effect of the anti-Rab3A and anti-Rab27 antibodies on the AR. We had previously shown that the inhibitory effect of anti-Rab3A antibodies in human sperm exocytosis is abolished when the antibodies are preblocked with recombinant Rab3A (33). Here we tested the effect of anti-Rab27 antibodies on the AR after blocking their active sites by preincubation with recombinant Rab27A before adding them to permeabilized sperm. Under these conditions, anti-Rab27 was no longer able to prevent the AR elicited by calcium (Fig. S2A, black bar), suggesting that its inhibitory effect is due to binding to endogenous Rab27. In contrast, preincubation of the anti-Rab27 antibody with recombinant Rab3A did not abolish the inhibitory effect (Fig. S2C, black bar). In the reverse condition, recombinant Rab27A was unable to prevent the exocytotic block caused by the anti-Rab3A antibody (Fig. S2B). These results confirm the specificity of the anti-Rab antibodies in sperm cells, in agreement with what we observed with recombinant proteins (Fig. S1A).
The acrosome behaves as an internal store of releasable calcium (41–44); efflux from this reservoir through inositol 1,4,5 triphosphate (IP3)-sensitive channels is required for the AR initiated by all inducers (32, 33, 41, 45). We had previously demonstrated that Rab3A acts in the AR before the release of calcium from the intracellular store (32). To assess when Rab27 is required with respect to intracellular calcium release, we conducted identical experiments, combining the anti-Rab27 antibodies and recombinant Slac2-b with the photolabile calcium chelator O-nitrophenyl EGTA-acetoxymethyl ester (NP-EGTA-AM). In the SLO-permeabilized human sperm model, NP-EGTA-AM crosses the plasma and outer acrosomal membranes, accumulates inside the acrosome, and prevents the AR triggered by all inducers by sequestering intra-acrosomal calcium for as long as the system is kept in the dark. UV photolysis of NP-EGTA-AM rapidly replenishes the acrosomal calcium pool, resuming exocytosis (36, 39, 41). In NP-EGTA-AM–loaded permeabilized sperm, anti-Rab27 and Slac2-b blocked exocytosis when added before, but not after, initiation of the AR by calcium (Fig. 2D). These data indicate that Rab27 acts in exocytosis before the start of intra-acrosomal calcium efflux.
Effects of AR Inducers on Rab3A and Rab27 Targeting to Membranes and Activation Status.
Having established that Rab3A and Rab27 are present in human sperm and play a role in the AR, we then asked whether these Rabs are activated during the onset of exocytosis. We first investigated whether challenging sperm to undergo the AR correlates with the subcellular localization of the secretory Rabs. To this end, we incubated sperm with 2-aminoethoxy-diphenylborate (2-APB), an IP3-sensitive calcium channel blocker, to prevent acrosomal loss due to exocytosis. We then added the exocytotic inducers calcium ionophore A23187 or 8-pCPT-2′-O-Me-cAMP, fractionated the cells, and assessed the levels of membrane-bound Rabs by Western blot analysis. We observed no differences in the amount of membrane-bound Rab27 from resting cells and from sperm treated with A23187 or 8-pCPT-2′-O-Me-cAMP (Fig. 3A). In contrast, treatment with either AR inducer increased the levels of membrane-bound Rab3A by ∼50% compared with untreated cells (Fig. 3A). These results indicate that targeting of Rab3A, but not (or to a lesser extent) of Rab27, to membranes appears linked to the initiation of exocytosis.
Fig. 3.
Initiation of the AR activates Rab27 and Rab3A. (A) Capacitated human sperm were incubated without (control) or with the AR inducers A23187 or 8pCPT, washed, and subjected to subcellular fractionation as described in SI Materials and Methods. Rab27 (Top) and Rab3A (Middle) were detected in the particulate (membrane) fractions by Western blot analysis using the rabbit polyclonal antibodies as probes. Anti-α-tubulin was used as a loading control (Bottom). Mr standards (× 103) are indicated on the left. (B) Quantification of 12 experiments (n = 3 for each panel) identical to those in A, with mean ± SEM from all replicates. Data were evaluated using the t-test for paired comparisons for means. NS indicates that statistical differences between control and treated samples were nonsignificant (P > 0.05). **P < 0.01. (C and D) Pull-down assays using GST-Slac2b-Sepharose (C) and GST-RIM-RBD-Sepharose (D). The levels of GTP-bound Rab27 (C) and Rab3A (D) were determined by Western blot analysis probed with the rabbit polyclonal anti-Rab27 and mouse monoclonal anti-Rab3A antibodies. Anti–α-tubulin was used as an input control. Mr standards (× 103) are indicated on the left. Shown are representative blots, with quantifications depicted as mean ± SEM from all replicates (n = 2 for Rab27; n = 4 for Rab3A) to the right of each blot. Data were evaluated using the t-test for paired comparisons for means. **P < 0.01.
We next applied a pull-down strategy to examine the effects of AR inducers on the GTP status of endogenous Rab27 in sperm. We first expressed Slac2-b fused to GST in Escherichia coli and conducted far-Western blot analyses to verify that our recombinant Slac2-b preparations were active. In brief, we ran GST and Slac2-b on SDS gels, then transferred the proteins to nitrocellulose and incubated with GTP-γ-S– or GDP-bound Rab27A, before continuing as for standard Western blot analysis, probing with anti-Rab27 and anti-GST antibodies. The far-Western blots showed much higher binding of Rab27A-GTP-γ-S than Rab27A-GDP to Slac2-b; neither bound GST (Fig. S1B). These results indicate that our Slac2-b preparation was able to discriminate between active and inactive Rab27. We used Slac2-b in pull-down assays as described previously (46) to determine the levels of Rab27-GTP in sperm. The threshold level of endogenous Rab27-GTP in unstimulated sperm was high; nevertheless, A23187 elicited an ∼1.5-fold increase in active Rab27 (Fig. 3C). Taken together, these results indicate that Rab27 is associated with membranes and significantly bound to GTP in resting human sperm. Initiation of the AR elicits further activation of this small GTPase.
We previously reported that sperm contain a GEF activity that exchanges GDP for GTP on Rab3A when challenged with calcium or 8-pCPT-2′-O-Me-cAMP (35, 47). We conducted those earlier experiments in SLO-permeabilized human sperm, but because we performed the Rab27 pull-down experiments reported in this paper with nonpermeabilized sperm, we wished to verify the Rab3A data under the same conditions. To proceed, we first expressed in E. coli the Rab3-binding domain (RBD) of Rab3-interacting molecule (RIM), an effector that specifically binds the GTP-bound form of Rab3, fused to GST. RIM-RBD pulled-down GTP-γ-S–loaded recombinant Rab3A; GDP-loaded Rab3A was pulled down to a substantially lesser extent (Fig. S1C). These results indicate that our RIM-RBD preparation was able to discriminate between active and inactive Rab3A; thus, we used it to verify the capacity of sperm to activate Rab3A during the AR. Given the difficulty of detecting endogenous Rab3A in sperm, we introduced a membrane-permeant version of Rab3A into the cells before performing the challenge with 8-pCPT-2′-O-Me-cAMP. We found that the levels of GTP-bound Rab3A increased by approximately twofold in response to the AR trigger (Fig. 3D). Taken together, our data indicate that the onset of the AR correlates with the activation of both Rab3A and Rab27.
Detecting the GTP Status of Endogenous Rab3A and Rab27 by Fluorescence Microscopy.
We were interested in determining the percentage of the sperm population that exhibit activated Rab3 and Rab27 in response to AR inducers, and in identifying where in the cell these active Rabs are localized. Unfortunately, pull-down assays are not suitable for addressing these issues (in addition to being lengthy to perform and only semiquantitative). Thus, we designed a protocol based on the principles underlining far-Western blot analysis, but with indirect immunofluorescence as the readout. We reasoned that if the interaction between Rab27 and Slac2-b survived the harsh conditions of SDS/PAGE and Western blot analysis (Fig. S1B), it also might be expected to survive the comparatively milder conditions applied in indirect immunofluorescence protocols. This was indeed the case. We first validated the assay to demonstrate that the probes can distinguish between GDP-bound and GTP-bound Rabs in this protocol. To do so, we incubated SLO-permeabilized sperm with EDTA to chelate endogenous Mg2+ cations and allow for the release of endogenous guanosine nucleotides. We then loaded the cells with GDP-β-S or GTP-γ-S to determine the minimum and maximum levels of active Rabs detectable by this assay. We smeared and fixed sperm on slides, overlaid with GST-RIM-RBD or GST-Slac2-b, washed the slides, and proceeded as for standard immunostaining, using anti-GST antibodies to detect the activity probes. The anti-GST antibodies immunodecorated the acrosomal region in 70% of the cells loaded with GTP-γ-S and overlaid with RIM-RBD, whereas only 29% of sperm treated with GDP-β-S gave a detectable signal (Fig. S3). Likewise, Slac2-b bound the acrosomal region in 23% of GTP-γ-S–treated sperm, but in only 6% of the GDP-β-S–treated sperm (Fig. S3). In the control condition, overlaying with GST and probing with the anti-GST antibodies did not accomplish immunostaining (Fig. S3). Thus, we validated our protocol by demonstrating that RIM-RBD and Slac2-b can distinguish between GDP-bound and GTP-bound Rab3A and Rab27. Our results indicate that GTP-bound Rab3A and Rab27 localize to the acrosomal region in human sperm, as expected for proteins with a role in exocytosis and consistent with indirect immunofluorescence data for anti-Rab3A (28) and anti-Rab27 (Fig. S3, Lower) antibodies.
Rab27 and Rab3A Act Sequentially During Human Sperm AR.
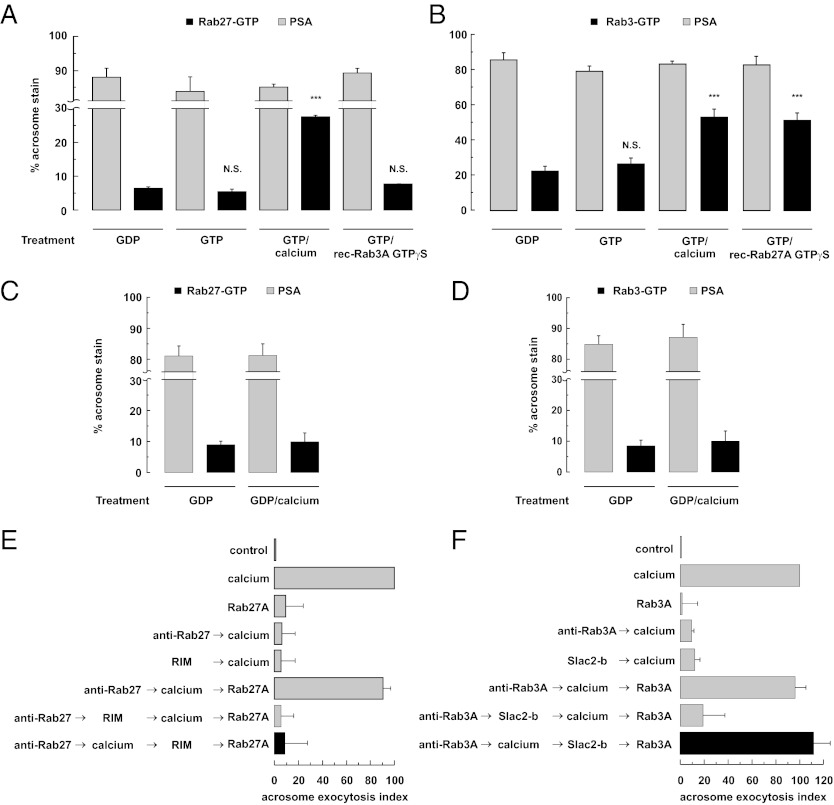
The coexistence of more than one Rab GTPase in the same membrane raises the question of how their functions are coordinated. Different models have been proposed to address this question based on data from yeast and mammalian endomembranes (for recent reviews, see refs. 7, 11, 48, 49). How Rab3 and Rab27 cooperate to achieve dense-core granule exocytosis is a question that has not yet been addressed in any model system. We investigated this issue in the AR by applying our recently developed immunofluorescence assay. First, we pretreated the cells with 2-APB to prevent loss of the acrosomal granule due to exocytosis; thus, the percentage of acrosome-reacted sperm was low under all conditions (Fig. 4 A and B, gray bars). Second, we loaded endogenous sperm GTPases with GDP and stabilized the bound nucleotide with high Mg2+ concentrations. Third, when indicated, we bathed the cells in a buffer containing GTP. We found the same percentage of cells depicting Rab27-GTP whether we treated with GDP alone or with GDP followed by GTP; in contrast, when we added calcium to the GTP-containing medium, the number of cells harboring Rab27-GTP in the acrosomal region increased by more than fourfold (Fig. 4A). These results suggest that sperm do not exchange GDP for GTP on Rab27 spontaneously; in other words, sperm Rab27-GEF is not constitutively active. Likewise, the percentage of cells exhibiting Rab3-GTP in the acrosomal region more than doubled when cells were exposed to GTP/calcium compared with GDP or GTP alone (Fig. 4B).
Fig. 4.
Rab27 and Rab3A play sequential roles during exocytosis; active Rab27 increases the level of Rab3A-GTP. (A–D) SLO-permeabilized human sperm treated as described in Materials and Methods were overlaid with GST-Slac2-b to detect active Rab27 (A and C) and with GST-RIM-RBD to detect active Rab3 (B and D) and then triple-stained with the anti-GST antibody to visualize the activity probes, with FITC-PSA to confirm that the AR was effectively prevented by 2-APB (PSA, gray bars), and with Hoechst 33342 to visualize all cells in each field (not shown). The percentage of cells immunodecorated in the acrosomal region by the anti-GST antibodies is represented by the black bars. The Tukey–Kramer post hoc test was used for pairwise comparisons. (A and B) The population of active Rabs increased on incubation with GTP plus CaCl2 compared with controls incubated with GDP (***P < 0.001; cell counts: 170 cells probed with Slac2-b and 484 cells probed with RIM-RBD). Note that by itself, GTP (cell counts: 220 cells probed with Slac2-b and 405 cells probed with RIM-RBD) was unable to increase the number of cells depicting active Rabs compared with those treated with GDP (NS, statistically nonsignificant; cell counts: 219 cells probed with Slac2-b and 406 cells probed with RIM-RBD). Recombinant Rab27A augmented the number of cells depicting endogenous GTP-Rab3A in the acrosomal region (B, NS with respect to GTP/CaCl2; 396 cells counted; ***P < 0.001 compared with GDP). The converse was not true; recombinant Rab3A did not influence the number of cells with active Rab27 in the head; (A, NS compared with controls loaded with GDP; 180 cells counted). The data represent mean ± SEM of at least three independent experiments. (C and D) Control experiments in which the cells were maintained in GDP instead of in GTP when calcium was added. Shown are quantifications (mean ± SEM, three independent experiments) of GTP-bound Rab27 (C; we counted 201 cells loaded with GDP and 192 cells loaded with GDP/calcium) and GTP-bound Rab3 (D; we counted 206 cells loaded with GDP and 205 cells loaded with GDP/calcium). (E) SLO-permeabilized spermatozoa were loaded with 7 nM anti-Rab27 antibodies for 8 min at 37 °C to block the signaling pathway in which Rab27 is required. The AR was subsequently initiated by adding 0.5 mM CaCl2. After an 8-min incubation at 37 °C to allow exocytosis to proceed to the Rab27-sensitive step, sperm were treated with 67 nM GST-RIM-RBD and incubated for an additional 8 min at 37 °C. Finally, we added 14 nM recombinant GST-Rab27A (unprenylated and not loaded with nucleotides) to rescue the anti-Rab27 antibody block and incubated as before (black bar). (F) Same as E, except that the reversible pair was 70 nM anti-Rab3A mouse monoclonal antibody rescued by 140 nM GST-Rab3A and that 140 nM GST-Slac2-b substituted for GST-RIM-RBD. We ran several controls in parallel (gray bars), including background AR in the absence of any stimulation (control), AR stimulated by 0.5 mM CaCl2 (calcium), lack of effect of 140/14 nM recombinant Rab3A/27A alone (Rab3A/27A), inhibition by 70/7 nM anti-Rab3A/27 antibodies (anti-Rab3A/27→calcium) and by 67 nM GST-RIM-RBD or 140 nM GST-Slac2-b (RIM/Slac2-b→calcium), rescue of the anti-Rab3A/27 antibodies by their cognate Rab3A/27A proteins (anti-Rab3A/27→calcium→Rab3A/27), and the inhibitory effect of the cassettes when present throughout the experiment (anti-Rab3A/27→Slac2-b/RIM→calcium→Rab3A/27). Sperm were fixed, and AR was measured by FITC-PSA binding as described in Materials and Methods. The data represent mean ± SEM of at least three independent experiments.
To rule out the possibility that calcium might influence GST-Slac2-b/RIM binding to the acrosomal region per se (in a Rab/GTP-independent manner), we conducted an additional control experiment in which cells were offered calcium but no GTP. The numbers of cells decorated with the Slac2-b cassette were indistinguishable regardless of whether we loaded cells with GDP or with GDP/calcium (Fig. 4C, black bars). Likewise, the active Rab3 probe labeled the same number of cells in the acrosomal region in both conditions (Fig. 4D, black bars). The percentage of acrosome-reacted sperm was low in all conditions (Fig. 4 C and D, gray bars). Taken together, our data suggest that sperm contain Rab27-GEF and Rab3-GEF activities that are stimulated in response to the AR inducer calcium.
To determine whether the two secretory Rabs act sequentially to achieve the AR, we ran experiments similar to those described above but replacing calcium with exogenous, persistently activated Rabs. The number of cells decorated with Slac2-b in the acrosomal region was similar in cells treated with GDP and cells treated with recombinant Rab3A-GTP-γ-S plus GTP (Fig. 4A). In contrast, the addition of recombinant Rab27A-GTP-γ-S to SLO-permeabilized sperm doubled the number of cells displaying Rab3-GTP in the acrosomal region (Fig. 4B). These results indicate that active Rab27 stimulates/recruits—directly or indirectly—a Rab3-GEF in human sperm. More importantly, they suggest that these Rabs act sequentially, rather than in a loop, during the AR.
Having established that Rab27 somehow activates Rab3 in human sperm, we reasoned that endogenous Rab27 must act upstream of Rab3 during the AR cascade put into motion by calcium. To test this hypothesis, we needed to set up functional assays in which anti-Rab antibodies halted the AR reversibly. Coincubation of anti-Rab antibodies with the corresponding Rabs abolished the inhibitory effect of the former (33) (Fig. S2). To assess the reversibility of the antibody block, we investigated whether recombinant Rabs could rescue exocytosis when added at the end of the sequence of additions, and found that in fact they did (Fig. 4 E and F; anti-Rab → calcium → Rab). We believe that this rescue stems from reversal of the antibodies’ effect rather than from reconstitution of the systems by functional recombinant Rabs, because they are neither prenylated nor loaded with GTP and thus should be inactive by themselves (Fig. 4 E and F). In addition, inert Rab27A (heat-inactivated) as well as the Rab3A mutant V55E, which does not bind the effectors Rabphilin or RIM (50, 51) and is inactive in PC12 cell secretion (52), rescued the corresponding antibody block (Fig. S2 D and E, black bars).
When added after the AR inducer calcium in the presence of anti-Rab27 antibodies (rescued at the end of the incubation by recombinant Rab27A), the Rab3-sequestering cassette RIM-RBD, which does not bind Rab27 (53), prevented the AR (Fig. 4E, black bar). These results indicate that RIM blocks the AR by sequestering Rab3-GTP at a stage downstream of Rab27. In a parallel experiment, we loaded sperm with an anti-Rab3A antibody, challenged with calcium, and offered Slac2-b, which does not bind Rab3 (54), before rescuing the antibody block with recombinant Rab3A. Under these conditions, Slac2-b failed to inhibit exocytosis (Fig. 4F, black bar). These findings suggest that by the time we added Slac2-b, the system had already traversed the step catalyzed by Rab27-GTP. Given that this was achieved in the presence of anti-Rab3 antibodies, we conclude that the action of Rab27 in the AR occurs upstream of that of Rab3.
Rab27 Recruits a Rab3 GEF Activity.
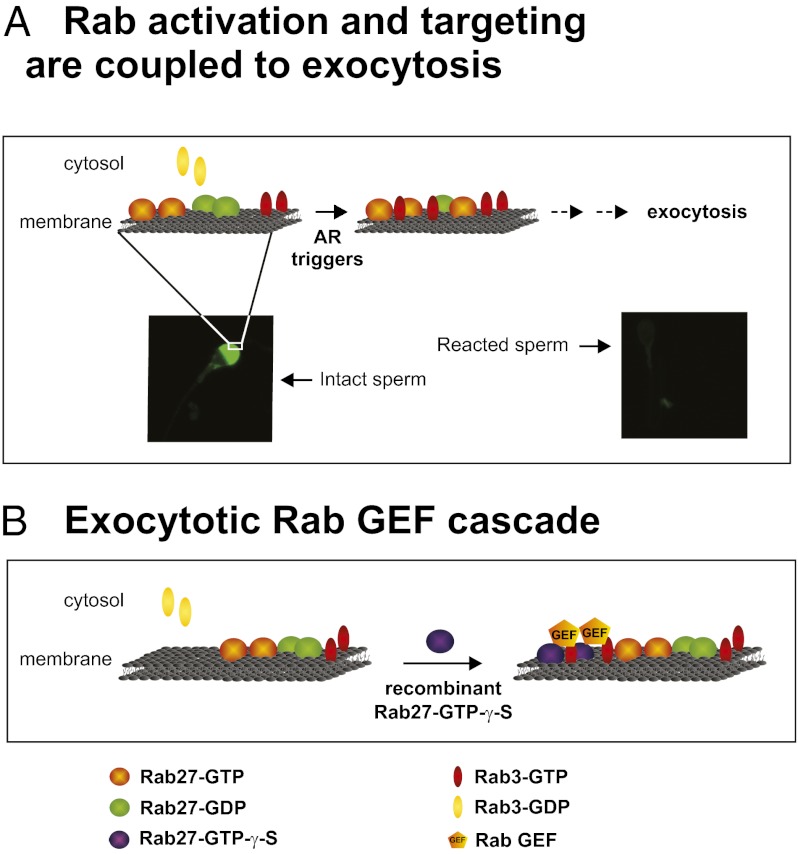
What molecular mechanism drives the Rab27/Rab3 cascade? As mentioned earlier, some Rabs activate later-acting Rabs through GEF cascades. To test whether a similar cascade might be involved in regulated dense-core granule exocytosis, we loaded human sperm extracts on GST-Rab27A-GTP-γ-S immobilized on glutathione-Sepharose beads. After washing, we incubated the beads with recombinant His6-Rab3A-GDP in the presence of GTP (a schematic of the protocol is provided in Fig. 5A). To maximize the probability of finding detectable levels of active Rab3A, we conducted these experiments with Rab3A Q81L, which has a 3.2-fold higher koff (GDP) value and an eightfold lower koff (GTP) value than WT. In addition, this mutant has undetectably low basal GTPase activity, and that stimulated by Rab3A GAP is only 12% of that of WT Rab3A (55). His6-Rab3A Q81L-GTP was pulled down at the end of the assay by GST-RIM-RBD (Fig. 5B, Top). The GDP for GTP exchange on recombinant Rab3A was catalyzed by the Rab3 GEF activity present in human sperm extracts; the enzyme was retained on the Rab27A column. In contrast to the high levels of Rab3A activated by the GEF immobilized by Rab27, little active Rab3A was detected in the negative control (immobilized GST). These background levels were likely due to spontaneous (nonenzymatic) exchange, given that we used a mutant that is GTPase-deficient and does not release GTP easily. These results support the idea that Rab27 and Rab3 represent a Rab cascade involved in regulated exocytosis. Because Rab27 recruits a Rab3 GEF, our findings establish an example of a Rab-GEF cascade.
Fig. 5.
Rab27 recruits a Rab3 GEF. (A) Schematic of the protocol. Beads represent glutathione-Sepharose; blue spheres, geranylgeranylated GST-Rab27A-GTP-γ-S; orange pentagons, Rab3 GEF isolated from human sperm extracts; red sphere, His6-Rab3A-GDP; green sphere, His6-Rab3A-GTP. (B) GST and GST-Rab27A-GTP-γ-S were immobilized on glutathione-Sepharose (i; anti-GST blot shows equal amounts of immobilized proteins), incubated with human sperm extracts (ii; anti–α-tubulin blot of the unbound fraction shows equal loading), and washed as described in Materials and Methods. The GEF activity from human sperm retained by immobilized Rab27 was assessed by incubating the beads with His6-Rab3A-GDP. After centrifugation, His6-Rab3A-GTP was pulled down from the supernatants with the GST-RIM-RBD cassette (iii; anti-GST blot shows equal amounts of immobilized cassette) and probed with the anti-Rab3A monoclonal antibody. Mr standards (× 103) are indicated on the left. Shown is an experiment representative of two repetitions.
Discussion
A central challenge in the field of reproductive biology is to understand how sperm regulate their secretion machinery to release their acrosomal contents only when they encounter the egg. At the most reductionist level, the first step in addressing this challenge is to delineate the cellular and molecular processes by which sperm mount an exocytotic response at the appropriate time. Our laboratory has contributed to the understanding of the molecular mechanisms that drive human sperm exocytosis by identifying and characterizing the role and point of action of a number of components of the fusion machinery (reviewed in refs. 24 and 25). Here we focus on the role of two secretory Rabs, Rab3A and Rab27, in the release of the sperm single dense-core secretory granule. Over the last several years, we have published several papers that demonstrate the positive role of Rab3A in sperm exocytosis (32, 33); in this paper, we describe the presence of Rab27 in sperm and its role in the AR. Rab3A and Rab27A are phylogenetically similar (54); Rab27A and Rab27B have only moderate homology to other human Rab sequences, with the greatest homology (41–44%) to members of the Rab3 subfamily (40). Rab27A and Rab27B share 66% identity at the nucleotide level, and 71% identity and 81% similarity at the amino acid sequence level. Rab27A is expressed in many mouse and human tissues and tumor cell lines, whereas Rab27B has a more limited expression pattern. Rab27B is present in human and mouse testis (19, 40, 56). Probing of whole human sperm extracts with the anti-Rab27 antibody on Western blot analysis revealed a single protein band corresponding to Rab27 (Fig. 1). Because Rab27A and Rab27B have very similar electrophoretic mobility, and because the anti-Rab27 antibody that we used is supposed to recognize both isoforms, we cannot distinguish which of the two is present in sperm. By introducing specific anti-Rab antibodies that sequester the endogenous proteins or recombinant cassettes that selectively bind the active forms into SLO-permeabilized human sperm, we found that both Rab3A (32, 35) (Fig. 4 and Fig. S2) and Rab27 (Figs. 2 and 4 and Fig. S2) are necessary for regulated exocytosis. In short, our findings reinforce the idea that Rab3A plays a positive role in sperm secretion and point to a similar positive role for Rab27.
Do these secretory Rabs cycle constantly, as described for Rab families that drive constitutive membrane traffic? We mined the literature for data on the coupling of subcellular localization and activation status of secretory Rabs to exocytosis stimuli in an attempt to unveil a unifying picture, but failed to find such a synthesis. We trust that the results reported here using the AR as a model system provide insight into these issues, given that we compared both Rab3 and Rab27 targeting to membranes and their activation status in resting and stimulated human sperm. Our findings can be summarized as follows: (i) The levels of membrane-associated Rab27 were similar in resting and stimulated sperm (Fig. 3A); (ii) in contrast to Rab27, the levels of membrane-bound Rab3A in untreated cells were lower than those in sperm treated with AR inducers (Fig. 3A); and (iii) challenging sperm to undergo exocytosis increased the proportion of GTP-bound Rabs (Fig. 3 C and D). In other words, inducing the AR activated both Rabs and targeted Rab3 to sperm membranes, whereas Rab27 was mainly membrane-bound under all conditions. Our findings pose the fundamental but as-yet unanswered question of how Rab27, which is already predominantly attached to membranes and in large proportion bound to GTP in resting sperm, relays extracellular signals to the intracellular fusion cascade to achieve exocytosis only when cells are stimulated. Like all exocytosis, that of the acrosome is a multistage process comprising the opening of ion (mainly calcium) channels, activation of multiple signaling pathways, orchestration of numerous protein–protein interactions to put the fusion machinery in place, and other steps. Morphologically, this process relies on the swelling of the dense-core granule and dramatic remodeling of the acrosomal membrane that lead to the latter contacting the plasma membrane at the points of eventual fusion. It would appear that the mere presence of active and membrane-bound endogenous Rab27 is not sufficient to accomplish exocytosis by itself.
How are the actions of Rab3A and Rab27 coordinated to accomplish the AR? Are they part of a single signal transduction pathway or separate pathways? In an attempt to answer these questions, we introduced the Rab-GTP–binding domains of RIM and Slac2-b into SLO-permeabilized sperm, and found that these Rabs act sequentially to accomplish the secretion of the sperm dense-core granule, with Rab27 regulating the earliest step (Fig. 4 E and F). It has been suggested that Rab3 and Rab27 exhibit opposite effects (Rab3 inhibitory and Rab27 stimulatory) on insulin exocytosis, based on results from overexpression of constitutively active mutants of these two GTPases (see ref. 57 for a review). However, a study conducted in PC12 cells demonstrated the need for caution when interpreting data derived from overexpression experiments; transfection with high levels of Rab3A or Rab27A plasmids was found to substantially inhibit evoked exocytosis, an effect not observed when transfecting with smaller amounts of DNA (18). Thus, we stress the importance of our findings, which were obtained by scrutinizing the roles of the endogenous Rabs and working with primary cultures instead of immortalized cell lines.
We have developed an indirect immunofluorescence assay to determine the subcellular localization of GTP-bound Rabs. We are excited about the potential applications of this assay. It is important to bear in mind that localization of active Rabs is otherwise impossible to ascertain, because pull-down assays report the activation status of these proteins, but not the location in the cell where they are activated or how many cells respond to the stimuli. The only attempt to address the issue of subcellular localization of active Rabs reported in the literature deals with the overexpression of GTPase-deficient mutants, which entails the inherent problem of overexpression itself, compounded by the inability of these mutants to cycle as the endogenous small G proteins do. Using our assay, we were able to detect the presence of active Rabs in the acrosomal region of human sperm, a localization expected of proteins relevant for exocytosis. We found an increasing percentage of cells expressing acrosomal Rab3-GTP and Rab27-GTP after challenging sperm with calcium to initiate the AR (Fig. 4 A and B). These findings were expected, because they lend support to our model indicating that the onset of the AR relies on the activation of Rab proteins, and also demonstrate the robustness of our assay in detecting this activation. We are currently designing experiments aimed at determining whether calcium stimulates Rab-GEFs and/or inhibits Rab-GAPs. Currently, we lack information on the molecular identities of these proteins in sperm, except for Rab3-GAPs 1 and 2, which were detected in a murine haploid germ cell proteome (58). Finally, the assay described in this paper allowed us to obtain one of the most relevant findings that we report here: an increasing number of cells depicting endogenous GTP-bound Rab3 in the acrosomal region in response to the introduction of recombinant geranylgeranylated and persistently active Rab27A into SLO-permeabilized human sperm (Fig. 4B). These results reinforce the idea that Rab27 acts upstream of Rab3A during the exocytotic cascade, leading to acrosomal release. The literature contains several examples of how small GTPases form networks to coordinate transport pathways, such as GAP cascades, Rab GEF cascades, and positive feedback loops (48). We show here that the molecular mechanism that governs the activation of Rab3 by Rab27 acts through a Rab GEF cascade, in which active Rab27A recruits, directly or indirectly, a Rab3A GEF activity (Fig. 5B). This report directly demonstrates the existence of sequential roles for these exocytotic Rabs, establishing an example of a Rab GEF cascade in regulated secretion.
Materials and Methods
Human Sperm Sample Preparation.
Human semen samples were obtained from normal healthy donors. Semen was allowed to liquefy for 30–60 min at 37 °C. After a swim-up protocol to isolate highly motile cells, sperm concentrations were adjusted to 7 × 106/mL before incubation for at least 2 h under capacitating conditions [Human Tubal Fluid media (as formulated by Irvine Scientific) supplemented with 0.5% BSA (HTF medium) HTF, 37 °C, 5% (vol/vol) CO2/95% (vol/vol) air]. Sperm were washed twice with PBS and resuspended in cold PBS containing 2.1 U/mL SLO for 15 min at 4 °C. Cells were washed once with PBS resuspended in ice-cold sucrose buffer (250 mM sucrose, 0.5 mM EGTA, 20 mM Hepes-K; pH 7) containing 2 mM DTT. For AR assays, inhibitors and stimulants were added sequentially, with incubation for 8–15 min at 37 °C after each addition. When indicated, SLO-permeabilized sperm was preloaded with photosensitive NP-EGTA-AM before incubation in the presence of inhibitors and/or calcium, with all procedures performed in the dark. Photolysis was induced after the last incubation by two exposures (1 min each) to a UV transilluminator (FBTIV-614; Fisher Scientific), and exocytosis resumed by mixing, and incubation for 5 min at 37 °C. Sperm were spotted on Teflon-printed slides, air-dried, and fixed/permeabilized in ice-cold methanol for 20 s. An alternative fixation protocol (described in Indirect Immunofluorescence) produced identical results. Acrosomal status was evaluated by staining with FITC-coupled Pisum sativum agglutinin (FITC-PSA, 25 μg/mL in PBS) for 40 min at room temperature, followed by a 20-min wash in water (59). We scored at least 200 cells per condition using an upright Nikon Optiphot II microscope equipped with epifluorescence optics. Basal (“control,” no stimulation) and positive (“calcium,” 0.5 mM CaCl2, corresponding to 10 μM free calcium estimated by MAXCHELATOR, a series of programs for determining the free metal concentration in the presence of chelators; available at http://maxchelator.stanford.edu/) controls were included in all experiments. Acrosomal exocytosis indices were calculated by subtracting the number of spontaneously reacted spermatozoa (basal control without stimulation, ranging from ∼8% to 20% before normalization) from all values and expressing the results as a percentage of the AR observed in the positive control (ranging from ∼18% to 40% before normalization; assigned 100% of responsive cells for normalization). Our analysis included only results derived from experiments that produced similar responses and in which the difference between basal and calcium-stimulated conditions was of at least 8–10 percentage points. Samples with more than 20% of spontaneously reacted sperm were excluded from our analysis. Data were evaluated using the Tukey–Kramer post hoc test for pairwise comparisons. Differences were considered significant at the P < 0.05 level.
Indirect Immunofluorescence.
Between 2 and 3.5 × 105 capacitated sperm were attached to poly-l-lysine–coated (stock 0.1%, diluted 1:20 in water; Sigma-Aldrich), 12-mm round coverslips by incubating for 30 min at room temperature in a moisturized chamber. The cells were then fixed in 2% (wt/vol) paraformaldehyde in PBS for 15 min at room temperature. The fixative was neutralized by overnight incubation at 4 °C in PBS containing 100 mM glycine. Next, the cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature and washed three times with PBS containing 0.1% polyvinylpyrrolidone (PBS/PVP; average MW = 40,000). Nonspecific staining was blocked by incubation in 5% (wt/vol) BSA in PBS/PVP for 1 h at 37 °C. Anti-Rab27 antibodies were diluted at 4 μg/mL in 3% (wt/vol) BSA in PBS/PVP, added to the coverslips, and incubated for 1 h at 37 °C in a moisturized chamber. After two washes for 10 min with 2% (wt/vol) saponin in PBS, Cy3-conjugated donkey anti-rabbit IgG (2.5 μg/mL in 1% BSA in PBS/PVP) was added, and the mixture was incubated for 1 h at room temperature while protected from light. Coverslips were washed six times for 6 min with PBS/PVP. Cells were subsequently stained for acrosomal contents as described earlier, but without air-drying, mounted with 1% propyl-gallate/50% (vol/vol) glycerol in PBS containing 2 μM Hoechst 33342, and stored at −20 °C in the dark until examination with an Eclipse TE2000 Nikon microscope equipped with a Plan Apo 40×/1.40 oil objective and a Hamamatsu digital C4742-95 camera operated with MetaMorph 6.1 software (Universal Imaging). Background was subtracted, and brightness/contrast were adjusted to render an all-or-nothing labeling pattern using ImageJ freeware.
In Vitro Geranylgeranylation of Recombinant Rab3A and Rab27A.
Recombinant His6-R-Rab3A and His6-Rab27A (Figs. 3D and 4 A and B) were prenylated in a solid-phase assay developed in our laboratory before being eluted from Ni-NTA-agarose beads. In brief, 10–20 μM Rabs immobilized on Ni-NTA-agarose were incubated in a geranylgeranylation mixture comprising 80 μM geranylgeranyl pyrophosphate, 200 μM GDP, 1 mM DTT, 3 mM MgCl2, 1 mg/mL BSA, 1 mg/mL bovine brain cytosol as a source of Rab geranylgeranyl transferase and Rab escort protein, a protease inhibitor mixture (Sigma-Aldrich P2714), and 20 mM Hepes-K (pH 7) for 2 h at 37 °C. After this incubation, we added four volumes of the same washing buffer (300 mM NaCl, 10 mM imidazole, 20 mM TrisHCl; pH 7.4) used during the purification of His6-fused proteins and incubated the mixture for 30 min at 4 °C. (This step favors the recapture of prenylated Rabs dissociated from the resin.) Beads were washed three times with His6-washing buffer and geranylgeranylated Rabs eluted with His6-elution buffer (300 mM NaCl, 400 mM imidazole, 20 mM TrisHCl; pH 7.4). GST-Rab27A was isoprenylated following a similar protocol except that it was immobilized on glutathione-Sepharose, and the buffers were those used in the purification of GST-fusion proteins (Fig. 5). Geranylgeranylation was confirmed by Triton X-114 partitioning. When indicated, various versions of Rab3A and Rab27A were loaded for 1 h at 37 °C with 0.125 mM of the appropriate guanosine nucleotide in a buffer containing 5 mM MgCl2, 0.03% Igepal or Nonidet-P40, 1 mM DTT, 25 mM EDTA, and 50 mM Hepes/KOH (pH 7.2). Rabs were separated from free nucleotides by gel filtration in Sephadex G25 minicolumns equilibrated in PBS containing 1% BSA added 1 mM MgCl2, and stored at −20 °C until use. Recombinant protein concentrations were determined by the Bradford method (Bio-Rad) using BSA as a standard in 96-well microplates, and quantified on a BioRad 3550 Microplate Reader or from the intensities of the bands in Coomassie blue-stained SDS/PAGE gels.
Detection of Nucleotide-Binding Status of Endogenous Rab3 and Rab27.
Capacitated, SLO-permeabilized sperm were incubated with 100 μM 2-APB for 10 min at 37 °C. Then the endogenous nucleotides were released by incubating sperm in 5 mM EDTA (which increases the off rate of bound nucleotides; ref. 60)/1 mM MgCl2 for 10 min at 37 °C. Cells were loaded with 40 μM GDP-β-S or GTP-γ-S for 10 min at 37 °C, and bound nucleotides were stabilized with 15 mM MgCl2 for 5 min at 37 °C (Fig. S3). A similar protocol was applied for the experiments depicted in Fig. 4 A and B and Fig. 3 C and D, except that cells were loaded with 40 μM GDP before the addition of high MgCl2 concentrations. After this step, aliquots were incubated with or without 200 μM GTP with and without 0.5 mM CaCl2 or 300 nM geranylgeranylated His6-Rab3A or His6-Rab27A loaded with GTP-γ-S. After a 15-min incubation at 37 °C, cell suspensions were fixed in 2% (wt/vol) paraformaldehyde, neutralized with 100 mM glycine, attached to poly-l-lysine–coated coverslips, and stored overnight at 4 °C in a moisturized chamber. Sperm membranes were permeabilized in 0.1% Triton X-100 in PBS for 10 min at room temperature, cells were washed three times for 6 min each with PBS/PVP, and nonspecific reactivity was blocked in 5% (wt/vol) BSA in PBS/PVP for 1 h at 37 °C. Slides were overlaid with 140 nM GST, GST-RIM-RBD, or GST-Slac2-b in 3% (wt/vol) BSA in PBS/PVP for 1 h at 37 °C. After three 6-min washes in PBS/PVP, the protocol proceeded as described in Indirect Immunofluorescence, except with anti-GST antibodies (31.5 μg/mL; 210 nM) substituted for anti-Rab27. The presence of immunostaining in the acrosomal region was scored by manually counting between 100 and 200 cells either directly in the fluorescence microscope view or in digital images from at least 10 fields. Data were evaluated using the Tukey–Kramer post hoc test for pairwise comparisons.
GST Pull-Down Assay.
Capacitated sperm (20–50 × 106 cells) were treated for 10 min at 37 °C with 100 μM 2-APB loaded (Fig. 3D) or not (Fig. 3C) with 10 nM (0.25 ng) His6-R-Rab3A, and finally challenged with 10 μM A23187 (Fig. 3C) or 50 μM 8pCPT (Fig. 3D) for 15 min at 37 °C. Cells were lysed in GST pull-down buffer [200 mM NaCl, 2.5 mM MgCl2, 1% (vol/vol) Igepal, 10% (vol/vol) glycerol, 1 mM PMSF plus the protease inhibitor mixture, and 50 mM TrisHCl; pH 7.4] by sonication on ice (two times for 15 s each). Proteins were allowed to diffuse into the lysis buffer for 15 min at 4 °C. These whole-cell detergent extracts were clarified by centrifugation at 14,000 × g for 5 min and used immediately.
Glutathione-Sepharose beads were washed twice with GST pull-down buffer and incubated with bacterial lysates containing GST-Slac2-b or GST-RIM-RBD for 1 h at 4 °C under constant rocking. Beads were washed twice with PBS and once with GST pull-down buffer and used immediately. Twenty μL of glutathione-Sepharose containing 5–10 μg of the appropriate fusion protein was added to sperm lysates in a total volume of 1 mL, and incubated by rotation at 4 °C for 30 min. The resin was recovered by centrifugation at 4 °C (2 min at 950 × g) and washed three times with ice-cold GST pull-down buffer. The resin-bound fractions were boiled in sample buffer and resolved by SDS/PAGE, and cellular Rab27-GTP and Rab3A-GTP levels were analyzed by immunoblotting as described below.
Recruitment of Human Sperm Rab3 GEF Activity by Immobilized Rab27.
Human sperm extracts were prepared as described in SI Materials and Methods, but with Triton X-100 substituted for Triton X-114. Nonspecific binding sites on glutathione-Sepharose beads were blocked by incubating three times with two volumes each of 0.1% BSA in 20 mM Hepes (pH 7.4). Then 2.5 μg of GST or geranylgeranylated GST-Rab27A-GTP-γ-S was incubated for 1 h at 4 °C with constant rotation with 20 μL of glutathione-Sepharose beads in binding buffer [20 mM Hepes (pH 7.4), 150 mM KAcO, 15 mM MgCl2, 0.05% Tween 20, 5 μM GTP, and protease inhibitors] in a final volume of 200 μL. Beads containing immobilized recombinant proteins were washed twice with 200 μL of binding buffer and incubated for 30 min at 4 °C under constant rotation with 0.5 mL of sperm extract (50 × 106 cells). Unbound sperm proteins were precipitated with CCl3H-CH3OH-H2O and dissolved in sample buffer (anti–α-tubulin Western blot; Fig. 5B). The beads containing the immobilized recombinant proteins plus any sperm proteins retained by them were used as the enzyme source in the subsequent Rab3A GEF assay. In brief, beads were washed twice with 0.5 mL of binding buffer and incubated for 10 min at 30 °C with 8 nM geranylgeranylated His6-Rab3A-GDP in 0.5 mL of binding buffer to promote the exchange of GDP for GTP. After centrifugation at 3,000 × g for 2 min at 4 °C, His6-Rab3A-GTP was pulled down from the supernatants with the GST-RIM-RBD cassette as described above.
SDS/PAGE and Western Blot Analysis.
Proteins were separated on Tris-tricine-SDS gels (61) or Tris-glycine-SDS gels (62) and transferred to 0.22-μm nitrocellulose membranes (Hybond; GE Healthcare). Nonspecific reactivity was blocked by incubation for 1 h at room temperature with 5% (wt/vol) skim milk dissolved in washing buffer [PBS (pH 7.6) and 0.2% Tween 20]. Blots were incubated with the anti-Rab27 (0.2 μg/mL), anti-Rab3A rabbit polyclonal (0.1 μg/mL; to detect endogenous Rab3A), anti-Rab3A mouse monoclonal (0.2 μg/mL; to detect recombinant Rab3A), or anti–α-tubulin (0.2 μg/mL) antibodies in blocking solution for 1 h at room temperature. Anti-GST antibodies were diluted to 40 ng/mL in washing buffer and incubated as for the other primary antibodies. HRP-conjugated goat anti-mouse IgG or goat anti-rabbit IgG were used as secondary antibodies (0.1 μg/mL in washing buffer) with 1-h incubations. Excess first and second antibodies were removed by washing five times for 10 min each in washing buffer. Detection was performed with a Millipore chemiluminescence system and a Fujifilm LAS-4000 Luminescent Image Analyzer. Quantification of signal intensities was performed with ImageJ.
Supplementary Material
Acknowledgments
We thank M. Furlán, N. Domizio, and A. Medero for excellent technical assistance; Drs. C. López, D. Munafó, R. Regazzi, R. Shirakawa, and P. Stahl for plasmids; Dr. S. Patterson for critical reading of the manuscript; and S. Laurito and Dr. F. Rodriguez for helpful suggestions. This work was supported by grants from the Argentinian Consejo Nacional de Investigaciones Científicas y Técnicas, Agencia Nacional de Promoción Cientifica y Tecnológica, and Secretaría de Ciencia y Técnica-Universidad Nacional de Cuyo (to C.N.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Author Summary on page 11910 (volume 109, number 30).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121173109/-/DCSupplemental.
References
- 1.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 2.Lang T, Jahn R. Core proteins of the secretory machinery. Handb Exp Pharmacol. 2008;184:107–127. doi: 10.1007/978-3-540-74805-2_5. [DOI] [PubMed] [Google Scholar]
- 3.Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci. 2011;12:127–138. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- 4.Südhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol. 2011;3:a005637. doi: 10.1101/cshperspect.a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laulagnier K, et al. Role of AP1 and Gadkin in the traffic of secretory endo-lysosomes. Mol Biol Cell. 2011;22:2068–2082. doi: 10.1091/mbc.E11-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- 7.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dulubova I, et al. A Munc13/RIM/Rab3 tripartite complex: From priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolmachova T, et al. A general role for Rab27a in secretory cells. Mol Biol Cell. 2004;15:332–344. doi: 10.1091/mbc.E03-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham ME, et al. A gain-of-function mutant of Munc18-1 stimulates secretory granule recruitment and exocytosis and reveals a direct interaction of Munc18-1 with Rab3. Biochem J. 2008;409:407–416. doi: 10.1042/BJ20071094. [DOI] [PubMed] [Google Scholar]
- 15.Pavlos NJ, et al. Quantitative analysis of synaptic vesicle Rabs uncovers distinct yet overlapping roles for Rab3a and Rab27b in Ca2+-triggered exocytosis. J Neurosci. 2010;30:13441–13453. doi: 10.1523/JNEUROSCI.0907-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuboi T, Fukuda M. The C2B domain of rabphilin directly interacts with SNAP-25 and regulates the docking step of dense core vesicle exocytosis in PC12 cells. J Biol Chem. 2005;280:39253–39259. doi: 10.1074/jbc.M507173200. [DOI] [PubMed] [Google Scholar]
- 17.Tsuboi T, Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J Cell Sci. 2006;119:2196–2203. doi: 10.1242/jcs.02962. [DOI] [PubMed] [Google Scholar]
- 18.Handley MT, Haynes LP, Burgoyne RD. Differential dynamics of Rab3A and Rab27A on secretory granules. J Cell Sci. 2007;120:973–984. doi: 10.1242/jcs.03406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi Z, et al. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol. 2002;22:1858–1867. doi: 10.1128/MCB.22.6.1858-1867.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrins MJ, Stuenkel EL. Kinetics of Rab27a-dependent actions on vesicle docking and priming in pancreatic beta-cells. J Physiol. 2008;586:5367–5381. doi: 10.1113/jphysiol.2008.158477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasai K, Fujita T, Gomi H, Izumi T. Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic. 2008;9:1191–1203. doi: 10.1111/j.1600-0854.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 22.Pavlos NJ, Jahn R. Distinct yet overlapping roles of Rab GTPases on synaptic vesicles. Small GTPases. 2011;2:77–81. doi: 10.4161/sgtp.2.2.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Florman HM, Ducibella T. In: Knobil and Neill's Physiology of Reproduction. Neill JD, editor. San Diego: Elsevier Academic; 2005. pp. 55–112. [Google Scholar]
- 24.Mayorga LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life. 2007;59:286–292. doi: 10.1080/15216540701222872. [DOI] [PubMed] [Google Scholar]
- 25.Tomes CN. Molecular mechanisms of membrane fusion during acrosomal exocytosis. Soc Reprod Fertil Suppl. 2007;65:275–291. [PubMed] [Google Scholar]
- 26.Tomes CN. In: Molecular Mechanisms of Exocytosis. Regazzi R, editor. New York: Landes Bioscience, Austin, TX and Springer; 2006. pp. 117–147. [Google Scholar]
- 27.Zitranski N, et al. The “acrosomal synapse”: Subcellular organization by lipid rafts and scaffolding proteins exhibits high similarities in neurons and mammalian spermatozoa. Commun Integr Biol. 2010;3:513–521. doi: 10.4161/cib.3.6.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yunes R, Michaut M, Tomes C, Mayorga LS. Rab3A triggers the acrosome reaction in permeabilized human spermatozoa. Biol Reprod. 2000;62:1084–1089. doi: 10.1095/biolreprod62.4.1084. [DOI] [PubMed] [Google Scholar]
- 29.Iida H, Yoshinaga Y, Tanaka S, Toshimori K, Mori T. Identification of Rab3A GTPase as an acrosome-associated small GTP-binding protein in rat sperm. Dev Biol. 1999;211:144–155. doi: 10.1006/dbio.1999.9302. [DOI] [PubMed] [Google Scholar]
- 30.Ward CR, Faundes D, Foster JA. The monomeric GTP binding protein, rab3a, is associated with the acrosome in mouse sperm. Mol Reprod Dev. 1999;53:413–421. doi: 10.1002/(SICI)1098-2795(199908)53:4<413::AID-MRD7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 31.Belmonte SA, et al. Cholesterol content regulates acrosomal exocytosis by enhancing Rab3A plasma membrane association. Dev Biol. 2005;285:393–408. doi: 10.1016/j.ydbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 32.De Blas GA, Roggero CM, Tomes CN, Mayorga LS. Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol. 2005;3:e323. doi: 10.1371/journal.pbio.0030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Branham MT, Mayorga LS, Tomes CN. Calcium-induced acrosomal exocytosis requires cAMP acting through a protein kinase A-independent, Epac-mediated pathway. J Biol Chem. 2006;281:8656–8666. doi: 10.1074/jbc.M508854200. [DOI] [PubMed] [Google Scholar]
- 34.Suhaiman L, et al. Sphingosine 1-phosphate and sphingosine kinase are involved in a novel signaling pathway leading to acrosomal exocytosis. J Biol Chem. 2010;285:16302–16314. doi: 10.1074/jbc.M109.072439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Branham MT, et al. Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. J Biol Chem. 2009;284:24825–24839. doi: 10.1074/jbc.M109.015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ackermann F, et al. The Multi-PDZ domain protein MUPP1 as a lipid raft-associated scaffolding protein controlling the acrosome reaction in mammalian spermatozoa. J Cell Physiol. 2008;214:757–768. doi: 10.1002/jcp.21272. [DOI] [PubMed] [Google Scholar]
- 37.Ackermann F, et al. CaMKIIalpha interacts with multi-PDZ domain protein MUPP1 in spermatozoa and prevents spontaneous acrosomal exocytosis. J Cell Sci. 2009;122:4547–4557. doi: 10.1242/jcs.058263. [DOI] [PubMed] [Google Scholar]
- 38.Hutt DM, Baltz JM, Ngsee JK. Synaptotagmin VI and VIII and syntaxin 2 are essential for the mouse sperm acrosome reaction. J Biol Chem. 2005;280:20197–20203. doi: 10.1074/jbc.M412920200. [DOI] [PubMed] [Google Scholar]
- 39.Hu XQ, et al. Acrosome formation-associated factor is involved in fertilization. Fertil Steril. 2010;93:1482–1492. doi: 10.1016/j.fertnstert.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Guo J, Miki T, Tachibana M, Gahl WA. Molecular cloning and characterization of rab27a and rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem Mol Med. 1997;60:27–37. doi: 10.1006/bmme.1996.2559. [DOI] [PubMed] [Google Scholar]
- 41.De Blas G, et al. The intraacrosomal calcium pool plays a direct role in acrosomal exocytosis. J Biol Chem. 2002;277:49326–49331. doi: 10.1074/jbc.M208587200. [DOI] [PubMed] [Google Scholar]
- 42.Herrick SB, et al. The acrosomal vesicle of mouse sperm is a calcium store. J Cell Physiol. 2005;202:663–671. doi: 10.1002/jcp.20172. [DOI] [PubMed] [Google Scholar]
- 43.Costello S, et al. Ca2+-stores in sperm: Their identities and functions. Reproduction. 2009;138:425–437. doi: 10.1530/REP-09-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darszon A, et al. Calcium channels and Ca2+ fluctuations in sperm physiology. Int Rev Cytol. 2005;243:79–172. doi: 10.1016/S0074-7696(05)43002-8. [DOI] [PubMed] [Google Scholar]
- 45.Lopez CI, Belmonte SA, De Blas GA, Mayorga LS. Membrane-permeant Rab3A triggers acrosomal exocytosis in living human sperm. FASEB J. 2007;21:4121–4130. doi: 10.1096/fj.06-7716com. [DOI] [PubMed] [Google Scholar]
- 46.Kondo H, et al. Constitutive GDP/GTP exchange and secretion-dependent GTP hydrolysis activity for Rab27 in platelets. J Biol Chem. 2006;281:28657–28665. doi: 10.1074/jbc.M603227200. [DOI] [PubMed] [Google Scholar]
- 47.Michaut M, Tomes CN, De Blas G, Yunes R, Mayorga LS. Calcium-triggered acrosomal exocytosis in human spermatozoa requires the coordinated activation of Rab3A and N-ethylmaleimide-sensitive factor. Proc Natl Acad Sci USA. 2000;97:9996–10001. doi: 10.1073/pnas.180206197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–659. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nottingham RM, Pfeffer SR. Defining the boundaries: Rab GEFs and GAPs. Proc Natl Acad Sci USA. 2009;106:14185–14186. doi: 10.1073/pnas.0907725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKiernan CJ, Brondyk WH, Macara IG. The Rab3A GTPase interacts with multiple factors through the same effector domain: Mutational analysis of cross-linking of Rab3A to a putative target protein. J Biol Chem. 1993;268:24449–24452. [PubMed] [Google Scholar]
- 51.Coppola T, et al. Disruption of Rab3-calmodulin interaction, but not other effector interactions, prevents Rab3 inhibition of exocytosis. EMBO J. 1999;18:5885–5891. doi: 10.1093/emboj/18.21.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johannes L, et al. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994;13:2029–2037. doi: 10.1002/j.1460-2075.1994.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2: Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J Biol Chem. 2003;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- 54.Kuroda TS, Fukuda M, Ariga H, Mikoshiba K. The Slp homology domain of synaptotagmin-like proteins 1-4 and Slac2 functions as a novel Rab27A binding domain. J Biol Chem. 2002;277:9212–9218. doi: 10.1074/jbc.M112414200. [DOI] [PubMed] [Google Scholar]
- 55.Brondyk WH, McKiernan CJ, Burstein ES, Macara IG. Mutants of Rab3A analogous to oncogenic Ras mutants: Sensitivity to Rab3A-GTPase activating protein and Rab3A-guanine nucleotide releasing factor. J Biol Chem. 1993;268:9410–9415. [PubMed] [Google Scholar]
- 56.Zhao S, Torii S, Yokota-Hashimoto H, Takeuchi T, Izumi T. Involvement of Rab27b in the regulated secretion of pituitary hormones. Endocrinology. 2002;143:1817–1824. doi: 10.1210/endo.143.5.8823. [DOI] [PubMed] [Google Scholar]
- 57.Waselle L, et al. Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis. Mol Biol Cell. 2003;14:4103–4113. doi: 10.1091/mbc.E03-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo X, et al. Proteomic analysis of proteins involved in spermiogenesis in mouse. J Proteome Res. 2010;9:1246–1256. doi: 10.1021/pr900735k. [DOI] [PubMed] [Google Scholar]
- 59.Mendoza C, Carreras A, Moos J, Tesarik J. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J Reprod Fertil. 1992;95:755–763. doi: 10.1530/jrf.0.0950755. [DOI] [PubMed] [Google Scholar]
- 60.Burstein ES, Macara IG. Interactions of the ras-like protein p25rab3A with Mg2+ and guanine nucleotides. Biochem J. 1992;282:387–392. doi: 10.1042/bj2820387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 62.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]