Abstract
Cellular senescence is widely believed to play a key role in tumor suppression, but the molecular pathways that regulate senescence are only incompletely understood. By using a secretome proteomics approach, we identified insulin-like growth factor binding protein 3 (IGFBP3) as a secreted mediator of breast cancer senescence upon chemotherapeutic drug treatment. The senescence-inducing activity of IGFBP3 is inhibited by tissue-type plasminogen activator-mediated proteolysis, which is counteracted by plasminogen activator inhibitor 1 (PAI-1), another secreted mediator of senescence. We demonstrate that IGFBP3 is a critical downstream target of PAI-1-induced senescence. These results suggest a role for an extracellular cascade of secreted proteins in the regulation of cellular senescence.
Keywords: chemotherapy, isotope-coded affinity tag, protease, protease inhibitor, protein secretion
Cellular senescence was originally described as irreversible proliferation arrest that limits the proliferation of human primary cells in culture (1). This phenomenon, “replicative senescence,” is caused by progressive shortening of telomeres. In addition, a variety of stressful or oncogenic stimuli can elicit a senescence response. These include DNA damage, oxidative stress, and expression of certain oncogenes such as ras and raf (2, 3). Accumulating evidence suggests that cellular senescence plays important roles in tumor suppression and organismal aging, but how senescence is regulated is largely unknown.
In addition to irreversible arrest of proliferation, senescent cells often display common phenotypes such as enlarged, flattened morphology, increased lysosome biogenesis, decreased protein synthesis and degradation, senescence-associated β-gal (SA β-gal) activity, and increased expression of cell cycle inhibitors (2, 3). Further, recent studies have begun to uncover profound alterations in protein secretion from senescent cells, which is collectively called the senescence-associated secretory phenotype (SASP) (4, 5) or senescence-messaging secretome (SMS) (6). These include increased secretion of inflammatory cytokines such as interleukins and chemokines, proteases, and growth regulators. These SASP or SMS factors may recruit immune cells for clearance of senescent cells, affect the architecture or function of surrounding tissues, modulate tumor progression, and contribute to aging and age-related diseases.
Most cancer cells express telomerase and do not undergo replicative senescence. However, cancer cells from a variety of tissues undergo senescence upon treatment with chemotherapeutic drugs or ionizing radiation (7, 8). Senescent cancer cells are characterized by proliferation arrest, flat and enlarged morphology, and SA β-gal activity, which is similar to the senescence phenotype of primary cells. A previous report demonstrated that naive MCF-7 breast cancer cells undergo senescence upon exposure to conditioned medium from senescent MCF-7 cells induced to senesce by treatment with doxorubicin, a widely used chemotherapeutic drug for breast cancer (9). This observation suggested the presence of secreted mediator(s) of senescence in the conditioned medium of senescent MCF-7 cells, which may contribute to the potent anticancer activity of doxorubicin. By using quantitative secretome proteomics, we identified insulin-like growth factor (IGF) binding protein 3 (IGFBP3) as a mediator of doxorubicin-induced senescence of MCF-7 cells. Extracellular IGFBP3 levels increased upon various senescence-inducing stimuli, and IGFBP3 induced senescence in several different cell types. We demonstrate that tissue-type plasminogen activator (t-PA)–plasminogen activator inhibitor-1 (PAI-1) system regulates the senescence-inducing activity of IGFBP3.
Results and Discussion
Increased Extracellular IGFBP3 upon Breast Cancer Senescence.
As reported previously (9), conditioned medium from doxorubicin-treated, senescent MCF-7 breast cancer cells inhibited the proliferation of naive MCF-7 cells (Fig. 1A) and induced senescence phenotype in these cells (Fig. 1B). We sought to identify the factor(s) mediating this “senescence bystander effect” by using a quantitative secretome proteomics approach. MCF-7 cells were induced to senesce by doxorubicin treatment, and the proteins secreted in the conditioned medium were identified and quantified (in comparison with nonsenescent MCF-7 cells) by isotope-coded affinity tag (ICAT) technology (10, 11). At the protein prophet probability score of 0.9 or higher (corresponding to a false identification rate of 0.8%), 1,020 proteins were identified and quantified. The list of proteins displaying more than twofold abundance changes (up or down) is shown in Datasets S1 and S2. After initial exploratory experiments and literature searches, we decided to focus on IGFBP3, which displayed 4.49-fold increased levels in senescent MCF-7–conditioned medium.
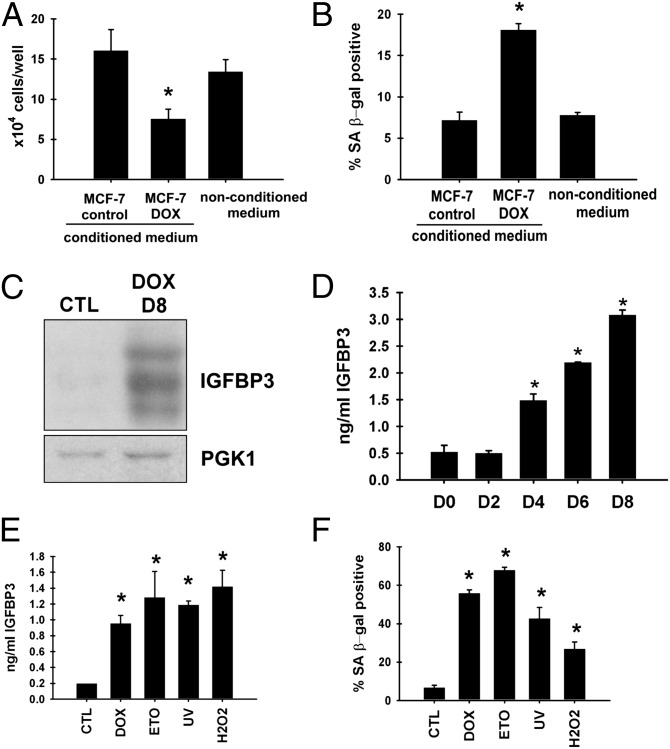
Fig. 1.
Increased extracellular IGFBP3 upon MCF-7 cell senescence. (A) Senescent MCF-7 conditioned medium inhibits cell proliferation. Naive MCF-7 cells were plated at 5 × 104 cells per well and treated with the conditioned medium from doxorubicin-treated, senescent MCF-7, or control MCF-7 cells or with nonconditioned medium for 3 d, and the cell numbers were determined (*P < 0.05 vs. control or nonconditioned medium). (B) Senescent MCF-7–conditioned medium induces senescence. SA β-gal staining was performed after 3-d treatment with each medium (*P < 0.05 vs. control or nonconditioned medium). (C) Increased extracellular IGFBP3 upon doxorubicin-induced senescence. Eight days after doxorubicin treatment of MCF-7 cells, conditioned medium was collected and concentrated. Conditioned medium from untreated cells was also included as control. Ten micrograms of each protein sample was analyzed for IGFBP3 levels by immunoblotting. Secreted PGK1 serves as a loading control. (D) Time course of IGFBP3 induction upon doxorubicin-induced senescence. On the indicated days after doxorubicin treatment of MCF-7 cells, extracellular IGFBP3 levels were determined by ELISA (*P < 0.05 vs. untreated cells; D0). (E) Increased extracellular IGFBP3 upon different stresses. MCF-7 cells were treated with 1 μM doxorubicin (DOX) for 2 h, 20 μM etoposide (ETO) for 48 h, 500 μM H2O2 for 2 h, or 2 J/m2 UV light. Four days after initiating each treatment, extracellular IGFBP3 levels were determined by ELISA (*P < 0.05 vs. control; CTL). (F) Senescence induction by different stresses. MCF-7 cells were treated as in E, and, 4 d later, cells were stained for SA β-gal (*P < 0.05 vs. control; CTL).
IGFBP3 is a major IGF-binding protein in blood and also functions as a cytokine acting in an autocrine and paracrine fashion (12, 13). The latter function of IGFBP3 is at least partly IGF-independent. IGFBP3 expression and secretion were shown to increase upon replicative senescence of primary fibroblasts (14, 15) or in response to DNA damage and hypoxia (16). We have confirmed the increased extracellular IGFBP3 levels upon doxorubicin-induced senescence of MCF-7 cells (Fig. 1 C and D). Although IGFBP3 was identified as a p53 target gene (17), IGFBP3 mRNA levels did not change upon doxorubicin treatment (see Fig. 5A), which suggested a posttranscriptional mechanism for IGFBP3 induction. Increased extracellular IGFBP3 levels were also observed upon different senescence-inducing stresses such as etoposide, UV, and hydrogen peroxide (Fig. 1 E and F).
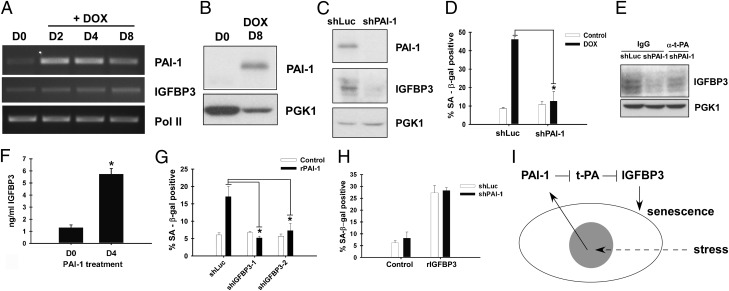
Fig. 5.
PAI-1–IGFBP3 cascade mediates senescence. (A) Induction of PAI-1 mRNA expression upon doxorubicin treatment. MCF-7 cells were treated with 1 μM doxorubicin for 2 h. On the indicated days after initiating the doxorubicin treatment, total RNA was isolated. Total RNA from untreated cells (D0) was also included as control. RT-PCR analysis was performed for mRNA levels of PAI-1, IGFBP3, and RNA polymerase II (Pol II; loading control). (B) Increased extracellular PAI-1 upon doxorubicin-induced senescence. Eight days after doxorubicin treatment of MCF-7 cells, the abundance of PAI-1 in the conditioned medium was examined by anti–PAI-1 immunoblotting. (C) PAI-1 knockdown results in concomitant decrease in IGFBP3 levels. MCF-7 cells were infected with lentiviruses expressing shRNA against luciferase or PAI-1 and treated with 1 μM doxorubicin. Eight days after doxorubicin treatment, the levels of PAI-1 and IGFBP3 in the conditioned medium were determined by immunoblotting. (D) PAI-1 knockdown inhibits doxorubicin-induced senescence. MCF-7 cells expressing shRNA against PAI-1 or luciferase were treated with 1 μM doxorubicin, and, 8 d later, stained for SA β-gal. (E) Anti–t-PA antibody alleviates IGFBP3 reduction upon PAI-1 knockdown. MCF-7 cells were infected with lentiviruses expressing shRNA against luciferase or PAI-1. The cells were treated with 1 μM doxorubicin and then cultured in the presence of 1 μg/mL anti-t-PA antibody (sc-5239; Santa Cruz Biotechnology) or IgG for 7 d. The levels of IGFBP3 and PGK1 in the conditioned medium were determined by immunoblotting. (F) PAI-1 increases extracellular IGFBP3 levels. MCF-7 cells were treated with 2.4 μg/mL of recombinant PAI-1 for 4 d, and the levels of extracellular IGFBP3 were determined by ELISA. (G) IGFBP3 knockdown abrogates PAI-1–induced senescence. MCF-7 cells were infected with lentiviruses expressing shRNAs against IGFBP3 or luciferase, treated with recombinant PAI-1 for 4 d, and stained for SA β-gal. (H) PAI-1 knockdown does not affect IGFBP3-induced senescence. MCF-7 cells were infected with lentiviruses expressing shRNA against luciferase or PAI-1. The cells were treated with 0.5 μg/mL recombinant IGFBP3 for 4 d or left untreated, and were stained for SA β-gal. (I) Role of PAI-1–t-PA–IGFBP3 cascade in stress-induced senescence.
IGFBP3 Mediates Senescence.
We then assessed the role of IGFBP3 in senescence induction. Lentiviral IGFBP3 expression in MCF-7 cells caused striking proliferation arrest as determined by the lack of BrdU incorporation and Ki-67 staining (Fig. 2 A and B), which was accompanied by the induction of SA β-gal activity (Fig. 2C). Further, addition of purified recombinant IGFBP3 protein to the culture medium of MCF-7 cells resulted in dose-dependent induction of SA β-gal activity (Fig. 2D), increased G1 and decreased S and G2/M fractions (Fig. S1A), reduced BrdU incorporation (Fig. S1B), and reduced mitotic phospho-histone H3 staining (Fig. S1C). Lentiviral IGFBP3 expression in IMR-90 human primary fibroblasts also resulted in abrogation of Ki-67 staining (Fig. S2A), induction of SA β-gal activity (Fig. S2B), and formation of senescence-associated heterochromatic foci (Fig. S2 C–E). Exogenous IGFBP3 was also shown to induce senescence in human primary vascular endothelial cells (18). These results suggest that IGFBP3 is sufficient for induction of senescence. We note that the concentration of recombinant IGFBP3 necessary to induce senescence phenotype (Fig. 2D) is higher than the IGFBP3 levels observed by ELISA upon doxorubicin-induced senescence (Fig. 1D). This disparity could be explained by the possibility that the effective concentration of autocrine and paracrine IGFBP3 to which the cells are exposed is likely higher than the concentration of IGFBP3 after diffusion in the culture medium, which is measured by ELISA. It is also possible that the recombinant IGFBP3 we used is not as potent as endogenously secreted IGFBP3 in inducing senescence.
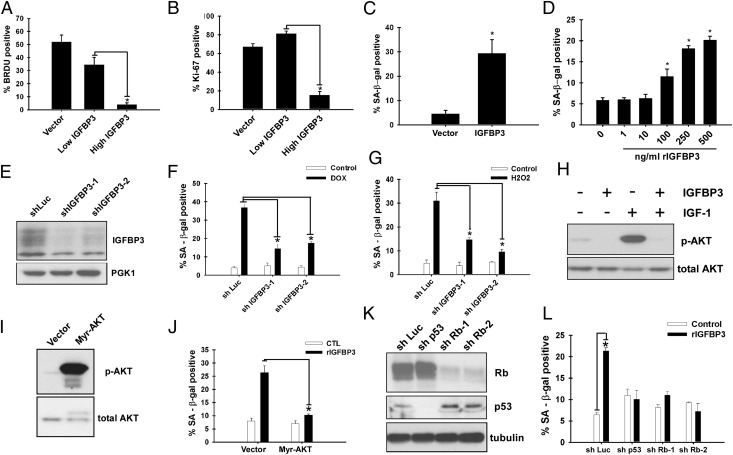
Fig. 2.
IGFBP3 mediates MCF-7 cell senescence. (A) IGFBP3 inhibits BrdU incorporation. MCF-7 cells were infected with empty lentiviral vector or IGFBP3-expressing lentivirus and were selected with 2 μg/mL puromycin. Four days after infection, the cells were labeled with BrdU for 24 h and stained with antibodies against BrdU and IGFBP3. Immunofluorescence microscopy was used to detect the cells displaying no or low IGFBP3 expression (low IGFBP3) and those displaying high IGFBP3 expression (high IGFBP3), and the percentage of BrdU-positive cells was scored (*P < 0.05). (B) IGFBP3 abrogates Ki-67 staining. MCF-7 cells were treated as in A, and the cells were stained for Ki-67 and IGFBP3 at 4 d after infection (*P < 0.05). (C) SA β-gal induction by lentiviral IGFBP3 expression. MCF-7 cells were infected with empty lentiviral vector or IGFBP3-expressing lentivirus and were stained for SA β-gal 4 d after infection (*P < 0.05 vs. vector). (D) SA β-gal induction by recombinant IGFBP3. MCF-7 cells were treated with the indicated concentration of IGFBP3 for 4 d and were stained for SA β-gal (*P < 0.05 vs. untreated control). (E) shRNA-mediated knockdown of IGFBP3. MCF-7 cells were infected with lentiviruses expressing shRNA against luciferase or IGFBP3. The levels of extracellular IGFBP3 were determined by immunoblotting. (F) IGFBP3 knockdown alleviates doxorubicin-induced senescence. The cells in E were treated with 1 μM doxorubicin, and, 8 d later, stained for SA β-gal. (G) IGFBP3 knockdown alleviates hydrogen peroxide-induced senescence. The cells in E were treated with 500 μM H2O2, and, 4 d later, stained for SA β-gal. (H) Effect of IGFBP3 on AKT phosphorylation. IGFBP3 and IGF1 were incubated together at equal molar ratios (0.5 μg/mL IGFBP3 and 0.12 μg/mL IGF-I) or alone for 30 min before addition to MCF-7 cells. The cells were treated overnight with IGFBP3 and/or IGF1. Phospho-AKT (Ser437) and total AKT levels were assessed by immunoblotting. (I) Expression of constitutively active AKT in MCF-7 cells. MCF-7 cells were infected with lentiviruses expressing constitutively active AKT (myr-AKT) or empty vector, and the levels of phospho-AKT and total AKT were analyzed by immunoblotting. (J) Constitutively active AKT abrogates IGFBP3-induced senescence. The cells in I were treated with IGFBP3 for 4 d and were stained for SA β-gal. (K) shRNA-mediated knockdown of p53 and Rb. MCF-7 cells were infected with lentiviruses expressing shRNA against luciferase (control), p53, or Rb. The levels of Rb and p53 were determined by immunoblotting. (L) Knockdown of p53 or Rb abrogates IGFBP3-induced senescence. The cells in K were treated with IGFBP3 for 4 d and were stained for SA β-gal.
Next, we examined the role of endogenous IGFBP3 in doxorubicin-induced senescence by shRNA-mediated knockdown. As shown in Fig. 2E, extracellular IGFBP3 was efficiently silenced by shRNAs, which also alleviated doxorubicin-induced senescence of MCF-7 cells (Fig. 2F), suggesting that IGFBP3 mediates doxorubicin-induced senescence in these cells. Doxorubicin treatment also increased the extracellular IGFBP3 levels of ZR-75-1 breast cancer cells (Fig. S3A) and ARPE-19 retinal pigment epithelial cells (Fig. S3C), and IGFBP3 knockdown alleviated doxorubicin-induced senescence in these cell types (Fig. S3 B and D). Further, oxidative stress by hydrogen peroxide treatment resulted in enhanced extracellular IGFBP3 levels (Fig. 1E) and the induction of senescence (Fig. 1F) in MCF-7 cells, which was diminished by IGFBP3 knockdown (Fig. 2G), suggesting that IGFBP3 also mediates oxidative stress-induced senescence.
IGFBP3 binds to IGFs and can function as an inhibitor of IGF signaling. In accordance with this finding, IGFBP3 suppressed the baseline and IGF-induced levels of phosphorylated AKT (Fig. 2H), which is a downstream target of IGF signaling. We then assessed the role of AKT in IGFBP3-induced senescence by using constitutively active AKT (i.e., myr-AKT). As shown in Fig. 2 I and J, myr-AKT expression in MCF-7 cells resulted in elevation of phosphorylated AKT levels and abrogation of IGFBP3-induced senescence, suggesting that IGFBP3 induces senescence through suppression of AKT activity. The p53 and Rb tumor suppressor pathways play key roles in different types of senescence. Senescence induced by IGFBP3 was abolished by knockdown of p53 or Rb (Fig. 2 K and L), which suggests the requirement of p53 and Rb tumor suppressor pathways for IGFBP3-induced senescence. Taken together, these results suggest that IGFBP3 functions as a secreted mediator of senescence, acting through suppression of AKT and requiring p53 and Rb.
t-PA Proteolyzes IGFBP3 and Inhibits Senescence Induction.
To identify the proteins interacting with IGFBP3 in the extracellular space, we expressed C-terminally FLAG-His–tagged IGFBP3 in 293T cells and isolated tagged IGFBP3 and its interacting proteins from the conditioned medium by nickel agarose purification followed by anti-FLAG immunoprecipitation. The sample was analyzed by mass spectrometry for protein identification. The list of identified proteins is shown in Dataset S3. One of the proteins identified was t-PA, a protease known to proteolyze plasminogen to generate plasmin. This finding raised a possibility that t-PA proteolyzes IGFBP3. We tested this possibility by coexpressing IGFBP3 and t-PA in 293T cells. As shown in Fig. 3A, coexpression of t-PA resulted in proteolysis of IGFBP3, but not SFRP1 (a secreted Wnt antagonist; control). Interestingly, urokinase [or urokinase-type plasminogen activator (u-PA)], a paralogue of t-PA that also proteolyzes plasminogen to plasmin, could not proteolyze IGFBP3 (Fig. 3A). Proteolysis of IGFBP3 by t-PA was also demonstrated by in vitro proteolysis assays using recombinant t-PA (Fig. 3B). Purified u-PA was incapable of proteolyzing IGFBP3 in vitro (Fig. 3B). A previous in vitro proteolysis study showed that t-PA and u-PA can proteolyze IGFBP3 (19), which is in partial disagreement with our in vitro and coexpression analyses. The reason for this discrepancy is not clear.
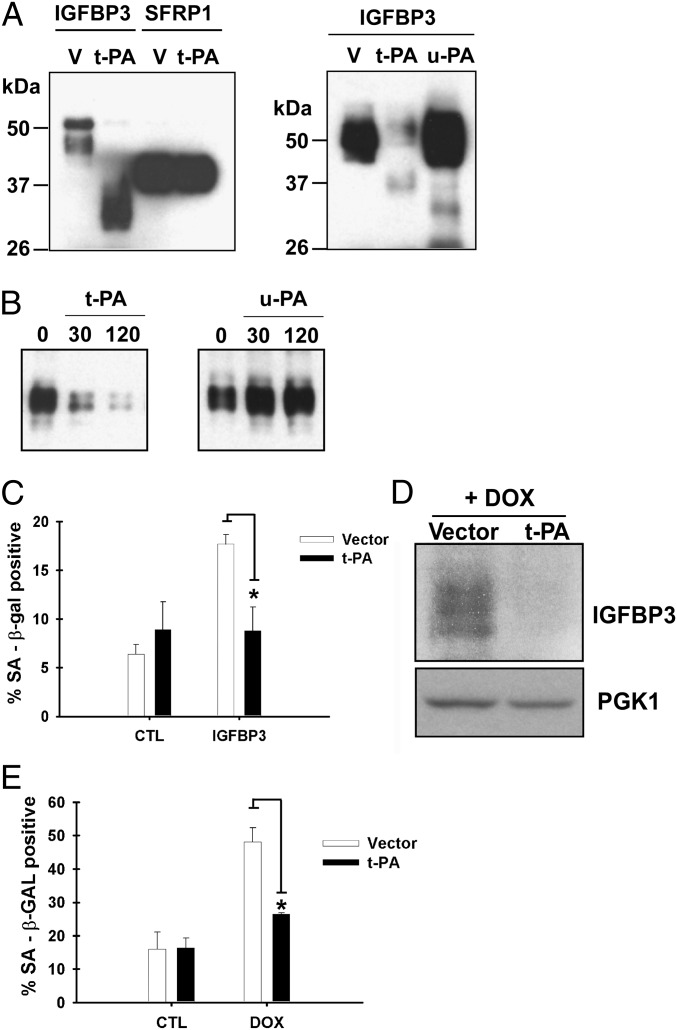
Fig. 3.
t-PA proteolyzes IGFBP3 and inhibits senescence induction. (A) IGFBP3 proteolysis by t-PA in the conditioned medium. (Left) 293T cells were transfected with IGFBP3 or SFRP1 (both C-terminally FLAG-His-tagged) together with t-PA or vector (“V”) as indicated. Secreted IGFBP3 or SFRP1 was purified from the conditioned medium by nickel agarose chromatography followed by anti-FLAG immunoprecipitation, and was detected by anti-FLAG immunoblotting. (Right) The effect of t-PA and u-PA on IGFBP3-FLAG-His was examined by cotransfection in 293T cells as described. (B) IGFBP3 proteolysis by t-PA in vitro. Purified C-terminally FLAG-His-tagged IGFBP3 was incubated with recombinant t-PA or u-PA (10 ng each) for 30 or 120 min at 37 °C, and detected by anti-FLAG immunoblotting. (C) t-PA abrogates IGFBP3-induced senescence. MCF-7 cells were infected with lentiviruses expressing t-PA or empty vector, treated with IGFBP3 for 4 d, and stained for SA β-gal. (D) t-PA reduces extracellular IGFBP3 levels upon doxorubicin treatment. MCF-7 cells were infected with lentiviruses expressing t-PA or empty vector and treated with 1 μM doxorubicin. Four days after doxorubicin treatment, the extracellular IGFBP3 levels were examined by anti-IGFBP3 immunoblotting. (E) t-PA alleviates doxorubicin-induced senescence. MCF-7 cells were infected with lentiviruses expressing t-PA or empty vector, treated with 1 μM doxorubicin, and, 8 d later, stained for SA β-gal.
We next analyzed the effect of t-PA on IGFBP3-mediated senescence. As shown in Fig. 3C, t-PA abolished IGFBP3-induced senescence of MCF-7 cells. Furthermore, t-PA reduced the extracellular IGFBP3 levels upon doxorubicin treatment (Fig. 3D) and abolished doxorubicin-induced senescence of MCF-7 cells (Fig. 3E), which is mediated by IGFBP3 (Fig. 2F). These results suggest that IGFBP3 proteolysis by t-PA resulted in the inactivation of senescence-inducing activity of IGFBP3.
PAI-1 Prevents IGFBP3 Proteolysis by t-PA and Induces Senescence.
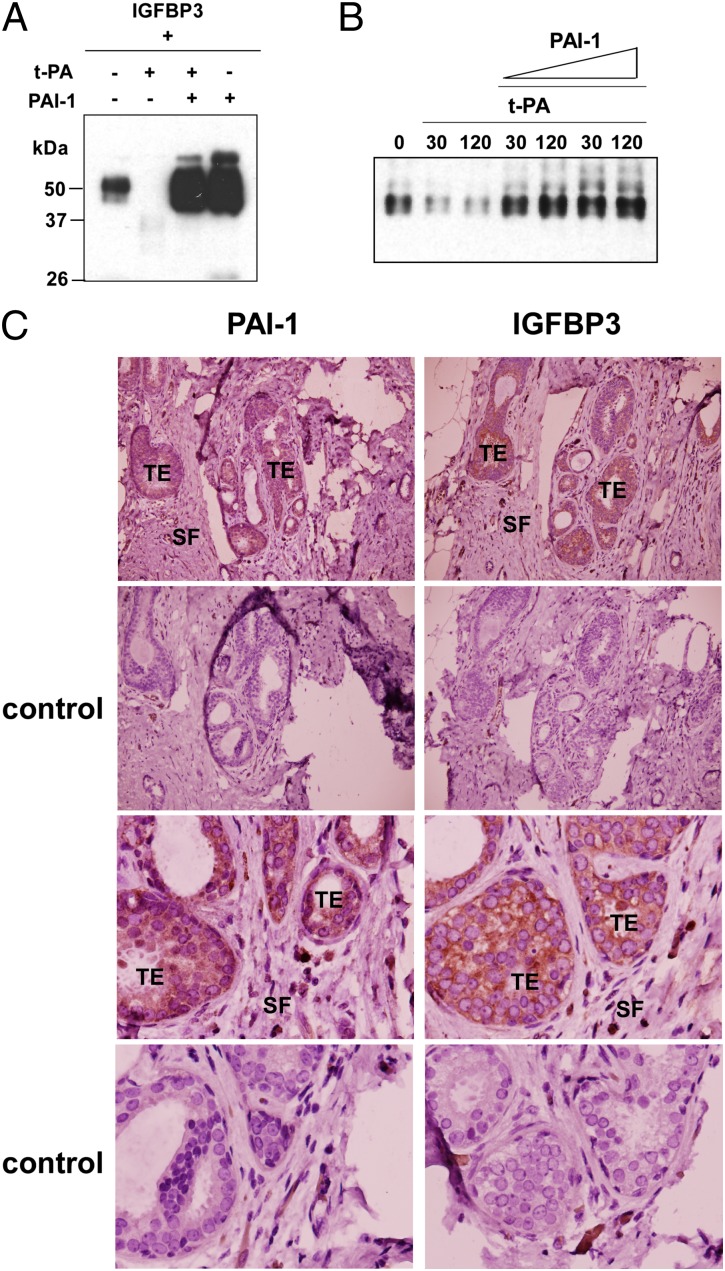
The protease activity of t-PA and u-PA is negatively regulated by a secreted inhibitor, PAI-1. As shown Fig. 4A, IGFBP3 proteolysis by t-PA in 293T cell-conditioned medium was abolished by coexpression of PAI-1. Coexpression of PAI-1 alone resulted in significant elevation of IGFBP3 levels (Fig. 4A, lane 4), which may be a result of inactivation of endogenously secreted t-PA by PAI-1. Purified recombinant PAI-1 was also able to inhibit IGFBP3 proteolysis by recombinant t-PA in vitro (Fig. 4B). These results suggest that PAI-1 protects IGFBP3 from proteolysis by t-PA. We also examined the expression of PAI-1 and IGFBP3 in human breast cancer and surrounding tissues by immunohistochemistry staining of consecutive tissue sections. We observed that, in 11 of 14 breast cancer cases analyzed, tumor epithelial cells stained positive for PAI-1 and IGFBP3 whereas stromal fibroblasts were negative (staining of five tumor cases shown in Fig. 4C and Fig. S4), suggesting a correlation between PAI-1 and IGFBP3 expression in vivo.
Fig. 4.
PAI-1 prevents IGFBP3 proteolysis by t-PA. (A) PAI-1 prevents IGFBP3 proteolysis in the conditioned medium. 293T cells were transfected with IGFBP3-FLAG-His together with t-PA or PAI-1 as indicated. Secreted IGFBP3-FLAG-His was detected by anti-FLAG immunoblotting as in Fig. 3A. (B) PAI-1 protects IGFBP3 from proteolysis by t-PA. Purified IGFBP3-FLAG-His was incubated with recombinant t-PA (10 ng) with or without recombinant PAI-1 (10 or 100 ng) for 30 or 120 min at 37 °C, and was detected by anti-FLAG immunoblotting. (C) Immunohistochemical analysis of PAI-1 and IGFBP3 in human breast carcinoma and surrounding tissues. Representative consecutive paraffin sections (case 8701) stained for PAI-1 (brown) or IGFBP3 (brown). The upper four images show lower magnification and the lower four show higher magnification. Control, negative control staining without primary antibody; SF, stromal fibroblasts; TE, tumor epithelial cells.
PAI-1 is a transcriptional target of p53 (20) and is known to be induced upon DNA damage (21). Consistent with this finding, doxorubicin treatment of MCF-7 cells dramatically increased PAI-1 mRNA levels (Fig. 5A) and extracellular PAI-1 protein levels (Fig. 5B). We note that mature PAI-1 lacks cysteines, which likely explains why PAI-1 was not detected in our ICAT proteomic analysis (Dataset S1). PAI-1 expression was also induced by other senescence-inducing stresses such as etoposide, hydrogen peroxide, and UV light (Fig. S5), suggesting a general role for PAI-1 in stress response. We then tested the effect of PAI-1 shRNA knockdown on extracellular IGFBP3 levels and senescence induction upon doxorubicin treatment of MCF-7 cells. As shown in Fig. 5 C and D, PAI-1 knockdown resulted in concomitant decrease in extracellular IGFBP3 levels and suppression of senescence induction. These results suggest that doxorubicin-induced DNA damage enhances the secretion of PAI-1, which in turn results in elevated extracellular IGFBP3 and induction of senescence in MCF-7 cells (Fig. 5I). The reduction of IGFBP3 levels upon PAI-1 knockdown was alleviated by the addition of anti–t-PA antibody to the culture medium (Fig. 5E), supporting the role for t-PA in mediating IGFBP3 reduction upon PAI-1 knockdown.
PAI-1 was previously identified as an inducer of senescence in human and mouse fibroblasts (22). We therefore tested whether senescence induced by PAI-1 is mediated by IGFBP3. As shown in Fig. 5 F and G, recombinant PAI-1 increased extracellular IGFBP3 levels and induced senescence in MCF-7 cells, which was abolished by knockdown of IGFBP3. IGFBP3 knockdown also abolished PAI-1–induced senescence in IMR-90 fibroblasts and ARPE-19 cells (Fig. S6). Consistent with IGFBP3 acting downstream of PAI-1, PAI-1 knockdown, which abolished doxorubicin-induced senescence (Fig. 5D), did not affect IGFBP3-induced senescence (Fig. 5H). These results suggest that IGFBP3 is a critical downstream mediator of PAI-1–induced senescence.
The PAI-1–t-PA cascade has a well-established role in the regulation of blood clot lysis through activation of plasminogen. t-PA has also been implicated in other biological processes such as synaptic plasticity and behavioral responses (23). This study identified IGFBP3 as a target of the PAI-1–t-PA cascade and revealed the role of the PAI-1–t-PA–IGFBP3 pathway in the regulation of stress-induced senescence (Fig. 5I). PAI-1 has long been considered as a marker of aging and senescence because its expression increases during organismal aging (24) and in cells undergoing replicative senescence (25, 26). It was shown, however, that PAI-1 is not only a marker of senescence, but also a critical downstream target of p53 in the induction of senescence (22). Ectopic expression of PAI-1 was sufficient to induce senescence in human and mouse fibroblasts (22). Our study extended these observations by identifying IGFBP3 as a critical downstream target of senescence induction by PAI-1. Regulation of senescence by an extracellular cascade of secreted proteins is noteworthy because it would allow non–cell-autonomous modulation of pro- or antisenescent state in a local microenvironment that is likely exposed to the same type and intensity of stresses.
IGFBP3 inhibits the proliferation of a number of cancer cell lines and the IGFBP3 gene is frequently silenced by promoter methylation in cancers of a variety of tissues such as colon, stomach, breast, ovary, and liver (12, 13), which led to the classification of IGFBP3 as a tumor suppressor. Our study identified IGFBP3 as a secreted mediator of stress-induced senescence and raised a possibility that IGFBP3 suppresses tumors by inducing senescence in preneoplastic cells. Extracellular IGFBP3 levels increased upon several different stimuli that induce DNA damage or oxidative insult (Fig. 1E), which are also mutagenic and hence potentially tumor-promoting. Inactivation of IGFBP3 by promoter methylation may allow preneoplastic cells to evade stress-induced senescence and accumulate further mutations to develop into a full-blown tumor. In addition, our results also implicate IGFBP3 in the response of established tumors to chemotherapy. A potent antitumor activity of doxorubicin was in part attributed to the senescence bystander effect (9), and our results suggest that this effect is mediated by IGFBP3. By spreading senescence to tumor cells that escaped the direct impact of chemotherapy, IGFBP3 may improve the treatment efficacy and prognosis. It is now worth considering IGFBP3 as a new type of cancer therapy that inhibits cancer proliferation by inducing senescence.
Materials and Methods
Cell Culture.
MCF-7 and ZR-75-1 cells were cultured in minimum essential medium supplemented with 10% (vol/vol) FCS and non-essential amino acids. IMR-90 fibroblasts were cultured in DMEM supplemented with 10% (vol/vol) FCS. ARPE-19 cells were cultured in DMEM/F-12 1:1 supplemented with 10% (vol/vol) FCS. 293T cells were cultured in DMEM supplemented with 10% (vol/vol) calf serum. Calcium phosphate coprecipitation was used for plasmid DNA transfection. The cells infected with lentiviruses were selected with 2 μg/mL puromycin for 48 h. Recombinant IGFBP3 was from R&D Systems. Recombinant His-PAI-1 was expressed and purified from Escherichia coli. Doxorubicin, hydrogen peroxide, and BrdU were from Sigma-Aldrich. Etoposide was from Calbiochem/EMD Biosciences. The target sequences for shRNAs are as follows: human IGFBP3 shRNA-1, GCACAGATACCCAGAACTT; IGFBP3 shRNA-2, GGGTGTCTGATCCCAAGTT; luciferase, GCACTCTGATTGACAAATACGATTT; Rb shRNA-1, GGTTGTGTCGAAATTGGATCA; Rb shRNA-2, CAGAGATCGTGTATTGAGATT; p53 shRNA, GACTCCAGTGGTAATCTACT; and PAI-1 shRNA, AAGTGAAGATCGAGGTGAA.
Senescence-associated β-gal assays were conducted as described previously (27).
Immunoblotting, Immunofluorescence, and ELISA.
Unless otherwise noted in the figure legends, 30 μg of whole cell lysate or 10 μg of conditioned medium was separated by SDS/PAGE and analyzed by immunoblotting as described previously (28). Immunofluorescence was performed as described previously (28). IGFBP3 ELISA was performed by using human IGFBP-3 Quantikine ELISA Kit (R&D Systems).
Immunohistochemistry.
Consecutive paraffin sections of invasive breast ductal carcinoma and surrounding tissues from patients who did not receive chemotherapy were stained for PAI-1 (mouse monoclonal anti–PAI-1; 3785; American Diagnostica) or IGFBP3 (mouse monoclonal anti-IGFBP3; MAB305; R&D Systems) by using VECTASTAIN ABC kit (Vector Laboratories) and 4plus HRP Universal Detection kit (Biocare Medical), and were counterstained with hematoxylin. Deidentified tumor samples were provided by the Cancer Therapy and Research Center Tumor Bank, and immunohistochemistry was performed by the Pathology Core Laboratory of the University of Texas Health Science Center at San Antonio.
In Vitro Proteolysis Assays.
C-terminally FLAG-His–tagged IGFBP3 purified from transfected 293T cell conditioned medium was incubated with indicated amount of recombinant t-PA (American Diagnostica), u-PA (GenWay Biotech), and PAI-1 (GenWay Biotech) in a reaction buffer (50 mM Tris, pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.01% Tween 20) at 37 °C and was analyzed by anti-FLAG immunoblotting.
RT-PCR.
Total cellular RNA was prepared by using TRIzol reagent (Invitrogen) and RT-PCR was performed as described previously (29). The following PCR primers were used: IGFBP3 5′ primer, TCTGCGTCAACGCTAGTGC, 3′ primer, GCTCTGAGACTCGTAGTCAACT; PAI-1 5′ primer, AGCTCCTTGTACAGATGCCG, 3′ primer, ACAACAGGAGGAGAAACCCA; and RNA polymerase II (Pol II) 5′ primer, GGATGACCTGACTCACAAACTG, 3′ primer, CGCCCAGACTTCTGCATGG.
Statistical Analysis.
WINKS statistical analysis software (Texasoft) was used. Data are expressed as mean ± SD. Statistical significance was determined by ANOVA and a post-hoc Newman–Keuls analysis. P values lower than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Mr. Barron Blackman for assistance with proteomics informatics and Dr. Martin Adamo for critical reading of the manuscript. This work was supported in part by National Institutes of Health Grants AG029587 (to Y.S.), CA125020 (to Y.S.), CA137568 (to Y.S.), and CA054174 (Cancer Therapy and Research Center at University of Texas Health Science Center at San Antonio, Mass Spectrometry Shared Resource and Pathology Core Laboratory).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120437109/-/DCSupplemental.
References
- 1.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Campisi J, d’Adda di Fagagna F. Cellular senescence: When bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 3.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppé JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 7.Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- 8.Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23:2919–2933. doi: 10.1038/sj.onc.1207518. [DOI] [PubMed] [Google Scholar]
- 9.Di X, et al. A chemotherapy-associated senescence bystander effect in breast cancer cells. Cancer Biol Ther. 2008;7:864–872. doi: 10.4161/cbt.7.6.5861. [DOI] [PubMed] [Google Scholar]
- 10.Gygi SP, et al. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 11.Shiio Y, Aebersold R. Quantitative proteome analysis using isotope-coded affinity tags and mass spectrometry. Nat Protoc. 2006;1:139–145. doi: 10.1038/nprot.2006.22. [DOI] [PubMed] [Google Scholar]
- 12.Jogie-Brahim S, Feldman D, Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr Rev. 2009;30:417–437. doi: 10.1210/er.2008-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada PM, Lee KW. Perspectives in mammalian IGFBP-3 biology: Local vs. systemic action. Am J Physiol Cell Physiol. 2009;296:C954–C976. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
- 14.Murano S, et al. Diverse gene sequences are overexpressed in Werner syndrome fibroblasts undergoing premature replicative senescence. Mol Cell Biol. 1991;11:3905–3914. doi: 10.1128/mcb.11.8.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein S, Moerman EJ, Jones RA, Baxter RC. Insulin-like growth factor binding protein 3 accumulates to high levels in culture medium of senescent and quiescent human fibroblasts. Proc Natl Acad Sci USA. 1991;88:9680–9684. doi: 10.1073/pnas.88.21.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimberg A, et al. p53-Dependent and p53-independent induction of insulin-like growth factor binding protein-3 by deoxyribonucleic acid damage and hypoxia. J Clin Endocrinol Metab. 2005;90:3568–3574. doi: 10.1210/jc.2004-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckbinder L, et al. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 18.Kim KS, et al. Regulation of replicative senescence by insulin-like growth factor-binding protein 3 in human umbilical vein endothelial cells. Aging Cell. 2007;6:535–545. doi: 10.1111/j.1474-9726.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- 19.Bang P, Fielder PJ. Human pregnancy serum contains at least two distinct proteolytic activities with the ability to degrade insulin-like growth factor binding protein-3. Endocrinology. 1997;138:3912–3917. doi: 10.1210/endo.138.9.5371. [DOI] [PubMed] [Google Scholar]
- 20.Kunz C, Pebler S, Otte J, von der Ahe D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res. 1995;23:3710–3717. doi: 10.1093/nar/23.18.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parra M, Jardí M, Koziczak M, Nagamine Y, Muñoz-Cánoves P. p53 Phosphorylation at serine 15 is required for transcriptional induction of the plasminogen activator inhibitor-1 (PAI-1) gene by the alkylating agent N-methyl-N’-nitro-N-nitrosoguanidine. J Biol Chem. 2001;276:36303–36310. doi: 10.1074/jbc.M103735200. [DOI] [PubMed] [Google Scholar]
- 22.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006;50:673–678. doi: 10.1016/j.neuron.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Mari D, Coppola R, Provenzano R. Hemostasis factors and aging. Exp Gerontol. 2008;43:66–73. doi: 10.1016/j.exger.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Mu XC, Higgins PJ. Differential growth state-dependent regulation of plasminogen activator inhibitor type-1 expression in senescent IMR-90 human diploid fibroblasts. J Cell Physiol. 1995;165:647–657. doi: 10.1002/jcp.1041650324. [DOI] [PubMed] [Google Scholar]
- 26.Martens JW, et al. Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb Haemost. 2003;89:393–404. [PubMed] [Google Scholar]
- 27.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiio Y, et al. Quantitative proteomic analysis of Myc oncoprotein function. EMBO J. 2002;21:5088–5096. doi: 10.1093/emboj/cdf525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiio Y, et al. Quantitative proteomic analysis of myc-induced apoptosis: A direct role for Myc induction of the mitochondrial chloride ion channel, mtCLIC/CLIC4. J Biol Chem. 2006;281:2750–2756. doi: 10.1074/jbc.M509349200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.