Abstract
The role of the central neuropeptide pigment-dispersing factor (PDF) in circadian timekeeping in Drosophila is remarkably similar to that of vasoactive intestinal peptide (VIP) in mammals. Like VIP, PDF is expressed outside the circadian network by neurons innervating the gut, but the function and mode of action of this PDF have not been characterized. Here we investigate the visceral roles of PDF by adapting cellular and physiological methods to the study of visceral responses to PDF signaling in wild-type and mutant genetic backgrounds. We find that intestinal PDF acts at a distance on the renal system, where it regulates ureter contractions. We show that PdfR, PDF's established receptor, is expressed by the muscles of the excretory system, and present evidence that PdfR-induced cAMP increases underlie the myotropic effects of PDF. These findings extend the similarities between PDF and VIP beyond their shared central role as circadian regulators, and uncover an unexpected endocrine mode of myotropic action for an intestinal neuropeptide on the renal system.
Keywords: intestine, tubule, enteric, nervous system
The gastrointestinal tract is an important signaling center that, through its production of systemic or neural signals, can have profound effects on other organs (1, 2). In some cases, the nature of these signals and their modes of action are beginning to be elucidated; for example, postprandial release of cholecystochinin hormone from the duodenum has been shown to reduce appetite through its action on the vagus nerve, and intestinal glucagon-like peptide 1 can regulate pancreatic insulin secretion in response to glucose intake (1, 3). However, the contribution of intestinal signals to the maintenance of homeostasis is likely to involve many more molecules and target tissues, as highlighted by our incomplete understanding of the amelioration of diabetes and hypertension resulting from bariatric surgery, or the contribution of deregulated intestinal signaling to conditions such as diabetes and obesity (1, 2).
Several mammalian families of hormones are conserved in insects (4, 5), some of which are present in the digestive tract, where they are produced by endocrine cells of the gut epithelium or by neurons innervating the gut (6, 7). Previous work has revealed myotropic effects for some of these peptides on explanted viscera (5, 7–9). These studies have typically made use of large insects that, although amenable to physiological study, cannot be easily manipulated genetically. This difficulty has precluded further investigation of the specific sites of release and cellular modes of action of these peptides.
Vasoactive intestinal polypeptide (VIP) is a 28-aa peptide hormone produced in many areas of the human body, including the brain and digestive tract (3, 10). VIP signals through two G protein-coupled receptors (GPCRs), VPAC1 and VPAC2, which are also widely expressed in the CNS and the smooth muscles of the digestive and urogenital tracts (11, 12). Genetic experiments have uncovered a broad range of functions for VIP and its receptors, including the regulation of smooth-muscle contraction in the digestive and excretory systems and an important role in circadian timekeeping in the suprachiasmatic nuclei of the hypothalamus (3, 10, 12). This latter function is strikingly similar to that of the 18-aa pigment-dispersing factor (PDF) in the Drosophila brain (13); the loss of PDF in flies and VIP in mice results in the inability to maintain strong, normally paced locomotor rhythms (14, 15), and both peptides mediate communication between neurons of the circadian clock network (16–20). These similarities are molecular as well as functional; VPAC2 and PdfR, the PDF receptor in flies, are both type II secretin-like receptors (21–23). The peripheral roles of Drosophila PDF and its receptor have not been investigated.
Here we investigate the visceral functions of PDF in Drosophila. By revealing a myotropic function for PDF and PdfR in the regulation of renal musculature, we uncover an endocrine mode of action for PDF and identify further similarities between PDF and mammalian VIP beyond the circadian clock. More generally, our findings point to an unexpected endocrine function for a gut-derived peptide in the regulation of renal function.
Results
Central Efferent PDF Neurons Innervate the Intestine of Drosophila Larvae and Adults.
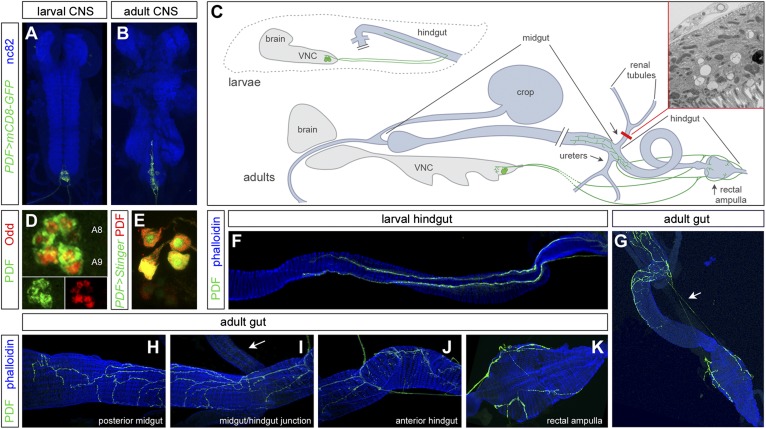
As part of our ongoing characterization of the signals exchanged between the nervous and digestive system in Drosophila (24), we turned our attention to the PDF neurites of the digestive tract, which project from neuronal cell bodies previously described for both the blowfly and Drosophila (7), but whose visceral targets have not been reported for adult Drosophila in detail (25). In addition to the expression of PDF by brain clock neurons, PDF is expressed by neurons in the ventral nerve cord (VNC) (25, 26) that do not express clock genes (7, 20). Expression analyses using a PDF-GAL4 reporter and an antibody against the PDF propeptide confirmed these previous reports, and revealed that the number of PDF+ efferent neurons is variable and age-dependent. Of the six to eight PDF+ neurons in the posterior abdominal segments of the larval VNC (Fig. 1A), four to eight persist in adults (Fig. 1B), wherein two populations of PDF+ neurons are apparent: three to four cell bodies that strongly express PDF, and one to four additional neurons displaying weak PDF expression (Fig. 1E). In young adults (<1 wk old), we find 3.6 ± 0.5 strongly expressing PDF neurons and 2.8± 2.3 weak ones. In older adults (>2 wk old), 3.9 ± 0.4 strongly expressing and only 0.9 ± 1.4 weakly expressing neurons are apparent.
Fig. 1.
The ab-PDF neurons innervate the larval and adult intestine. (A) Six to eight PDF-GAL4+ efferent neurons are apparent in the two posterior-most segments of the larval VNC. (B) Four to eight of these neurons persist in the adult VNC. In A and B, nc82 is used as a general neuropil marker. (C) Summary of the innervation of the larval and adult intestine by the ab-PDF neurons. The CNS is in gray, the digestive tract is displayed in light blue. Only the hindgut is shown in larvae and only half of the cell bodies are displayed. The electron micrograph boxed in red shows a cross-section of the ureters at the level indicated by the red line. A tracheal terminal and a muscle cell are apparent, but no neurites or neuronal cell bodies are present (see Fig. S1 for additional micrographs). (D) The ab-PDF neurons in the larval A8 and A9 posterior segments are Odd+. (E) In the adult VNC, four neurons strongly express both PDF (in red) and PDF-GAL4 (in green, visualized with the nuclear UAS-Stinger) (51). One to four weakly expressing neurons (posterior to the other neurons in this image) are apparent in some CNSs. (F) PDF innervation of the larval hindgut. Two parallel fibers extend along the hindgut muscles. (G–K) PDF innervation of the adult intestine. (G) Connection (arrow) between the rectal ampulla branches and the gut terminals. (H) Innervation of the posterior midgut. (I) Innervation of the midgut/hindgut junction. The ureter (arrow) is not innervated. (J) Innervation of the anterior hindgut. (K) PDF neurites on the rectal ampulla. In F–K, phalloidin is used to visualize the gut muscles. The images in A, B, F, and G were captured using a 10× objective; D, E, and H–K were captured using a 63× objective.
The size and position of the abdominal PDF (ab-PDF) neurons were reminiscent of the gut-innervating MP1 and dMP2 neurons, which express the transcription factor Odd-skipped (27, 28). An anti–Odd-skipped antibody confirmed that the ab-PDF neurons are the MP1 and dMP2 neurons of segments A8 and A9 (Fig. 1D). Analysis of adult ab-PDF axons revealed visceral innervation similar to that of the blowfly (25). Ab-PDF neurons form two nerves that reach the posterior hindgut and extend aborally to orally along the sides of the hindgut in larvae (Fig. 1 C and F). PDF+ varicosities are apparent throughout the length of these fibers. In adults, the two PDF+ nerves form complex arbors that populate the posterior midgut and anterior hindgut (Fig. 1 C and H–J). A subset of PDF neurites branch off from these main fibers to project to the rectum (Fig. 1 C, G, and K). Besides the CNS and digestive tract, no PDF immunoreactivity was apparent in other adult tissues. Absence of innervation of the renal tubules (Fig. 1I) was further confirmed using transmission electron microscopy (Fig. 1C and Fig. S1). Thus, the only visceral site of PDF innervation is the posterior intestine, which is supplied by the central neurosecretory MP1 and dMP2 lineages.
PDF Induces Ureter Contractions but Has No Acute Effects on Intestinal Motility.
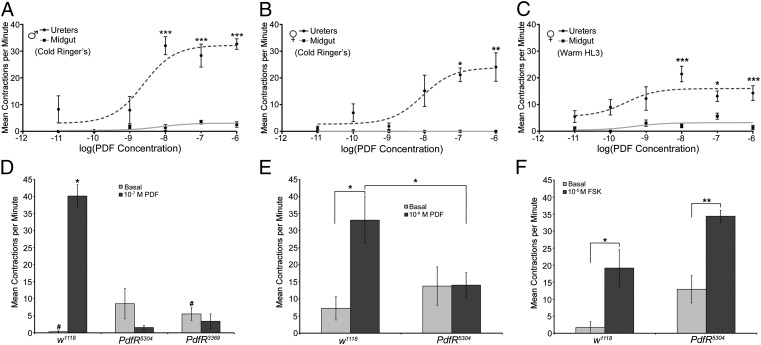
The intestinal innervation of the ab-PDF neurons prompted us to investigate if PDF modulates visceral contractions. We adapted the ex vivo motility assays used in larger insects to quantify contractions in the smaller adult viscera of Drosophila (Fig. S2), and asked if PDF caused changes in visceral contraction. Under our initial experimental conditions, viscera from wild-type Canton S flies displayed low basal contraction rates (Fig. 2D, Fig. S3A, and Movies S1 and S2), and a range of PDF concentrations resulted in no significant changes in midgut or hindgut contractions (Fig. 2 A and B, and Fig. S3A). Unexpectedly, PDF caused dose-dependent increases in ureter contraction rate (Fig. 2 A and B, and Movie S3), a neighboring tissue not directly innervated by PDF neurons (Fig. 1 C and I, and Fig. S1). In Drosophila, each set of renal tubules converge onto a muscular ureter, which controls the flow of urine to the gut (Fig. 1 C and I) (29, 30). For male ureters, PDF concentrations greater than 10−9 M caused contraction rates that were significantly higher than those of vehicle controls (Fig. 2A). For females, PDF concentrations greater than 10−8 M induced contraction rates that were significantly higher than vehicle controls (Fig. 2B). The EC50 of the PDF dose–response curve for ureter contraction rates was 2.406 × 10−9 M PDF for males and 7.214 × 10−9 M PDF for females.
Fig. 2.
PDF induces PdfR-dependent ureter contractions. (A) Dose–response curves for PDF on the contraction rates of male Canton S (wild-type) midguts and ureters dissected in cold Ringer’s with contractions expressed as contractions per minute (cpm ± SEM). Mean basal contraction rates were 3.09 ± 1.72 cpm for the ureters and 0 ± 0 cpm for midguts. Mean contraction rates for vehicle controls were 0.60 ± 0.34 cpm for ureters and 0 ± 0 cpm for midguts. The EC50 was 2.41 × 10−9 M PDF. (B) Dose–response curves for PDF on contraction rates of female Canton S midguts and ureters. Mean basal contraction rates were 2.38 ± 1.43 cpm for the ureters and 0.38 ± 0.28 cpm for midguts. Mean contraction rates for vehicle controls were 4.07 ± 1.82 cpm for ureters and 1.93 ± 1.20 cpm for midguts. The EC50 was 8.72 ± 10−9. (C) Dose–response curves for female wild-type midguts and ureters dissected in warm HL3. Mean basal contraction rates were 2.48 ± 0.58 cpm for the ureters and 0.45 ± 0.13 cpm for midguts. Mean contraction rates for vehicle controls were 2.08 ± 1.66 cpm for ureters and 0 ± 0 cpm for midguts. The EC50 was 2.71 × 10−10 M PDF. (D) Comparison of PDF effects on ureter contraction rates from wild-type (w1118) and PdfR mutant (PdfR5304 and PdfR3369) viscera prepared using cold Ringer’s solution. For wild-type ureters, the addition of 10−7 M PDF caused a significant increase in ureter contraction rate (P < 0.001). No significant increase in contraction rate was seen in either PdfR mutant (PdfR5304: P = 0.4688; PdfR3369: P = 0.4375). PdfR mutants displayed higher basal ureter contraction rates compared with w1118 ureters, but this difference was statistically significant only for PdfR3369 [indicated by pound (#) signs, PdfR5304: P = 0.0623; PdfR3369: P = 0.0497]. (E) The effects of 10−6 M PDF on the ureters of w1118 and PdfR5304 viscera prepared using warm HL3. PDF caused significant increases in the contractions rates of w1118 ureters compared with basal rates (P = 0.0313) and to PDF-treated PdfR5304 tubules (P = 0.0230). There was no significant difference in basal and PDF-treated contraction rates of PdfR5304 ureters (P = 0.9340). (F) The effects of FSK on the ureters of wild-type (w1118) and PdfR5304 viscera dissected using warm HL3. Wild-type ureters responded to 10 μM FSK with a significant increase in contraction rate compared with basal rates (P = 0.013), as did the ureters of PdfR5304 viscera (P = 0.0313). On all graphs the error bars = SEM. For A and B, significance was tested by a Mann–Whitney U test comparing the contraction rates of PDF-treated viscera to those of vehicle-treated viscera (0.1% DMSO). In D–F PDF-treated contraction rates were compared with basal rates using paired-sample Wilcoxon signed rank tests. *P < 0.05, **P < 0.01, and ***P < 0.001).
These experiments suggested that, although ab-PDF neurons innervate the digestive tract, PDF does not alter intestinal peristalsis, but rather stimulates ureter contraction. We repeated these experiments on viscera dissected from CO2 anesthetized flies into room temperature hemolymph-like saline (HL3), to rule out chilling and the relatively high K+ concentrations associated with the ice-cold Ringer’s as the reason for the lack of PDF effects on the gut. This increased basal visceral contraction rates (Fig. 2E and Fig. S3B), allowing us to test the ability of PDF to contract or relax the digestive tract. Under these conditions, PDF application neither increased nor reduced gut contraction rates (Fig. S3B) but significantly increased ureter contraction rates (Fig. 2C). Thus, PDF has a specific myotropic effect on the renal system, suggesting an endocrine mode of action for the gut-innervating ab-PDF neurons.
PdfR Is Expressed in Ureter Muscles and Is Required for PDF’s Effects on Ureters.
In its role as a regulator of circadian locomotor rhythms, PDF signals through PdfR, a GPCR encoded by CG13758 (21–23). To test if PdfR is required for PDF’s effects on ureters, we compared the PDF responses of adult viscera dissected from the wild-type w1118 strain and the PdfR mutants PdfR3369 and PdfR5304 (21). Ureters from w1118 control flies displayed large increases in contraction rates when treated with 10−7 M PDF. In contrast, neither PdfR mutant displayed increased ureter contraction rates in response to PDF (Fig. 2D). This same pattern held true for w1118 and PdfR5304 ureters dissected under warm HL3 (Fig. 2E).
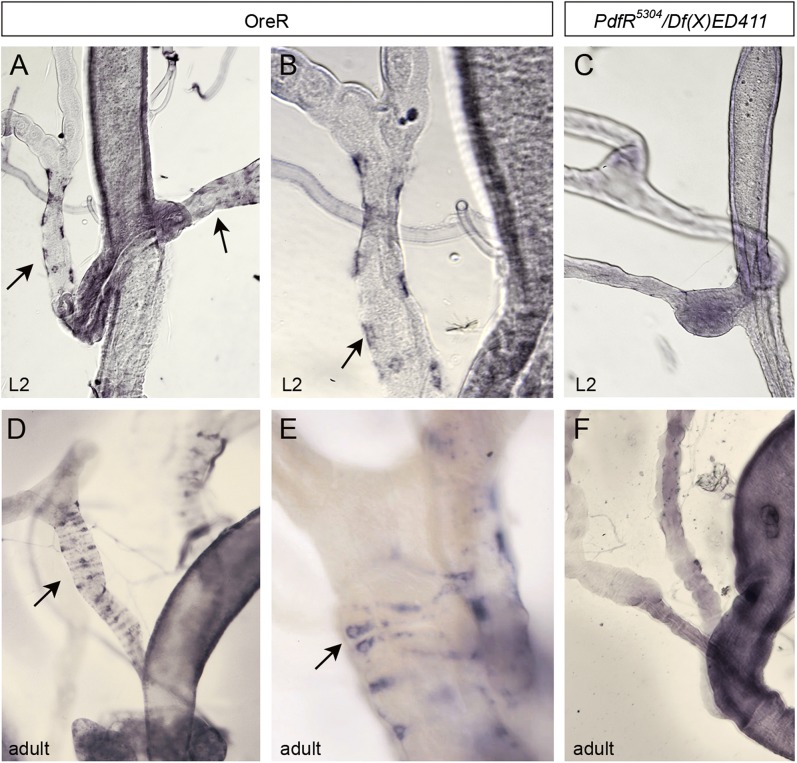
These results suggested that PdfR acts within the ureter to trigger PDF-induced contractions. We therefore mapped the visceral expression of PdfR using in situ hybridization, as anti-PdfR sera are unsuitable for immunocytochemistry (31). PdfR RNA is present in both larval and adult ureter circular muscles (Fig. 3 A, B, D, and E). Weaker expression in midgut and hindgut muscles could be observed in some, but not all, digestive tracts. These PdfR RNA signals were absent in PdfR5304 mutants (Fig. 3 C and F). We also compared PdfR RNA abundance relative to the housekeeping gene Rpl32 in distal renal tubules, ureters, and midguts using real-time PCR. Consistent with our in situ results, ureters expressed relatively high levels of PdfR RNA compared with distal renal tubules and midguts, although PdfR RNA expression was detectable in all three of these visceral tissues (Fig. S4). Thus, anatomical, molecular, and physiological evidence all indicate a role for PdfR acting in the ureter muscles to mediate the myotropic effects of PDF on the renal system.
Fig. 3.
PdfR mRNA is expressed in ureter circular muscles. (A and B) In situ hybridization reveals expression of PdfR RNA in wild-type (Oregon-R) larval ureters (A, arrows). The perinuclear and banded signal is characteristic of circular muscle expression (B, arrow). (C) No signal is apparent in PdfR mutant larval ureters. (D and E) PdfR expression in adult Oregon-R ureters (arrows). (F) The PdfR signal is absent in PdfR mutant adult ureters. A, C, D and F were captured using a 10× objective; B and E were captured using a 20× objective.
cAMP Increases Underlie the Visceral Effects of PDF.
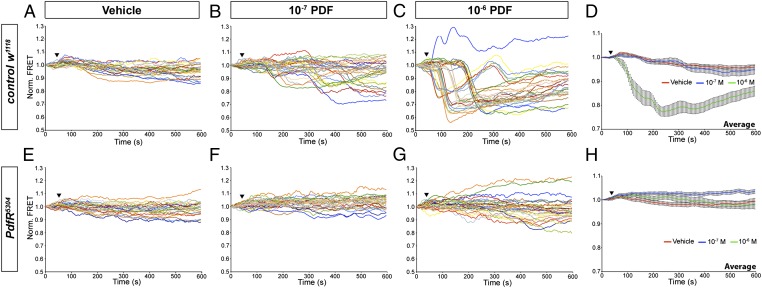
In central brain neurons, PdfR signals through increases in cAMP (23, 31). To investigate if ureter muscles display cAMP increases in response to PDF, we conducted cAMP imaging using the FRET sensor Epac1-camps, which has been used in the brain to measure PDF-induced cAMP increases (31). Epac1-camps displays a loss of YFP/CFP FRET when cAMP levels rise (32). We expressed UAS-Epac1-camps using Mef2-GAL4: an enhancer trap that reports the expression of myocyte enhancer factor 2, a transcription factor expressed by muscle cells (33), resulting in sensor expression in the ureter musculature (Fig. S5). The ratiometric nature of the sensor allowed for FRET measurements in moving ureters (Movies S4 and S5). Wild-type ureter muscles showed significant FRET loss in response to 10−6 M PDF (Fig. 4 A, C, and D), consistent with PDF-induced cAMP increases in the ureter muscles. At doses of 10−7 M PDF, a concentration that consistently elicits ureter contractions (Fig. 2 A and B), only a subset of ureter muscles responded with significant FRET changes (Fig. 4B). This discrepancy might reflect a limitation of the sensor whose affinity for cAMP must be high enough to respond to physiologically relevant cAMP concentrations, but low enough not to grossly interfere with cellular physiology and signaling. We next performed Epac1-camps imaging on the ureter muscles of PdfR5304;UAS-Epac1-camps/Mef2-GAL4 male flies. The muscles of PdfR5304 mutant ureters did not respond to 10−6 or 10−7 M PDF (Fig. 4 E–H). Thus, the cAMP response of the ureter muscles to PDF requires PdfR.
Fig. 4.
PDF causes cAMP increases in ureter muscles. Epac1-camps FRET imaged from the ureter muscles of adult male Drosophila. Decreases in YFP/CFP FRET indicate increases in cAMP. (A–C) The responses of wild-type (w1118) male ureter muscles to vehicle (0.1% DMSO), 10−7 M PDF, and 10−6 M PDF, respectively. The data for 10−6 and 10−7 M are based on six ureters from six w1118 flies with five regions of interest (ROIs) per ureter. The data for vehicle controls are based on five ureters from five flies with five ROIs per ureter. (D) Mean normalized FRET responses for the traces shown in A–C. The FRET values for the 10−6 M PDF treatment were significantly different from vehicle controls after 110 s (P < 0.001). No significant differences were observed between the vehicle and the 10−7 M PDF, although several individual muscles responded to this dose (B). (E–G) Responses of PdfR5304 mutant ureter muscles to vehicle, 10−7 M PDF, and 10−6 M PDF, respectively. The data for the 10−6 and 10−7 M PDF treatments are based on six ureters from six flies with five ROIs per ureter. The data for vehicle controls are based on five ureters from five flies with five ROIs per ureter. (H) The average normalized FRET responses for the traces shown in E–G. For PdfR5304 ureter muscles, neither peptide dose caused significant FRET changes compared with vehicle. There was a significant difference between PdfR5304 and w1118 when treated with 10−6 M PDF. By 115 s, the FRET ratios of w1118 ureter muscles were significantly lower than those of PdfR5304 (P < 0.001). In graphs D and H the error bars = SEM calculated from all muscles at each timepoint. Black triangles indicate time of bath application.
These results suggested that PDF affects ureter motility through cAMP increases. We asked if elevating cAMP would induce ureter contractions using forskolin (FSK), a direct activator of adenylate cyclases (34). FSK application caused significant increases in ureter contractions (Fig. 2F). Thus, increasing cAMP resulted in increased ureter contractions. FSK also caused significant increases in midgut contraction rates, but only in preparations dissected in warm HL3 buffer (Fig. S3C). This finding is consistent with previous work finding that cAMP signaling stimulates gut contractions in Acheta (35) and indicates that dissected guts were capable of contraction, lending further support to the lack of myotropic effects of PDF on the gut.
These results indicate that PDF-triggered PdfR activation in ureters results in cAMP increases, and support a model in which PdfR signaling in ureter muscles induces contractions through cAMP increases.
PdfR Expression in the Visceral Muscles of PdfR Mutants Rescues PDF-Induced Renal Motility.
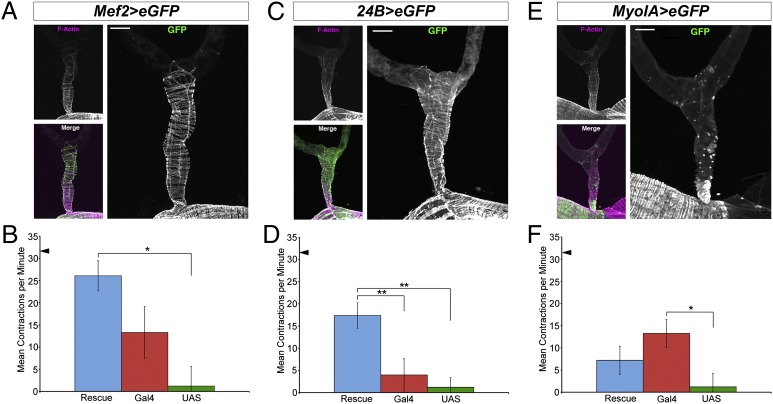
We next asked if PDF responsiveness could be conferred on PdfR5304 ureters by rescuing PdfR expression in visceral muscles using UAS-PdfR16L, which is capable of rescuing the geotaxic defects displayed by PdfR mutants (23) and confers PDF responsiveness to neurons that do not typically respond to PDF (31). We first used Mef2-GAL4 (Fig. 5A) to reinstate PdfR expression in ureter muscles. The PdfR5304;UAS-PdfR16L/Mef2-GAL4 rescue line displayed a significantly higher frequency of ureter contractions than PdfR5304;UAS-PdfR16L/+ controls in the presence of 10−6 M PDF (Fig. 5B) (P = 0.0021), with rates approaching those of wild-type ureters. The Mef2-rescue line also displayed mean contraction rates that were higher than those of PdfR5304;Mef2-GAL4/+ controls, but because of the relatively high contraction rate of a single PdfR5304;Mef2-GAL4/+ preparation, this difference was not statistically significant (P = 0.0861). The 24B-GAL4 line drives expression in the ureter musculature (Fig. 5C) and the PdfR5304;UAS-PdfR16L/+;24B-GAL4/+ rescue line displayed a significantly higher frequency of ureter contractions than the PdfR5304;;24B-GAL4/+ (P = 0.0087) and PdfR5304;UAS-PdfR16L/+ (P = 0.0032) controls in the presence of 10−6 M PDF (Fig. 5D). Thus, 24B-GAL4-driven PdfR expression rescued the PDF responsiveness of PdfR5304 ureters. No such rescue was observed when UAS-PdfR16L was expressed using MyoIA-GAL4, an enhancer trap for the myosin 1A gene, which is expressed in epithelial cells of the midgut and proximal tubule (36), but not in the muscles of the ureter (Fig. 5 E and F). Thus, the GPCR required for PDF’s role in central circadian timekeeping also mediates a peripheral action of this peptide.
Fig. 5.
Ureter muscle expression of PdfR rescues PDF-induced renal contractions in PdfR mutants. (A) Confocal micrograph of a ureter from a male w;UAS-eGFP/Mef2-GAL4 fly. (Upper Left) Visceral muscle F-actin visualized with fluorescently labeled phalloidin. (Right) GAL4-driven GFP expression in the ureter. (Lower Left) Merged image of phalloidin (magenta) and GFP (green). (B) The Mef2 rescue line (PdfR5304;UAS-PdfR/Mef2-GAL4, shown in blue, n = 7) displayed contraction rates in the presence of 10−6 M PDF that approached those of PDF-treated Canton S ureters (triangle in B). This rescue line showed significantly higher contraction rates than PdfR5304;UAS-PdfR/+ controls (green, P = 0.0079, n = 8). The Mef2 rescue line also displayed a higher mean ureter contraction rate than PdfR5304;Mef2-GAL4/+; controls (red, n = 6) but because of high contraction rates of a single PdfR5304;Mef2-GAL4/+ preparation, this difference was not statistically significant (P = 0.0861). (C) A confocal micrograph of a typical ureter from a male w;UAS-eGFP/+;24B-GAL4/+ fly. Micrographs arranged as for A. (D) The 24B rescue line (PdfR5304;UAS-PdfR/+;24B-GAL4/+; blue, n = 6) displayed significantly higher ureter contraction rates in the presence of 10−6 M PDF compared with PdfR5304;;24B-GAL4/+; (red, P = 0.0087, n = 6) and PdfR5304;UAS-PdfR/+; (green, P = 0.0032, n = 8) controls. (E) Confocal micrographs of a typical ureter from a male w;UAS-eGFP/+;MyoIa-GAL4/+ fly revealing expression of MyoIA-GAL4 in epithelia and a lack of GFP in ureter muscle. Panels arranged as in A. (F) The frequency of ureter contractions in the presence of 10−6 M PDF in the PdfR5304;UAS-PdfR/+;MyoIA-GAL4/+ rescue line (blue, n = 6) and the PdfR5304;;MyoIA-GAL4/+ (red, n = 6) and PdfR5304;UAS-PdfR/+ (green, n = 8) controls. There were no significant differences in ureter contraction rates between the rescue and control lines (P = 0.2403 for GAL4 control and P = 0.0597 for UAS control). There was a significant difference in contraction rate between the GAL4 control and the UAS control (P = 0.0051). (Scale bars, 50 μm in A, C, and E.) Black triangles in B, D, and F represent the frequency of ureter contractions displayed by wild-type Canton S ureters in the presence of 10−6 M PDF. For ease of comparison, the same data for PdfR/UAS-PdfR controls (n = 8) were included on all three histograms. *P < 0.05; **P < 0.01.
Excitation of Visceral PDF Axons Stimulates Ureter Contractions.
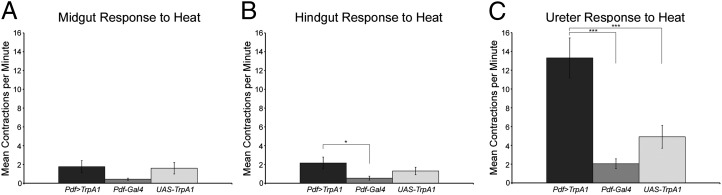
The lack of renal PDF innervation, together with the experiments described above, indicate that PDF from the ab-PDF neurons acts at a distance to stimulate ureter contractions. To test this model, we adapted the use of the Drosophila TrpA1 (dTrpA1) heat-sensitive channel, which renders neurons excitable by high temperature pulses (37), to excite visceral PDF axons in explanted viscera. Heat activation of PDF-GAL4-driven dTrpA1 did not affect gut peristalsis significantly, but led to a significant increases in ureter contraction rates (Fig. 6, and Movies S6 and S7). Because the axons of the abdominal gut-innervating PDF neurons were the only dTrpA1-expressing neurites present in this preparation, we conclude that excitation of the ab-PDF neurons is sufficient to stimulate ureter contractions without affecting midgut motility.
Fig. 6.
TrpA1-mediated excitation of ab-PDF neuron axons induces ureter contractions. Following 5 min of incubation at high temperature (31 °C), the contraction rates of explanted midguts (A) or hindguts (B) were not significantly different between w; PDF-GAL4/+;UAS-dTrpA1/+ and GAL4/+ or UAS/+ controls (for midguts, Friedman rank sum test, P = 0.004, asymptotic general independence test, P = 0.9, n = 20; for hindguts, Friedman rank sum test, P = 0.002, asymptotic general independence test, P = 0.02, pair-wise comparisons P = 0.02 compared with GAL4 control, P = 0.3 compared with UAS control, P = 0.4 between controls, n = 20). (C) The same conditions led to a significant increase in the contraction rate of ureters in w; PDF-GAL4/+;UAS-dTrpA1/+ (Friedman rank sum test, P < 0.001, asymptotic general independence test, P < 0.001, pair-wise comparisons P < 0.001 compared with GAL4 control, P < 0.001 compared with UAS control, P = 0.4 between controls, n = 20). Full genotypes are as follows: w;PDF-GAL4/+;UAS-dTrpA1/+ (PDF > dTrpA1); w;PDF-GAL4/+ (PDF-GAL4) and w;; +/UAS-dTrpA1 (UAS-dTrpA1). *P < 0.05; ***P < 0.001.
Discussion
Drosophila PDF and Mammalian VIP: Striking Similarities Beyond the Circadian Clock Network.
Striking similarities have been found between fly PDF and mammalian VIP in the generation of daily behavioral rhythms (10, 13). Outside the central clock, mammalian VIP and its related ligand PACAP (pituitary adenylate cyclase activating peptide) act on smooth-muscle receptors in a variety of internal organs, including those of the lower urinary tract (39–43). Our findings of broad visceral PdfR expression and a function for PDF signaling in the regulation of visceral muscle contraction extend the similarities between these peptides and their receptors beyond the central clock. The PdfR-expressing circular muscles of the ureter control the flow of urine into the terminal portion of the digestive tract (29, 30) in a manner analogous to the VPAC2R+ detrusor muscle of the mammalian bladder. Abnormal fluid regulation has also been described in mice lacking VIP or PACAP (40, 42, 43), so it will be of interest to establish if PDF signaling has related in vivo roles in flies. The presence of VIP/PACAP immunoreactivity in neural fibers of the lower urinary tract of mammals suggests a local, transmitter-like action on the excretory muscle receptors (12), and VIP is generally believed to act as a local neurotransmitter rather than as a circulating hormone (44, 45). However, systemic effects of gut-derived VIP cannot be ruled out. Indeed, VIP plasma levels increase after an oral osmotic load or intravenous application of cholinesterase inhibitors (46, 47). In light of our findings, which reveal a role for circulating PDF on the renal system, a possible systemic function of VIP deserves further investigation using, for example, transgenic mice lacking the peptide in specific (enteric vs. renal) neuronal populations.
What the Gut Tells the Kidney.
Previous work has made use of the larger viscera of locusts or crickets to establish that some neuropeptides induce renal tubule movements (35, 48). Our results using Drosophila indicate that renal regulators are secreted from an unexpected source, the gut-innervating ab-PDF neurons, to act as endocrine regulators of the renal muscles, which are entirely devoid of innervation (Fig. 1C and Fig. S1) (24). Indeed, our dTrpA1 experiments strongly suggest that the normal ligand for visceral PdfR is released from the gut-innervating ab-PDF neurons, because the only other site of PDF production in mature adults are the clock interneurons in the brain, which are thought to be chemically insulated from the hemolymph by the blood-brain barrier (49) and were not present during our dTrpA1 excitation experiments. Consistent with this idea, extracts of the abdominal ganglia of another insect, Carausius morosus, have a potent stimulatory action on renal tubule writhing, the active factors in which were suspected to be peptides (50).
The directionality of this endocrine signal from the gut to the renal system is unexpected and may be of relevance to mammals, where functional links between the digestive and excretory systems are well established with regard to absorption and secretion, and evidence suggests that communication between the two systems might facilitate their concerted action (44). An enterorenal axis, whereby unidentified gut-derived signals affect kidney function directly, has recently been proposed on the basis of two observations: the activity of duodenal mucosa homogenates on kidney ion secretion and the differential effect of intravenously versus orally administered sodium loads on renal sodium excretion (44).
In summary, our findings indicate a role for PDF in the control of visceral physiology in the fly, thereby extending the similarities between fly PDF and mammalian VIP beyond their shared role in circadian timekeeping. These findings have also revealed a peptidergic enterorenal axis in Drosophila, the evolutionary conservation and significance of which deserves further investigation.
Methods
Fly Stocks and Rearing.
A detailed list of fly stocks and a description of fly rearing conditions are described in SI Methods.
Imaging, Anatomy, and Physiology.
Detailed methods for all imaging, anatomical, and physiological experiments are described in SI Methods.
Citations relevant to fly stocks and experimental methods are listed in SI Methods.
Supplementary Material
Acknowledgments
We thank Helen Skaer and Jeremy Skepper for electron microscopy advice and assistance; Paola Cognigni and Robert Denver for critical reading of the manuscript; Michael Bate and Jimena Berni for the water-heated chamber for the Drosophila TrpA1 experiments; Vivek Mehta for conducting the blind quantifications of visceral contraction movies; Katherine Lelito, Ann Marie Macara, Zepeng Yao, and Qi Zhang for assisting with visceral dissections for real-time PCR, and Mohammad Samie for real-time PCR advice and assistance; and Bruce Edgar, Manfred Frasch, Matthias Landgraf, Stefan Pulver, Iris Salecker, Jim Skeath, Ralf Stanewsky, Paul Taghert, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank. I.M-A. thanks her family for babysitting while she worked on this manuscript. This work was funded by a Wellcome Trust Research Career Development Fellowship WT083559 (to I.M.-A.); National Institutes of Health Grant National Institute of Neurological Disorders and Stroke R00NS62953 (to O.T.S.); a Spanish Ministry of Education fellowship (to D.P.); and a Wellcome Trust doctoral studentship (to G.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200247109/-/DCSupplemental.
References
- 1.Field BC, Chaudhri OB, Bloom SR. Bowels control brain: Gut hormones and obesity. Nat Rev Endocrinol. 2010;6:444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- 2.Rubino F, R’bibo SL, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: The role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6:102–109. doi: 10.1038/nrendo.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LR, editor. Phsyiology of the Gastrointestinal Tract. Burlington: Elsevier Academic; 2006. [Google Scholar]
- 4.Dockray G. Physiology of enteric neuropeptides. In: Johnson L, editor. Physiology of the Gastrointestinal Tract. Vol 1. New York: Raven Press; 1987. pp. 41–66. [Google Scholar]
- 5.Schoofs L. Structure, function, and distribution of insect myotropic peptides. In: Davey KG, Peter RE, Tobe SS, editors. Perspectives in Comparative Endocrinology. Ottawa: National Research Council of Canada; 1994. pp. 155–156. [Google Scholar]
- 6.Veenstra JA, Agricola HJ, Sellami A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008;334:499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- 7.Nässel DR, Winther ÅME. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Brown BE, Starratt AN. Isolation of proctolin, a myotropic peptide, from Periplaneta americana. J Insect Physiol. 1975;21:1879–1881. [Google Scholar]
- 9.Taghert PH, Veenstra JA. Drosophila neuropeptide signaling. Adv Genet. 2003;49:1–65. doi: 10.1016/s0065-2660(03)01001-0. [DOI] [PubMed] [Google Scholar]
- 10.Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reubi JC. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann N Y Acad Sci. 2000;921:1–25. doi: 10.1111/j.1749-6632.2000.tb06946.x. [DOI] [PubMed] [Google Scholar]
- 12.Yoshiyama M, de Groat WC. The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract. J Mol Neurosci. 2008;36:227–240. doi: 10.1007/s12031-008-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 14.Colwell CS, et al. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- 15.Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 16.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown TM, Colwell CS, Waschek JA, Piggins HD. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. J Neurophysiol. 2007;97:2553–2558. doi: 10.1152/jn.01206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Im SH, Li W, Taghert PH. PDFR and CRY signaling converge in a subset of clock neurons to modulate the amplitude and phase of circadian behavior in Drosophila. PLoS ONE. 2011;6:e18974. doi: 10.1371/journal.pone.0018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lear BC, Zhang L, Allada R. The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS Biol. 2009;7:e1000154. doi: 10.1371/journal.pbio.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shafer OT, Taghert PH. RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: The anatomical basis of a neuropeptide’s circadian functions. PLoS ONE. 2009;4:e8298. doi: 10.1371/journal.pone.0008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyun S, et al. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Lear BC, et al. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Mertens I, et al. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nässel DR, Shiga S, Mohrherr CJ, Rao KR. Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: Immunocytochemistry and partial characterization. J Comp Neurol. 1993;331:183–198. doi: 10.1002/cne.903310204. [DOI] [PubMed] [Google Scholar]
- 26.Helfrich-Förster C. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol. 1997;380:335–354. doi: 10.1002/(sici)1096-9861(19970414)380:3<335::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Miguel-Aliaga I, Thor S. Segment-specific prevention of pioneer neuron apoptosis by cell-autonomous, postmitotic Hox gene activity. Development. 2004;131:6093–6105. doi: 10.1242/dev.01521. [DOI] [PubMed] [Google Scholar]
- 28.Spana EP, Kopczynski C, Goodman CS, Doe CQ. Asymmetric localization of numb autonomously determines sibling neuron identity in the Drosophila CNS. Development. 1995;121:3489–3494. doi: 10.1242/dev.121.11.3489. [DOI] [PubMed] [Google Scholar]
- 29.Dow JT, Davies SA. The Drosophila melanogaster Malpighian tubule. In: Evans PD, editor. Advances in Insect Physiology. San Diego: Academic; 2001. pp. 1–83. [Google Scholar]
- 30.Wessing A, Eichelberg D. Malpighian tubules, rectal papillae and excretion. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. Vol 2c. New York: Academic; 1978. pp. 1–42. [Google Scholar]
- 31.Shafer OT, et al. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 33.Ranganayakulu G, Schulz RA, Olson EN. Wingless signaling induces nautilus expression in the ventral mesoderm of the Drosophila embryo. Dev Biol. 1996;176:143–148. doi: 10.1006/dbio.1996.9987. [DOI] [PubMed] [Google Scholar]
- 34.de Souza NJ, Dohadwalla AN, Reden J. Forskolin: A labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med Res Rev. 1983;3:201–219. doi: 10.1002/med.2610030205. [DOI] [PubMed] [Google Scholar]
- 35.Blake PD, Kay I, Coast GM. Myotropic activity of Acheta diuretic peptide on the foregut of the house cricket, Acheta domesticus (L.) J Insect Physiol. 1996;42:1053–1059. [Google Scholar]
- 36.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helfrich-Förster C, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol. 1993;337:177–190. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- 39.Bitar KN, Makhlouf GM. Relaxation of isolated gastric smooth muscle cells by vasoactive intestinal peptide. Science. 1982;216:531–533. doi: 10.1126/science.6176025. [DOI] [PubMed] [Google Scholar]
- 40.Girard BM, Malley SE, Braas KM, May V, Vizzard MA. PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium. J Mol Neurosci. 2010;42:378–389. doi: 10.1007/s12031-010-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harmar AJ, et al. Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology. 2004;145:1203–1210. doi: 10.1210/en.2003-1058. [DOI] [PubMed] [Google Scholar]
- 42.May V, Vizzard MA. Bladder dysfunction and altered somatic sensitivity in PACAP-/- mice. J Urol. 2010;183:772–779. doi: 10.1016/j.juro.2009.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Studeny S, et al. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)-/- mice. J Mol Neurosci. 2008;36:175–187. doi: 10.1007/s12031-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michell AR, Debnam ES, Unwin RJ. Regulation of renal function by the gastrointestinal tract: Potential role of gut-derived peptides and hormones. Annu Rev Physiol. 2008;70:379–403. doi: 10.1146/annurev.physiol.69.040705.141330. [DOI] [PubMed] [Google Scholar]
- 45.Unwin RJ, Ganz MD, Sterzel RB. Brain-gut peptides, renal function and cell growth. Kidney Int. 1990;37:1031–1047. doi: 10.1038/ki.1990.83. [DOI] [PubMed] [Google Scholar]
- 46.Porter JP, Ganong WF. Vasoactive Intestinal Peptide. Advances in Peptide Hormone Research. New York: Raven Press; 1982. pp. 285–297. [Google Scholar]
- 47.Porter JP, Thrasher TN, Said SI, Ganong WF. Vasoactive intestinal peptide in the regulation of renin secretion. Am J Physiol. 1985;249:F84–F89. doi: 10.1152/ajprenal.1985.249.1.F84. [DOI] [PubMed] [Google Scholar]
- 48.Coast GM. The influence of neuropeptides on Malpighian tubule writhing and its significance for excretion. Peptides. 1998;19:469–480. doi: 10.1016/s0196-9781(97)00461-0. [DOI] [PubMed] [Google Scholar]
- 49.Carlson SD, Juang JL, Hilgers SL, Garment MB. Blood barriers of the insect. Annu Rev Entomol. 2000;45:151–174. doi: 10.1146/annurev.ento.45.1.151. [DOI] [PubMed] [Google Scholar]
- 50.Pilcher DEM. Stimulation of movements of Malpighian tubules of Carausius by pharmacologically active substances and tissue extracts. J Insect Physiol. 1971;17:463–470. [Google Scholar]
- 51.Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726, 728, 730, 732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.