Abstract
Thellungiella salsuginea, a close relative of Arabidopsis, represents an extremophile model for abiotic stress tolerance studies. We present the draft sequence of the T. salsuginea genome, assembled based on ∼134-fold coverage to seven chromosomes with a coding capacity of at least 28,457 genes. This genome provides resources and evidence about the nature of defense mechanisms constituting the genetic basis underlying plant abiotic stress tolerance. Comparative genomics and experimental analyses identified genes related to cation transport, abscisic acid signaling, and wax production prominent in T. salsuginea as possible contributors to its success in stressful environments.
Keywords: genome sequence, halophyte, gene duplication, stress response
Abiotic stresses such as salinity, drought, or temperature extremes greatly impair plant growth and development and crop yield. The need to cultivate marginal lands to increase food production in the future will expose crops to adverse conditions and exacerbate agricultural problems. Thus, enormous value will come from a better understanding of the mechanisms through which plant tolerance of abiotic stresses is achieved. Most studies on plant response mechanisms leading to stress tolerance have been conducted with the model plant Arabidopsis, which has a relatively low capacity to survive abiotic stresses. However, the Arabidopsis model and work on a variety of other species have provided clues about enhanced stress tolerance based on individual genes in a number of pathways. Unfortunately, in nearly all cases, genes with a stress-alleviating quality under controlled conditions have failed to generate stress protection in the field. This lack of success argues for developing models that can provide crucial insights into mechanisms that confer high levels of stress tolerance in species that exhibit natural tolerance (1, 2).
The crucifer Thellungiella salsuginea (Pallas), a close relative of Arabidopsis originally classified as Thellungiella halophila, is a halophyte with exceptionally high resistance to cold, drought, and oxidative stresses as well as salinity (1–6). T. salsuginea is exemplary by its short life cycle, self-fertility, and being genetically transformable (3). These characteristics make the species an excellent model for unraveling the factors that constitute abiotic stress tolerance (1–8). Further advantages are its relatively small genome size [approximately twice that of A. thaliana (3)] and the availability of ecotypes that show a range of stress responses (8).
High-throughput studies of T. salsuginea thus far have been restricted largely to the characterization of its transcriptome (5, 7, 8). In addition, comparisons of transcriptome stress responses in T. salsuginea and Arabidopsis thaliana highlighted different regulation of well-known pathways as well as unstudied stress-related genes (4, 6). The recent publication of the genome sequence of the congeneric species Thellungiella parvula has enabled consideration of the genomic and evolutionary basis of stress adaptation with the improved resolution provided by a comparative approach (9).
Here we present the genome sequence and overall chromosome structure of T. salsuginea and use comparative genomics and experimental approaches to identify genes in T. salsuginea that contribute to its success in stressful environments.
Results
Sequence and Assembly.
We sequenced the genome of T. salsuginea (Shandong ecotype) using the paired-end Solexa sequencing method (Illumina GA II system). Based on flow cytometry of isolated nuclei stained with propidium iodide (3), we expected a genome size of ∼260 Mb (SI Appendix, Table S1). Thus, with a total of 34.8 Gb of high-quality sequences, the genome was covered ∼134-fold (SI Appendix, Table S2). The final length of the assembled sequences amounted to ∼233.7 Mb, covering about 90% of the estimated genome size. The assembly consists of 2,682 scaffolds, the 10 longest of which range from 1.9–6.8 Mb (SI Appendix, Table S1) and represent 17% of the assembled genome.
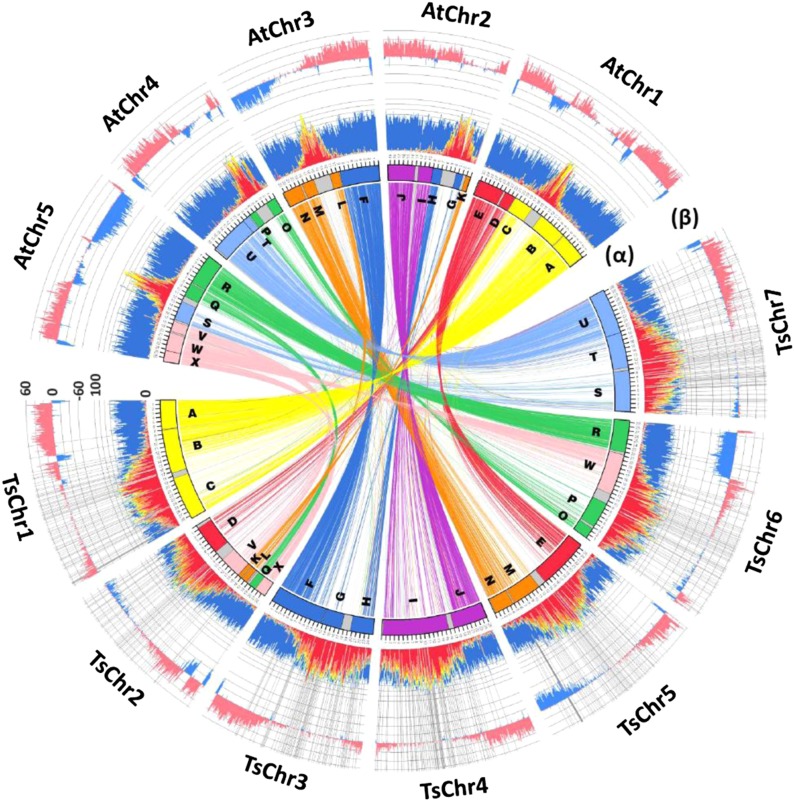
In the absence of genetic and physical markers, we assigned many remaining scaffolds to blocks (chromosome segments) identified by Lysak and coworkers (10, 11) by comparative chromosome painting, which represents the ancestral karyotype in the crucifers. By tracing these blocks, we anchored 515 scaffolds onto seven chromosomes, with a total size of 186 Mb (about 80% of the total assembled genome; Fig. 1).
Fig. 1.
The genome of T. salsuginea. The assembled seven chromosomes of T. salsuginea are shown in a comparison with A. thaliana. Ancestral karyotype blocks A–X (10) are shown in different colors. Sequences with >70% similarity over the length of 2 kb are connected by links of the same colors as the ancestral karyotype blocks. Histogram α represents the distribution of TEs and predicted genes. Class I retrotransposons, class II DNA transposons, and unclassified repetitive sequences are indicated by red, orange, and yellow colors, respectively, and the predicted genes are shown in blue. The outer histogram β shows the percentage of sequences that can be aligned between the two species with >70% identity. Alignments longer than 500 bp were counted, and their percentages per 100-Kb windows are presented, with the alignments in opposite directions in the two genomes shown in blue and the alignments in the same direction shown in pink. Scales in the y-axes of the histograms are in percentage. Radial lines indicate the boundaries of the scaffolds used in the T. salsuginea genome assembly.
Repetitive Sequences.
The size of the T. salsuginea genome is approximately twice that of A. thaliana, largely reflecting a proliferation of transposable elements (TEs). A repetitive-sequence database search combined with detection of TEs identified 121 Mb of repetitive sequences (SI Appendix) representing ∼52% of the genome (SI Appendix, Tables S1 and S3). This percentage is much higher than the 13.2% and 7.5% TE contents of A. thaliana (12) and T. parvula (13), respectively. Like most of higher plant genomes, class I TEs (retrotransposons), especially LTR retrotransposons, account for a comparatively high percentage (36%) of the T. salsuginea genome. Among these, gypsy and copia are the two most abundant TE families.
Gene Space.
A total of 28,457 protein-coding regions were predicted in the sequenced T. salsuginea genome using a combination of homologous sequence searches, ab initio gene predictions, and transcriptome data comparisons with the genome sequence (SI Appendix, Table S1 and Dataset S1). In addition, 447 tRNAs, 11 rRNAs, 432 snRNAs, and 162 microRNAs (including 126 conserved ones) were identified (SI Appendix, Tables S1 and S4). The overall ORF length distribution of T. salsuginea is comparable to that of A. thaliana, with a slightly higher proportion of ORFs shorter than 1,000 bp identified in T. salsuginea (SI Appendix, Fig. S1). The average exon length of T. salsuginea and A. thaliana genes is similar (228 and 224 bp, respectively), whereas the average intron length of T. salsuginea is ∼30% larger than that of A. thaliana (200 and 157 bp, respectively) (SI Appendix, Table S1) (12).
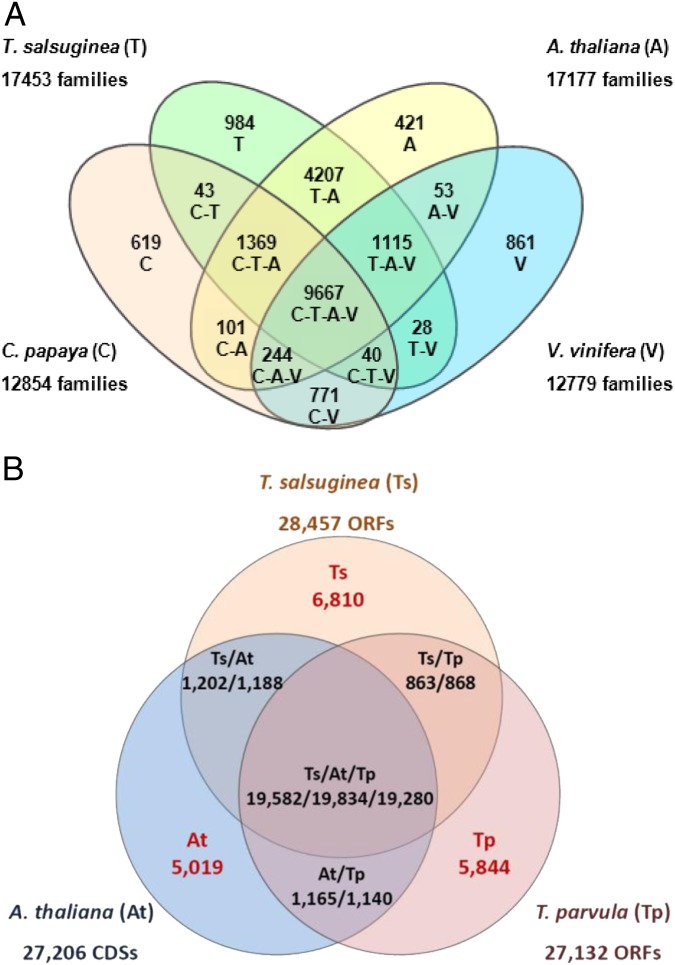
About 93% of the predicted coding regions showed at least partial similarity with known protein sequences and can be annotated (SI Appendix, Table S1). Comparative genomic analysis identified 984 T. salsuginea unique gene families and 9,667 families shared by T. salsuginea, A. thaliana, Carica papaya, and Vitis vinifera (Fig. 2A). Consistent with their close evolutionary relationships, 16,358 gene families were shared by T. salsuginea and A. thaliana, representing 93.7% and 95.2% of all gene families, respectively (Fig. 2A). The protein-coding gene models were compared with A. thaliana and T. parvula (13), and orthologous gene models were identified. Thellungiella species share comparable numbers of orthologs with each other and with A. thaliana. Both Thellungiella species contain large numbers of “orphan” genes for which no orthologs exist in A. thaliana (Fig. 2B, indicated by red color). Among all orphan gene models, 54.7%, 62.8%, and 36.5% in T. salsuginea, T. parvula, and A. thaliana, respectively, lacked any Gene Ontology (GO) annotation and hence are annotated as functionally unknown (Dataset S2).
Fig. 2.
Comparison of orthologous genes and gene groups. (A) Shared orthologous gene clusters among the T. salsuginea, A. thaliana, C. papaya, and V. vinifera genomes. Program OrthoMCL was applied to identify orthologous groups among the T. salsuginea (T), A. thaliana (A), C. papaya (C), and V. vinifera (V) genomes. (B) Shared orthologous genes among crucifers T. salsuginea (Ts), T. parvula (Tp), and A. thaliana (At). Orthologs were identified using OrthoMCL. Genes from different species were considered as orthologs if the shared homology in their deduced amino acid sequences (BlastP, e < 0.00001) was more than 50% of the size of the genes being compared. Numbers of orphan genes lacking an ortholog in the other two species are shown in red. Lists of genes and their GO annotations in each category are given in Dataset S2.
Evolutionary History.
Phylogenetic analyses (SI Appendix, Fig. S2) indicate a time of divergence between T. salsuginea and A. thaliana of 7–12 Mya, following the split of the Arabidopsis and Brassica lineages (14). A similar time has been suggested for the A. thaliana and T. parvula split (9). Previous studies have suggested that the A. thaliana genome shows signatures of the paleohexaploidy whole-genome duplication (WGD) event γ proposed at the base of eudicot divergence and two recent WGD events, β and α, within the crucifer lineage (14). Similarly, two peaks representing the β and α events [∼0.28 and ∼0.6 fourfold degenerative third-codon transversion (4dTv) distance] were identified in T. salsuginea (SI Appendix, Fig. S3), suggesting that the divergence of T. salsuginea and A. thaliana occurred after the two most recent WGD events.
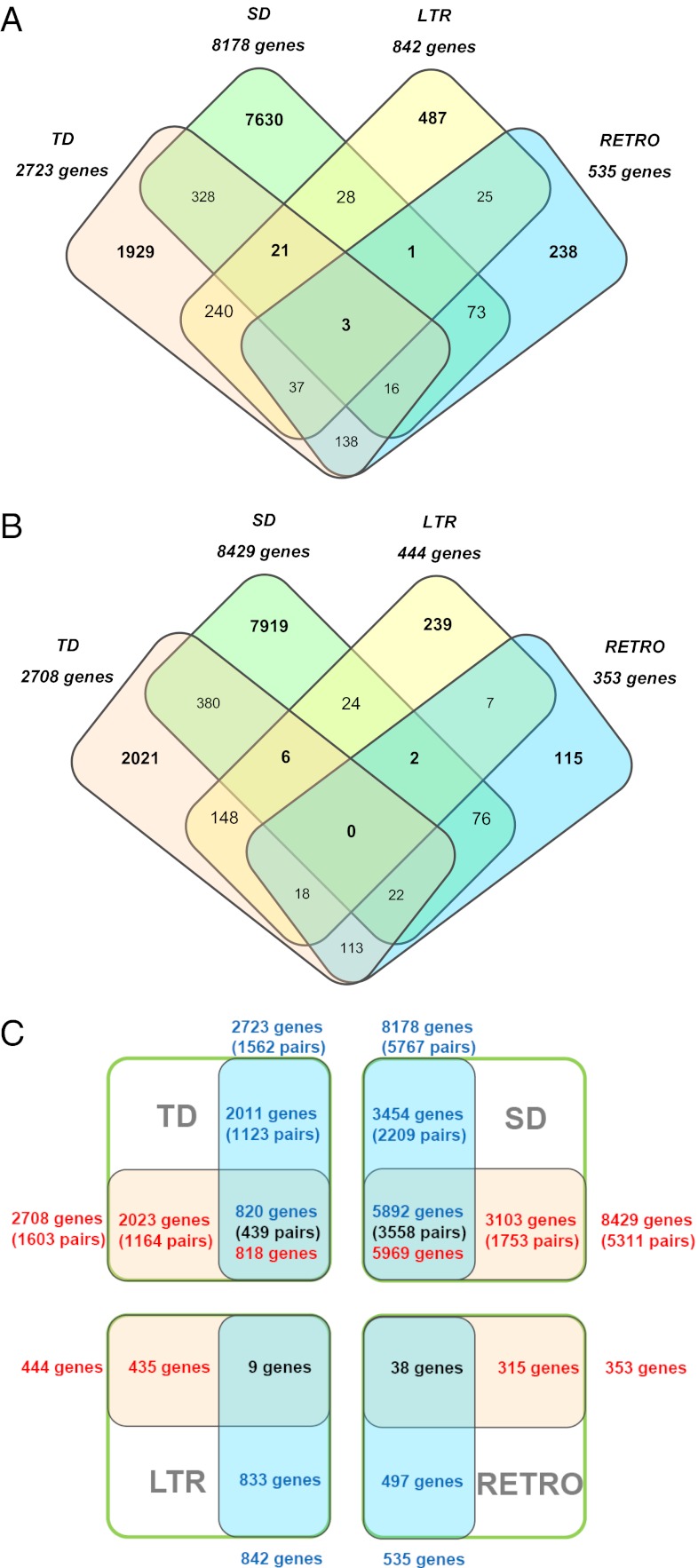
Tandem duplication, segmental duplication, and retrotransposition-directed duplications (SI Appendix) were analyzed to weigh their contribution to the variation in gene copy number and to probe for a possible bias in gene functional enrichment in T. salsuginea and A. thaliana. The total numbers of tandemly duplicated genes and segmentally duplicated genes are similar in the two species. In contrast, genes carrying an LTR transposon and retrogenes, although they only accounted for a small proportion of genes in total, were significantly more expanded in T. salsuginea (Fig. 3 A and B), probably reflecting the high abundance of TEs, especially LTR retrotransposons. About 30% of the tandemly duplicated genes and 60% of segmentally duplicated genes were shared between T. salsuginea and A. thaliana. Very few duplication events seemingly mediated by retrogene action, especially those based on LTRs, were conserved, indicating that those events occurred after the divergence of the two species. GO analysis revealed that both tandem and segmental duplications tended to affect genes in similar functional categories (SI Appendix, Table S5).
Fig. 3.
Comparison of tandem duplication (TD), segmental duplication (SD), and retrotransposition (LTR and RETRO) events in the T. salsuginea and A. thaliana genomes. (A and B) Assembled venn plots show shared and specific genes among different types of gene duplications in T. salsuginea (A) and A. thaliana (B). (C) Comparisons of each type of gene duplication in the two species. Numbers of duplicated genes in T. salsuginea are in red; numbers in A. thaliana are in blue. LTR, LTR retrotransposon carrying genes; RETRO, retrogenes; SD, segmental duplicated genes; TD, tandem duplicated genes.
Genome Designed for Stress Response Capacity.
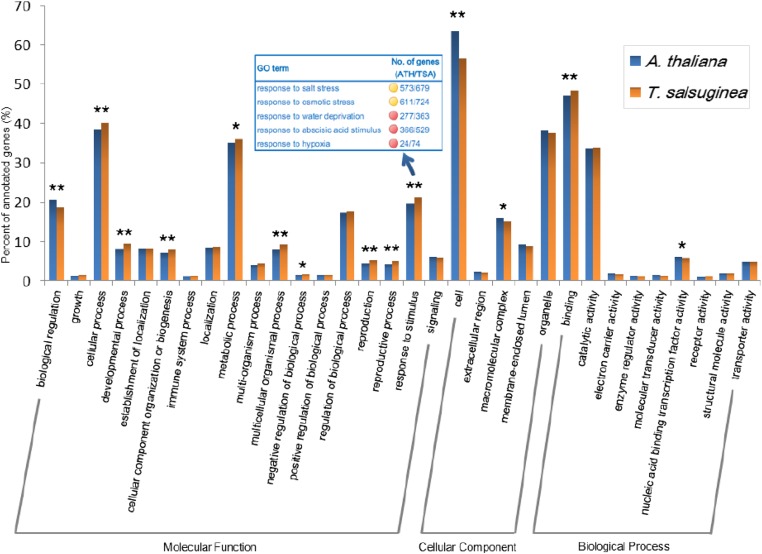
GO terms were assigned to the T. salsuginea predicted ORFs and A. thaliana annotated genes using the Blast2GO pipeline (15). The term “response to stimulus” was identified among the GO categories that differed significantly between T. salsuginea and A. thaliana, with more genes in the T. salsuginea genome classified into this category (Fig. 4). This divergence represents the contribution of the combinatory effect of four major duplication events (SI Appendix, Table S5). The same trend also was observed in the T. parvula genome (13). Detailed analysis revealed that genes related to “response to salt stress,” ”osmotic stress,” “water deprivation,” “ABA stimulus,” and “hypoxia” were expanded in the “response to stimulus” category in T. salsuginea compared with A. thaliana (Fig. 4). As a genome signature, this difference may be caused by and could contribute to the high salinity- and drought-tolerant phenotype of T. salsuginea.
Fig. 4.
GO comparison of T. salsuginea and A. thaliana. Blast2GO results of protein-coding regions from T. salsuginea and A. thaliana were mapped to categories in the second level of GO terms. Fisher’s exact test was used to evaluate the significance of differences in GO category enrichment in the two species. GO terms that contain more than 1% of total genes were included in the graph; those with P values below 0.01 and 0.05 are marked by double stars and stars, respectively, on the histogram. Subcategories of the term “response to stimulus” that differ significantly in the two species are shown in the box.
A total of 21 transcription factor families were found to be expanded in the T. salsuginea genome compared with A. thaliana (SI Appendix, Table S6). These expansions may be associated with the adaptation of T. salsuginea to extreme environments, because individual members of some families in A. thaliana have been linked previously with stress resistance. For example, the RAV gene family, which had been reported to respond to high salt and cold stresses (16, 17), expanded from six members in the A. thaliana genome to nine in T. salsuginea. Other gene families with known functions in abiotic stress response that expanded in numbers in T. salsuginea include the NF-X1, GRAS, HSF, and Trihelix families. It has been shown that one NF-X1 family member, AtNFXL1, is required for growth of Arabidopsis under salinity stress (18), and RGL3 in the GRAS family can be up-regulated transiently by cold stress (19). HSFA2, the most abundant member of the heat-shock response HSF family, also is induced by salinity in Arabidopsis, and its overexpression enhances salt and osmotic stress tolerance (20). The GTgamma subfamily in the Trihelix family contains three genes induced by most abiotic stresses in rice (21). Overexpression of two soybean Trihelix family genes in Arabidopsis greatly enhanced salt, drought, and cold tolerance (22).
Expansion of Genes Related to the Maintenance of Ion Equilibrium.
Effective establishment of ionic and osmotic equilibrium is important for plant salinity and drought tolerance. Comparison of gene families involved in ion transport in T. salsuginea and A. thaliana indicated that gene families providing ionic stress protection, including HKT, CNGC, PPa, ACA, AVP, ATBGL, CIPK, and CDPK (23–25), have more members in T. salsuginea (SI Appendix, Table S7). One group, the HKT gene family, encodes Na+/K+ transporters that may provide key components affecting or determining salt tolerance in plants (26–29). Recently, two HKT1 transcripts have been reported in T. salsuginea (30); however, the genome annotation revealed a third homolog (Ts6g08740/TsHKT1;3). The three TsHKT1 genes exist in a tandem gene array, similar to the tandem duplication of two HKT1 genes in T. parvula (13); only one copy is present in A. thaliana (SI Appendix, Table S7). Based on phylogenetic analysis, Ts6g08650/TsHKT1;1 is clustered with AtHKT1, whereas the other two TsHKT1 cluster with the two TpHKT1 genes in another group (SI Appendix, Fig. S4A). All three T. salsuginea HKT1 genes were found to be expressed, with the expression of TsHKT1;2 (Ts6g08730) being significantly higher than the expression of the other two genes (SI Appendix, Fig. S4B).
Stress Tolerance-Supportive Genes and Pathways.
Reduction of water loss by epicuticular wax is a strategy used by plants to defend themselves against abiotic stresses (31). Throughout its development, T. salsuginea exhibits highly glaucous leaves indicative of complex epicuticular wax organization. We found a tandem duplicated gene in T. salsuginea encoding cytochrome P450-dependent midchain hydroxylase MAH1/CYP96A15, which currently is the only known enzyme in the wax-producing–related alkane-forming pathway. This gene is also tandemly duplicated in the T. parvula genome (13) but is not duplicated in A. thaliana (SI Appendix, Fig. S5 and Table S8), perhaps explaining the previous finding that the wax content was much higher in T. salsuginea than in A. thaliana leaves (32). Genes involved in hormone pathways may serve as another example: The ZEP, AAO, and CYP707A families, all of which are involved in the abscisic acid (ABA) biosynthesis pathway, show an expansion of gene numbers in the T. salsuginea genome (SI Appendix, Table S9). This expansion may lead to a more complex regulation of ABA biogenesis, contributing to stress tolerance; the induction of gene expression by ABA in Arabidopsis is slower than in T. salsuginea until much higher stress levels have been reached (4, 6). The rapid ABA response in T. salsuginea under high salt conditions may confer a higher salinity-tolerance capacity by slowing down its growth rate. Further experiment evidence is necessary to confirm this hypothesis.
Additional salt stress-related gene families expanded in the T. salsuginea genome are summarized in SI Appendix, Table S10. Among them, SAT32 is interesting because it is expanded from one gene in A. thaliana and T. parvula to six members in the T. salsuginea genome (SI Appendix, Fig. S6). AtSAT32 is homologous to human IFN-related developmental regulator (IFRD) and is reported to be involved in salt-stress response (33). It is possible that these expanded family members give T. salsuginea more flexibility in response to salinity stress.
Discussion
With the increasing availability of second-generation sequencing, plant genome sequences are appearing in increasing numbers. Because of a desire to understand and improve agronomical important species, crops are an obvious target. A second group includes putative keystone species, i.e., models that might elucidate the evolutionary dimension of the genetic diversity essential to colonization of nearly every climate zone on earth. The third category is plants chosen for their close relationship to existing genomic and genetic models with the goal of expanding potential comparisons relevant at the biochemical and physiological level in particular environments. T. salsuginea is such a plant. On the one hand, it is phylogenetically and developmentally similar to the prototypical model, A. thaliana. It is a plant with halophytic characters and exceptionally high abiotic stress tolerance, including salinity, cold and freezing temperatures, and the ability to grow in poor soils. During the last decade it also has received considerable attention as a model of physiological and molecular defense against salinity stress (1–8). With the genome sequence presented in this study and with reference to the recently sequenced genome of the congeneric T. parvula (13), both juxtaposed with Arabidopsis, we have expanded the exploration of gene complement and allele structures that favored extremophile adaptations.
By tracing differences in genome structure and their evolutionary history, we have been able to point to processes that generated two species with extremely divergent adaptations within a time span of 7–12 million years (SI Appendix, Fig. S2). Although the gene spaces show extensive colinearity (Fig. 1), and the number of predicted gene models is similar to A. thaliana (SI Appendix, Fig. S1), selective expansions of seemingly stress-related gene families were observed in the T. salsuginea genome (Fig. 4 and SI Appendix, Tables S7–S10). Copy number variations of orthologs are largely caused by tandem and segmental duplication events that are unique to each species (Fig. 3C), similar to observations in the T. parvula genome (13).
However, the T. salsuginea genome is characterized by a dramatically higher content of TEs as compared with A. thaliana and T. parvula, and this greater number of TEs is largely responsible for its enlarged genome size (Fig. 1). Genes contained in LTR and retroelements are more abundant in T. salsuginea, with significantly higher numbers of these elements showing tandem duplications than in A. thaliana (455 vs. 307 genes). This observation confirms the role of TEs in tandem duplication events (Fig. 3 A and B). In addition, gene duplications have led to changes in gene dosage. Following sub- and neo-functionalization, functional diversification ensues, and duplicated copies that include favorable characters are retained in the process of natural selection. Duplicates lacking clear advantages for the organism turn into pseudogenes that eventually disappear (34). The stressful environment to which T. salsuginea has been exposed seems to have resulted in or to have contributed to the particular population of gene duplications that were retained. This view is supported by the presence of a comparable number but different suite of species-specific duplications in the A. thaliana genome (Fig. 3C). We also observed a number of translocation events for individual and small groups of genes relative to the Arabidopsis and T. parvula genomes, although it is not yet possible to assign a particular functional significance to these translocation events. Another outstanding character is the frequency of alterations in the sequences and cis-element structures of promoters for orthologous genes in the three species. These alterations can result in a complete rewiring of gene regulation, as exemplified by the expression of the duplicated HKT1 genes (SI Appendix, Fig. S4) as well as for other stress-related genes (9).
Another level of complexity is present in the form of a substantial number of orphan genes that are specific to T. salsuginea. These genes do not have an ortholog in A. thaliana or in T. parvula (Fig. 2B) and frequently have no annotation based on sequence similarity. They may represent unique means of adaptation by providing domains with alternative functions, or they may be involved in shuffling of known protein domains. Compared with Arabidopsis, T. salsuginea is characterized by a dramatically different lifestyle, a unique gene complement, significant differences in the expression of orthologs, and a larger genome size. The T. salsuginea genome provides a tool for comparison and contrast to the well-established model, Arabidopsis. The resolution provided by the comparison between the two species is exceptionally high. Such resolution is not achievable by comparing plant genomes that are evolutionarily more distant. Multispecies comparative genomics strategies now can focus in detail on gene duplications in stress-related functions, neo-functionalization of duplicated genes, the consequences of translocation events, and orphan gene functions. The divergent regulation of gene expression in development and in communication with stressful environments now can be probed with the support of global transcript profiles. Because fundamental differences in handling stress are emerging (34), it seems that pathways and functions related to stress observed in Arabidopsis could be different in evolutionarily stress-tolerant plants. The genome of T. salsuginea will be a useful tool in exploring mechanisms of adaptive evolution.
Methods
DNA Library Construction and Sequencing.
Short-insert DNA libraries (170 bp, 300 bp, and 800 bp) and long-insert DNA libraries (2 kb, 5 kb, and 10 kb) were built following protocols described previously (35). All libraries were subjected to paired-end sequencing runs, following the manufacturer’s user guide (Illumina). A total of 12 DNA libraries were built and sequenced to ensure the randomness of clones. The raw sequence reads with base-calling duplicates, adapter contamination, PCR duplicates, and low-quality sequences were cleaned from the initial sequencing output using custom scripts.
Genome Assembly.
We used a hybrid assembly and a hierarchical assembly approach with multiple assembly programs to build gap-free contigs, contigs combined to scaffolds, and scaffolds ordered into pseudochromosomes. At the first level of assembly, we used ABySS (36) and SOAPdenovo (35) followed by minimus2 (37) for meta-assembly of the primary contigs and scaffolds. Contigs were generated with a minimum of 10 overlapping high-quality mate pairs in stringent assembly parameters using 41- to 64-bp-long k-mers in search of high-quality contigs in the primary assemblies. Contigs with lengths less than 100 bp were discarded. In the initial assembly, 50 and 90% of the total length of 174,275,254 bp was covered by contigs larger than 3.23 kb and 149 bp, respectively (contig N50 = 3.23 kb, N90 = 149 bp). Scaffolds were assembled by adding all the paired-end reads to the initial contig assembly, followed by meta-assembly using minumus2. The assembled scaffolds were aligned to both the T. parvula (13) and A. thaliana (12) genome sequences using Nucmer (38). Scaffolds that could be aligned unambiguously to an ancestral karyotype block (39) in either T. parvula or A. thaliana were mapped to the karyotype model for the subclade Eutremae identified by comparative chromosome painting (10). Directions of mapped scaffolds were visualized further and corrected using the comparative genome visualization tool MAUVE (40), consulting both T. parvula and A. thaliana genomes. Scaffolds that could not be aligned unambiguously were labeled as unaligned.
More methods and details of data collection are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Beijing Genomics Institute staff members Ying Huang, Na An, Chunfang Peng, Yinqi Bai, Jianwen Li, Qingle Cai, Shiping Liu, Min Xie, Wei Fan, Bo Wang, Sheng Tang, Yuxiang Liu, Juan Wang, Kui Wu, Chuyu Lin, Yalin Huang, Kang Yi, Fei Teng, Fengjie Yu, Haibo Lin, Ruiqiang Li, Zhi Jiang, XiaoJu Qian, Hailong Luo, and Junjie Liu for their sequencing support. This research was supported by Grants 31030047, 30921061, 30825029, and 90917016 from the National Science Foundation of China, Grants 973 2012CB114300 and 2012CB114200 from the National Basic Research Program of China, and by Grant 2009A0714-05 from the State Key Laboratory of Plant Genomics of China. D.-H.O., S.Y.L., and H.J.B. are supported by World Class University Program R32–10148 at Gyeongsang National University, Republic of Korea and the Next-generation BioGreen21 Program SSAC, PJ009030, Rural Development Administration, Republic of Korea.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence for the Thellungiella salsuginea genome reported in this paper has been deposited in the Data Bank of Japan (DDBJ)/European Molecular Biology Laboratory (EMBL)/GenBank database, http://www.ncbi.nlm.nih.gov/bioproject/?term=txid72664 (accession no. AHIU00000000; PID 80723).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209954109/-/DCSupplemental.
References
- 1.Amtmann A. Learning from evolution: Thellungiella generates new knowledge on essential and critical components of abiotic stress tolerance in plants. Mol Plant. 2009;2:3–12. doi: 10.1093/mp/ssn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bressan RA, et al. Learning from the Arabidopsis experience. The next gene search paradigm. Plant Physiol. 2001;127:1354–1360. [PMC free article] [PubMed] [Google Scholar]
- 3.Inan G, et al. Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol. 2004;135:1718–1737. doi: 10.1104/pp.104.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taji T, et al. Comparative genomics in salt tolerance between Arabidopsis and aRabidopsis-related halophyte salt cress using Arabidopsis microarray. Plant Physiol. 2004;135:1697–1709. doi: 10.1104/pp.104.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong CE, et al. Transcriptional profiling implicates novel interactions between abiotic stress and hormonal responses in Thellungiella, a close relative of Arabidopsis. Plant Physiol. 2006;140:1437–1450. doi: 10.1104/pp.105.070508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong Q, Li P, Ma S, Indu Rupassara S, Bohnert HJ. Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J. 2005;44:826–839. doi: 10.1111/j.1365-313X.2005.02587.x. [DOI] [PubMed] [Google Scholar]
- 7.Taji T, et al. Large-scale collection and annotation of full-length enriched cDNAs from a model halophyte, Thellungiella halophila. BMC Plant Biol. 2008;8:115. doi: 10.1186/1471-2229-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CE, et al. Expressed sequence tags from the Yukon ecotype of Thellungiella reveal that gene expression in response to cold, drought and salinity shows little overlap. Plant Mol Biol. 2005;58:561–574. doi: 10.1007/s11103-005-6163-6. [DOI] [PubMed] [Google Scholar]
- 9.Oh DH, et al. Genome structures and halophyte-specific gene expression of the extremophile Thellungiella parvula in comparison with Thellungiella salsuginea (Thellungiella halophila) and Arabidopsis. Plant Physiol. 2010;154:1040–1052. doi: 10.1104/pp.110.163923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandáková T, Lysak MA. Chromosomal phylogeny and karyotype evolution in x=7 crucifer species (Brassicaceae) Plant Cell. 2008;20:2559–2570. doi: 10.1105/tpc.108.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lysak MA, et al. Mechanisms of chromosome number reduction in Arabidopsis thaliana and related Brassicaceae species. Proc Natl Acad Sci USA. 2006;103:5224–5229. doi: 10.1073/pnas.0510791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Initiative TAG. Arabidopsis Genome Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 13.Dassanayake M, et al. The genome of the extremophile crucifer Thellungiella parvula. Nat Genet. 2011;43:913–918. doi: 10.1038/ng.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lysak MA, Koch MA. Phylogeny, Genome, and Karyotype Evolution of Crucifers (Brassicaceae) In: Schmidt R, Bancroft I, editors. Genetics and Genomics of the Brassicaceae. New York: Springer; 2011. pp. 1–31. [Google Scholar]
- 15.Götz S, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler SG, Cook D, Thomashow MF. Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol. 2005;137:961–968. doi: 10.1104/pp.104.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohn KH, Lee SC, Jung HW, Hong JK, Hwang BK. Expression and functional roles of the pepper pathogen-induced transcription factor RAV1 in bacterial disease resistance, and drought and salt stress tolerance. Plant Mol Biol. 2006;61:897–915. doi: 10.1007/s11103-006-0057-0. [DOI] [PubMed] [Google Scholar]
- 18.Lisso J, Altmann T, Müssig C. The AtNFXL1 gene encodes a NF-X1 type zinc finger protein required for growth under salt stress. FEBS Lett. 2006;580:4851–4856. doi: 10.1016/j.febslet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 19.Achard P, et al. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell. 2008;20:2117–2129. doi: 10.1105/tpc.108.058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa D, Yamaguchi K, Nishiuchi T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J Exp Bot. 2007;58:3373–3383. doi: 10.1093/jxb/erm184. [DOI] [PubMed] [Google Scholar]
- 21.Fang Y, Xie K, Hou X, Hu H, Xiong L. Systematic analysis of GT factor family of rice reveals a novel subfamily involved in stress responses. Mol Genet Genomics. 2010;283:157–169. doi: 10.1007/s00438-009-0507-x. [DOI] [PubMed] [Google Scholar]
- 22.Xie ZM, et al. Soybean Trihelix transcription factors GmGT-2A and GmGT-2B improve plant tolerance to abiotic stresses in transgenic Arabidopsis. PLoS ONE. 2009;4:e6898. doi: 10.1371/journal.pone.0006898. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Volkov V, Amtmann A. Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, has specific root ion-channel features supporting K+/Na+ homeostasis under salinity stress. Plant J. 2006;48:342–353. doi: 10.1111/j.1365-313X.2006.02876.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun ZB, et al. Overexpression of a Thellungiella halophila CBL9 homolog, ThCBL9, confers salt and osmotic tolerances in transgenic Arabidopsis thaliana. J Plant Biol. 2008;51(1):25–34. [Google Scholar]
- 25.Lv S, et al. Overexpression of an H+-PPase gene from Thellungiella halophila in cotton enhances salt tolerance and improves growth and photosynthetic performance. Plant Cell Physiol. 2008;49:1150–1164. doi: 10.1093/pcp/pcn090. [DOI] [PubMed] [Google Scholar]
- 26.Vera-Estrella R, Barkla BJ, García-Ramírez L, Pantoja O. Salt stress in Thellungiella halophila activates Na+ transport mechanisms required for salinity tolerance. Plant Physiol. 2005;139:1507–1517. doi: 10.1104/pp.105.067850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rus A, et al. Natural variants of AtHKT1 enhance Na+ accumulation in two wild populations of Arabidopsis. PLoS Genet. 2006;2:e210. doi: 10.1371/journal.pgen.0020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren ZH, et al. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- 29.Byrt CS, et al. HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol. 2007;143:1918–1928. doi: 10.1104/pp.106.093476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali Z, et al. TsHKT1;2, a HKT1 homolog from the extremophile Arabidopsis relative Thellungiella salsuginea, shows K(+) specificity in the presence of NaCl. Plant Physiol. 2012;158:1463–1474. doi: 10.1104/pp.111.193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosma DK, et al. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009;151:1918–1929. doi: 10.1104/pp.109.141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teusink RS, Rahman M, Bressan RA, Jenks MA. Cuticular waxes on Arabidopsis thaliana close relatives Thellungiella halophila and Thellungiella parvula. Int J Plant Sci. 2002;163(2):309–315. [Google Scholar]
- 33.Park MY, et al. Isolation and functional characterization of the Arabidopsis salt-tolerance 32 (AtSAT32) gene associated with salt tolerance and ABA signaling. Physiol Plant. 2009;135:426–435. doi: 10.1111/j.1399-3054.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- 34.Oh DH, Dassanayake M, Bohnert HJ, Cheeseman JM. Life at the extreme: Lessons from the genome. Genome Biol. 2012;13:241. doi: 10.1186/gb-2012-13-3-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R, et al. The sequence and de novo assembly of the giant panda genome. Nature. 2010;463:311–317. doi: 10.1038/nature08696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson JT, et al. ABySS: A parallel assembler for short read sequence data. Genome Res. 2009;19:1117–1123. doi: 10.1101/gr.089532.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer DD, Delcher AL, Salzberg SL, Pop M. Minimus: A fast, lightweight genome assembler. BMC Bioinformatics. 2007;8:64. doi: 10.1186/1471-2105-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurtz S, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schranz ME, Lysak MA, Mitchell-Olds T. The ABC’s of comparative genomics in the Brassicaceae: Building blocks of crucifer genomes. Trends Plant Sci. 2006;11:535–542. doi: 10.1016/j.tplants.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Darling AC, Mau B, Blattner FR, Perna NT. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.