Abstract
Sensory circuits are shaped by experience in early postnatal life and in many brain areas late maturation of inhibition drives activity-dependent development. In the newborn spinal dorsal horn, activity is dominated by inputs from low threshold A fibers, whereas nociceptive C-fiber inputs mature gradually over the first postnatal weeks. How this changing afferent input influences the maturation of dorsal horn inhibition is not known. We show an absence of functional glycinergic inhibition in newborn dorsal horn circuits: Dorsal horn receptive fields and afferent-evoked excitation are initially facilitated by glycinergic activity due, at least in part, to glycinergic disinhibition of GAD67 cells. Glycinergic inhibitory control emerges in the second postnatal week, coinciding with an expression switch from neonatal α2 homomeric to predominantly mature α1/β glycine receptors (GlyRs). We further show that the onset of glycinergic inhibition depends upon the maturation of C-fiber inputs to the dorsal horn: selective block of afferent C fibers in postnatal week 2, using perisciatic injections of the cationic anesthetic QX-314, lidocaine, and capsaicin, delays the maturation of both GlyR subunits and glycinergic inhibition, maintaining dorsal neurons in a neonatal state, where tactile responses are facilitated, rather than inhibited, by glycinergic network activity. Thus, glycine may serve to facilitate tactile A-fiber–mediated information and enhance activity-dependent synaptic strengthening in the immature dorsal horn. This period ceases in the second postnatal week with the maturation of C-fiber spinal input, which triggers postsynaptic changes leading to glycinergic inhibition and only then is balanced excitation and inhibition achieved in dorsal horn sensory circuits.
Keywords: somatosensory, neonate, pain
Newborn mammals are strikingly sensitive to tactile and noxious mechanical stimulation of the body surface. At birth, cutaneous flexion withdrawal reflexes are greater in amplitude and duration and are poorly directed compared with adults, and individual spinal dorsal horn cells in young rats have lower cutaneous sensory thresholds and larger receptive fields (RFs) (1–4). This sensitivity is especially directed toward tactile stimulation and the developmental refinement and dampening of responses to touch coincides with a reduction of A fiber inputs to the superficial dorsal horn and a strengthening of C-fiber synaptic contacts (4, 5).
Because the intrinsic excitability of spinal sensory neurons does not change over this period (6), the gradual postnatal reduction in excitability of spinal sensory circuits is thought to reflect the maturation of functional inhibitory connections in the dorsal horn. Whereas some aspects of GABAergic neurotransmission are postnatally regulated in the dorsal horn (7–9), the robust GABAergic inhibition of spinal sensory circuits observed from birth makes this an unlikely explanation for the excitability of neonatal cutaneous spinal sensory circuits (6, 10). Glycinergic transmission is also postnatally regulated (6) but remains to be investigated in neonatal dorsal horn circuits. In adults, Aβ fibers synapse directly onto glycinergic interneurons (11, 12) and glycine receptor (GlyR) antagonists enhance dorsal horn cell sensitivity to A-fiber inputs (13, 14), analogous to that seen in the healthy developing neonate. We therefore hypothesized that the cutaneous sensitivity of neonates arises from an absence of glycinergic inhibition in the newborn dorsal horn.
The late development of inhibitory processes is a feature of many sensory systems, most notably in the visual cortex (15) and allows them to be shaped by postnatal sensory experience. The correct balance of excitation to inhibition established over a “critical period” of circuit maturation and synaptic strengthening arises through activity-dependent processes. The gradual decrease in dorsal horn sensory excitability is known to be NMDA dependent (4), suggesting the need for neural activity in shaping receptive fields and connectivity. In the first week of life, A-fiber tactile inputs arising from spontaneous movements are required if afferent connections and nociceptive reflexes are to develop normally (16, 17), highlighting the functional importance of delayed maturation of inhibitory control of A-fiber input. The increase in strength of C-fiber inputs in the second postnatal week may play a key role here as it coincides with the decline in dorsal horn sensory excitability. We therefore further hypothesized that the onset of glycinergic inhibition depends upon activity from C fibers in the second week of life.
Results
Postnatal Laminar Reorganization of Dorsal Horn Glycinergic Terminals.
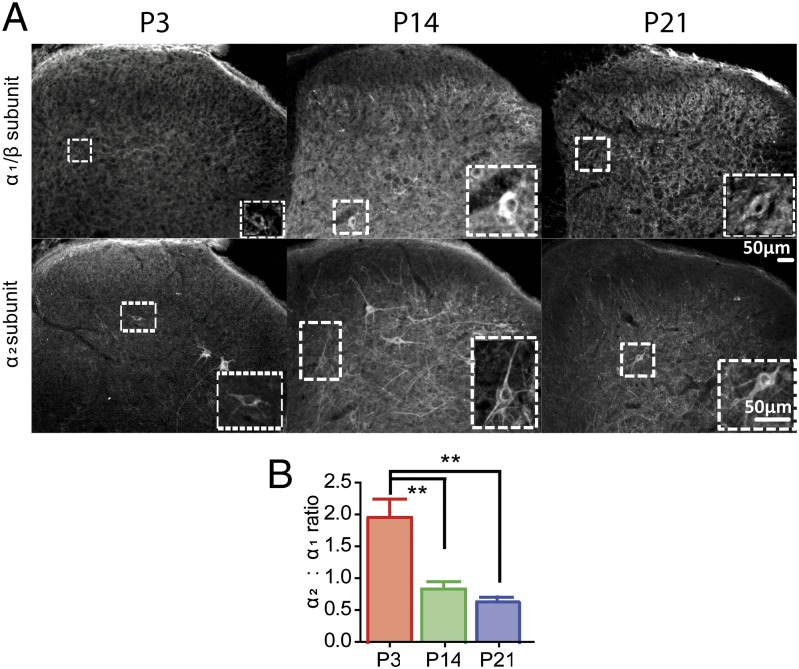
The GlyR is composed of α and β subunits arranged around a central pore. To date there are four known α subunit isoforms, (α1–α4) and a single β subunit isoform (18). Over the first two postnatal weeks, α1 and α2 subunit expression are inversely correlated: α2 expression decreases and that of α1 increases (19). To examine the postnatal development of glycinergic transmission, we first mapped the postnatal development of postsynaptic α1/β and α2 glycine receptor subunits and presynaptic glycinergic terminals in the spinal dorsal horn. At postnatal day 3 (P3), the ratio of α2 GlyR immunopositive neurons to heteromeric α1/β GlyR+ neurons in lamina III was significantly higher than at P14 or P21 (Fig. 1). In addition, presynaptic terminal GlyT2 immunolabeling revealed clear postnatal developmental regulation of terminal patterns in laminae IIi and III, only achieving adult patterns by P14 (20) (Fig. S1 A and B). These results suggest that both glycinergic inputs and postsynaptic sites mature in the second postnatal week.
Fig. 1.
Glycine receptor subunits undergo developmental expression changes over the first three postnatal weeks. L4 spinal sections were stained for both the α1/β heteromeric receptor (Upper) and the α2 subunit (Lower) of the glycine receptor. (Insets) Immunopositive cells used for analysis. (B) Ratio of α2+:α1/β+ neurons. At P3, expression of α2 is greater than α1/β in the dorsal horn, but the ratio reverses at P14 and P21. Data presented as mean ± SEM, *P < 0.05, **P < 0.01. n = 4 animals per group, five sections per animal. Identical section appears in Upper and Lower panels at each age.
Postnatal Onset of Tonic Glycinergic Inhibition of Dorsal Horn Receptive Fields.
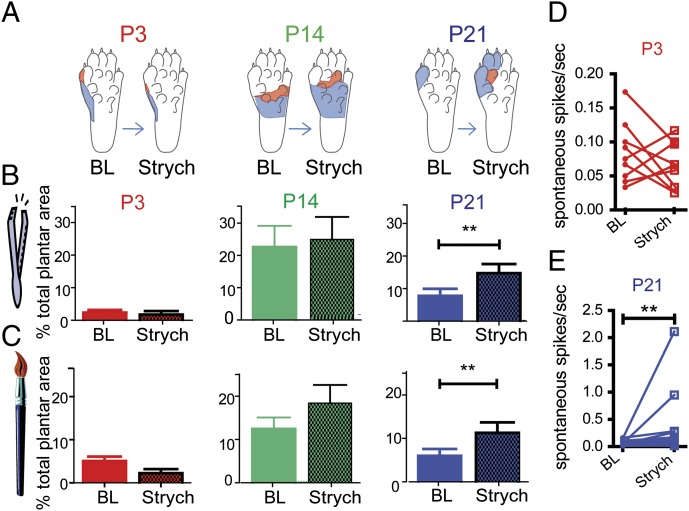
Next we investigated the postnatal development of glycinergic control of receptive fields in the intact dorsal horn using the glycine receptor antagonist, strychnine (strych). Hindpaw receptive fields of single wide dynamic range (WDR) dorsal horn neurons were mapped with cutaneous brush and pinch stimuli at baseline (BL), and 20 min after application of strychnine to the cord surface [165 ng/g; P3: 1.65 μg (4.9 nmol); P14: 3.3 μg (9.8 nmol); P21: 9.9 μg (29.4 nmol)]. Fig. 2 shows that at P21, strychnine increased WDR dorsal horn neuron receptive field size by nearly twofold from baseline (mean pinch RF BL: 7.8 ± 2%, strych: 14.8 ± 2.6%, P < 0.01; mean brush RF BL: 6.0 ± 1.5%, strych: 11.3 ± 2.2%, P < 0.01), as expected following disinhibition of tonic glycine activity. However, at younger ages, P3 and P14, strychnine application had no effect on receptive field size, mapped with either brush or pinch stimulation, indicating the absence of tonic glycinergic regulation of receptive field size in the dorsal horn until rats are over 14 d old. In addition, spinal strychnine had no effect on spontaneous activity in the absence of any cutaneous stimulation at P3 but increased spontaneous activity at P21 (mean increase 0.26 ± 0.16 spikes/s from baseline; n = 14; P < 0.01; Fig. 2 D and E).
Fig. 2.
Glycinergic blockade increases receptive field size of P21 dorsal horn neurons but not at P3 or P14. (A) Representative pinch only (red) and brush- and pinch-sensitive (blue) RFs of single recorded neurons of P3 (Left), P14 (Center), and P21 (Right) neurons at baseline and 20 min following strychnine. (B) Histograms of mean pinch-sensitive RF size as a function of plantar hindpaw surface, pre- and poststrychnine application to the spinal cord. (C) Histograms of mean brush-sensitive RF size. (D and E) Effect of strychnine on the spontaneous activity of individual spinal dorsal horn neurons of (D) P3 and (E) P21 rats. **P < 0.01. P3: n = 9; P14: n = 10; P21: n = 14. Error bars represent SEM, *P < 0.05, **P < 0.01.
Glycine Facilitation of Dynamic Tactile Dorsal Horn Activity in the Newborn Dorsal Horn.
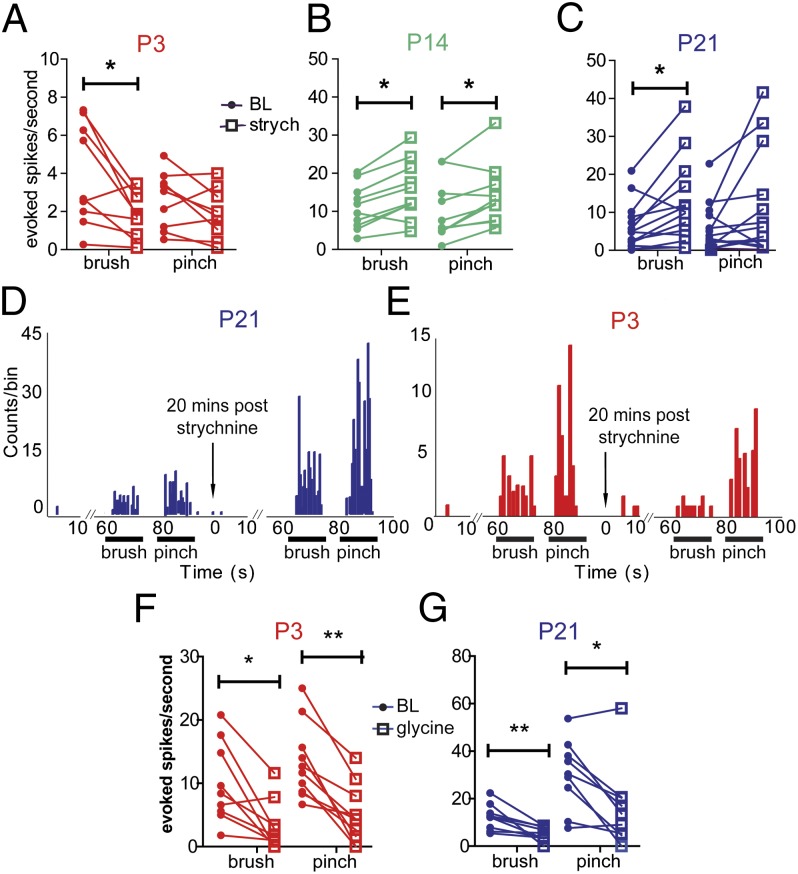
To determine the influence of glycinergic control of tactile and nociceptive evoked activity in the developing dorsal horn, evoked responses of dorsal horn WDR neurons to dynamic, tactile (brush) stimulation and to noxious pinch of the receptive field were recorded at BL and after strychnine application to the spinal cord (Fig. 3). As expected from previous studies (14, 21, 22), brush-evoked activity at P21 was significantly increased following the application of strychnine to the spinal cord, resulting in a mean increase of 5.7 ± 1.9 spikes/s (n = 14; P < 0.05). Pinch-evoked activity also tended to increase but the effects were more variable. The same was true at P14, where strychnine enhanced WDR cell brush-evoked firing by 3.2 ± 0.9 spikes/s from baseline, and pinch-evoked firing by 2.8 ± 1 spikes/s (n = 10; P < 0.05). The pattern was quite different at P3, however, as at this age strychnine had no effect on pinch-evoked activity but inhibited brush-evoked activity by a mean of 2.2 ± 0.7 spikes/s (n = 9; P < 0.05).
Fig. 3.
Strychnine reveals a glycinergic facilitation of brush-evoked activity at P3, which becomes inhibitory by P14. (A, B, and C) Brush-evoked and pinch-evoked action potential firing in response to strychnine application to the spinal cord of (A) P3 (n = 9), (B) P14 (n = 10), and (C) P21 neurons (n = 14). (D and E) Poststimulus time histograms of representative WDR neurons at (D) P21, and (E) P3 at baseline, and 20 min poststrychnine. Note that within the same cell at P3, strychnine selectively inhibits brush responses, although not affecting those to pinch. (F and G) Brush- and pinch-evoked firing of (F) P3 (n = 9) and (G) P21 neurons (n = 9) in response to glycine administration to the cord. *P < 0.05, **P < 0.01.
These results show that glycinergic inhibition of cutaneous-evoked activity is not present until the second postnatal week and that before that time a transient, modality-specific glycinergic facilitation is activated by innocuous dynamic tactile stimulation.
Exogenously Applied Glycine Inhibits Dorsal Horn Activity at All Ages.
In contrast to the glycine facilitation revealed by strychnine, over 89% of WDR dorsal horn cells at both P3 and P21 were inhibited from 10 min after spinal application of exogenous glycine (400 μg) and no cells were excited. Glycine application to the surface of the dorsal horn resulted in a mean decrease of brush-evoked activity by 6.8 ± 1.8 spikes/s in P21 neurons (P < 0.01), and a mean decrease of 6.6 ± 1.8 spikes/s in P3 neurons (Fig. 3 F and G; P < 0.05). Pinch-evoked activity was similarly reduced in dorsal horn neurons recorded at both ages (P3: mean decrease of 8.3 ± 1.5 spikes/s from baseline at 10 min after glycine application; P < 0.01; P21: mean decrease 13.7 ± 4.1 spikes/s, P < 0.05). Thus, dorsal horn glycine receptors are capable of producing inhibition of neuronal activity in response to exogenous glycine at P3, but this is not evident when endogenous glycine is released within the P3 network.
Spinal blockade of glycine transport by the GlyT2 blocker, ALX-1393 (100 μg) at P21 significantly reduced brush- and pinch-evoked activity after 20 min, as would be expected from an accumulation of synaptic glycine (brush: mean decrease of 4.4 ± 1.2 spikes/s from baseline, P < 0.05; pinch: mean decrease of 3.8 ± 1.2 spikes/s, P < 0.05; Fig. S2). At P3, however, ALX application did not alter activity (brush: P = 0.057; pinch: P = 0.098, n = 10), suggesting that there was insufficient glycine accumulation at this age or that receptors are not located near enough to the site of release to be activated by endogenous glycine release.
Maturation of Spinal Glycinergic Inhibition Is Dependent upon Peripheral C-Fiber Activity.
The postnatal onset of glycinergic inhibition in the dorsal horn suggests that the maturation is dependent on sensory activity, particularly activity in peripheral C fibers, whose synaptic inputs mature slowly over the same postnatal period (23).
To study the impact of C fibers on the development of glycinergic inhibition, we used the impermeable lidocaine derivative QX-314. QX-314 does not permeate the resting cell membrane, but requires for permeation the opening of a wide pore ion channel such as transient receptor potential cation channel subfamily V member 1 (TRPV1) (24). Thus, by injecting a mixture of QX-314, the local anesthetic lidocaine, followed by capsaicin (both TRPV1 channel agonists) into the perisciatic space, TRPV1+ nociceptive C-fiber primary afferents can be selectively targeted and silenced (25). Here we show that injection of 2% lidocaine, 0.2% QX-314, and 0.05% capsaicin into the perisciatic area of P10 pups results in blockade of noxious reflexes, assessed by the behavioral pinch nociceptive reflex assay (26) for 8–14 h postinjection (Fig. 4A). These injections were administered every 8 h for 3 d to ensure a full blockade of C fibers over P10 to P13.
Fig. 4.
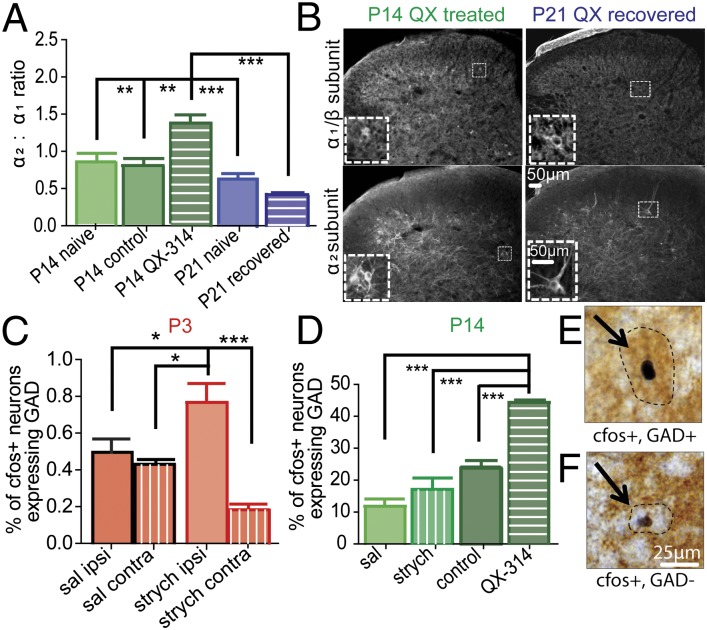
Blocking C-fiber activity in early postnatal life delays the maturation of glycinergic inhibition of spinal dorsal horn neurons. (A) QX-314 mixture sciatic nerve blockade at P10 lasts 8 h (n = 6–15). (B) Effect of strychnine on brush- and pinch-evoked firing of WDRs of QX-314–treated P14 pups (Left; n = 9) and in pups 1 wk after recovery from QX-314 (P21; right; n = 8). (C) Representative raw traces of single neurons at P14 QX-314–treated (Left) and P21 QX-314–recovered (Right) animals in response to brush before and 20 min after strychnine. (D) Histograms of mean brush-sensitive RF size of P14 QX-314–treated (Left) and P21 QX-314–recovered (Right) WDRs at baseline and poststrychnine. (Insets) Representative pinch only (red) and brush- and pinch-sensitive (blue) RFs for single P14 QX-314–treated (Left) and P21 QX-314–recovered (Right) WDRs at baseline and 20 min following strychnine. *P < 0.05, **P < 0.01. Error bars represent SEM.
WDR cells from P14 pups, treated with a QX-314 mixture or saline from P10 to P13, were recorded from the dorsal horn of intact anesthetized rats and their evoked activity in response to brush and pinch was recorded. Baseline responses of these neurons to brush and pinch were not different from naive P14 neurons (one-way ANOVA Kruskall–Wallis test with Dunn’s multiple comparison posttest comparing P14 naive baseline, P14 saline-treated baseline, and P14 QX-314–treated baseline brush: P = 0.84; pinch: P = 0.17). The presence of glycinergic inhibition was tested using spinal application of strychnine, as described above. Fig. 4 shows that selective blockade of C-fiber activity with QX-314 mixture from P10–P13 prevented the maturation of glycinergic inhibition of brush-evoked activity at P14. In C-fiber blocked P14 animals, strychnine did not result in increased WDR activity as in naive animals, but instead inhibited brush- and pinch-evoked activity (brush: mean decrease of 13.9 ± 4.9 spikes/s from baseline; pinch: mean decrease of 12.1 ± 3.3 spikes/s from baseline, 20 min poststrychnine; Fig. 4B). This decrease in activity was similar to that observed in naive P3 rat pups (Fig. 3). In addition to this inhibition of neuronal firing, strychnine also significantly decreased WDR receptive field size in QX-314–treated rats (mean decrease of 9.5% ± 3.1 of total plantar surface of hindpaw; Fig. 4 D and E). This effect was only observed when C fibers were blocked over P10–P13, a period of C-fiber synapse maturation. QX-314 treatment from P17 to P19, resulted in normal mature dorsal horn neuron strychnine facilitation of brush at P21 (Fig. S3), implying that C-fiber input is required for the maturation of glycinergic inhibition, but not for its maintenance.
A subset of animals was allowed to grow up for a further 7 d to examine any long-term effects of QX-314 mixture treatment on the physiology of WDR neurons. These neurons showed full recovery, with clear strychnine-induced facilitation of brush-evoked firing (mean increase of 12.3 ± 3.5 spikes/s from baseline; Fig. 4B, Right column) and pinch-evoked firing (mean increase 12.3 ± 2.9 spikes/s from baseline), suggesting that maturation of glycinergic inhibition had been delayed rather than permanently prevented.
Peripheral C-Fiber Blockade Delays the Maturation of GlyR Subunit Expression and of GAD67+ Cell Activity in Lamina III.
The functional integrity of central C-fiber terminals and glycinergic terminals were unaffected by QX-314 treatment as shown by IB4 staining (Fig. S4) and GlyT2 expression (Fig. S5), respectively. However, the pattern of GlyR subunit expression was altered by C-fiber blockade. The ratio of α2 homomeric GlyR immunopositive neurons to heteromeric α1/β GlyR+ neurons in lamina III was significantly higher at P14 following QX-314 treatment, compared with saline controls (Fig. 5A), resembling the α2/α1 ratio normally observed at P3 (Fig. 1B). By P21, the ratio in QX-314 recovered animals was not significantly different from P21 naive controls.
Fig. 5.
Immature glycinergic signaling is characterized by inefficient targeting of inhibitory interneurons and a shift in postsynaptic receptor subunit expression. (A) C-fiber blockade prevents the normal maturation of the postsynaptic switch in glycine receptor subunit expression. (B) L4 spinal sections of P14 QX-314–treated (Left) or P21 pups 1 wk after QX-314 recovery (Right) were stained for the α1/β heteromeric receptor (Upper) and the α2 subunit of the glycine receptor (Lower). (Insets) Immunopositive cells used for analysis. (C) Percentage of lamina III c-fos+ neurons that are also GAD+ increases significantly after i.t. strychnine and brush stimulation of the paw in P3 rats. (D) C-fiber blockade results in an increase in c-fos+ GAD+ neurons at P14 following i.t. strychnine and brushing of the paw, which reflects that seen in the neonate. (E and F) Sample images of (E) c-fos+ and GAD+ and (F) c-fos+ and GAD− neurons in the P14 spinal dorsal horn. *P < 0.05, **P < 0.01, ***P < 0.001. n = 4 animals per group, five sections per animal for both experiments. Identical section appears in Upper and Lower panels at each age.
In addition, QX-314 treatment altered the pattern of glycinergic control of inhibitory interneurones. Fig. 5 E and F show examples of c-fos+ GAD67+ (c-fos+, GAD+) and c-fos+ GAD67− (c-fos+, GAD−) neurons evoked after intrathecal (i.t.) injection of strychnine and brush of the hindpaw. A significant shift in strychnine and brush-evoked fos activation toward GAD+ cells in lamina III was observed in QX-314 C-fiber blocked animals at P14 (Fig. 5D). This shift mimics the pattern of activation normally seen at P3 (Fig. 5C). The mean number of c-fos+ cells remained the same in P3, P14, and P14 QX groups after i.t. strychnine (Fig. S6), indicating that the developmental decrease in GAD+ cell activation is due to a shift, rather than a loss, of glycinergic control of interneurons, from a broad control of inhibitory (and excitatory) interneurons at birth to a more selective control of GAD−, excitatory interneurons in the adult. Thus, blockade of C-fiber activity at a critical period of development results in delayed GlyR maturation and delayed organization of glycinergic control of inhibitory interneurones.
Discussion
Here we have shown that a major form of inhibition in the dorsal horn of the spinal cord, glycinergic inhibition, is not functional until the second week of life. Furthermore the maturation of this inhibition is dependent upon postnatal activity in peripheral C fibers. Thus, whereas newborn spinal sensory circuits are known to be under strong GABAergic inhibitory control (6, 10), they are free of glycinergic influence, which slowly matures over the first two postnatal weeks. Before that time, not only is there no glycinergic inhibition, but there is evidence of glycinergic facilitation of dorsal horn wide dynamic range neurons, particularly to low-threshold, dynamic brushing of the skin. The switch from glycinergic facilitation within the sensory network to inhibition occurs around P14, coinciding with the strengthening of C-fiber synapses within the superficial dorsal horn (3). C-fiber activity has been implicated in the organization of several sensory circuits within the dorsal horn (27, 28) but to date, no study has successfully silenced C fibers during development without use of a systemic toxin or irreversible damage to the entire nociceptive system. Using a combination of QX-314, lidocaine, and capsaicin, we have blocked activity in C fibers in a single peripheral nerve over a defined period of development and have established the critical role of this activity in the maturation of glycinergic inhibition. Silencing C fibers over 3 d from P10 to P13 delays the maturation of glycinergic inhibition, such that dorsal horn sensory neurons at P14 remain free of glycine inhibition and display glycine facilitation, similar to that normally seen at the earlier age of P3.
There are a number of possible mechanisms underlying the absence of glycinergic inhibition in the neonate. Here we show that glycinergic terminals, as labeled by GlyT2, are diffusely distributed throughout the deeper spinal dorsal horn until the second postnatal week, after which expression patterns begin to be restricted to lamina III, as seen in the adult (20). However, this distribution is not affected by QX-314 treatment, which delays the onset of inhibition, suggesting that it is not critical to the maturation of functional inhibition. The absence of glycinergic inhibition in the neonate could be due to insufficient glycine release: although glycine is present in the spinal cord from embryonic days 16–18 (29, 30), the levels may not be sufficient to activate glycine receptors in the early postnatal period. The anion reversal potential matures early (6, 9) and is unlikely to be an issue here, but postsynaptic glycinergic miniature inhibitory postsynaptic current frequency increases in the dorsal horn over the first three postnatal weeks, suggesting a developmental increase in presynaptic quantal release of glycine and/or of glycinergic terminals.
Because the results show that exogenously applied glycine is inhibitory from birth, the delayed endogenous glycinergic inhibition is likely due to immature synaptic connections. Our results point to the ratio of α2/α1 subunits as a key mechanism for absence of inhibition, because the high ratio observed at P3 but normally down-regulated by P14, was still evident at P14 following QX-314 treatment. The high α2/α1 ratio suggests fewer synaptically anchored glycinergic receptors in the neonate, lowering the likelihood of precise inhibitory signaling. Binding of the anchoring protein, gephyrin to the β subunit is necessary for GlyR cluster formation thus only α/β heteromeric GlyRs will aggregate at inhibitory synapses. β Subunits are absent in neonatal α2 homomeric glycine receptors, unlike the predominant α1 heteromers found in the adult (31, 32). However, whereas the high α2/α1 ratio may explain lack of inhibition, it does not explain glycine facilitation of brush-evoked activity observed here. One possibility is that the little glycinergic inhibition that exists in the neonate is not precisely targeted within the dorsal horn network. We tested this hypothesis by examining the pattern of fos activation in lamina III interneurones following strychnine activation in normal and QX-314–treated rats. The shift in fos activation in lamina III toward GAD+ inhibitory neurons in normal P3 and QX-314–treated animals, compared with P14 untreated animals suggests a wider pattern of inhibitory interneuronal control in the neonate that is refined by C-fiber activity in the second postnatal week. That there is no detectable difference in the number of brush-activated c-fos+ neurons after i.t. strychnine observed between P14 untreated, P3 neonatal, and P14 C-fiber blocked pups suggests a shift from a broad glycinergic control that includes inhibitory interneurons to a more selective control of excitatory interneurons rather than an overall change in total interneuronal activity over this developmental period.
The absence of glycine inhibition is likely to be a major contributor to the excitability of the neonatal dorsal horn and in particular to the strength of myelinated A-fiber–evoked responses, which are normally under strong glycinergic control in the adult (13, 18, 33). A-fiber inputs dominate neonatal dorsal horn activity before the second postnatal week (1, 5, 34) and Aβ fibers form a greater number of monosynaptic inputs to superficial dorsal horn neurons during the first weeks of life relative to C-fiber input (5, 34). Neonatal lamina I and II neurons express c-fos in response to innocuous low threshold input, in contrast to the adult, which requires noxious stimulation (1, 2). Innocuous tactile information appears to enable the organization and strengthening synapses in the neonatal dorsal horn (17, 35) and the absence of glycinergic inhibition would undoubtedly serve to maximize these activity-dependent developmental processes.
In contrast to A fibers, C-fiber synaptic input matures slowly over a number of postnatal weeks. A lower percentage of neonatal dorsal horn neurons respond to noxious stimulation of the receptive field than in the adult, and C-fiber input into the dorsal horn is weak until the end of the first postnatal week (3). Primary afferent fiber input to lamina II shifts over the weeks after birth from a predominantly Aβ fiber to predominantly Aδ- and C-fiber innervation (5, 34). Our finding that the buildup of nociceptive C-fiber input is critical for the development of glycinergic inhibition of cutaneous activity in the dorsal horn explains early reports of somatotopic disorganization and lack of A-evoked inhibition, now known to be glycine mediated, of C-fiber responses in the adult dorsal horn following neonatal systemic capsaicin (4, 27, 28).
A picture emerges from this study of a sensory dorsal horn that is shaped by afferent activity. In the immediate neonatal period, the predominant input is low threshold, from A fibers, and this input is important for organizing terminal fields and refining nociceptive reflexes (35). The absence of glycinergic inhibition and presence of some facilitation at this time could be advantageous in maximizing A-fiber–evoked activity. However, in the second postnatal week, C-fiber central synapses strengthen and C-fiber activity begins to drive the maturation of glycinergic inhibition, through changes in glycinergic receptor subunit expression and synaptic reorganization. Although the exact mechanisms of this receptor subunit reorganization still remain to be elucidated, it seems plausible that these may be NMDA receptor mediated. This inhibition particularly targets A-fiber–evoked activity, dampening down the excitability of the dorsal horn, reducing receptive field size, and maximizing the relative influence of C fibers (18). Thus, the balance of activity from peripheral sensory A fibers and C fibers drives the balance of excitatory and inhibitory circuits within the dorsal horn over a critical postnatal period.
Materials and Methods
Additional materials and methods are given in SI Materials and Methods and Figs. S1–S6.
In Vivo Extracellular Recordings.
Rats were anesthetized, tracheotomized, and artificially ventilated under isoflurane anesthesia [maintenance of 1.8% (vol/vol) in medical O2]. A laminectomy was performed to expose the lumbar spinal cord L4 and L5, and individual WDRs were recorded extracellularly using glass-coated tungsten microelectrodes. WDR neurons were selected such that each neuron tested responded to light touch and to noxious pinch applied to the RF. The RF of a cell was characterized and mapped using pinch and brush and drawn out in detail onto a representative image of the paw.
Pharmacology.
Once baseline responses were established, 100 μL drug or vehicle was applied to the cord. Maximum activity of strychnine (165 ng/g in 0.9% NaCl) was observed at 20 min; this time point was therefore used for all subsequent graphing and analysis. In glycine experiments (400 μg in 0.9% NaCl) maximum activity was observed at 10 min.
Sciatic Nerve Blockade.
P10 (or P17) pups were anesthetized under isoflurane, and 50 μL (P17:100 μL) of a solution containing 0.2% QX-314, 2% (wt/vol) lidocaine (Sigma-Aldrich), or saline was injected at the left sciatic notch. Ten minutes thereafter, 20 μL (P17:50 μL) of 0.05% capsaicin or saline was injected into the same area. Injections were repeated every 8 h (P17:12 h) for 3 d. Sciatic blockade was assessed using a superficial nociceptive pinch behavioral test described elsewhere (26).
Immunohistochemistry.
For α1/β and α2, sections were incubated in anti-α1/β (Synaptic Systems) and visualized using TSA and FITC conjugate. A secondary stain of goat anti-α2 (Santa Cruz) was then visualized using Alexa 594. α2+ or α1/β+ cells were counted within lamina III and expressed as a ratio of α2:α1β. For c-fos and GAD67 stain, rats were anesthetized with urethane and i.t. injected with strychnine or saline, followed 20 min later by brush of one hindpaw for 10 min. Two hours later, the cord was removed and sections were incubated in primary anti–c-fos (Calbiochem), and visualized using nickel DAB amplification. Sections were then incubated in anti-GAD67 (Chemicon) and developed using brown DAB. C-fos only and c-fos/GAD+ cells were counted within LIII. Counts were performed on five sections chosen at random per animal, with four animals per group for both α subunit and c-fos experiments.
Supplementary Material
Acknowledgments
We thank Dr. Francisco Zafra for the kind gift of the GlyT2 antibody used on naive animals. This work has been supported by the Medical Research Council (United Kingdom).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118960109/-/DCSupplemental.
References
- 1.Fitzgerald M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J Physiol. 1985;364:1–18. doi: 10.1113/jphysiol.1985.sp015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coggeshall RE, Jennings EA, Fitzgerald M. Evidence that large myelinated primary afferent fibers make synaptic contacts in lamina II of neonatal rats. Brain Res Dev Brain Res. 1996;92:81–90. doi: 10.1016/0165-3806(95)00207-3. [DOI] [PubMed] [Google Scholar]
- 3.Jennings E, Fitzgerald M. Postnatal changes in responses of rat dorsal horn cells to afferent stimulation: A fibre-induced sensitization. J Physiol. 1998;509:859–868. doi: 10.1111/j.1469-7793.1998.859bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–1258. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakatsuka T, Ataka T, Kumamoto E, Tamaki T, Yoshimura M. Alteration in synaptic inputs through C-afferent fibers to substantia gelatinosa neurons of the rat spinal dorsal horn during postnatal development. Neuroscience. 2000;99:549–556. doi: 10.1016/s0306-4522(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 6.Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci. 2004;24:4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordero-Erausquin M, Coull JA, Boudreau D, Rolland M, De Koninck Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: Impact of chloride extrusion capacity. J Neurosci. 2005;25:9613–9623. doi: 10.1523/JNEUROSCI.1488-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingram RA, Fitzgerald M, Baccei ML. Developmental changes in the fidelity and short-term plasticity of GABAergic synapses in the neonatal rat dorsal horn. J Neurophysiol. 2008;99:3144–3150. doi: 10.1152/jn.01342.2007. [DOI] [PubMed] [Google Scholar]
- 9.Keller AF, Coull JAM, Chery N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. J Neurosci. 2001;21:7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremner L, Fitzgerald M, Baccei M. Functional GABA(A)-receptor-mediated inhibition in the neonatal dorsal horn. J Neurophysiol. 2006;95:3893–3897. doi: 10.1152/jn.00123.2006. [DOI] [PubMed] [Google Scholar]
- 11.Narikawa K, Furue H, Kumamoto E, Yoshimura M. In vivo patch-clamp analysis of IPSCs evoked in rat substantia gelatinosa neurons by cutaneous mechanical stimulation. J Neurophysiol. 2000;84:2171–2174. doi: 10.1152/jn.2000.84.4.2171. [DOI] [PubMed] [Google Scholar]
- 12.Todd AJ. An electron microscope study of glycine-like immunoreactivity in laminae I-III of the spinal dorsal horn of the rat. Neuroscience. 1990;39:387–394. doi: 10.1016/0306-4522(90)90275-9. [DOI] [PubMed] [Google Scholar]
- 13.Sherman SE, Loomis CW. Strychnine-sensitive modulation is selective for non-noxious somatosensory input in the spinal cord of the rat. Pain. 1996;66:321–330. doi: 10.1016/0304-3959(96)03063-1. [DOI] [PubMed] [Google Scholar]
- 14.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: Effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 15.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 16.Petersson P, Waldenström A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- 17.Granmo M, Petersson P, Schouenborg J. Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci. 2008;28:5494–5503. doi: 10.1523/JNEUROSCI.0651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeilhofer HU, Wildner H, Yévenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spike RC, Watt C, Zafra F, Todd AJ. An ultrastructural study of the glycine transporter GLYT2 and its association with glycine in the superficial laminae of the rat spinal dorsal horn. Neuroscience. 1997;77:543–551. doi: 10.1016/s0306-4522(96)00501-5. [DOI] [PubMed] [Google Scholar]
- 21.Miraucourt LS, Moisset X, Dallel R, Voisin DL. Glycine inhibitory dysfunction induces a selectively dynamic, morphine-resistant, and neurokinin 1 receptor- independent mechanical allodynia. J Neurosci. 2009;29:2519–2527. doi: 10.1523/JNEUROSCI.3923-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: Disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 24.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 25.Binshtok AM, et al. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111:127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung YC, et al. Ephedrine blocks rat sciatic nerve in vivo and sodium channels in vitro. Anesthesiology. 2005;103:1246–1252. doi: 10.1097/00000542-200512000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Wall PD, Fitzgerald M, Nussbaumer JC, Van der Loos H, Devor M. Somatotopic maps are disorganized in adult rodents treated neonatally with capsaicin. Nature. 1982;295:691–693. doi: 10.1038/295691a0. [DOI] [PubMed] [Google Scholar]
- 28.Cervero F, Plenderleith MB. C-fibre excitation and tonic descending inhibition of dorsal horn neurones in adult rats treated at birth with capsaicin. J Physiol. 1985;365:223–237. doi: 10.1113/jphysiol.1985.sp015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berki AC, O’Donovan MJ, Antal M. Developmental expression of glycine immunoreactivity and its colocalization with GABA in the embryonic chick lumbosacral spinal cord. J Comp Neurol. 1995;362:583–596. doi: 10.1002/cne.903620411. [DOI] [PubMed] [Google Scholar]
- 30.Allain AE, Baïri A, Meyrand P, Branchereau P. Expression of the glycinergic system during the course of embryonic development in the mouse spinal cord and its co-localization with GABA immunoreactivity. J Comp Neurol. 2006;496:832–846. doi: 10.1002/cne.20967. [DOI] [PubMed] [Google Scholar]
- 31.Kirsch J, Meyer G, Betz H. Synaptic targeting of ionotropic neurotransmitter receptors. Mol Cell Neurosci. 1996;8:93–98. doi: 10.1006/mcne.1996.0048. [DOI] [PubMed] [Google Scholar]
- 32.Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: Where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS ONE. 2007;2(11):e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JS, Nakatsuka T, Nagata K, Higashi H, Yoshimura M. Reorganization of the primary afferent termination in the rat spinal dorsal horn during post-natal development. Brain Res Dev Brain Res. 1999;113:29–36. doi: 10.1016/s0165-3806(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 35.Waldenström A, Thelin J, Thimansson E, Levinsson A, Schouenborg J. Developmental learning in a pain-related system: Evidence for a cross-modality mechanism. J Neurosci. 2003;23:7719–7725. doi: 10.1523/JNEUROSCI.23-20-07719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.