Abstract
Depression is a debilitating mental illness and is often comorbid with metabolic disorders such as type 2 diabetes. Adiponectin is an adipocyte–derived hormone with antidiabetic and insulin-sensitizing properties. Here we show that adiponectin levels in plasma are reduced in a chronic social-defeat stress model of depression, which correlates with decreased social interaction time. A reduction in adiponectin levels caused by haploinsufficiency results in increased susceptibility to social aversion, “anhedonia,” and learned helplessness and causes impaired glucocorticoid-mediated negative feedback on the hypothalamic–pituitary–adrenal (HPA) axis. Intracerebroventricular (i.c.v.) injection of an adiponectin neutralizing antibody precipitates stress-induced depressive-like behavior. Conversely, i.c.v. administration of exogenous adiponectin produces antidepressant-like behavioral effects in normal-weight mice and in diet-induced obese diabetic mice. Taken together, these results suggest a critical role of adiponectin in depressive-like behaviors and point to a potential innovative therapeutic approach for depressive disorders.
Keywords: adipose tissue, adipokines, tail suspension test, forced swim test, dexamethasome suppression test
Depression is a chronic and debilitating mental illness. An association between depression and diabetes has been well recognized as the prevalence of depression is doubled in type 2 diabetics compared with the general population (1–3). However, treatment with currently available antidepressant drugs can increase the risk of developing type 2 diabetes among those at high risk for the disease (4–6). Thus, characterization of the biological factors for comorbidity of depression and diabetes and identification of innovative therapeutic targets for the treatment of depression, especially for those with comorbid diabetes, are urgently needed.
Adiponectin, a circulating hormone exclusively secreted from adipocytes, is a biomarker of type 2 diabetes and possesses antidiabetic and insulin-sensitizing properties (7). Plasma levels of adiponectin are positively correlated with insulin sensitivity and inversely associated with obesity and type 2 diabetes (8–11). Adiponectin is present in plasma as either a full-length or a globular form that is generated by proteolytic cleavage of full-length adiponectin. Full-length adiponectin is the predominant circulating form and can be found as a trimer, hexamer, or high molecular weight (HMW) oligomer (10, 12), whereas globular adiponectin exists as a trimer (13). Evidence shows that adiponectin enters the brain from circulation (14–17). Intraventricular (i.v.) injection of adiponectin leads to a rise in cerebrospinal fluid (CSF) adiponectin levels in mice and rats (15, 18), and trimeric and hexameric forms of adiponectin are detected in the CSF of adiponectin knockout mice after i.v. injection (16). Previous studies have demonstrated the presence of adiponectin receptors, AdipoR1 and AdipoR2, in the central nervous system (16, 18–21); however, their expression patterns in discrete brain areas remain to be determined.
Emerging clinical evidence suggests an association between circulating adiponectin levels and depression but with inconsistent results, perhaps as a result of small sample sizes, sex differences, and age ranges covered in these studies (22–26). Questions remain to be answered as to whether adiponectin plays a role in the pathophysiology of depression and whether adiponectin modulates depressive behaviors. In this study, we used multiple experimental stress paradigms to induce depressive-like symptoms and investigated the relationship between endogenous adiponectin levels and the development of depressive-like behaviors. Furthermore, we examined whether exogenous adiponectin has antidepressant-like properties.
Results
Chronic Social Defeat Reduces Circulating Adiponectin Concentrations.
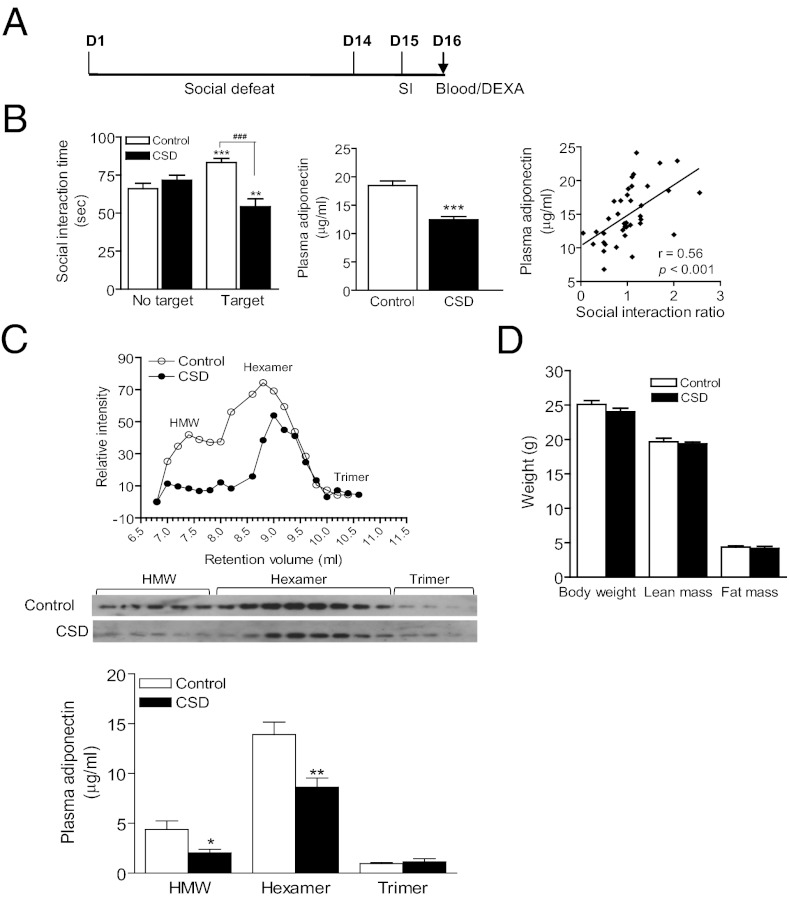
First, we determined whether circulating adiponectin levels are altered in an animal model of depression. Chronic social-defeat stress to male C57BL/6J mice induces a range of symptoms of human depression including social withdrawal and anhedonia; thus it has been used as a model of depression (27–29). In this study, mice were exposed to 14 d of repeated social-defeat stress (29), and plasma adiponectin concentrations were determined 24 h or 48 h after the last social-defeat session. Total adiponectin levels in plasma were decreased after social defeat (24 h: control 18.37 ± 0.87, defeat 12.61 ± 0.96, t(19) = 4.467, P < 0.001; 48 h: control 18.46 ± 1.15, defeat 13.11 ± 0.61, t(20) = 4.538; P < 0.001). In attempting to correlate changes in adiponectin levels with depressive behavior, a separate cohort of mice was exposed to 14 d of social-defeat stress, and social interaction and plasma adiponectin levels were examined in the same individual mice (Fig. 1A). A decline in social interaction was found to strongly correlate with a reduction in total plasma adiponectin levels (Fig. 1B, Right, r = 0.56; P < 0.001).
Fig. 1.
Chronic social defeat reduces circulating adiponectin concentrations. (A) Timeline of the experimental procedure. D1–16, day1–16; SI, social interaction; DEXA, dual energy X-ray absorptiometry. (B) Chronic social defeat (CSD) for 14 consecutive days induces reduction of social interaction time (Left; F(3,72) = 8.914, P < 0.001) and decreases total plasma levels of adiponectin (Center; t(36) = 6.239, P < 0.0001). Nondefeat control, n = 16; CSD, n = 22. **P < 0.01, ***P < 0.001 compared with their respective no target conditions; ###P < 0.001 compared control mice in the presence of a social target. There is a significant correlation between total plasma adiponectin levels and social interaction ratio (time spent in the interaction zone in the presence of a social target/ time spent in interaction zone without a social target) (r = 0.56; P < 0.001). (C) Analysis of the oligomeric complex distribution of adiponectin. (Upper) Representative distribution of adiponectin oligomeric complexes from socially defeated mice in comparison with nondefeated control mice. Adiponectin was separated by gel filtration chromatography followed by immunoblotting. (Lower) Concentrations of oligomeric complexes of adiponectin in plasma that was calculated on the basis of the relative ratio of adiponectin oligomeric complexes revealed by gel filtration and total adiponectin levels quantified by ELISA. CSD decreases relative levels of HMW (t(8) = 2.583: P < 0.05) and hexamers (t(8) = 3.420, P < 0.01) without affecting trimers (t(8) = 0.5851, P > 0.5). n = 5 per group. (D) Body weight and body composition measured by DEXA at the end of the experiments. CSD has no significant effect on body weight (t(14) = 1.438 P = 0.172), fat mass (t(14) = 0.5138, P = 0.615) or lean mass (t(14) = 0.5682, P = 0.579). n = 8 per group. All data are expressed as mean ± SEM.
Recent studies suggest that different multimeric forms of adiponectin can influence its ability to pass the blood–brain barrier and its biological activity (14–17). Next, we examined the effects of social-defeat stress on the distribution of oligomeric complexes of adiponectin using gel filtration chromatography followed by immunoblotting. The levels of both HMW and hexameric adiponectin were significantly reduced in defeated mice. The amount of trimeric adiponectin was low in plasma and showed no significant change after social defeat (Fig. 1C). As adipose tissue is the primary source of adiponectin, we asked whether reduced plasma adiponectin levels were due to changes in fat mass. Body composition (fat mass and lean mass), body weight, and plasma adiponectin levels were measured in mice at 48 h following 14 d of social defeat (Fig. 1D). Fat mass and body weight were unchanged, although a significant reduction in total plasma adiponectin levels were observed in these defeated mice (control 16.22 ± 1.11, defeat 9.91 ± 0.93; t(14) = 4.365, P < 0.001), suggesting that reduced total plasma adiponectin levels are unlikely to be secondary to a change in adipose mass. Next, adiponectin mRNA levels in adipose tissue were measured by quantitative real-time PCR and showed no significant difference between defeated and nondefeated control groups (control 100 ± 5.8, defeat 108 ± 11.3; t(17) = 1.072, P = 0.298).
Adiponectin Haploinsufficiency Increases Susceptibility to Depression-Like Behaviors.
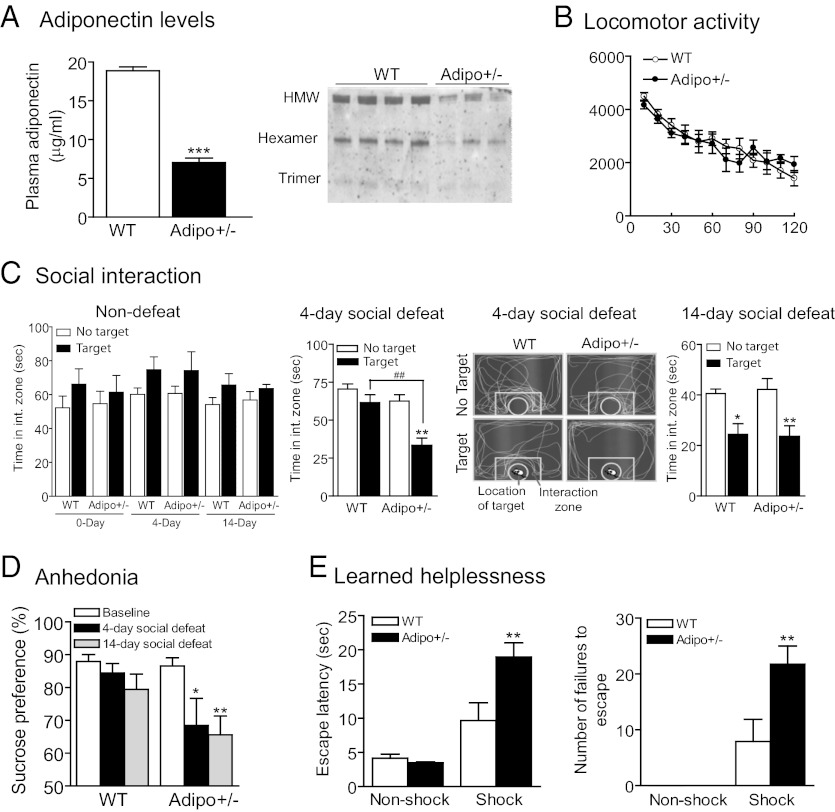
To determine the functional consequence of reduced circulating adiponectin levels on the development of depressive symptoms, we used adiponectin haploinsufficient (Adipo+/−) mice with an ∼60% reduction in total plasma adiponectin levels (Fig. 2A, Left), mimicking the condition of socially defeated wild-type mice. Also, Adipo+/− mice showed reduction in both hexamers and HMW oligomers (Fig. 2A, Right). Adipo+/− mice exhibited normal locomotor activity (Fig. 2B) and sensory functions including visual and olfactory functions (Fig. S1). To determine the effect of adiponectin haploinsufficiency on social-defeat–induced depressive behaviors, mice were subjected to social-defeat stress for 4 or 14 d and subsequently tested for social interaction. Baseline social interaction in Adipo+/− mice and wild-type littermate controls without exposure to social defeat was similar (Fig. 2C, Left). A short-term (4 d) social defeat significantly increased social aversion, i.e., reduced social interaction time, in Adipo+/− mice but not in wild-type littermate controls (Fig. 2C, Center). After 14 d of social defeat, both Adipo+/− mice and wild-type littermate controls showed social aversion to an equivalent extent (Fig. 2C, Right). Chronic social defeat also induces “anhedonia,” a core symptom of depression, which can be measured using the sucrose preference test in mice (28, 29). Baseline sucrose preference was indistinguishable between Adipo+/− mice and littermate controls. A significant reduction in sucrose preference, an anhedonic-like phenotype, was seen in Adipo+/− mice after exposure to 4- or 14-d social defeat (Fig. 2D), whereas wild-type mice showed a decreasing trend with increasing exposure to social defeat, but did not reach statistical significance (Fig. 2D). These results indicate that Adipo+/− mice are more prone to social-defeat–induced depressive symptoms. Mice were also tested in a learned helplessness paradigm, a depression model in which mice are exposed to inescapable and unpredictable stress, e.g., foot shock, and subsequently develop deficits in coping capability in an aversive but escapable situation (29). After two consecutive days of exposure to inescapable foot shock, Adipo+/− mice exhibited longer escape latency and a greater number of escape failures than wild-type littermate controls (Fig. 2E). Under the nonshock control condition (exposure to the shock chamber without delivering foot shock), Adipo+/− mice and wild-type littermates were similar in their escape performance (Fig. 2E). To rule out altered pain sensitivity as a confounding factor in the learned helplessness test, mice were tested in the hot plate test and showed no genotype difference in the hot plate response latency (Adipo+/− 34.9 ± 6.92 s, wild-type littermate 38.0 ± 7.50 s; P > 0.5), suggesting that the results in the learned helplessness test reflect true deficits in stress coping. Taken together, these results suggest that adiponectin haploinsufficiency increases susceptibility to stress-induced depression-like behaviors.
Fig. 2.
Adiponectin haploinsufficiency increases susceptibility to stress-induced depression-like behaviors. (A, Left) Total plasma adiponectin levels in Adipo+/− mice and wild-type littermates. n = 5–6 per group. ***P < 0.001. (Right) Adiponectin oligomers as determined by gel elctrophoresis under nonreducing and nonheat-denaturing conditions. (B) Locomotor activity. There is no significant difference in locomotor performance within 2 h between Adipo+/− mice and wild-type littermate controls (F(1,188) = 0.073, P = 0.788). n = 8 per group. (C) Social-defeat–induced social withdrawal. Left graph, nondefeated Adipo+/− mice and wild-type littermate mice show no difference in social interaction measured at 0, 4, and 14 d after housing in cages identical to defeated mice (genotype F(1,82) = 0.004, P = 0.948; target F(1,82) = 7.350, P < 0.01; time F(2,82) = 1.752, P = 0.180; genotype × target F(1,82) = 0.282, P = 0.597; genotype × time F(2,82) = 0.0128, P = 0.987; genotype × target × time F(2,82) = 0.449, P = 0.956). Center graph and image, Adipo+/− mice display significant social aversion following 4 d of social defeat (genotype F(1,48) = 15.384, P < 0.001; target F(1,48) = 17.194, P < 0.001; genotype × target F(1,48) = 4.860, P < 0.05). Right graph, both Adipo+/− mice and wild-type littermate controls exhibit social aversion after 14 d of social defeat (genotype F(1,38) = 0.008, P = 0.929; target F(1,38) = 20.486, P < 0.001; genotype × target F(1,38) = 0.108, P = 0.744). Nondefeat control n = 7–9 per group; defeat, n = 10–15 per group. **P < 0.01 compared with the respective no-target conditions; ##P < 0.01 compared with wild-type littermates with the presence of a target. (D) Social-defeat–induced anhedonia. Adipo+/− mice show significant reduction in sucrose preference following social-defeat stress (genotype F(1,46) = 6.785, P = 0.01; defeat F(2,46) = 4.936, P = 0.01). n = 8–9 per group. *P < 0.05, **P < 0.01 compared with presocial-defeat conditions. (E) Inescapable foot-shock–induced learned helplessness. There are significant effects of genotype, shock stress, and genotype × shock stress interaction on escape latency (Left, genotype F(1,32) = 4.248, P < 0.05; shock stress F(1,32) = 25.015, P < 0.001; genotype × stress interaction F(1,32) = 5.636, P < 0.05) and number of failure to escape (Right, genotype F(1,32) = 4.545, P < 0.05; shock stress F(1,32) = 20.831, P < 0.001; genotype × stress interaction F(1,32) = 4.545, P < 0.05). Wild-type, n = 6 for nonshock and n = 10 for shock treatment; Adipo+/−, n = 8 for nonshock and n = 12 for shock treatment. *P < 0.05, **P < 0.01 compared with wild-type littermates exposed to foot-shock stress. All data are expressed as mean ± SEM.
Adiponectin Haploinsufficiency Impairs Glucocorticoid-Mediated Negative Feedback on the Hypothalamic–Pituitary–Adrenal Axis.
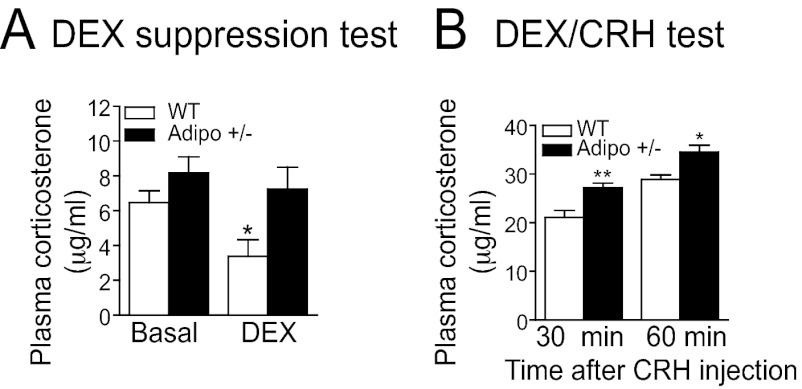
A hyperactive hypothalamic–pituitary–adrenal (HPA) axis is one of the most consistent features of major depression. Depressed patients show marked resistance to dexamethasone (DEX) suppression and exaggerated response to a combination of DEX suppression and corticotrophin releasing hormone (CRH) stimulation (30, 31). Using the same tests, we measured the sensitivity of the HPA axis of Adipo+/− mice to glucocorticoid-induced negative feedback. For the DEX suppression test, mice received i.p. injection of DEX (50 μg/kg) 6 h before corticosterone was measured. DEX treatment significantly decreased plasma corticosterone levels in wild-type mice, but failed to do so in Adipo+/− mice (Fig. 3A). For the combined DEX/CRH test, mice received an injection of DEX (50 μg/kg, i.p.) followed by a CRH challenge (75 μg/kg, i.p.). The rise of corticosterone concentrations in plasma at 30 and 60 min after CRH stimulation was significantly higher in Adipo+/− mice than wild-type littermate controls (Fig. 3B). These results reflect a deficit in negative feedback control of the HPA axis in Adipo+/− mice, mimicking what occurs in depressed patients (32, 33).
Fig. 3.
Adiponectin haploinsufficiency results in resistance to glucocorticoid-induced feedback inhibition of hypothalamic–pituitary–adrenal axis. (A) Dexamethasone (DEX) suppression test. DEX-induced suppression of corticosterone secretion is impaired in Adipo+/− mice (treatment F(1,30) = 8.394, P < 0.01; genotype F(1,30) = 14.176, P < 0.001; treatment × genotype F(1,30) = 3.366; P = 0.077). Wild-type, n = 10 for basal and n = 9 for DEX treatment; Adipo+/−, n = 10 for basal and n = 7 for DEX treatment. *P < 0.05 compared with basal levels. (B) Combined DEX/CRH test. Adipo+/− mice show enhanced corticosterone response to CRH challenge (F(1,40) = 12.303, P = 0.001). Wild-type, n = 10; Adipo+/−, n = 11. *P < 0.05, **P < 0.01 compared with wild-type littermate controls. All data are expressed as mean ± SEM.
Expression of AdipoR1 and AdipoR2 in Mood-Related Brain Areas.
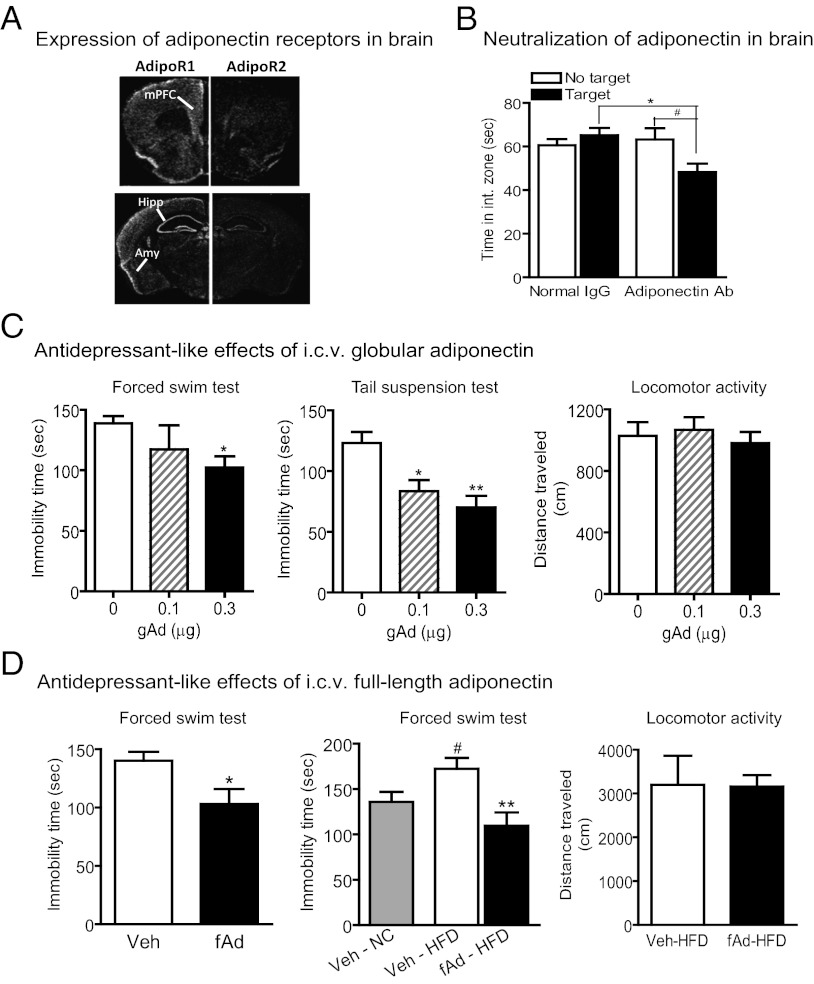
Although previous studies have indicated the presence of AdipoR1 and AdipoR2 in the brain (16, 20, 21), the distribution of these receptors in discrete brain areas implicated in mood regulation remain unclear. In situ hybridization with specific cRNA probes revealed that AdipoR1 mRNA was highly expressed in various brain regions, such as the medial prefrontal cortex (mPFC), hippocampus, and amygdala (Fig. 4A). AdipoR2 mRNA expression was restricted to a few brain areas including the hippocampus and certain hypothalamic nuclei (Fig. 4A).
Fig. 4.
Central adiponectin action modulates depression-like behaviors. (A) In situ hybridization showing expression patterns of AdipoR1 and AdipoR2 mRNA in the forebrain. mPFC, medial prefrontal cortex; Hipp, hippocampus; Amy, amygdala. (B) Effect of endogenous adiponectin in the brain on defeat-induced social aversion. Mice received i.c.v. injection of monoclonal adiponectin antibody (0.6 μg) or normal IgG 1 h before each social-defeat episode and exhibited significant social aversion after 4 d of social defeat (F(3,36) = 3.766, P < 0.05). n = 10 per group. #P = 0.01 compared with the no-target condition; *P < 0.01 compared with control mice received normal IgG. (C) Antidepressant-like properties of i.c.v. infusion of globular adiponectin (gAd) in normal chow-diet–fed mice at 9–11 wk of age. I.c.v. injection of globular adiponectin decreases immobility time in the forced swim test (Left; control n = 16, gAd (0.1 μg) n = 10, gAd (0.3 μg) n = 16; F(2,39) = 3.236, P < 0.05) and the tail suspension test (Center; control n = 18, gAd (0.1 μg) n = 9, gAd (0.3 μg) n = 16; F(2,40) = 9.267, P < 0.001). *P < 0.05, **P < 0.01) compared with vehicle-treated control mice without affecting locomotor activity measured in the home cage (Right, control n = 14, gAd (0.1 μg) n = 8, gAd (0.3 μg) n = 13; F(2,32) = 0.238, P = 0.789). (D) Antidepressant-like effect of i.c.v. infusion of full-length adiponectin (fAd) in normal chow diet (NC) or high-fat diet (HFD)-fed mice. Left, i.c.v. injection of full-length adiponectin (1 µg) significantly decreases immobility time in the forced swim test in NC fed mice at 9–11 wk of age (t(14) = 2.503, P < 0.05; n = 8 per group). *P < 0.05 compared with vehicle (veh)-treated controls. Center, i.c.v. infusion of fAd (1 μg) produces a significant reduction in immobility in the forced swim test in HFD (60% of kcal from fat)-fed mice at 4 wk of age for 16 wk (Left, t(17) = 3.397, P < 0.01; n = 9–10 per group) without affecting locomotor activity measured in an open box (Right, t(8) = 0.0531, P = 0.959; n = 5 per group). #P < 0.05 compared with vehicle (Veh)-treated NC-fed mice; **P < 0.01 compared with Veh-treated HFD-fed mice. All data are expressed as mean ± SEM.
Neutralizing Adiponectin in the Brain Increases Susceptibility to Depression-Like Behavior.
The expression of AdipoRs in mood-related brain areas suggests a role of central adiponectin in depressive behaviors. To test this idea, endogenous adiponectin in the brain was neutralized by intracerebroventricular (i.c.v.) injection of an adiponectin monoclonal antibody. Mice received an i.c.v. injection of the adiponectin neutralizing antibody or normal mouse serum IgG (control) 1 h before each social-defeat episode. Mice treated with the adiponectin antibody displayed significant social aversion after 4 d of social defeat compared with mice treated with normal IgG (Fig. 4B). This finding suggests that endogenous adiponectin in the brain plays a role in determining the susceptibility to stress-induced social withdrawal.
Intracerebroventricular Administration of Adiponectin Produces an Antidepressant-Like Effect.
We next examined whether administration of exogenous adiponectin to the brain produces antidepressant-like effects. Both globular and full-length adiponectin were tested for their antidepressant-like properties in adult mice. The forced swim test and tail suspension test are widely used for screening antidepressant drugs (34, 35). I.c.v. injection of recombinant globular adiponectin to normal-chow–fed mice significantly decreased immobility time in the forced swim test as well as in the tail suspension test, indicating an antidepressant-like effect (Fig. 4C, Left and Center). Locomotor activity was not affected by i.c.v. injection of globular adiponectin (Fig. 4C, Right), confirming that antidepressant-like behavioral effects of globular adiponectin were not a nonspecific consequence of hypolocomotion. A decrease in immobility in the forced swim test was also observed following i.c.v. injection of full-length adiponectin to normal-chow–fed mice (Fig. 4D, Left). These data suggest that both globular and full-length adiponectin have antidepressant-like activity.
The C57BL/6 mouse strain chronically fed a high-fat diet (HFD) is an established rodent model of diet-induced obesity and type 2 diabetes. Mice were fed with a HFD (60 kcal% fat) for 16 wk to induce an obese, diabetic phenotype. Their response to the behavioral effect of adiponectin in the forced swim test was evaluated. Mice fed a HFD exhibited increased immobility time compared with age-matched, normal-chow–fed mice (Fig. 4D, Center). I.c.v. injection of full-length adiponectin in HFD-fed mice significantly reduced immobility time without affecting locomotor activity (Fig. 4D, Center and Right). The HFD-fed mice were confirmed to have developed severe obesity, hyperglycemia, and hyperinsulinemia. Plasma adiponectin levels were reduced in the HDF-fed mice (Fig. S2).
Discussion
Adiponectin is well known for its ability to regulate metabolic function. In this study, we addressed the unique role of adiponectin in depression-related behaviors. We demonstrated that circulating adiponectin levels were reduced in a chronic social-defeat model of depression and were inversely correlated with the social interaction ratio. Adiponectin haploinsufficiency increased the susceptibility to stress-induced depressive behaviors and impaired HPA function. AdipoR1 and AdipoR2 were highly expressed in depression-related brain areas, and administration of adiponectin to the brain elicited antidepressant-like behavioral effects in normal-weight mice and in obese diabetic mice. Our results suggest that adiponectin levels are critical in determining susceptibility to depressive behaviors and adiponectin could be considered as a potential innovative therapeutic target for depression treatment.
It is well established that adiponectin regulates glucose and lipid metabolism (7). Circulating adiponectin levels are reduced by chronic high-fat diets and are inversely associated with adipose mass, insulin resistance, and hyperglycemia (36–38). A unique and important finding from this study is that circulating adiponectin levels are reduced by chronic social-defeat stress, independent of changes in adipose mass. This reduction in circulating adiponectin levels correlated with social withdrawal, a common symptom in psychiatric disorders such as depression and posttraumatic stress disorder. The mechanisms underlying social-defeat–induced reduction in circulating adiponectin levels are not yet known. However, studies have shown that glucocorticoid stress hormones inhibit adiponectin gene expression and secretion both in vitro and in vivo (39–42). It seems reasonable to speculate that social-defeat–induced decrease in plasma adiponectin levels may be attributed to stress-induced glucocorticoid surge. Analysis of adipose tissue gene expression revealed no change in adiponectin mRNA levels after social defeat. Low circulating adiponectin levels induced by social defeat could occur at translational/posttranslational modification levels.
Further studies suggest that decreased adiponectin levels in defeated mice contribute to the development of depressive behaviors. Following a short period of social-defeat stress (4 d), adiponectin haploinsufficient mice exhibited significant social aversion and anhedonia, whereas wild-type mice only showed social aversion after 14 d of social defeat, suggesting that adiponectin insufficiency increases the susceptibility to social-defeat stress. Similar effects of adiponectin haploinsufficiency were also seen in the learned helplessness model of depression. Adiponectin haploinsufficient mice displayed increased helplessness after exposure to inescapable and unpredictable electric foot shocks. These depression-like behavioral phenotypes may be due, at least in part, to an impairment of glucocorticoid negative feedback functions as these mutant mice exhibited insensitivity to glucocorticoid negative feedback in the DEX suppression test and exaggerated response to CRH stimulation. This could lead to overactivation of the HPA axis, a key factor in the development of depression (43–45).
Adiponectin undergoes oligomerization and different oligomeric complexes possess distinct biological activities. The exact isoforms of adiponectin involved in depressive behaviors remain unclear. As plasma hexameric adiponectin, a form that can pass the blood–brain barrier (16), is decreased by social defeat and in adiponectin haploinsuffcient mice, the endogenous hexameric isoform may be involved in mediating the depressive-like behavioral phenotype. By contrast, i.c.v. infusion of recombinant globular adiponectin or recombinant full-length adiponectin produces antidepressant-like behavioral effects in normal weight mice. Adiponectin also elicits antidepressant-like effects in obese diabetic mice. This is of great importance as current antidepressant medications increase the risk for type 2 diabetes (4–6). So far, only about one-third of the patients suffering from major depressive disorder can be successfully treated with antidepressant drugs. Adiponectin with its antidiabetic and insulin-sensitizing properties would serve as an innovative therapeutic target for depression, especially for those individuals with comorbid diabetes or prediabetes and those treatment-resistant to current antidepressant medications. Compounds that increase endogenous adiponectin levels may be proven to have beneficial effects on treatment of depression. Administration of peroxisome proliferator-activated receptor-γ (PPARγ) agonists increases plasma levels of adiponectin by two- to threefold (46–49). Interestingly, it has been recently reported that PPARγ agonists, i.e., pioglitazone and rosiglitazone, produce antidepressant effects (50, 51). We speculate that the antidepressant-like effects of PPARγ agonists may be mediated via elevating endogenous adiponectin levels.
The functional mechanisms underlying adiponectin actions in the central nervous system are so far poorly understood. The distinct expression of AdipoR1 and AdipoR2 in the limbic forebrain areas suggest different physiological roles for these receptors. It has been reported that AdipoR1 has greater binding affinity to globular adiponectin, whereas adipoR2 has an intermediate affinity for both globular and full-length adiponectin (19). Activation of AdipoR1 and AdipoR2 can stimulate the activities of AMP-activated protein kinase (AMPK) and p38 mitogen-activated protein kinase (p38MAPK). We have recently shown that the p38MAPK signaling pathway mediates adiponectin-induced phosphorylation at the inhibitory Ser389 site of glycogen synthase kinase 3β (GSK-3β) (19). Substantial evidence has linked active GSK-3β to increased susceptibility to mood disorders and inhibition of GSK-3β to therapeutic outcomes of antidepressants (52–54). We propose that adiponectin-induced inhibition of GSK-3β may underlie the mechanisms of the antidepressant-like effects of adiponectin. Moreover, hippocampal neurogenesis has been implicated in the mechanisms of action of antidepressant medications (55, 56). Adiponectin stimulates hippocampal neurogenesis (19), which may contribute to its antidepressant-like behavioral effects. Biosynthesis, secretion, transport of adiponectin, and the interaction between adiponectin and its receptors are complex processes. Future studies will be required to identify the factors that mediate stress-induced reduction in circulating adiponectin and determine the receptor subtype(s) and neuronal circuits that are responsible for the antidepressant-like effects of adiponectin.
Materials and Methods
Animals.
Adult wild-type male C57BL/6J mice fed regular chow diet or a high-fat diet (60% kcal from fat; Research Diets) were purchased from Jackson Laboratory and maintained on the same diets. Male CD1 retired breeders, purchased from Charles River, were used as the resident mice for the social-defeat paradigm. Heterozygous adiponectin-deficient mice on a C57BL/6J genetic background were intercrossed to generate adiponectin-haploinsufficient mice and wild-type littermates (57). For further details see SI Materials and Methods.
Chronic Social Defeat.
A resident-intruder paradigm was used as previously reported (29). Briefly, each male mouse on C57BL/6J genetic background was individually introduced to the home cage of an unfamiliar aggressive CD1 resident mouse for 10 min and physically defeated. After the defeat, the resident CD1 mouse and the intruder mouse were housed in one-half of the cage separated by a perforated plastic divider to allow visual, olfactory, and auditory contact for the remainder of the 24-h period. Mice were exposed to a new resident CD1 mouse cage and subjected to social defeat each day for 4 or 14 consecutive days. Nondefeated control mice were housed two per cage in cages identical to those used for the socially defeated mice.
Analysis of Distribution of Adiponectin Oligomeric Complexes.
Body Composition Assessment.
The body composition was assessed by dual-energy X-ray absorptiometry (DEXA) (GE Medical Systems) at 48 h after social defeat.
cRNA Probe Synthesis and in Situ Hybridization.
RNA Extraction from Adipose Tissue and Real Time-PCR Analysis.
Dexamethasone Suppression Test.
Mice received an i.p. injection of DEX (50 μg/kg body weight). Blood samples were collected by tail nick 6 h after DEX injection. For the combined DEX/CRH test, mice received an injection of dexamethasone (50 μg/kg body weight, i.p.). Six hours after administration of dexamethasone, mice were injected with 75 μg/kg of CRH (American Peptide). Blood samples were collected 30 and 60 min after CRH injection and corticosterone concentrations were determined by RIA (SI Materials and Methods).
Plasma Glucose, Insulin, and Adiponectin Measurement.
Cannula Implantation and Central Microinjection.
Behavioral Testing.
All of the behavioral tests were performed in adult male mice at 9–12 wk of age, except the experiments using mice fed a high-fat diet for 16 wk.
Social interaction test was performed as described previously (29, 58). The test was conducted in a 40 × 40 cm open field arena enclosed in a dark testing chamber and illuminated with infrared light. Mice were tested for two 2.5-min sessions in an open arena with a 9-cm round wire basket located at one end of the arena in the interaction zone (Fig. 2C). For experimental details, see SI Materials and Methods.
Behavioral tests, including sucrose preference, learned helplessness, hot plate, tail suspension test, forced swim test, locomotor activity, visual cliff, and olfactory function, were performed as described previously (58–60). For experimental details, see SI Materials and Methods.
Statistical Analysis.
All results are expressed as mean ± SEM. Statistical analyses were performed using two-tailed t tests, one-way, or multiway ANOVAs followed by a post hoc Bonferroni/Dunn or Tukey/Kramer (for unequal n) test. Correlations were determined by linear regression analysis.
Supplementary Material
Acknowledgments
The authors thank Dr. Alan Frazer for critical reading of an early version of the manuscript. This work was supported by a National Alliance for Research on Schizophrenia and Depression Independent Investigator Award (to X.-Y.L.) and Grants MH096251 and MH076929 from the National Institutes of Health (to X.-Y.L.), DK76902 (to FL), and DK55758 (to P.E.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202835109/-/DCSupplemental.
References
- 1.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 2.Knol MJ, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 4.Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166:591–598. doi: 10.1176/appi.ajp.2008.08071065. [DOI] [PubMed] [Google Scholar]
- 5.Brown LC, Majumdar SR, Johnson JA. Type of antidepressant therapy and risk of type 2 diabetes in people with depression. Diabetes Res Clin Pract. 2008;79:61–67. doi: 10.1016/j.diabres.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Rubin RR, et al. Diabetes Prevention Program Research Group Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care. 2008;31:420–426. doi: 10.2337/dc07-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trujillo ME, Scherer PE. Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 8.Cnop M, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 9.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 10.Waki H, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 11.Weyer C, et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 12.Pajvani UB, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 14.Kusminski CM, et al. Adiponectin complexes in human cerebrospinal fluid: Distinct complex distribution from serum. Diabetologia. 2007;50:634–642. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 15.Neumeier M, et al. Detection of adiponectin in cerebrospinal fluid in humans. Am J Physiol Endocrinol Metab. 2007;293:E965–E969. doi: 10.1152/ajpendo.00119.2007. [DOI] [PubMed] [Google Scholar]
- 16.Kubota N, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Ebinuma H, et al. Improved ELISA for selective measurement of adiponectin multimers and identification of adiponectin in human cerebrospinal fluid. Clin Chem. 2007;53:1541–1544. doi: 10.1373/clinchem.2007.085654. [DOI] [PubMed] [Google Scholar]
- 18.Qi Y, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Guo M, Zhang W, Lu XY. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3β (GSK-3β)/β-catenin signaling cascade. J Biol Chem. 2011;286:44913–44920. doi: 10.1074/jbc.M111.310052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamauchi T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 21.Fry M, et al. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci. 2006;26:9695–9702. doi: 10.1523/JNEUROSCI.2014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leo R, et al. Decreased plasma adiponectin concentration in major depression. Neurosci Lett. 2006;407:211–213. doi: 10.1016/j.neulet.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Narita K, et al. Plasma levels of adiponectin and tumor necrosis factor-alpha in patients with remitted major depression receiving long-term maintenance antidepressant therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1159–1162. doi: 10.1016/j.pnpbp.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Lehto SM, et al. Serum adiponectin and resistin levels in major depressive disorder. Acta Psychiatr Scand. 2010;121:209–215. doi: 10.1111/j.1600-0447.2009.01463.x. [DOI] [PubMed] [Google Scholar]
- 25.Zeugmann S, Quante A, Heuser I, Schwarzer R, Anghelescu I. Inflammatory biomarkers in 70 depressed inpatients with and without the metabolic syndrome. J Clin Psychiatry. 2010;71:1007–1016. doi: 10.4088/JCP.08m04767blu. [DOI] [PubMed] [Google Scholar]
- 26.Cizza G, et al. POWER Study Group Low 24-hour adiponectin and high nocturnal leptin concentrations in a case-control study of community-dwelling premenopausal women with major depressive disorder: The Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression (POWER) study. J Clin Psychiatry. 2010;71:1079–1087. doi: 10.4088/JCP.09m05314blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Yu T, et al. Cognitive and neural correlates of depression-like behaviour in socially defeated mice: An animal model of depression with cognitive dysfunction. Int J Neuropsychopharmacol. 2011;14:303–317. doi: 10.1017/S1461145710000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardeleben U, Holsboer F. Cortisol response to a combined dexamethasone-human corticotrophin-releasing hormone challenge in patients with depression. J Neuroendocrinol. 1989;1:485–488. doi: 10.1111/j.1365-2826.1989.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 31.Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Arch Gen Psychiatry. 1976;33:1051–1058. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- 32.Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 33.Holsboer F, Bender W, Benkert O, Klein HE, Schmauss M. Diagnostic value of dexamethasone suppression test in depression. Lancet. 1980;2:706. doi: 10.1016/s0140-6736(80)92755-5. [DOI] [PubMed] [Google Scholar]
- 34.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 35.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 36.Chang HP, Wang ML, Chan MH, Chiu YS, Chen YH. Antiobesity activities of indole-3-carbinol in high-fat-diet-induced obese mice. Nutrition. 2011;27:463–470. doi: 10.1016/j.nut.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 37.He M, et al. Reversal of obesity and insulin resistance by a non-peptidic glucagon-like peptide-1 receptor agonist in diet-induced obese mice. PLoS ONE. 2010;5:e14205. doi: 10.1371/journal.pone.0014205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takasawa K, Kubota N, Terauchi Y, Kadowaki T. Impact of increased PPARgamma activity in adipocytes in vivo on adiposity, insulin sensitivity and the effects of rosiglitazone treatment. Endocr J. 2008;55:767–776. doi: 10.1507/endocrj.k08e-018. [DOI] [PubMed] [Google Scholar]
- 39.Fallo F, et al. Effect of glucocorticoids on adiponectin: A study in healthy subjects and in Cushing’s syndrome. Eur J Endocrinol. 2004;150:339–344. doi: 10.1530/eje.0.1500339. [DOI] [PubMed] [Google Scholar]
- 40.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 41.Makimura H, Mizuno TM, Bergen H, Mobbs CV. Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. Am J Physiol Endocrinol Metab. 2002;283:E1266–E1271. doi: 10.1152/ajpendo.00227.2002. [DOI] [PubMed] [Google Scholar]
- 42.Degawa-Yamauchi M, et al. Regulation of adiponectin expression in human adipocytes: Effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obes Res. 2005;13:662–669. doi: 10.1038/oby.2005.74. [DOI] [PubMed] [Google Scholar]
- 43.Pariante CM, Lightman SL. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Holsboer F, Von Bardeleben U, Gerken A, Stalla GK, Müller OA. Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor in depression. N Engl J Med. 1984;311:1127. doi: 10.1056/NEJM198410253111718. [DOI] [PubMed] [Google Scholar]
- 45.Gold PW, et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s disease. Pathophysiologic and diagnostic implications. N Engl J Med. 1986;314:1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- 46.Yu JG, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 47.Yang WS, et al. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care. 2002;25:376–380. doi: 10.2337/diacare.25.2.376. [DOI] [PubMed] [Google Scholar]
- 48.Miyazaki Y, et al. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:4312–4319. doi: 10.1210/jc.2004-0190. [DOI] [PubMed] [Google Scholar]
- 49.Pajvani UB, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 50.Eissa Ahmed AA, Al-Rasheed NM, Al-Rasheed NM. Antidepressant-like effects of rosiglitazone, a PPARγ agonist, in the rat forced swim and mouse tail suspension tests. Behav Pharmacol. 2009;20:635–642. doi: 10.1097/FBP.0b013e328331b9bf. [DOI] [PubMed] [Google Scholar]
- 51.Sepanjnia K, Modabbernia A, Ashrafi M, Modabbernia MJ, Akhondzadeh S. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: Randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 56.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 57.Nawrocki AR, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 58.Guo M, et al. Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Transcult Psychiatry. 2012;2:e83. doi: 10.1038/tp.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J, Perez SM, Zhang W, Lodge DJ, Lu XY. Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic-like behavior and increases dopaminergic activity in amygdala. Mol Psychiatry. 2011;16:1024–1038. doi: 10.1038/mp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, et al. Acute administration of leptin produces anxiolytic-like effects: A comparison with fluoxetine. Psychopharmacology (Berl) 2010;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.