Schizophrenia is a devastating brain disease that affects ∼1% of the population worldwide and is characterized by a complex array of positive (delusions and hallucinations), negative (apathy and social withdrawal), and cognitive (deficits in attention and working memory) symptoms. Clinical features of schizophrenia frequently arise during late adolescence or early adulthood, which places this disease as the most chronic of the psychotic disorders and as one of the leading causes of disability and premature mortality (1). Increasing evidence suggests that cognitive impairment is at the core of schizophrenia and precedes the manifestation of psychosis (2). Furthermore, no pharmacological treatment is currently available for cognitive deficits, which are among the most debilitating symptoms of the disorder. The identification of novel treatments to ameliorate the cognitive symptoms of schizophrenia thus seems crucial to improve the quality of life in these patients.
Schizophrenia is thought to be a neurodevelopmental disorder with highly heritability (3). Genetics studies over the past few years have found a strong association between polymorphisms, common genetic variation, and structural deletions in the gene encoding for neuregulin-1 (NRG1) and its receptor ERBB4 with risk for schizophrenia (4–8). In particular, risk polymorphisms in ERBB4 that predict increased expression of the ERBB4 cytoplasmic isoform-1 (CYT-1) receptor isoform and GABA levels in human cortex have attracted a lot of attention (7, 9, 10). The CYT-1 isoform of ERBB4 contains a phosphatidylinositol 3-Kinase (PI3K)-binding site and triggers activation of the PI3K pathway (Fig. 1). Genetic manipulations of NRG1/ERBB4 signaling in animal models lead to abnormal GABA neural circuit development and plasticity (11–13) and cause behavior alterations associated with attention and cognitive deficits, such as prepulse inhibition (PPI) deficits, working memory impairment, and hyperactivity (11, 13). PI3K function has also been linked to neural circuit development, synaptic plasticity, and cognitive function (14, 15).
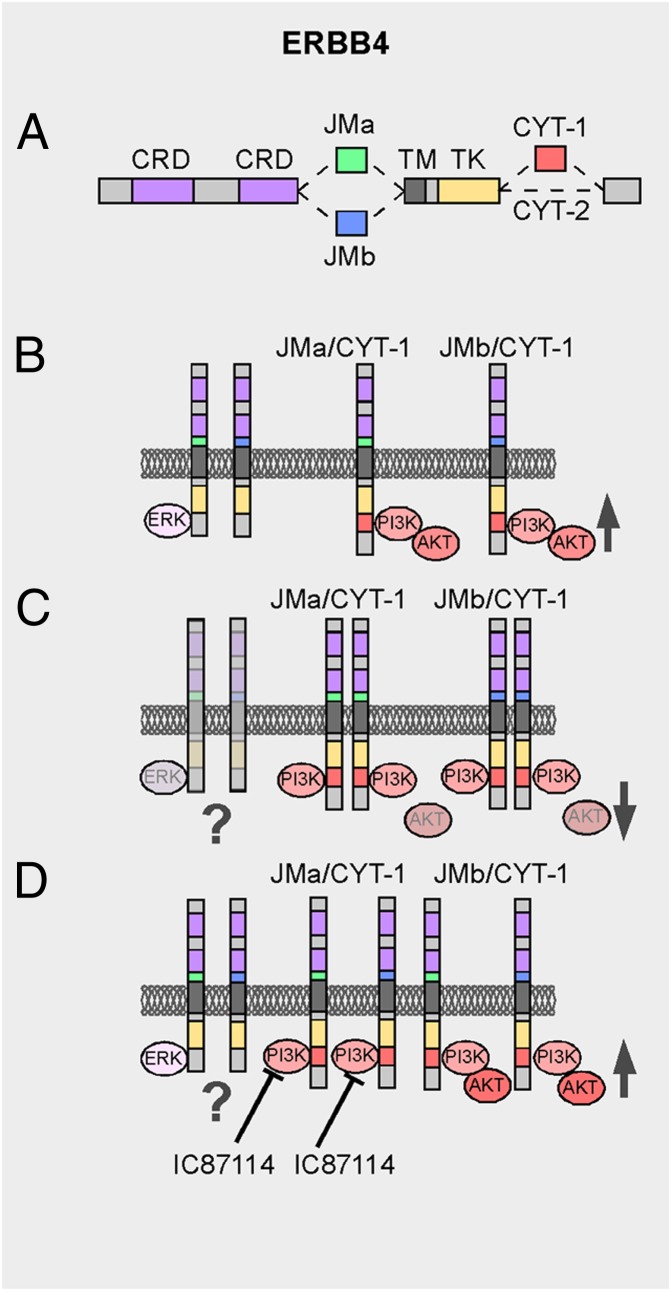
Fig. 1.
Model of ERBB4 signaling in schizophrenia: a unique candidate target. (A) Schematic drawing of alternative ERBB4 splice variants. (B) Normal expression of ERBB4 isoforms. (C) Increased expression of ERBB4/CYT-1 isoforms in schizophrenia. (D) Specific inhibition of p110δ catalytical isoform of PI3K by IC87114 restores AKT activation. CRD, cysteine-rich domain; CYT, cytoplasmic isoform; JM, juxtamembrane isoform, TK, tyrosine kinase domain; TM, transmembrane domain.
Thus, human genetics and experimental models point out the potential relevance of NRG1/ERBB4 signaling in the regulation of different aspects of schizophrenia, including cognitive function, but what we are still missing in the field is a more rationale use of this information to identify putative targets and design potential novel drugs to alleviate some of these symptoms.
In PNAS, Law et al. (16) provide unique insights into the mechanisms that link NRG1/ERBB4 signaling and schizophrenia. They first found that an ERBB4 risk haplotype (AGG; rs7598440; rs839523; rs707284) associated with elevated ERBB4 CYT-1 transcription in prefrontal cortex and hippocampus is expressed in patient-derived lymphoblastoid B-cell lines (LCLs). Using these cells, they searched for changes in downstream signaling partners of ERBB4-juxtamembrane isoform-a/CYT-1. They found that the expression of phosphatidylinositol 3-kinase isoform D (PIK3CD), which encodes one of the catalytical subunits of this enzyme (p110δ), changes in association with schizophrenia and ERBB4 genetic variation. PIK3CD transcripts were found to be increased in LCLs, prefrontal cortex, and hippocampus. In contrast, no changes were detected in the ERK-MAPK pathway, which is also linked to ERBB4 signaling. Surprisingly, increased PIK3CD levels did not enhance PI3K function but, instead, dampened PI3K signaling (16) (Fig. 1C). These results thus reveal a complex deregulation of the NRG1/ERBB4/PI3K pathway in schizophrenia that has a negative impact on PI3K signaling. These unexpected results raise the question of whether altered expression in NRG1/ERBB4 signaling in schizophrenia causes gain or loss of function, or even any specific deregulation, within this signaling pathway. It is worth mentioning that down-regulation and overexpression of NRG1 cause similar behavioral phenotypes, including impaired recognition memory, hyperactivity, and enhanced exploratory phenotype (17–19). Some of these behavioral deficits are also found in ERBB4 loss-of-function experimental models (11, 13). These results draw our attention to the need to be cautious in our interpretation of the mouse models. Does any unbalance in the expression of NRG1 and ERBB4 have the same functional consequences? Detailed analysis of NRG1 and ERBB4 expression in human postmortem brains of subjects carrying these polymorphisms and functional analyses in mouse models with specific overexpression or deletions of the different isoforms of NRG1 and ERBB4 will improve our understanding of the consequences of disrupting the expression of these genes in the etiology of schizophrenia.
Antipsychotic drugs modulate positive symptoms but have many side effects, in addition to the lack of impact on negative and cognitive symptoms. To test whether manipulation of the PIK3CD cascade reverses some of the deficits of well-known schizophrenia experimental models, Law et al. (16) use a specific inhibitor of PIK3CD, the small molecule IC87114. Remarkably, this compound dramatically blocked amphetamine-induced hyperlocomotion in a mouse model of psychosis-like behavior (16). In addition, IC87114 reversed PPI deficits in a neurodevelopmental animal model of schizophrenia based on a ventral hippocampal lesion that causes deficits in sensorimotor gating. These defects in sensorimotor gating have been associated with attention and cognitive deficits. The specific action of IC87114 on the PI3K pathway also influences the activation of thymoma viral proto-oncogene 1 (AKT) (Fig. 1D).
PI3K signaling has been shown to be involved in neural circuit development and synaptic plasticity (14, 20). Because of this, one would intuitively expect that inhibition of PI3K signaling would result in decreased cognitive performance. In contrast, Law et al. (16) find that specific inhibition of the catalytical subunit of PI3K restores attention and cognitive impairment, as measured by PPI deficits. How do we can reconcile these contradictory ideas? First, IC87114 function seems to be specific to the catalytical subunit in one of the PIK3 isoforms. Second, increased ERBB4/CYT-1 receptor expression may cause a molecular shift toward an attenuated NRG1/ERBB4/PI3K signaling system, perhaps acting as a dominant-negative form. In this context, inhibition of this alteration may restore the cascade to relatively normal signaling levels (Fig. 1D). It would be interesting to know the extent of the inhibition of p110δ,
Law et al. provide unique insights into the mechanisms that link NRG1/ERBB4 signaling and schizophrenia.
and whether the alternative signaling cascades are decreased in humans carrying polymorphisms on ERBB4/CYT-1.
Do we have a unique candidate target for the treatment of neuropsychiatric disorders? The IC87114 inhibitor seems very specific because it exerts its action only through the catalytical isoform of the PIK3. Moreover, when tested in experimental animal models, it does not show side effects; thus, it is a promising candidate. It would be interesting, however, to extend these initial observations to additional animal models. Furthermore, we ignore the mechanism of action through which inhibition of PI3K signaling improves cognition. Because this pathway has also been shown to be involved in neural circuit development and synaptic plasticity (14, 20), it is possible that chronic inhibition of this pathway has deleterious consequences, and this should be explored.
Further studies on the specific deregulation of ERBB4 signaling cascades in humans should contribute to increase our understanding of the role of NRG1/ERBB4 in schizophrenia. The development of new animal models based on these discoveries, and their subsequent analysis, should also fuel discoveries in this area. Together, these studies are beginning to pave the way for a more logical design of strategies for searching new druggable targets.
Acknowledgments
B.R. is a European Molecular Biology Organization (EMBO) Young Investigator. Work on neuregulins and ERBB4 signaling in the B.R. laboratory is supported by grants from the Spanish Government: SAF2010-21723, CONSOLIDER CSD2007-00023, and the Alicia Koplowitz Foundation.
Footnotes
The author declares no conflict of interest.
See companion article on page 12165.
References
- 1.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 2.Barch DM, Ceaser A. Cognition in schizophrenia: Core psychological and neural mechanisms. Trends Cogn Sci. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapoport JL, Giedd JN, Gogtay N. 2012. Neurodevelopmental model of schizophrenia: Update 2012. Mol Psychiatry, 10.138.mp.2012.23. [DOI] [PMC free article] [PubMed]
- 4.Law AJ, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh T, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 6.Nicodemus KK, et al. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Mol Psychiatry. 2006;11:1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- 7.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: Association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(2):142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 8.Norton N, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(2):96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- 9.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16(2):129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 10.Marenco S, et al. Genetic association of ErbB4 and human cortical GABA levels in vivo. J Neurosci. 2011;31:11628–11632. doi: 10.1523/JNEUROSCI.1529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YJ, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci USA. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazzari P, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 13.Shamir A, et al. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci. 2012;32:2988–2997. doi: 10.1523/JNEUROSCI.1899-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuesto G, et al. Phosphoinositide-3-kinase activation controls synaptogenesis and spinogenesis in hippocampal neurons. J Neurosci. 2011;31:2721–2733. doi: 10.1523/JNEUROSCI.4477-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JI, et al. PI3Kγ is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat Neurosci. 2011;14:1447–1454. doi: 10.1038/nn.2937. [DOI] [PubMed] [Google Scholar]
- 16.Law AJ, et al. Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110δ inhibition as a potential therapeutic strategy. Proc Natl Acad Sci USA. 2012;109:12165–12170. doi: 10.1073/pnas.1206118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefansson H, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy L, Cappas E, Lai D, Boucher AA, Karl T. Cognition in transmembrane domain neuregulin 1 mutant mice. Neuroscience. 2010;170:800–807. doi: 10.1016/j.neuroscience.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Kato T, et al. Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PLoS ONE. 2010;5:e14185. doi: 10.1371/journal.pone.0014185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arendt KL, et al. PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane. Nat Neurosci. 2010;13:36–44. doi: 10.1038/nn.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]