Abstract
Background
Bone marrow has been shown to be superior to peripheral blood, as a stem cell source, in young patients (<20 years of age) with acquired aplastic anemia undergoing a matched sibling transplant. The aim of this study was to test whether this currently also holds true for older patients with acquired aplastic anemia.
Design and Methods
We analyzed 1886 patients with acquired aplastic anemia who received a first transplant from a human leukocyte antigen identical sibling between 1999 and 2009, with either bone marrow (n=1163) or peripheral blood (n=723) as the source of stem cells.
Results
In multivariate Cox analysis negative predictors for survival were: patient’s age over 20 years (RR 2.0, P<0.0001), an interval between diagnosis and transplantation of more than 114 days (RR 1.3, P=0.006), no anti-thymocyte globulin in the conditioning (RR 1.6, P=0.0001), a conditioning regimen other than cyclophosphamide (RR=1.3, P=0.008) and the use of peripheral blood as the source of stem cells (RR 1.6, P<0.00001). The survival advantage for recipients of bone marrow rather than peripheral blood was statistically significant in patients aged 1–19 years (90% versus 76% P<0.00001) as well as in patients aged over 20 years (74% versus 64%, P=0.001). The advantage for recipients of bone marrow over peripheral blood was maintained above the age of 50 years (69% versus 39%, P=0.01). Acute and chronic graft-versus-host disease were more frequent in peripheral blood transplants. Major causes of death were graft-versus-host disease (2% versus 6% in bone marrow and peripheral blood recipients, respectively), infections (6% versus 13%), and graft rejection (1.5% versus 2.5%).
Conclusions
This study shows that bone marrow should be the preferred stem cell source for matched sibling transplants in acquired aplastic anemia, in patients of all age groups.
Keywords: aplastic anemia, allogeneic transplantation, bone marrow transplants, peripheral blood transplants
Introduction
Graft-versus-host disease (GvHD) and graft rejection have been major issues for successful allogeneic bone marrow transplantation in patients with acquired severe aplastic anemia.1 With the advent of peripheral blood (PB) as a stem cell source, it was thought that rejection would be reduced: however, in 2007, a first comparison by the European Group for Blood and Marrow Transplantation (EBMT)/Center for International Blood and Marrow Transplant Research (CIBMTR) between bone marrow (BM) and PB failed to confirm this hypothesis, and showed a significant survival advantage for recipients of BM rather than PB.2 In that study, which included 692 patients, the survival advantage for BM recipients was statistically significant for patients under the age of 20, but not for older patients, although the survival advantage was similar: this diversity was, perhaps, due to smaller numbers of patients in the older groups.
A second study by the CIBMTR recently compared different stem cell sources – granulocyte-colony stimulating factor (G-CSF)-stimulated BM (n=78), unstimulated BM (n=547), or PB progenitor cells (n=134) in sibling grafts between 1997 to 2003.3 Grade II–IV and III–IV acute GvHD, and chronic GvHD were significantly more frequent after PB transplants than after BM ones. The mortality rate was lower after transplantation of unstimulated BM than after transplantation of G-CSF-stimulated BM or PB progenitor cells. This study concluded that BM is the preferred stem cell source for HLA-matched sibling transplants for severe aplastic anemia.3
Despite these results, PB is still being used as a source of stem cells for patients with acquired aplastic anemia: a recent survey by the Aplastic Anemia Working Party of the EBMT (WPSAA-EBMT) found that, in 2009, 40% of transplants were from PB (unpublished data). This is difficult to understand, particularly because of the significantly greater risks of both acute and chronic GvHD following PB grafts,4 which may have short-and long-term consequences on morbidity and mortality, without the potential added value of a graft-versus-leukemia effect.5 The justification for the use of PB, despite the higher risk of GvHD, is the propensity of patients with severe aplastic anemia to reject their grafts, and it is thought that PB may reduce this risk. However, there is little evidence that this is the case: indeed, in the 2007 study,2 the risks of primary graft failure (9% versus 9%) and secondary graft failure (6% versus 7%) were identical in patients receiving BM or PB grafts. Based on data available in the literature, it would currently seem that PB grafts expose patients with severe aplastic anemia to a higher risk of mortality because of more GvHD, without the benefit of lowering the risk of rejection.
To assess whether more recent findings confirm or disprove these data, we have now analyzed 1,886 patients with severe aplastic anemia transplanted between 1999 and 2009 and report our findings here.
Design and Methods
Patients
Patients were treated in 303 EBMT Centers (see Appendix) and their data entered into the WPSAA-EBMT Registry in accordance with standard procedures. Patients were selected as having acquired aplastic anemia, a first transplant from an HLA identical sibling between 1999–2009, with the graft consisting of stem cells from BM or PB. PB stem cells were collected from the sibling donors after mobilization with G-CSF according to standard procedures. Patients receiving combined BM/PB or BM/cord blood transplants were excluded, as were those with constitutional marrow failure and with donors other than HLA-identical siblings.
GvHD prophylaxis, was heterogeneous: however, the major aggregations were cyclosporine alone, cyclosporine + other agents, cyclosporine + methotrexate, cyclosporine + methotrexate + another agent. Because cyclosporine + methotrexate was the predominant regimen, and because in univariate analysis it proved to offer a survival advantage, patients were ultimately divided into those receiving cyclosporine + methotrexate and those receiving all other regimens.
Conditioning regimens were also heterogeneous: cyclophosphamide 200 mg/kg was the regimen used in the large majority of patients; radiation was used in 114 patients as total lymphoid irradiation, in 31 as total body irradiation, and in 7 as thoraco-abdominal irradiation.
The severity of the aplasia was recorded in 487 patients as very severe (n=192), severe (n=114) and non-severe (n=181).
Table 1 presents the clinical characteristics of the patients. The percentages of patients receiving PB grafts in the 10-year period were as follows: 36.2% in 1999–2001, 36.8% in 2002–2003, 39.3% in 2004–2005, 40.5% in 2006–2007 and 38.8% in 2008–2009.
Table 1.
Clinical characteristics of the patients.

There were significant differences between recipients of BM or PB grafts (Table 1): the latter were older, were grafted more recently, more frequently had cytomegalovirus-positive donors, were grafted after a longer interval from diagnosis, more frequently received a conditioning regimen other than cyclophosphamide 200 mg/kg (including regimens with radiation), more frequently had GvHD prophylaxis other than cyclosporine + methotrexate, and were less likely to have been given anti-thymocyte globulin (ATG) in the conditioning regimen; they also had a shorter follow-up (Table 1).
Acute and chronic GvHD were diagnosed according to standard criteria and recorded.
Statistical analysis
The patients’ data were analyzed with the NCSS package. Comparisons were carried out using the χ2 test for categorical variables and the non-parametric Mann-Whitney test for continuous variables. The end-point for the survival analysis was death from any cause. Univariate and multivariate survival analyses were performed out using the Cox proportional hazard model.
Results
Engraftment
It was reported that engraftment did not occur in 5.5% and 7.2% of the BM and PB recipients, respectively (P=0.1); the BM and PB transplants engrafted in 90.7% and 89.9% of cases, respectively (P=0.5), and 1.3% and 1.4% of the BM and PB recipients lost their grafts (P=0.2).
The median day for neutrophil engraftment was day 20 (range, 3–156) for BM recipients and day 15 (range, 5–68) for patients given a PB graft (P<0.0001). The median day for platelet engraftment was day 27 (range, 4–305) for BM recipients and day 15 (range, 5–68) (P<0.0001) for the PB recipients.
Graft-versus-host disease
Acute grade II–IV GvHD developed in 11% and 17% of patients receiving BM and PB grafts, respectively (P=0.001), while the corresponding percentages for acute grade III–IV GvHD were 4% and 7%, respectively (P=0.005). Chronic GvHD developed in 11% of BM recipients and in 22% of the patients given a PB graft (P<0.00001). Extensive chronic GvHD developed in 4% and 8% of the BM and PB recipients, respectively (P=0.0004) (Figure 1).
Figure 1.
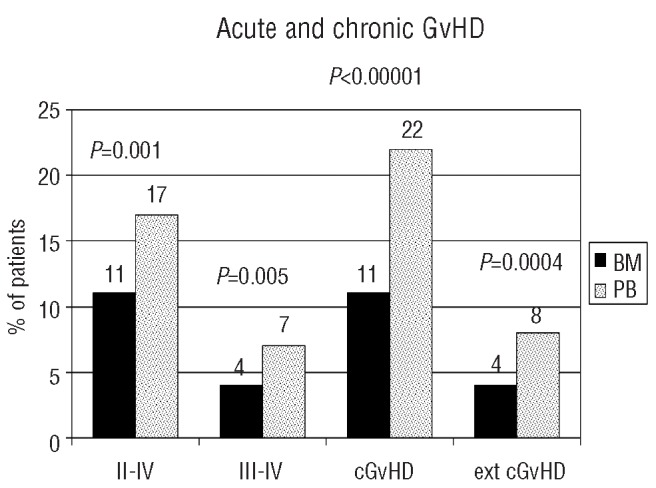
Incidences of acute and chronic GvHD expressed as percentages. Patients receiving peripheral blood (PB-hashed bars) transplants had significantly higher risks of acute GvHD grade II–IV, III–IV, overall chronic GvHD and extensive chronic GvHD, as compared to patients receiving bone marrow (BM-black bars).
The use of anti-thymocyte globulin reduced the risk of acute grade II–IV GvHD from 16% to 8% (P=0.0001) and that of chronic GvHD from 17% to 12% (P=0.001).
Univariate analysis
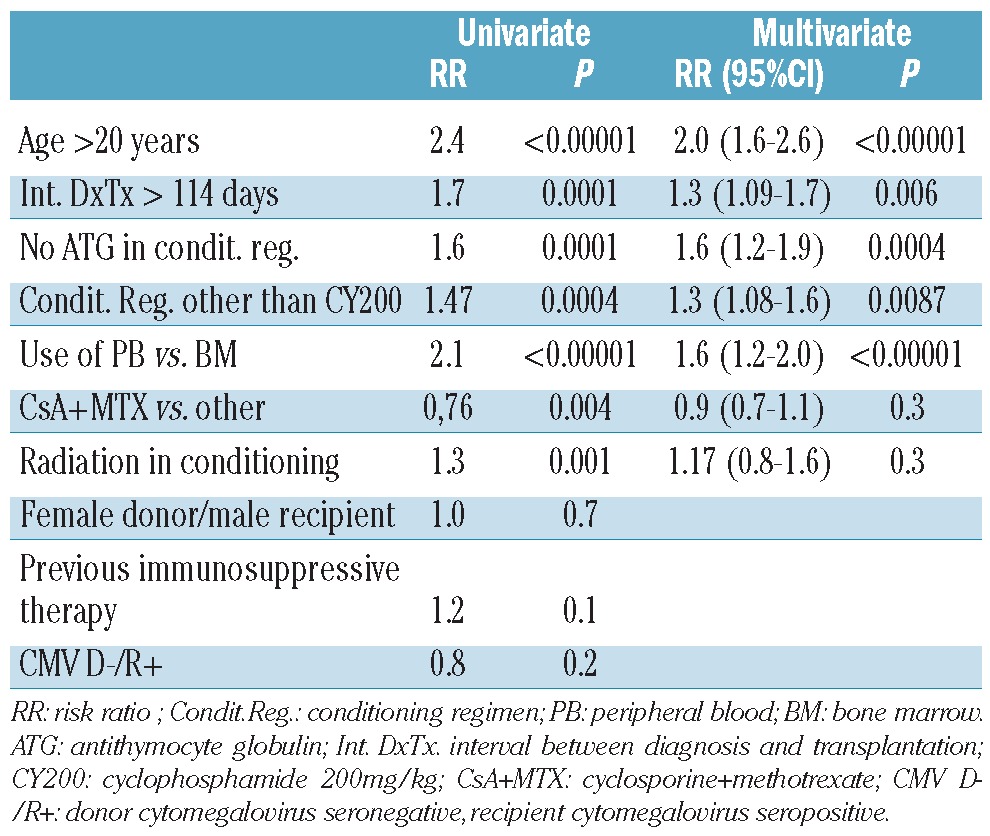
In univariate analysis there were six negative predictors of survival in the whole cohort of patients: age ≥ 20 years, an interval between diagnosis and transplantation of more than 114 days (the median), a preparative regimen without anti-thymocyte globulin, a conditioning regimen other than cyclophosphamide 200 mg/kg, peripheral blood as the stem cell source, GvHD prophylaxis with a regimen other than cyclosporine + methotrexate and the use of radiation in the conditioning regimen (Table 2). Donors’ age and gender, recipients’ gender, including female donors for male recipients, previous immunosuppressive therapy, cytomegalovirus status and year of transplant, were not statistically significant.
Table 2.
Univariate and multivariate analyses of survival.
The overall actuarial 10-year survival for the entire population was 74%. Survival rates for patients transplanted within 144 days of diagnosis or after were 84% and 72%, respectively (P<0.0001) (Figure 2A); those for patients aged 1–20 years or over 20 years were 87% and 70%, respectively (P<0.00001) (Figure 2B). With regards to the preparation regimen, the survival rates in patients given cyclophosphamide 200 mg/kg or other regimens were 80% and 72%, respectively (P=0.0004) (Figure 2C). Survival rates were higher in patients receiving anti-thymocyte globulin in the condition regimen that in those not (84% versus 74%, respectively; P=0.0001) (Figure 2D) and in patients given cyclosporine + methotrexate compared to those give other GvHD prophylaxis (81% versus 75%, respectively; P=0.004), whereas they were lower in patients who received irradiation than in those who did not (61% versus 78%, respectively; P<0.001).
Figure 2.
Actuarial survival for the whole cohort of patients stratified, in univariate analysis, according to: (A) the interval between diagnosis and transplantation (</≥ 114 days, median) (B) age (</≥ 20 years); (C), use of antithymocyte globulin (ATG) (yes/no) (D) and type of preparative regimen (cyclophosphamide 200 mg/kg/other). A significant difference in survival can be seen for all four variables.
Age and stem cell source
In univariate analysis the actuarial survival of patients receiving BM or PB was 84% versus 68%, respectively (P<0.00001): the survival or patients stratified according to age (< or ≥ 20 years) and stem cell source is depicted in Figure 3. Under the age of 20 the advantage conferred by BM over PB was demonstrated by the survival rates of 90% versus 76%, respectively (P<0.00001); the advantage was still present in patients aged ≥20 years (74% versus 64%, respectively; P=0.001) and in patients over the age of 50, the advantage of BM (n=58) over PB (n=76) was 69% versus 39% (P=0.01).
Figure 3.
Actuarial survival of patients receiving peripheral blood (PB) or bone marrow (BM) transplants, and stratified by age: (A) <20 years or (B) ≥20 years of age. The difference is statistically significant in both groups.
Survival: multivariate analysis
Variables significant in univariate analysis were entered into a multivariate model, and five proved to be independent negative predictors (Table 2): older age, longer interval between diagnosis and transplantation, no anti-thymocyte globulin in the conditioning regimen, a regimen other than cyclophosphamide 200 mg/kg and PB as the source of stem cells.
Survival: identification of different risk groups
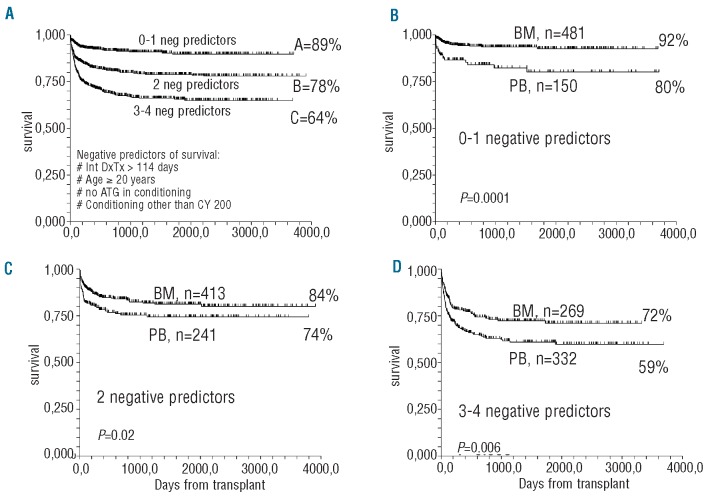
We then wanted to assess the effect of stem cell source in patients with different risks of mortality: for this purpose the four negative predictors, diagnosis–transplant interval, age, anti-thymocyte use and conditioning regimen, were used to identify three groups of patients with significantly different outcomes: low-risk (0–1 negative predictor; n=631; actuarial survival 89%), medium-risk (2 negative predictors; n=654; survival 78%) and high-risk (3–4 negative predictors; n=601; survival 64%) (Figure 4A). The actuarial survival rates of BM versus PB graft recipients in the low-risk group were 92% versus 80% (P=0.0001) (Figure 4B), in the intermediate-risk group they were 84% versus 74% (P=0.02) (Figure 4C) and in the high-risk group they were 72% versus 59% (P=0.006) (Figure 4D).
Figure 4.
(A) Actuarial survival of all patients stratified by negative predictors of survival: interval diagnosis-transplant (Dx-Tx) ≥114 days, recipients’ age ≥20 years, no anti-thymocyte globulin (ATG) in the conditioning, a conditioning regimen other than cyclophosphamide 200 mg/kg. Three groups were identified: low risk (survival 89%), intermediate risk (survival 78%) and high risk (survival 64%). (B) Actuarial survival for BM vs PB graft recipients in the low risk group (P=0.0001); (C) Actuarial survival for BM vs. PB graft recipients in the intermediate risk group (P=0.02); (D) Actuarial survival for BM vs. PB graft recipients in the high risk group (P=0.006).
Causes of death
Table 3 presents the causes of death in patients receiving BM or PB grafts: in the PB group there were more deaths due to GvHD (2% versus 6%, P=0.00001) and infections (6% versus 13%, P<0.00001). Rates of mortality related to rejection were comparable, with a trend for a greater risk in PB graft recipients (1.5% versus 2.5%, respectively; P=0.07) (Table 3).
Table 3.
Causes of death.

Centers and stem cell source
Three hundred and five Centers contributed data for this study (see Appendix): 116 Centers did not use PB as a stem cell source in any of their transplants, 38 used PB in 1–25% of their transplants, 53 Centers used PB in 26–50% and 98 Centers used PB for more than 50% of their grafts. The average crude survival rate in Centers never using PB was 84%, for Centers using PB grafts in 1–25% of cases it was 80%, for Centers using PB in 26–50% of cases it was 78%, and in Centers using PB in more than 50% of cases it was 72%. We then looked at patients contributed by each Center and use of PB grafts: of the large Centers contributing data from more than 20 patients each, only one Center never used PB and 30% of these Centers used PB grafts in more than 50% of cases, irrespective of their size. Sixty-two Centers never used BM as a source of stem cells for their transplants.
Discussion
In this large, EBMT registry-based study, we have shown that patients with acquired aplastic anemia, grafted from HLA identical siblings, have a survival advantage when given BM as compared to PB transplants. This holds true for all age groups, suggesting that BM should be the preferred source of stem cells for these patients. We have also shown, or confirmed, that other predictors of survival are patients’ age, the use of anti-thymocyte globulin in the conditioning regimen, a longer interval between diagnosis and transplantation and the use of a conventional cyclophosphamide 200 mg/kg conditioning regimen.
In the previous joint EBMT/CIBMTR study on stem cell source,2 the survival advantage for BM over PB was 12% in patients under 20 years of age, and 12% in patients over 20 years, but was statistically significant only in the younger patients. In the present analysis the difference is similar, 14% in young patients and 10% in older patients, but highly significant in both groups because of the large number of patients.
There were differences in the characteristics of the BM and PB recipients, which would argue for a selection of more difficult patients in the PB group: indeed recipients of PB grafts were older, were grafted later after diagnosis, and had more frequently received a conditioning regimen other than cyclophosphamide 200 mg/kg, including radiation; on the other hand they had been transplanted more recently. In order to determine the effect of these differences we first conducted univariate and multivariate analyses showing that unfavorable predictors of outcome were older age, no anti-thymocyte globulin in the conditioning regimen, more than 114 days (the median interval) between diagnosis and transplantation, and the use of a conditioning regimen other than cyclophosphamide 200 mg/kg. We then assigned a score of 1 for each of these unfavorable factors: three risk groups could be identified, with 0–1, 2 and 3–4 risk factors, with significantly different actuarial survival rates (89%, 78%, and 64%, respectively). Having identified the patients who would be expected to do worse, we then looked at the use of BM and PB in these different risk groups: the survival advantage for BM recipients was evident in all three groups, thus not supporting the hypothesis that PB grafts produce better results in difficult patients. We also looked at the very old patients (>50 years of age), and also here BM recipients did significantly better than PB recipients. These results were confirmed in a multivariate Cox model, in which stem cell source was included as a variable: in this model, after correcting for known negative predictors, the relative risk of death of PB transplant recipients was 1.6 (P<0.00001). The recent CIBMTR study3 confirms these results. When looking at a Center effect, we could subdivide Centers into four groups on the basis of their use of PB as the stem cell source: never (0%), in 1–25% of cases, in 26–50% of cases and in 51–100% of cases. The worst survival rates were seen in patients treated in the Centers in the last group, using a high proportion of PB grafts, while the best survival rates were found in the first group. However, we could not show that large Centers were using more BM, since only one Center in the ten reporting data from over 20 patients never used PB. Therefore, a large number of small Centers, contributing one or two patients each, used BM only. In our opinion this makes the result still more striking.
But why should PB transplant recipients do worse than BM transplant recipients? We found more deaths due to GvHD, and infections, which are often combined causes of failure. Interestingly, despite faster neutrophil and platelet engraftment, we found no differences in the proportions of patients recorded as having engrafted, in the proportions of patients who lost their graft, or in a reduction of deaths classified as due to rejection (1.5% in BM recipients and 2.5% in PB recipients), in keeping with previously reported data from the EBMT/CIBMTR study.2 Thus PB grafts fail to reduce the incidence and mortality due to graft failure, but increase the risk of GvHD, thus exposing the patient to increased morbidity and mortality. In particular, we found twice as much chronic GvHD in PB graft recipients than in BM recipients (22% versus 11%), without the need for a graft-versus-leukemia effect. This being the case, it is likely that unrelated PB transplants will also yield similar inferior results when compared to BM transplants for aplastic anemia: a preliminary analysis of 451 BM and 153 PB grafts suggests a 20% survival advantage for BM recipients (68% versus 48%) in the period 1999–2009 (WPSAA-EBMT Registry 2011; unpublished data). In keeping with these results, the CIBMTR has recently published a paper showing that BM grafts offer a survival advantage over grafts of stem cells derived from PB in patients with aplastic anemia receiving transplants from unrelated donors.5
There is, therefore, currently no good reason to collect PB cells for transplants in patients with aplastic anemia: there are no advantages for the donors, exposed to a significantly greater risk of severe adverse events when donating PB,6 or for the recipients. It is, therefore hoped that the current propensity of Centers to collect PB, both related and unrelated, for this particular indication may be overcome by the evidence provided in this report as well as in others.
In addition to stem cell source, transplant strategies are also relevant to the optimal outcome: in this study the use of anti-thymocyte globulin in the conditioning regimen was associated with statistically improved survival in univariate and multivariate analyses, possibly because of the known effects of anti-thymocyte globulin on reducing acute and chronic GvHD and improving engraftment.8–11 It should be noted that a previous prospective randomized trial had failed to show a significant advantage from the use of anti-thymocyte globulin in the conditioning regimen:12 the anti-thymocyte globulin-related survival advantage was 6% in that trial, whereas in our study it is 10%, but in a larger number of patients, and is, therefore, highly significant. Thus, the conventional use of cyclophosphamide 200 mg/kg and anti-thymocyte globulin remains the standard of care, as advocated many years ago by the Seattle group.1 and confirmed with long-term follow-up:13,14 this is also true for transplants in developing countries.15 An alternative to anti-thymocyte globulin is alemtuzumab, as shown in a recent retrospective multicenter study16 in which alemtuzumab, combined with fludarabine and low-dose cyclophosphamide, produced a low incidence of chronic GVHD and very encouraging survival rates. Of note, also in that study the overall survival was worse in patients who received PB grafts than in those who received BM grafts.
The hypothesis that conditioning regimens, including fludarabine,17 will overcome the negative effect of age,18 should be tested prospectively before one can advocate their use as a standard of care: the Seattle group has recently published encouraging results on conventional cyclophosphamide conditioning in patients over 40 years of age.19
Finally, patients grafted earlier, in our case within 4 months of diagnosis (114 days being the median interval between diagnosis and transplantation), do better than patients grafted later. This is an important result which has emerged repeatedly in many analyses of transplantation for aplastic anemia,20 but it should not be underestimated: in this study the longer the interval the poorer the outcome, with survival rates for patients grafted beyond 1 year being 25% lower than those for patients grafted within 4 months. The general hematologist should be aware that early referral of these rare patients to a transplant center will improve the chances of cure for a given patient: this is probably particularly important when the patient has an HLA identical sibling willing to donate stem cells.
In conclusion, this study shows that BM is the stem cell source of choice for patients of any age with acquired severe aplastic anemia. It also proves that prophylaxis of acute and chronic GvHD with anti-thymocyte globulin, and the use of cyclophosphamide 200 mg/kg significantly improve survival. Whether newer conditioning regimens, with low-dose fludarabine and cyclophosphamide, can reduce mortality in the older age group remains to be proven in prospective trials.
Footnotes
Funding: this work was partly supported by the European Group for Blood and Marrow Transplantation (EBMT), Associazione Italiana Ricerca contro il Cancro (A.I.R.C.), Milano and Fondazione Ricerca Trapianto Midollo Osseo (FARITMO) Genova.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Storb R, Thomas ED, Buckner CD, Clift RA, Johnson FL, Fefer A, et al. Allogeneic marrow grafting for treatment of aplastic anemia. Blood. 1974;43(2):157–80. [PubMed] [Google Scholar]
- 2.Schrezenmeier H, Passweg JR, Marsh JC, Bacigalupo A, Bredeson CN, Bullorsky E, et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood. 2007;110(4):1397–400. doi: 10.1182/blood-2007-03-081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu R, Brazauskas R, Kan F, Bashey A, Bredeson C, Camitta B, et al. Comparison of outcomes after transplantation of G-CSF-stimulated bone marrow grafts versus bone marrow or peripheral blood grafts from HLA-matched sibling donors for patients with severe aplastic anemia. Biol Blood Marrow Transplant. 2011;17(7):1018–24. doi: 10.1016/j.bbmt.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY, et al. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncol. 2010;11(4):331–8. doi: 10.1016/S1470-2045(09)70352-3. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Le Rademacher J, Antin JH, Champlin RE, Carreras J, Fay J, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118(9):2618–21. doi: 10.1182/blood-2011-05-354001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halter J, Kodera Y, Ispizua AU, Greinix HT, Schmitz N, Favre G, et al. Severe events in donors after allogeneic hematopoietic stem cell donation. Haematologica. 2009;94(1):94–101. doi: 10.3324/haematol.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringdén O, Karlsson H, Olsson R, Omazic B, Uhlin M. The allogeneic graft-versus-cancer effect. Br J Haematol. 2009;147(5):614–33. doi: 10.1111/j.1365-2141.2009.07886.x. [DOI] [PubMed] [Google Scholar]
- 8.Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98(10):2942–7. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 9.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 10.Deeg HJ, Storer BE, Boeckh M, Martin PJ, McCune JS, Myerson D, et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant. 2006;12(5):573–84. doi: 10.1016/j.bbmt.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 11.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–54. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 12.Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109(10):4582–5. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storb R, Leisenring W, Anasetti C, Appelbaum FR, Buckner CD, Bensinger WI, et al. Longterm follow-up of allogeneic marrow transplants in patients with aplastic anemia conditioned by cyclophosphamide combined with antithymocyte globulin. Blood. 1997;89(10):3890–1. [PubMed] [Google Scholar]
- 14.Deeg HJ, Leisenring W, Storb R, Nims J, Flowers ME, Witherspoon RP, et al. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood. 1998;91(10):3637–45. [PubMed] [Google Scholar]
- 15.Ladeb S, Abdelkefi A, Torjman L, Ben Neji H, Lakhal A, Kaabi H, et al. Allogeneic hematopoietic stem cell transplantation for acquired aplastic anemia using cyclophosphamide and antithymocyte globulin: a single center experience. Bone Marrow Transplant. 2009 Jul 27; doi: 10.1038/bmt.2009.175. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Marsh JC, Gupta V, Lim Z, Ho AY, Ireland R, Hayden J, et al. Alemtuzumab with fludarabine and cyclophosphamide reduces chronic graft versus host disease after allogeneic stem cell transplantation for acquired aplastic anemia. Blood. 2011;118(8):2351–7. doi: 10.1182/blood-2010-12-327536. [DOI] [PubMed] [Google Scholar]
- 17.Maury S, Bacigalupo A, Anderlini P, Aljurf M, Marsh J, Socié G, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94(9):1312–5. doi: 10.3324/haematol.2009.006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta V, Eapen M, Bajorunaite R, Carreras J, Aljurf M, Gale RP, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95(12):2119–25. doi: 10.3324/haematol.2010.026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangiolo D, Storb R, Deeg HJ, Flowers ME, Martin PJ, Sandmaier BM, et al. Outcome of allogeneic hematopoietic cell transplantation from HLA-identical siblings for severe aplastic anemia in patients over 40 years of age. Biol Blood Marrow Transplant. 2010;16(10):1411–8. doi: 10.1016/j.bbmt.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locasciulli A, Oneto R, Bacigalupo A, Socié G, Korthof E, Bekassy A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade a report from the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2007;92(1):11–8. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]