Abstract
Background
Systemic activation of hemostasis is frequently observed in cancer patients, even in the absence of thrombosis. Moreover, this activation has been implicated in tumor progression, angiogenesis and metastatic spread. Increased levels of D-dimer, which is a degradation product of cross-linked fibrin, indicate a global activation of hemostasis and fibrinolysis.
Design and Methods
In a prospective and observational cohort study, we assessed the prognostic value of D-dimer levels for overall survival and mortality risk in 1178 cancer patients included in the Vienna Cancer and Thrombosis Study (CATS). Patients were followed over 2 years at regular intervals until occurrence of symptomatic venous thromboembolism or death. D-dimer levels were measured with a quantitative D-dimer latex agglutination assay
Results
The main solid tumors were malignancies of the lung (n=182), breast (n=157), lower gastrointestinal tract (n=133), pancreas (n=74), stomach (n=50), kidney (n=37), prostate (n=133), and brain (n=148); 201 of the patients had hematologic malignancies; 63 had other tumors. During a median follow-up of 731 days, 460 (39.0%) patients died. The overall survival probabilities for patients with D-dimer levels categorized into four groups based on the 1st, 2nd and 3rd quartiles of the D-dimer distribution in the total study population were 88%, 82%, 66% and 53% after 1 year, and 78%, 66%, 50% and 30% after 2 years, respectively (P<0.001). The univariate hazard ratio of D-dimer (per double increase) for mortality was 1.5 (95% confidence interval: 1.4–1.6, P<0.001) and remained increased in multivariable analysis including tumor subgroups, age, sex and venous thromboembolism.
Conclusions
High D-dimer levels were associated with poor overall survival and increased mortality risk in cancer patients.
Keywords: prognosis, D-dimer, hemostasis, tumor, thrombosis, venous thromboembolism
Introduction
Both experimental and clinical studies have evidenced an association between cancer and hemostasis.1 Interestingly, a systemic activation of blood coagulation and procoagulant changes in the hemostatic system have frequently been observed in cancer patients, even in the absence of venous thromboembolism (VTE).2,3
Moreover, coagulation activation, in particular thrombin generation and fibrin formation and dissolution, have been implicated in angiogenesis, tumor cell invasion, tumor progression, and metastatic spread. Thrombin is a pivotal enzyme in the process of blood coagulation and leads to the conversion of fibrinogen to fibrin, which is the end product of blood coagulation and finally results in the formation of a fibrin clot. Tumor cells also possess strong procoagulant activities that induce local activation of the coagulation system and deposition of fibrin, which has an important role in the formation of tumor stroma and hematogenous spread of tumor cells.4,5 The interaction of fibrin, platelets and tumor cells leads to the formation of platelet-fibrin-tumor-cell aggregates that promote endothelial adhesion and metastatic spread, as well as tumor cell growth and tumor cell survival.6 In addition, fibrin degradation products have been shown to display strong angiogenic properties.7
D-dimer is a biomarker that globally indicates the activation of hemostasis and fibrinolysis. It is a degradation product of fibrin, which is produced when cross-linked fibrin is degraded by plasmin-induced fibrinolytic activity. As D-dimer plasma levels are elevated after clot formation, the measurement of D-dimer is routinely used in conjunction with clinical parameters in the initial assessment of suspected acute VTE.8 Elevated D-dimer levels may also be observed in other clinical settings, such as cancer, pregnancy and infectious diseases or following trauma and surgery.9 Recently, high D-dimer levels were reported to be predictive of the occurrence of VTE in cancer patients.10–12 In patients with unprovoked VTE, D-dimer levels have been demonstrated to predict recurrence of VTE after discontinuation of oral anticoagulant therapy.8 In a prospective, interventional study in patients without cancer who had unprovoked VTE and who had received vitamin K antagonists, D-dimer levels were measured 1 month after discontinuation of anticoagulation to determine the duration of anticoagulation therapy, suggesting a prolonged anticoagulation for prevention of recurrent VTE in patients with elevated D-dimer levels.13
Cancer is frequently associated with activation of the hemostatic system and the extent of this activation has been reported to correlate with a more advanced tumor stage, with unfavorable outcomes and the patient’s prognosis in small studies of patients with breast,14–16 colorec-tal17,18 and lung cancer.19–21 Thus, we sought to determine the role of the activation of hemostasis and fibrinolysis, reflected by plasma levels of D-dimer, in the prognosis of patients with various types of cancer in a large prospective study.
Design and Methods
Study design and study population
This study was performed in the framework of the ongoing Vienna Cancer and Thrombosis Study (CATS), a prospective and observational cohort study of cancer patients initiated in 2003. It is a single center study that has been conducted at the Vienna General Hospital of the Medical University of Vienna after approval by the local ethics committee and in accordance with the Declaration of Helsinki. The principal objective of CATS is to investigate and establish risk factors predictive of the occurrence of VTE in cancer patients and to improve risk assessment of VTE in patients diagnosed with cancer. The methodology of CATS and the exact inclusion and exclusion criteria have previously been described in detail.10,22,23 Briefly, patients were included if they met the following criteria: (i) a newly diagnosed cancer of the brain (i.e. high-grade glioma), breast, lung, upper or lower gastrointestinal tract, pancreas, kidney, prostate or other site (mainly of the female genital system and sarcoma), hematologic malignancies (myeloma and lymphoma); or progression of disease after complete or partial remission; (ii) histological confirmation of diagnosis; (iii) age over 18 years; (iv) willingness to participate; and (v) written informed consent. Exclusion criteria for all participants were: (i) overt bacterial or viral infection; (ii) venous or arterial thromboembolism within the preceding 3 months; and (iii) continuous anticoagulation with vitamin K antagonists or low-molecular-weight heparins. For patients with progression of disease, additional exclusion criteria were surgery or radiotherapy within the preceding 2 weeks and chemotherapy within 3 months prior to study inclusion. At the time of study enrollment a blood sample was drawn for D-dimer measurement.
The observation period started at the time of blood sampling and a regular follow-up was performed approximately every 3 months. Patients were followed prospectively either for a maximum of 2 years, or until the occurrence of VTE or death, loss of follow-up, or withdrawal of consent. In the case of sudden death, autopsy was requested. Available autopsy protocols were checked for VTE. Once a year the Austrian Mortality Registry was searched for entries concerning study participants. All deaths were included in the analysis of the current study.
All clinical parameters, complete follow-up information and plasma samples for D-dimer measurement were available for 1178 patients who had been enrolled between October 2003 and March 2010 for analyses of D-dimer levels as a prognostic parameter for overall survival and risk of mortality.
Outcome measure
The outcome measure of the current analysis was death from any cause within 2 years after entry into the study.
Blood sampling and laboratory analysis of D-dimer levels
Venous blood samples were collected into Vacutainer citrate tubes (Vacuette; Greiner-Bio-One; containing 1/10 volume sodium citrate stock solution at 0.129 mmol/L) by sterile, atraumatic venipuncture. Samples were centrifuged to obtain platelet-poor plasma, and aliquots were stored at −80°C until testing was performed in series. D-dimer levels were measured by a quantitative latex assay (STA-LIAtest D-DI; Diagnostica-Stago, Asnieres, France) on an STA-R analyzer (Diagnostica-Stago) according to the manufacturer’s instructions.
Statistical analysis
The characteristics of the patients were described by median values and 25th–75th percentiles (because of non-normally distributed continuous variables) and by frequencies and percentages for categorical variables. To compare the distribution of D-dimer levels for patients with cancer in different sites a Kruskal-Wallis test was applied. The median of the follow-up distribution was estimated by the Kaplan-Meier method with the sense of the status indicator reversed.24 Kaplan-Meier analysis was used to visualize the association between categorized levels of D-dimer and overall survival. D-dimer was categorized into four groups based on the 1st, 2nd and 3rd quartiles of the D-dimer distribution in the total study population. Univariate and multivariable Cox-regression analyses were used for calculating the risk of mortality from study inclusion until last follow-up, the patient’s death or the maximal length of follow-up of 2 years. Multivariable Cox-regression analysis comprised D-dimer level, our variable of main interest, age, sex, four different tumor groups (hematologic malignancy, brain tumor, solid tumor without metastasis and solid tumor with metastasis) and VTE. Hazard ratios for D-dimer, a continuous variable, are given per double increase and hazard ratios for age, another continuous variable, are given per each 10-year increase. VTE was modeled as a time-dependent covariate, which means that a patient was associated with the VTE group starting with the time point of the VTE occurrence. The multivariable Cox-regression model was tested for all pair-wise interactions and interactions with log(time) by means of candidate variables. An interaction was considered statistically significant if its P value was less than 0.01. As a significant interaction was found between D-dimer level and the four tumor groups (P<0.001), this interaction was included in the final multivariable Cox-model. An interaction between D-dimer level and the four tumor groups results in four different hazard ratios for each double increase of D-dimer level for each of the tumor groups. For hypothesis tests we considered P<0.05 as statistically significant and all tests were two-sided. All statistical computations were performed with SAS System V9.2 (SAS Institute, Cary, NC, USA). Figure 1 was produced with R 2.10.25
Figure 1.
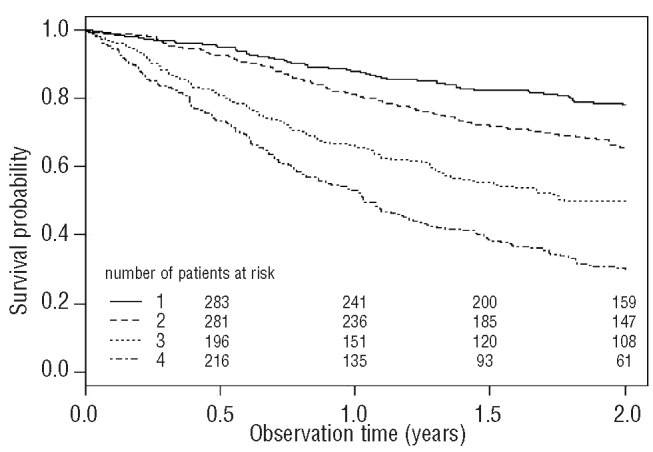
Cumulative probability of overall survival in the total study population (n=1178). (1) Patients in the 1st group with D-dimer levels ranging from minimum to 1st quartile [0.03 to 0.34 μg/mL] of levels in the total study population are compared to (2) those with D-dimer levels in the 2nd group with levels between the 1st and 2nd quartiles [0.34 to 0.71 μg/mL], (3) those in the 3rd group with levels between the 2nd and 3rd quartiles [0.71 to 1.33 μg/mL] and (4) the 4th group with D-dimer levels ranging from the 3rd quartile to maximum [1.33 to 45.1 μg/mL] of D-dimer levels in the study population. Total numbers of patients with D-dimer levels in the 1st, 2nd, 3rd and 4th groups at study inclusion were 304, 327, 253 and 294, respectively.
Results
Baseline characteristics of the study population
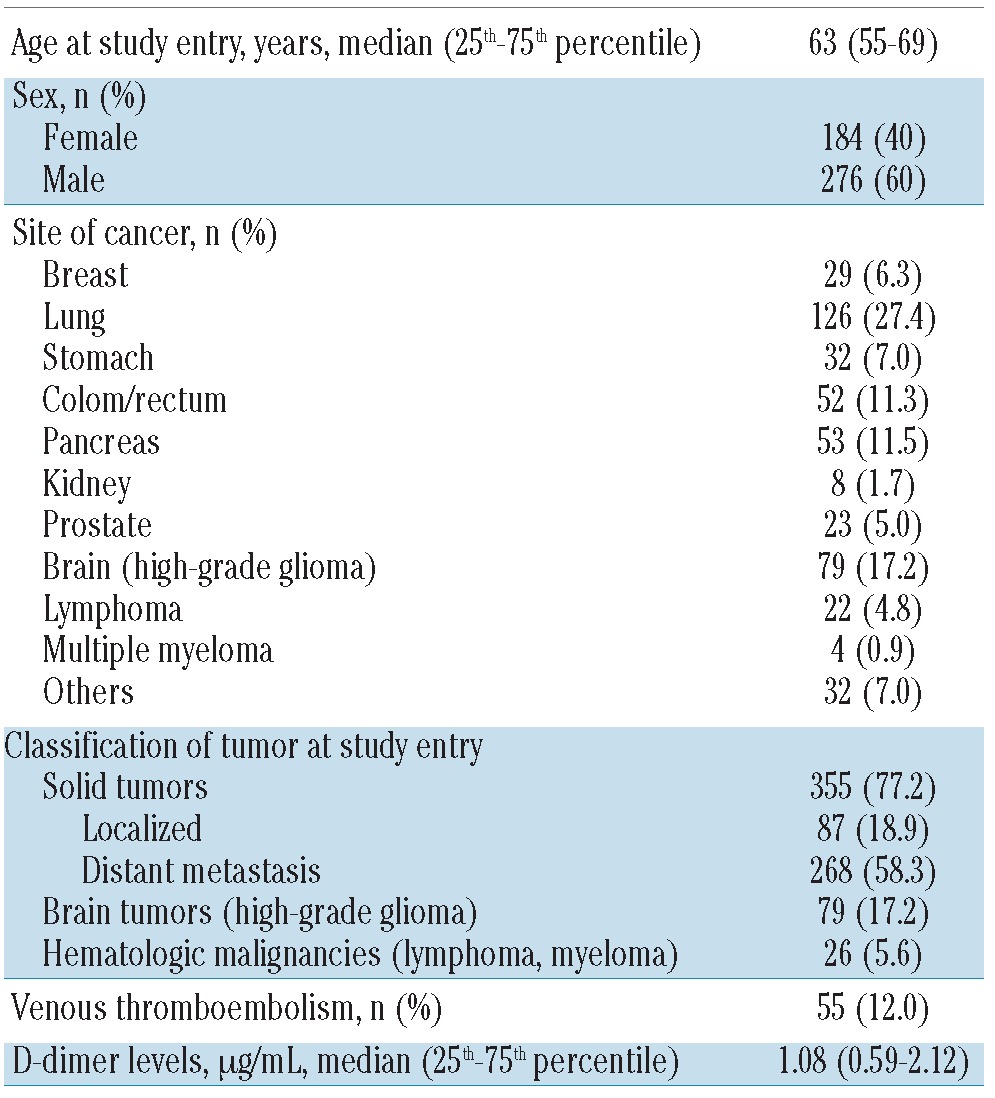
In total, 1178 prospectively followed patients with cancer were included. Their detailed characteristics are listed in Table 1. The median observation time of the study population was 731 days. Eight-hundred and twenty-nine (70.4%) patients had a solid tumor, 148 (12.6%) patients had a brain tumor and 201 (17.0%) patients had a hematologic malignancy. At the time of study inclusion, distant metastases were present in 414 patients with a solid tumor. A total of 627 (53.2%) cancer patients were newly diagnosed, whereas 551 (46.8%) patients had progressive disease after a complete or partial remission. Four-hundred and sixty patients (39.0%) died during the follow-up period. Detailed information on these patients is shown in Table 2.
Table 1.
Baseline characteristics of the total study population (n=1178).
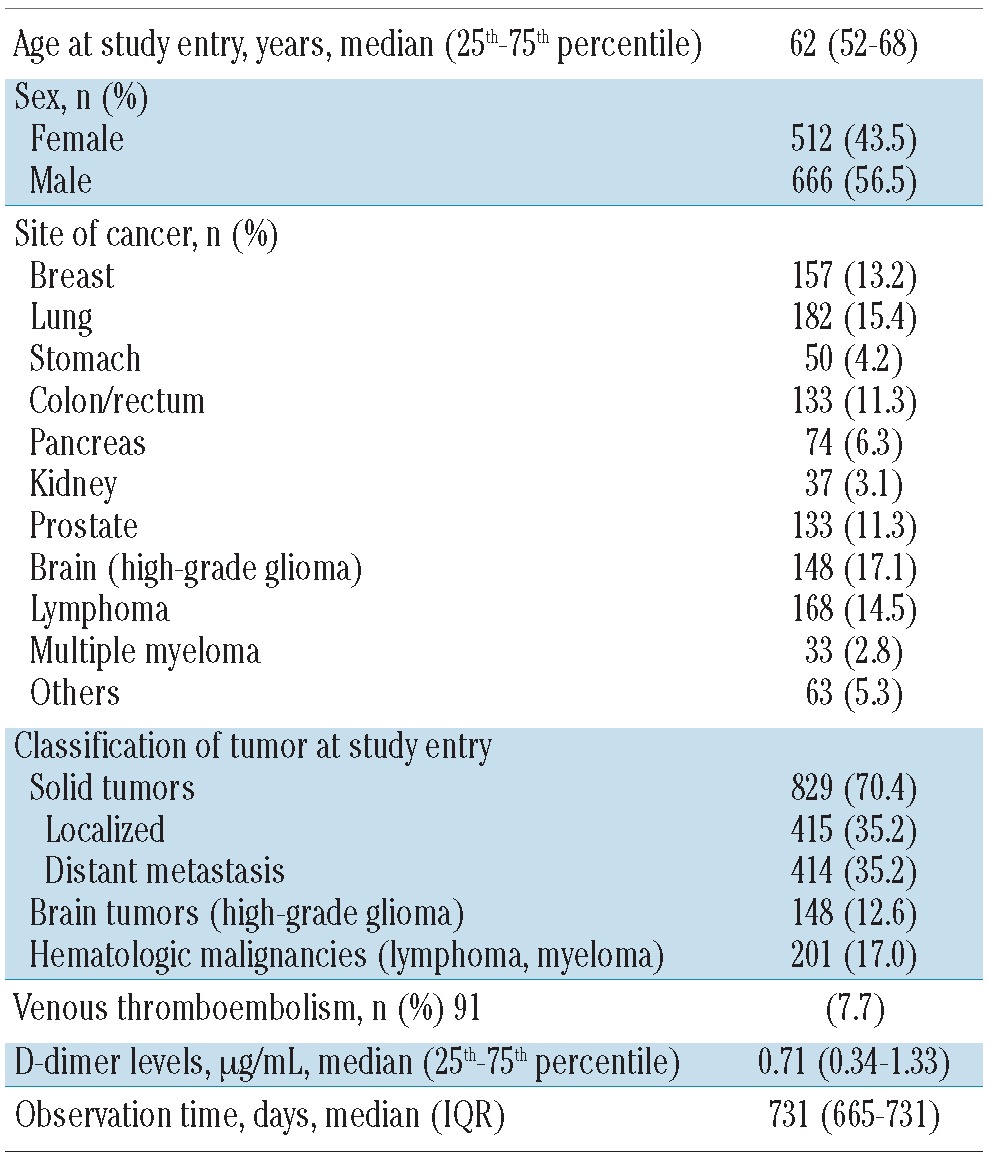
Table 2.
Characteristics of patients who died during the observation period (n=460).
VTE occurred in 7.7% of the study population (n=91, 36 females and 55 males; median [interquartile range] age, 62 [67–80] years) during follow-up. The site of VTE was an isolated deep vein thrombosis (DVT) of the lower extremity in 40 patients, an isolated pulmonary embolism in 36 patients and a combined DVT of the lower extremity with pulmonary embolism in 4 patients. In 11 patients other sites of VTE were recorded. Pulmonary embolism was documented to be fatal in four patients.
Distribution of D-dimer levels in the study population and in subgroups
In the total study population, the median D-dimer level (μg/mL) at study inclusion was 0.71 (25th–75th percentile: 0.34–1.33). The median D-dimer level in patients with brain tumors was 0.66 (25th–75th percentile: 0.34–1.33) and in those with hematologic malignancies it was 0.59 (25th–75th percentile: 0.34–1.09). The median D-dimer level was higher in patients with solid tumors with distant metastasis than in those without distant metastasis, being 0.99 (25th–75th percentile: 0.55–2.01) and 0.50 (25th–75th percentile: 0.34–0.91), respectively. Patients who died during the observation period had significantly higher D-dimer levels at baseline compared to those who were alive at the end of the study or at last follow-up: 1.08 (25th–75th percentile: 0.59–2.12) and 0.71 (25th–75th percentile: 0.34–1.33), respectively.
D-dimer levels and probability of survival
Figure 1 shows the Kaplan-Meier estimates for overall survival according to D-dimer levels. Elevated D-dimer levels were significantly associated with shorter overall survival (log-rank test: P<0.001). For Kaplan-Meier analysis, patients were categorized in four groups according to their D-dimer levels: 1st group (D-dimer levels ranging from minimum to 1st quartile of D-dimer levels in the total study population: 0.03–0.34 μg/mL), 2nd group (D-dimer levels from 1st quartile to 2nd quartile: 0.34–0.71 μg/mL), 3rd group (D-dimer levels from 2nd quartile to 3rd quartile: 0.71–1.33 μg/mL) and 4th group (D-dimer levels from 3rd quartile to maximum: 1.33–45.1 μg/mL).
In the total study population, the probabilities of overall survival for patients with D-dimer levels in the 1st, 2nd, 3rd and 4th groups were 88%, 82%, 66% and 53% after 1 year, and further declined to 78%, 66%, 50% and 30% after 2 years, respectively.
Risk of mortality in relation to D-dimer levels
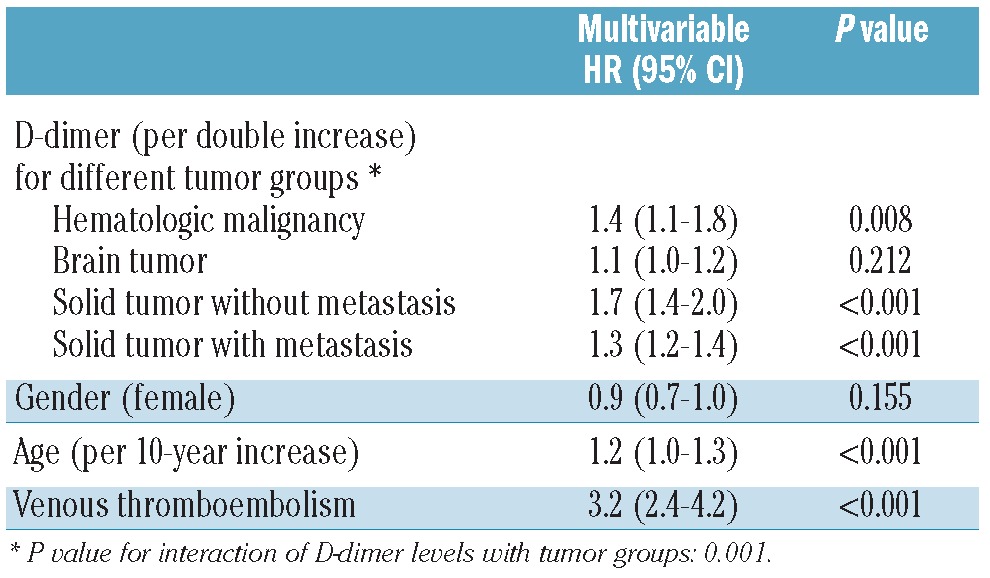
In univariate Cox-regression analysis, each doubling of D-dimer level was associated with a 1.5-fold (95% CI: 1.4–1.6, P<0.001) increase in the hazard ratio for mortality, and this association remained significantly increased in multivariable analysis after adjustment for age, sex, VTE and different tumor groups (considering brain, hematologic malignancies and solid tumors with and without distant metastasis). Table 3 shows the multivariable analysis and hazard ratios of mortality for elevated D-dimer levels in subgroups of patients divided by tumor group, age, sex and VTE. In an additional multivariable analysis we adjusted for patients with newly diagnosed cancer or progression of disease after remission and the results shown in Table 3 remained unchanged (see Online Supplementary Table S1).
Table 3.
Multivariable analysis and hazard ratios (95% confidence interval) of mortality for D-dimer levels in different subgroups of tumors, age, sex and VTE.
Compared to patients with D-dimer levels in the 1st group, the univariate hazard ratio for mortality of those with D-dimer levels in the 2nd group was 1.7 (95% CI 1.2–2.3, P=0.002), for those in the 3rd group it was 3.0 (95% CI 2.2–4.1, P<0.001) and for those in the 4th group it was 4.9 (95% CI 3.7–6.6, P<0.001).
D-dimer level and mortality risk according to site of tumor
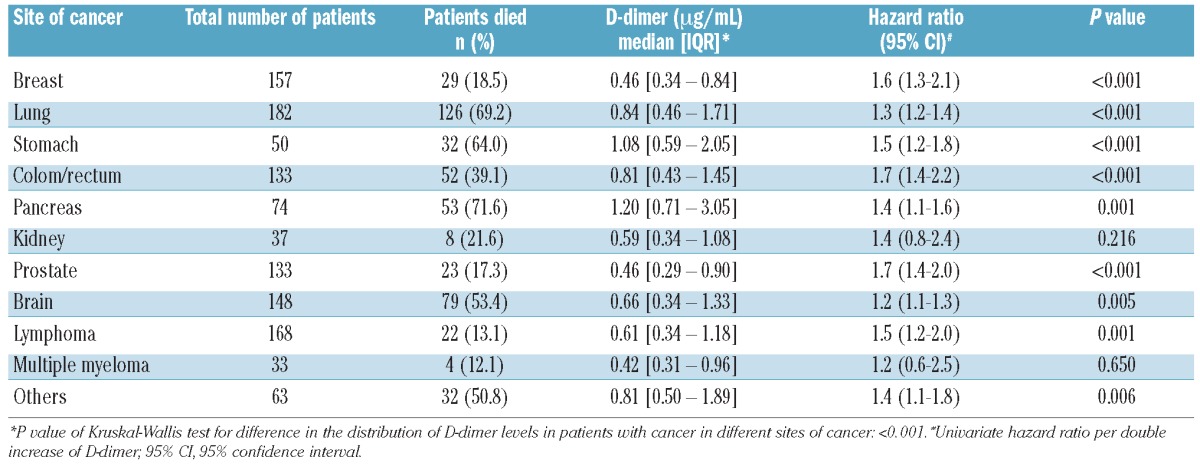
The association of D-dimer levels with risk of mortality was analyzed separately for each cancer site. In univariate analyses, an elevated D-dimer level qualified as a prognostic parameter associated with increased mortality risk in patients with brain tumors, lymphomas and in those with breast, lung, stomach, colorectal, pancreatic and prostate cancers. Table 4 summarizes the distribution of D-dimer levels and their association with mortality risk in patients with the various types of cancer. Kaplan-Meier survival analyses for each cancer site are provided in the online supplementary appendix (Online Supplementary Figures S1–S11).
Table 4.
Distribution of D-dimer levels in different sites of cancer and association of D-dimer with mortality risk per double increase of D-dimer levels.
Discussion
In this large prospective cohort study of patients diagnosed with a broad range of malignancies, we found that high plasma levels of D-dimer are associated with increased risk of mortality. After 2 years, the probability of overall survival in cancer patients with the highest D-dimer levels of the total study population (D-dimer levels ranging from 3rd quartile to the maximum level) was just 30% as opposed to that of 78% in cancer patients with lower levels (D-dimer levels ranging from the minimum to the 1st quartile). The mortality risk in patients with elevated D-dimer remained independently increased in different subgroups after adjustment for age, sex, and VTE.
These are novel findings that concur with previous observations in small studies including patients with tumors in single sites such as breast,14–16 colon and rectum,17,18 lung19–21 and brain.26 Whereas these small studies did not analyze whether the association of high D-dimer levels with poor prognosis was independent of VTE, we also recorded the occurrence of VTE during a relatively long follow-up time of up to 2 years, as this was the primary aim of our study.
VTE is a frequent complication of cancer and is among the leading causes of death in cancer patients. In epidemiological studies, VTE was associated with a poor prognosis and with a more than 3-fold increased risk of mortality in the general cancer population.27–29 Consistently, in our present study the occurrence of VTE was a prognostic parameter for shorter survival and resulted in a 3.2-fold increased risk of mortality. The association of D-dimer and VTE with poor prognosis of cancer was independent of each other in hematologic malignancies and solid tumors with and without metastases. This observation is of particular interest, because high D-dimer levels have previously been reported to predict VTE in cancer patients.10–12 Here, we could show that enhanced activation of coagulation and fibrinolysis, as reflected by high levels of D-dimer, is independently associated with an unfavorable prognosis in patients with solid cancers and hematologic malignancies and is not necessarily mediated by the increased risk of VTE in patients with elevated D-dimer levels. In this respect, it is of interest that anticoagulation, in particular that provided by low molecular weight heparins, has been suggested to have a favorable effect on survival of cancer patients.30 It would, therefore, be useful to investigate the use of low molecular weight heparins in cancer patients with elevated D-dimer levels for prevention of VTE and improvement of survival.
As expected, age was also associated with an increased mortality risk in our study. D-dimer levels are known to increase with age.31 In multivariable analysis, after adjustment for age, we could confirm that the increased risk of mortality seen in cancer patients with elevated D-dimer levels was independent of our patients’ age. The presence of metastases in patients with solid tumors included in our study, as expected, was another predictive parameter linked to poor prognosis. Previously, D-dimer levels had been reported to be increased in patients with tumors in more advanced stages. In a small study of 40 patients with colorectal cancer, D-dimer levels were reported to be associated with tumor stage and shorter post-operative survival even after curative resection.17 Similarly, D-dimer levels correlated with clinical stage, lymphovascular invasion and axillary lymph node involvement in operable breast cancer and D-dimer was suggested to be a biomarker for predicting early tumor metastases.14 In metastatic breast cancer, D-dimer levels were significantly associated with tumor load determined by imaging techniques.16 Interestingly, plasma D-dimer levels have been shown to correlate with tumor markers such as CA-125 in ovarian cancer32,33 and carcinoembryonic antigen in colorectal cancer.18 The latter study demonstrated that high D-dimer levels predicted disease progression and overall survival better than carcinoembryonic antigen did, indicating that assays of D-dimer may serve as a useful strategy for disease monitoring in patients undergoing therapy. In our study, baseline D-dimer levels were higher in cancer patients with metastases. We adjusted the association of elevated D-dimer level with overall survival and mortality for the presence or absence of metastases and found that the poor prognosis of patients with high D-dimer levels was independent of the presence of metastases suggesting that D-dimer might be a clinically important marker for the prognosis of cancer in general.
Together with the previous observations, our study points towards a relation between activation of hemostasis, reflected by plasma levels of D-dimer, and a more aggressive tumor biology leading to poor clinical outcomes. Mechanistically, this association is explained in experimental studies that have demonstrated the significance of abnormalities in hemostasis and fibrinolysis for the pathogenesis of the malignant process. The activation of coagulation is mainly considered to be the result of increased expression of tissue factor, which is the primary trigger in the initial activation of the clotting cascade, ultimately leading to fibrin deposition. Tissue factor is also expressed by tumor cells and contributes to a variety of pathological processes, such as VTE, metastatic spread, tumor growth, and tumor angiogenesis.34 It has been hypothesized that tissue factor on circulating tumor cells also leads to the fibrin coating of the cells that enables the cells to be captured within the microvasculature and facilitates hematogenous metastasis.34 In addition, thrombin, a central enzyme in the clotting cascade, which proteolyses fibrinogen to fibrin and activates platelets, leads to the formation of platelet-fibrin thrombi. Interestingly, decreased tumor metastasis has been demonstrated in an animal model of fibrinogen-deficient mice.4,35 Furthermore, the formation of a clot around the tumor cells in the circulation also prevents the tumor cells from being killed by natural-killer cells.36 D-dimer levels, as a result of fibrin deposition and subsequent degradation can be easily measured with standardized methods and are routinely used in the clinical setting. They may be a global surrogate marker of the association between cancer and the activation of hemostasis and fibrinolysis, with elevated D-dimer levels representing the pathogenesis of a more aggressive malignant process associated with poor prognosis.
There are some limitations concerning the present study. D-dimer levels were measured only once at study inclusion. Future longitudinal studies with serial measurements of D-dimer levels would be necessary to analyze their correlation with disease progression or remission and to investigate the influence of anti-cancer treatment. Furthermore, the prognosis of cancer patients is largely dependent on tumor stage. In our study, we did not have information on the exact tumor stage in patients with solid tumors, although we did record the presence or absence of distant metastases in all patients with solid tumors at their inclusion into the study. In multivariable analysis, the association of elevated D-dimer levels with increased risk of mortality was independent of the presence of distant metastasis. Another limitation of the study is that we recorded only symptomatic VTE events during the observation period. The prevalence of asymptomatic VTE in cancer patients has been reported to be significant.37–39 We cannot exclude that asymptomatic VTE was present in some patients and impaired their disease course and survival. Since autopsy is not performed on a routine basis in cancer patients and autopsy protocols were not, therefore, always available, fatal pulmonary embolism may have been missed in some cases. However, the incidence of VTE events in our study corresponds very well with the incidences reported from other prospective studies.27,40,41 As CATS was originally designed to investigate predictive parameters for the occurrence of cancer-associated VTE, detailed data on anti-cancer treatment was collected until the occurrence of VTE, death or for a maximum of 2 years. We did not, therefore, have complete information on anti-cancer treatment after the occurrence of VTE and could not adjust the association of elevated D-dimer level with mortality for the type of anti-cancer treatment. Finally, we only had data on overall survival and did not have detailed information on the causes of death and could not, therefore, restrict our analyses specifically to deaths from cancer as the outcome measure.
In a previous study including 705 patients with solid tumors, we found significant associations of C-reactive protein and soluble P-selectin with increased risk of mortality.42 We propose extending our prospective cohort study, which is still ongoing, and recruiting more patients in order to allow analyses of other biomarkers and direct comparison between the predictive values of the different biomarkers on survival in cancer patients.
In conclusion, the estimation of a cancer patient’s prognosis is of utmost clinical interest and may help when making decisions on the type and intensity of anti-cancer treatment. Our results suggest that D-dimer is a promising prognostic biomarker associated with poor overall survival in the general cancer population. Further prospective studies are needed to establish the role of D-dimer levels with regard to clinical outcomes in cancer patients, such as disease-free, cancer-specific and overall survival.
Acknowledgments
We thank all the people who supported us in recruiting patients for the Vienna Cancer and Thrombosis Study (CATS) and who served in the adjudication committee. We are also grateful to Tanja Altreiter for proof-reading this manuscript.
Footnotes
Funding: this study was supported by a grant from the Jubiläumsfonds of the Austrian National Bank (project numbers 10935 and 12739) and by an unrestricted grant from Pfizer Austria.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol. 2009;27(29):4821–6. doi: 10.1200/JCO.2009.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards RL, Rickles FR, Moritz TE, Henderson WG, Zacharski LR, Forman WB, et al. Abnormalities of blood coagulation tests in patients with cancer. Am J Clin Pathol. 1987;88(5):596–602. doi: 10.1093/ajcp/88.5.596. [DOI] [PubMed] [Google Scholar]
- 3.Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol. 2009;22(1):49–60. doi: 10.1016/j.beha.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96(10):3302–9. [PubMed] [Google Scholar]
- 5.Malik G, Knowles LM, Dhir R, Xu S, Yang S, Ruoslahti E, et al. Plasma fibronectin promotes lung metastasis by contributions to fibrin clots and tumor cell invasion. Cancer Res. 2010;70(11):4327–34. doi: 10.1158/0008-5472.CAN-09-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30(12):2362–7. doi: 10.1161/ATVBAHA.110.207514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson WD, Smith EB, Stirk CM, Marshall FI, Stout AJ, Kocchar A. Angiogenic activity of fibrin degradation products is located in fibrin fragment E. J Pathol. 1992;168(1):47–53. doi: 10.1002/path.1711680109. [DOI] [PubMed] [Google Scholar]
- 8.Pabinger I, Ay C. Biomarkers and venous thromboembolism. Arterioscler Thromb Vasc Biol. 2009;29(3):332–6. doi: 10.1161/ATVBAHA.108.182188. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G, Franchini M, Targher G, Favaloro EJ. Help me, Doctor! My D-dimer is raised. Ann Med. 2008;40(8):594–605. doi: 10.1080/07853890802161015. [DOI] [PubMed] [Google Scholar]
- 10.Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, et al. D-dimer and pro-thrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2009;27(25):4124–9. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 11.Arpaia G, Carpenedo M, Verga M, Mastrogiacomo O, Fagnani D, Lanfredini M, et al. D-dimer before chemotherapy might predict venous thromboembolism. Blood Coagul Fibrinolysis. 2009;20(3):170–5. doi: 10.1097/MBC.0b013e32831bc2de. [DOI] [PubMed] [Google Scholar]
- 12.Ay C, Pabinger I. Tests predictive of thrombosis in cancer. Thromb Res. 2010;125(Suppl 2):S12–5. doi: 10.1016/S0049-3848(10)70005-0. [DOI] [PubMed] [Google Scholar]
- 13.Palareti G, Cosmi B, Legnani C, Tosetto A, Brusi C, Iorio A, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355(17):1780–9. doi: 10.1056/NEJMoa054444. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell K, Haroon Z, Broadwater G, Berry D, Harris L, Iglehart JD, et al. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000;18(3):600–8. doi: 10.1200/JCO.2000.18.3.600. [DOI] [PubMed] [Google Scholar]
- 15.Batschauer AP, Figueiredo CP, Bueno EC, Ribeiro MA, Dusse LM, Fernandes AP, et al. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol. 2010;21(6):1267–72. doi: 10.1093/annonc/mdp474. [DOI] [PubMed] [Google Scholar]
- 16.Dirix LY, Salgado R, Weytjens R, Colpaert C, Benoy I, Huget P, et al. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer. 2002;86(3):389–95. doi: 10.1038/sj.bjc.6600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31(8):388–94. doi: 10.1093/jjco/hye075. [DOI] [PubMed] [Google Scholar]
- 18.Blackwell K, Hurwitz H, Liebérman G, Novotny W, Snyder S, Dewhirst M, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer. 2004;101(1):77–82. doi: 10.1002/cncr.20336. [DOI] [PubMed] [Google Scholar]
- 19.Altiay G, Ciftci A, Demir M, Kocak Z, Sut N, Tabakoglu E, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol (R Coll Radiol) 2007;19(7):494–8. doi: 10.1016/j.clon.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Buccheri G, Torchio P, Ferrigno D. Plasma levels of D-dimer in lung carcinoma: clinical and prognostic significance. Cancer. 2003;97(12):3044–52. doi: 10.1002/cncr.11432. [DOI] [PubMed] [Google Scholar]
- 21.Taguchi O, Gabazza EC, Yasui H, Kobayashi T, Yoshida M, Kobayashi H. Prognostic significance of plasma D-dimer levels in patients with lung cancer. Thorax. 1997;52(6):563–5. doi: 10.1136/thx.52.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) Blood. 2008;112(7):2703–8. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 23.Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–82. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 24.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–6. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 25.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. http://www.R-project.org. [Google Scholar]
- 26.Hoke M, Dieckmann K, Koppensteiner R, Schillinger M, Marosi C, Mlekusch W. Prognostic value of plasma d-dimer levels in patients with glioblastoma multiforme-Results from a pilot study. Wien Klin Wochenschr. 2011;123(7–8):199–203. doi: 10.1007/s00508-011-1556-9. [DOI] [PubMed] [Google Scholar]
- 27.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous throm-boembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 28.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25(1):70–6. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 29.Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24(7):1112–8. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 30.Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27(29):4902–11. doi: 10.1200/JCO.2009.22.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schutgens RE, Haas FJ, Biesma DH. Reduced efficacy of clinical probability score and D-dimer assay in elderly subjects suspected of having deep vein thrombosis. Br J Haematol. 2005;129(5):653–7. doi: 10.1111/j.1365-2141.2005.05515.x. [DOI] [PubMed] [Google Scholar]
- 32.Mirshahi SS, Pujade-Lauraine E, Soria C, Mirshahi M, Fretault J, Bernadou A, et al. D-dimer and CA 125 levels in patients with ovarian cancer during antineoplastic therapy. Prognostic significance for the success of anti-cancer treatment. Cancer. 1992;69(9):2289–92. doi: 10.1002/1097-0142(19920501)69:9<2289::aid-cncr2820690914>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Rose PG, Terrien JM, Baker S. Plasma D-dimer and peritoneal CA-125 levels as predictors of disease status in ovarian carcinoma. J Surg Oncol. 1994;56(3):168–71. doi: 10.1002/jso.2930560309. [DOI] [PubMed] [Google Scholar]
- 34.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27(29):4834–8. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104(2):397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 36.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 37.Cronin CG, Lohan DG, Keane M, Roche C, Murphy JM. Prevalence and significance of asymptomatic venous thromboembolic disease found on oncologic staging CT. AJR Am J Roentgenol. 2007;189(1):162–70. doi: 10.2214/AJR.07.2067. [DOI] [PubMed] [Google Scholar]
- 38.Gladish GW, Choe DH, Marom EM, Sabloff BS, Broemeling LD, Munden RF. Incidental pulmonary emboli in oncology patients: prevalence, CT evaluation, and natural history. Radiology. 2006;240(1):246–55. doi: 10.1148/radiol.2401051129. [DOI] [PubMed] [Google Scholar]
- 39.Dentali F, Ageno W, Pierfranceschi MG, Imberti D, Malato A, Nitti C, et al. Prognostic relevance of asymptomatic venous thromboembolism in patients with cancer. J Thromb Haemost. 2011;9(5):1081–3. doi: 10.1111/j.1538-7836.2011.04259.x. [DOI] [PubMed] [Google Scholar]
- 40.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339–46. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 41.Blom JW, Vanderschoot JP, Oostindiër MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4(3):529–35. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 42.Kanz R, Vukovich T, Vormittag R, Dunkler D, Ay C, Thaler J, et al. Thrombosis risk and survival in cancer patients with elevated C-reative protein. J Thromb Haemost. 2011;9(1):57–63. doi: 10.1111/j.1538-7836.2010.04069.x. [DOI] [PubMed] [Google Scholar]


