Abstract
Background
Hemophilia is caused by deficiencies in coagulation factor VIII or IX, resulting in direct blockade of the intrinsic tenase complex and indirect blockade of the extrinsic tenase complex which is rapidly inhibited upon binding of factor Xa to tissue factor pathway inhibitor. We evaluated the ability of Gla-domainless factor Xa, a truncated form of factor Xa devoid of procoagulant properties, to bind to tissue factor pathway inhibitor and to alleviate the physiological inhibition of the extrinsic tenase.
Design and Methods
Using a thrombin generation assay triggered by a low concentration of tissue factor, we evaluated the ability of Gla-domainless factor Xa to restore blood coagulation in plasma from hemophilia A and B patients without and with inhibitors. We then compared its efficacy to generate thrombin to depletion of antithrombin or tissue factor pathway inhibitor by specific antibodies. Finally, we compared the kinetics of neutralization of factor Xa and Gla-domainless factor Xa by antithrombin and tissue factor pathway inhibitor.
Results
Gla-domainless factor Xa was able to restore thrombin generation in plasma samples from hemophiliacs. This effect was observed for plasma from hemophilia A patients without or with inhibitors and for plasma from hemophilia B patients. Gla-domainless factor Xa had a lower affinity than factor Xa for tissue factor pathway inhibitor whereas the affinities of both proteins for antithrombin were similar. Finally, despite a short half-life in plasma, the effect of Gla-domainless factor Xa on thrombin generation was sustained for at least 1 hour.
Conclusions
As Gla-domainless factor Xa was able to restore thrombin generation in plasma from hemophilia patients, our results suggest that it may be an effective alternative to current treatments for hemophilia with or without an inhibitor.
Keywords: Gla-domainless factor Xa, FXa, TFPI, thrombin generation, hemophilia, inhibitor
Introduction
Hemophilia is an X-linked bleeding disorder characterized by dysfunction of the intrinsic tenase complex because of a deficiency in coagulation factor VIII (hemophilia A) or IX (hemophilia B).1 In recent years, the treatment of patients with severe hemophilia has improved considerably because of the greater availability of concentrates, allowing widespread adoption of prophylaxis to prevent bleeding episodes.2 However, for hemophilia A in particular, the development of antibodies inhibiting the activity of the therapeutic clotting factor is the most serious and costly complication of replacement therapy.3 Inhibitor formation is observed in 10 to 30% of hemophilia A patients, depending on the nature of the concentrate used,4 and in 1.5 to 3% of hemophilia B patients.5 The first aim in treating the inhibitor is to eradicate it permanently by immune tolerance induction in order to be able to resume replacement therapy.6,7 For patients in whom this immune tolerance cannot be achieved and who have a high titer of inhibitors [> 5 Bethseda units (BU)/mL)], bypassing agents are needed. FEIBA (factor VIII inhibitor-bypassing activity), an activated prothrombin complex, and NovoSeven, a recombinant activated factor VII (rFVIIa), are both extensively used to treat hemorrhagic episodes in patients with inhibitors.8,9 However, both these products have limitations. A randomized comparison of the two products showed that a substantial number of patients do not respond to these bypassing agents.10 Moreover, rare thrombotic adverse events are observed with both rFVIIa and FEIBA.11 These problems highlight the need to develop alternative therapeutic strategies.
As emphasized by Tuddenham, the pathways involved in bypassing a blocked tenase converge towards the tissue factor (TF)-dependent complex that initiates coagulation.12 One possible way of bypassing a blocked tenase is to increase the availability of TF through generation of TF-bearing microparticles, such as is observed following infusion of P-selectin/immunoglobulin chimera protein (as proposed by Hrachovinovà et al).13 Another possibility is to neutralize the activity of tissue factor pathway inhibitor (TFPI). In fact, anti-TFPI immunoglobulin shortens the coagulation time of plasma from hemophiliacs14 and the bleeding time in rabbits with antibody-induced hemophilia A.15 In addition, other approaches to neutralizing TFPI have been shown to be active ex vivo and in animal models.16–19
Here, we propose a new approach to unlock the tenase complex of hemophilia patients with or without inhibitor. In contrast to activated factor X (FXa), Gla-domainless FXa (GDXa) is unable to bind to procoagulant phospholipids and is almost completely devoid of procoagulant activity.20 However, as GDXa retains the capacity to bind TFPI21 and the GDXa-TFPI complex is unable to inhibit the FVIIa-TF complex,22 GDXa may compete with FXa and induce a decrease in the generation of the TF-FVIIa-FXa-TFPI quaternary complex that blocks the extrinsic tenase. In this study we, therefore, investigated the ability of GDXa to restore thrombin generation in plasma from patients with hemophilia.
Design and Methods
Materials
A pool of frozen plasma from normal subjects and individual plasma samples from patients with hemophilia A or hemophilia B, phospholipids TGT, Prionex, corn trypsin inhibitor, chromogenic substrate PNAPEP 1025, human FXa, human des-Gla-factor Xa (GDXa), and human TFPI sheep antibody were obtained from Cryopep (Montpellier, France). Recombinant human TFPI was obtained from Sino Biological Inc. (Beijing, China), whereas the relipidated recombinant human TF (Innovin) came from Siemens Healthcare Diagnostics (Puteaux, France). For thrombin generation assays, we used the Thrombin calibrator, FluCaKit and 96-well, round-bottomed microtiter plates (Immulon 2HB, U-bottom plate) from Diagnostica Stago (Asnières, France), whereas for enzymatic experiments, we used 96-well, flat-bottomed microtiter plates from Greiner (Frickenhausen, Germany). Antithrombin sheep antibody came from Affinity Biologicals (Sandhill Drive, Canada). We used the Actichrom TFPI activity assay from American Diagnostica (Stamford, USA) to determine TFPI activity. The antithrombin activity assay (STA-Stachrom antithrombin III) was from Diagnostica STAGO. Enzymatic calculations were realized with PRISM 5.0 software.
Thrombin generation assays
Thrombin generation was measured according to Hemker’s method using 1 pM of TF to activate coagulation23 in the presence of 30 μg/mL corn trypsin inhibitor to prevent the activation of the contact phase of coagulation during the incubation period.24 Briefly, a 20-μL mixture of TF, 4 μM phospholipids, and 80 μL of plasma were manually pipetted in triplicate into a microtiter plate. Twenty microliters of thrombin calibrator with 80 μL of plasma were also pipetted in triplicate into the plate. The plate was then inserted into a Varioskan (Thermofisher, Illkirch, France) set at an excitation wavelength of 390 nm, with an emission wavelength of 460 nm and a bandwidth of 10 nm. Twenty microliters of FluCaKit (2.5 mM fluorogenic substrate (Z-Gly-Gly-Arg-AMC, ZGGR-AMC) with 0.1 M CaCl2) were automatically injected into all of the wells, starting the reaction. The fluorescence signal was read every 20 sec for 60 min. Raw data on fluorescence intensities were exported to Sigmaplot® 9.0 for mathematical calculations using the previously described three-wave method.25 The parameters determined from a thrombin generation assay are: the endogenous thrombin potential, which corresponds to the area under the thrombin generation curve; the peak height, which corresponds to the maximal level of thrombin; the lag time, which corresponds to the time taken to reach 2 nM thrombin; and the peak time, which corresponds to the time taken to reach the peak height.
GDXa, FXa or Novoseven was diluted, at pH 7.35, in 1% Prionex, 18 mM HEPES and 135 mM sodium chloride buffer (buffer A) and added at various concentrations to samples of hemophilic plasma pretreated with corn trypsin inhibitor.
Neutralization of antithrombin and tissue factor pathway inhibitor by specific antibodies
For neutralization of antithrombin, a plasma sample from a patient with severe hemophilia A was spiked and incubated for 1 h at 25°C with different concentrations of anti-human antithrombin sheep IgG (1.8, 3, 5, and 7.5 g/L) before being tested in the thrombin generation assay. In parallel, antithrombin activity was measured on a STAR coagulometer (Diagnostica Stago) with STA-Stachrom antithrombin III reagents.
For neutralization of TFPI, the same hemophilia A plasma was spiked with different concentrations of anti-human TFPI sheep immunoglobulins (2.5, 5, 10, and 50 mg/L) before being tested in the thrombin generation assay. In parallel, TFPI activity was determined with the Actichrom TFPI activity assay according to the manufacturer’s instructions. Briefly, 20 μL of plasma diluted 20-fold were incubated at 37°C in the presence of 20 μL TF/FVIIa for 30 min. FX was then added and incubated at 37°C for 15 min before EDTA and Spectrozyme FXa were added. The reaction was stopped 5 min later by adding glacial acetic acid and absorbance was read at 405 nm.
Chromogenic assays
Determination of the kinetic constants of Gla-domainless activated factor X and normal activated factor X
In order to determine GDXa or FXa activity, 0.3 nM of enzyme were incubated for 5 min at 37°C in buffer A at pH 8.4. Then, PNAPEP 1025 chromogenic substrate was added at concentrations of 0.33, 0.50, 1, 1.5, and 2.0 mM, and the variation of absorbance was recorded at 405 nm.
Enzymatic inhibition of antithrombin
For the assay of antithrombin inhibition, FXa or GDXa (1.25 nM) was incubated at 37°C in buffer A in the presence of increasing concentrations of antithrombin (0 to 500 nM). Aliquots of 200 μL of the mixture were removed at different time intervals, up to 90 min. Then, 50 μL of 6 mM PNAPEP 1025 chromogenic substrate were added and the variation of absorbance was recorded.
Enzymatic inhibition of tissue factor pathway inhibitor
Inhibition of GDXa or FXa activity by TFPI was analyzed by incubating 0.25 nM of enzyme for 3 h at 25°C in buffer A in the presence of increasing concentrations of TFPI (0 to 30 nM for GDXa and 0 to 10 nM for FXa) in a final volume of 200 μL. Then, 50 μL of 2.5 mM PNAPEP 1025 chromogenic substrate were added, and the variation of absorbance was recorded. Ki* was determined as previously described.26,27
Determination of the plasma half-life of Gla-domainless activated factor X and normal activated factor X
The half-lives of GDXa and FXa in plasma were determined by spiking normal plasma with 50 nM GDXa or FXa. The mixture was then incubated at 37°C. Aliquots were removed from 0 to 60 min and immediately diluted 25 times in buffer A before addition of 1.5 mM PNAPEP 1025 chromogenic substrate and determination of residual amidolytic activity, as previously described.
Results
Kinetic parameters of Gla-domainless activated factor X and normal activated factor X
Before analyzing the effect of GDXa on thrombin generation, we characterized it together with FXa by PNAPEP 1025 chromogenic substrate cleavage. GDXa showed a similar affinity (Km=0.75±0.05 mM) to FXa (Km=0.64±0.03 mM) and similar catalytic properties (kcat=290±8 sec−1) to FXa (kcat=375±5 sec−1) (Table 1). These results are consistent with previous observations obtained with the S2222 chromogenic substrate.20
Table 1.
Enzymatic properties of GDXa and FXa. Data are representative of two independent measurements performed in triplicate with PNAPEP 1025 chromogenic substrate.

Influence of Gla-domainless activated factor X and normal activated factor X on thrombin generation
At a concentration of 1 pM, TF was unable to induce thrombin generation in plasma from patients with severe hemophilia A, as shown by the dotted line in Figure 1A. However, in the presence of 50 nM GDXa, clear restoration of thrombin generation was observed (Figure 1B). GDXa normalized all parameters related to thrombin generation including endogenous thrombin potential, lag time, peak height, and peak time (Figure 1B, Table 2). The observed thrombin generation was not a direct effect of GDXa on plasma, as no thrombin was generated in the absence of TF (Figure 1B, dotted line). This was observed in all the hemophilia plasmas tested. Furthermore, in contrast to GDXa, FXa triggered thrombin generation even in the absence of TF (Figure 1C), as it is able to convert prothrombin to thrombin directly.
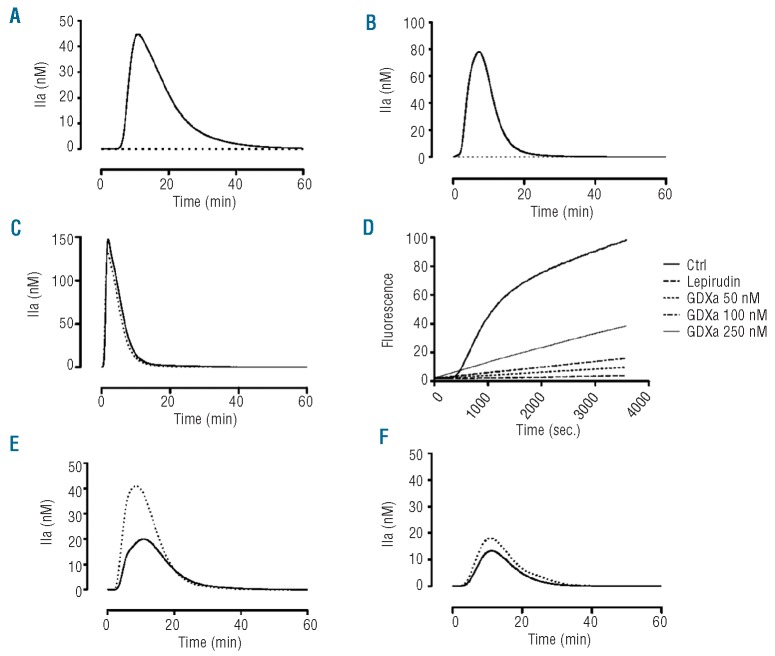
Figure 1.
Influence of GDXa or FXa on thrombin (IIa) generation. (A) Thrombin generation assays in the presence of phospholipids and TF in normal pooled plasma (solid line) and in severe hemophilia A plasma (dotted line). (B) Hemophilic plasma spiked with GDXa (50 nM) in the presence of phospholipids with TF (solid line) and without TF (dotted line). (C) Hemophilic plasma spiked with FXa (50 nM) in the presence of phospholipids with TF (solid line) and without TF (dotted line). (D) Cleavage of the ZGGR-AMC substrate by GDXa. Normal corn trypsin inhibitor-pretreated plasma was tested in the presence of phospholipids and TF both with and without (Ctrl) lepirudin 6 μg/mL in the absence or presence of various concentrations of GDXa. (E) Thrombin generation in the presence of phospholipids and TF in hemophilic plasma spiked with 10 nM (solid line) or 20 nM (dotted line) GDXa and (F) with 40 nM (thick line) or 200 nM (dotted line) rFVIIa. Data are representative of the experiments in at least, three different severe hemophilia A plasma samples.
Table 2.
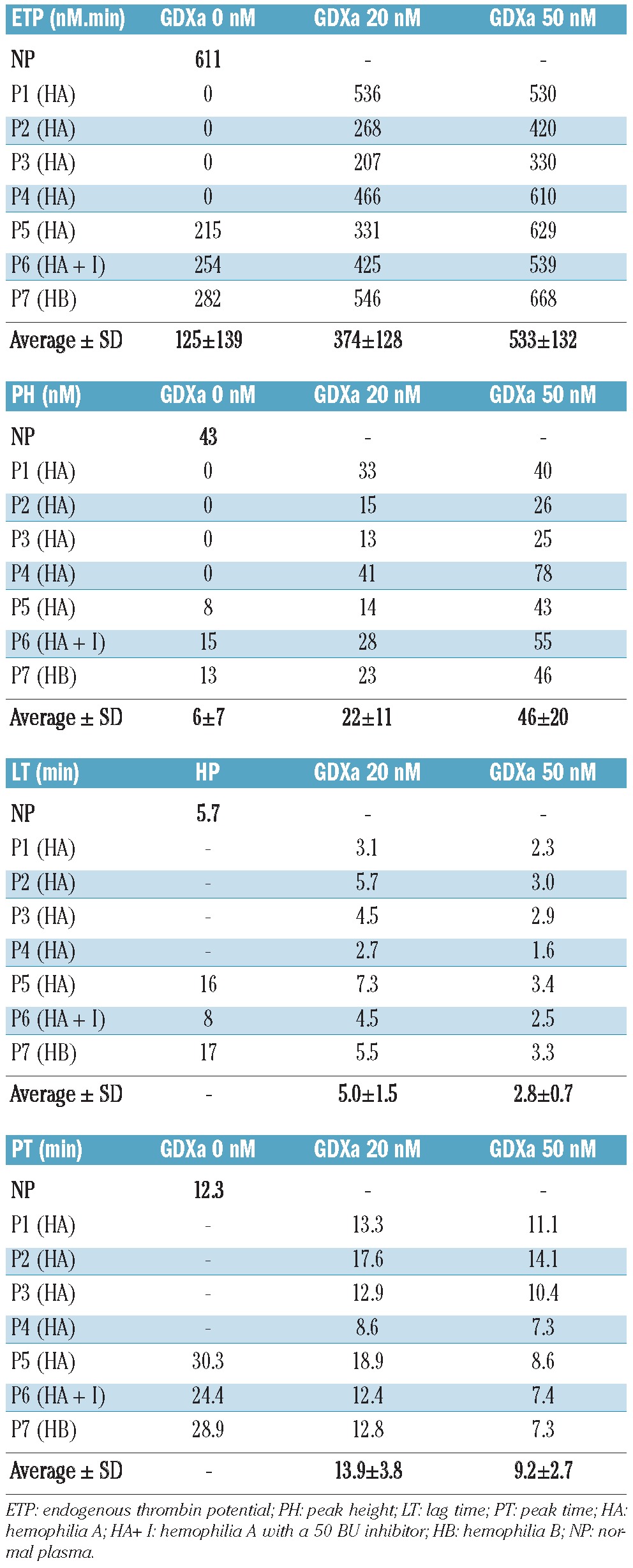
Influence of GDXa on thrombin generation in five different severe hemophilia A plasmas with and without inhibitor and in one severe B hemophilia plasma. Hemophilia plasmas were spiked with 20 or 50 nM GDXa and immediately tested in thrombin generation assays.
To quantify any interference caused by direct cleavage of the ZGGR-AMC substrate by GDXa, increasing amounts of the enzyme were added in the presence of a saturating amount (6 μg/mL) of the thrombin inhibitor lepirudin. As shown in Figure 1D, the raw fluorescence signal was totally abolished in the presence of lepirudin. Under these conditions, GDXa cleaved the ZGGR-AMC substrate proportionally to its concentration. At a concentration of 50 nM, the final signal was 8% of that generated in the absence of lepirudin; at a concentration of 250 nM, the signal was roughly 40% of the total fluorescence. However, because the signal was linear, it was mathematically included into the α2-macroglobulin – thrombin complex signal in the three-wave method used to calculate the thrombin concentration curves and did not affect the thrombin generation results.25
We then evaluated the minimal amount of GDXa capable of restoring thrombin generation in plasma from patients with severe hemophilia A. We found that a concentration of 20 nM GDXa yielded a thrombin generation curve in this plasma (Figure 1E) similar to that observed for normal plasma (Figure 1A). In addition, we observed that 10 nM GDXa generated a slightly higher signal than the one obtained in the presence of 200 nM rFVIIa (Figure 1F).
Effect of antibodies against both antithrombin and tissue factor pathway inhibitor on thrombin generation
Anti-antithrombin antibody was able to increase endogenous thrombin potential massively, with a lower impact on kinetic parameters (Table 3 and Figure 2A). At 7.5 g/L of anti-antithrombin antibody (9% antithrombin residual activity), endogenous thrombin potential increased dramatically to 2716 nM.min whereas the peak height reached 76 nM. These results contrast with those of the kinetic parameters (lag time = 6.6 min, peak time = 31.3 min).
Table 3.
Influence of anti-antithrombin and anti-TFPI antibodies on thrombin generation. Severe hemophilia A plasma was spiked with different concentrations of anti-human antithrombin or anti-human TFPI antibodies before the thrombin generation assay. Antithrombin and TFPI residual activities were measured according to the method described in the Design and Methods section.
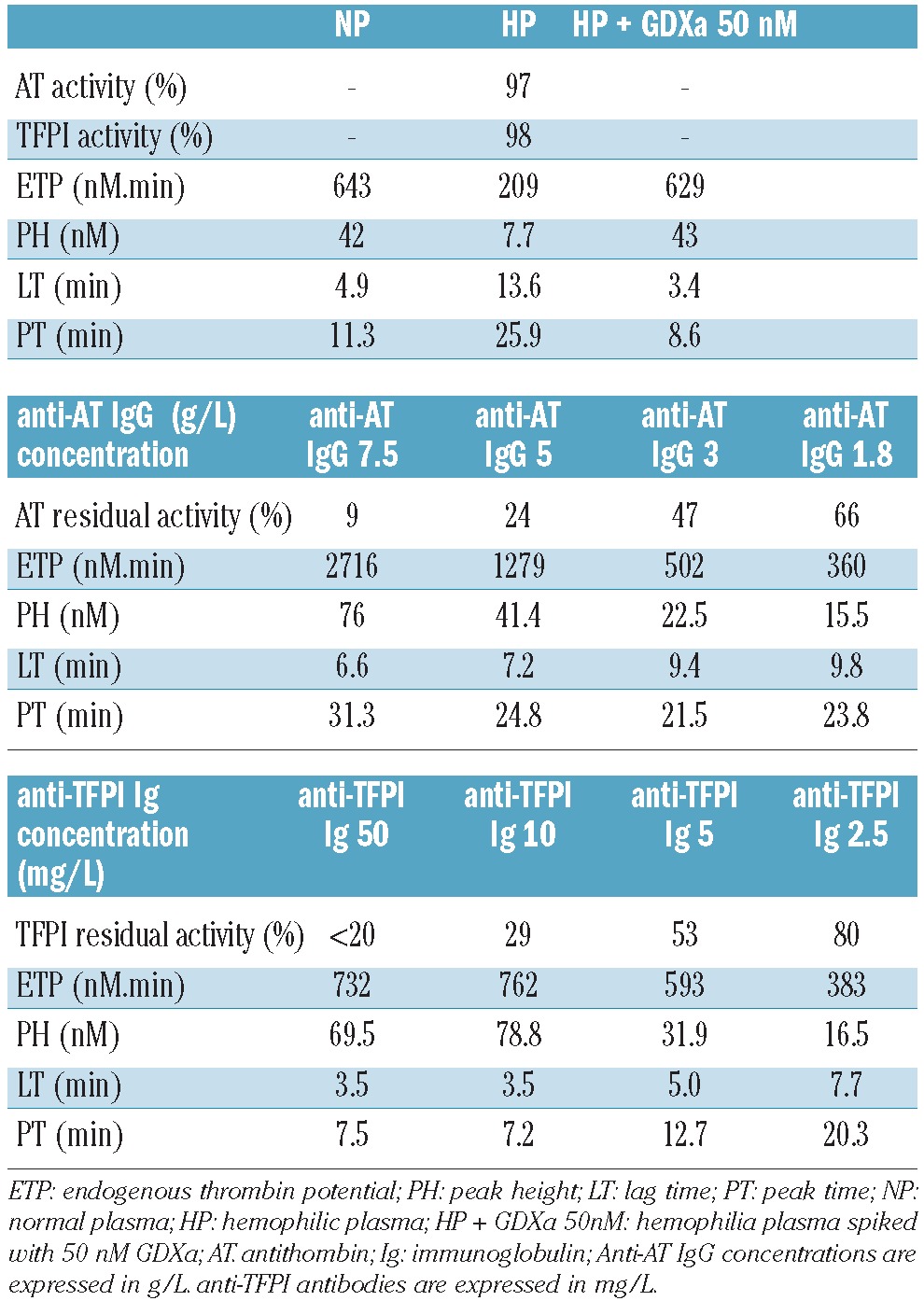
Figure 2.
Influence of anti-antithombin (AT) and anti-TFPI antibodies on thrombin generation in hemophilia plasma. A severe hemophilia A plasma was spiked with different concentrations of anti-human antithrombin (A) or anti-human TFPI (B) antibodies before the thrombin generation assay. Concentrations of antibodies are expressed in g/L for anti-antithrombin and mg/L for anti-TFPI. The concentration of GDXa is in nM. HP stands for hemophilia plasma; NP: normal plasma.
Likewise, as previously reported by Erhardtsen et al.,15 an anti-TFPI antibody was also able to restore coagulation of plasma from hemophiliacs. At concentrations above 10 mg/L of anti-TFPI antibody (residual TFPI activity < 30%), all the parameters of the thrombin generation assay were corrected in hemophilia plasma (Table 3). At 10 mg/L (Table 3 and Figure 2B), endogenous thrombin potential and peak height increased respectively from 209 to 762 nM.min and from 8 to 79 nM. The lag time and peak time decreased respectively from 13.6 to 3.5 min and from 25.9 to 7.2 min.
Enzymatic inhibition of Gla-domainless activated factor X and normal activated factor X by tissue factor pathway inhibitor and antithrombin
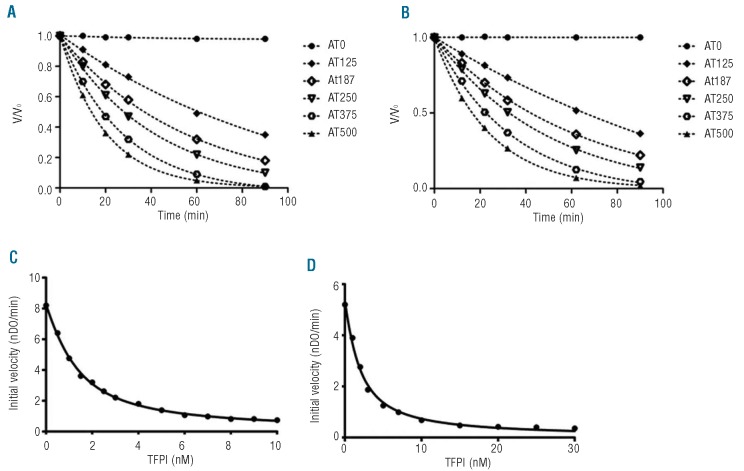
For irreversible enzymatic inhibition by antithrombin (Table 1), the inhibition profiles of GDXa (k2=1.50±0.04×103 M−1.s−1, Figure 3B) and FXa were identical (k2 = 1.57±0.08×103 M−1.s−1, Figure 3A). TFPI is a slow, tight binding inhibitor of FXa26,27 and, at a low concentration, a weak inhibitor of GDXa.28–30 We, therefore, compared TFPI inhibition of FXa and GDXa (Table 1). GDXa showed a lower affinity for TFPI (Ki* = 0.31±0.04 nM, Figure 3D) compared to FXa (Ki* = 0.17±0.03 nM, Figure 3C). In addition, the achievement of equilibrium in this experiment was suggested by the identity of the titration curves following incubation for up to 18 h.
Figure 3.
Enzymatic inhibition of GDXa and FXa by TFPI or antithrombin (AT). (A–B) For antithrombin inhibition, 1.25 nM of FXa (A) or GDXa (B) were incubated in the presence of increasing concentrations of antithrombin (0 to 500 nM) at 37°C. Aliquots were removed at different times (0 to 90 min) and added to PNAPEP 1025 chromogenic substrate. (C–D) Inhibition of FXa (C) or GDXa (D) activity by TFPI was analyzed by incubating 0.25 nM of the enzyme for 3 h at 25°C in buffer A in the presence of increasing concentrations of TFPI (0 to 30 nM for GDXa and 0 to 10 nM for FXa). Data are representative of two different experiments.
Half-life of Gla-domainless activated factor X and normal activated factor X in plasma
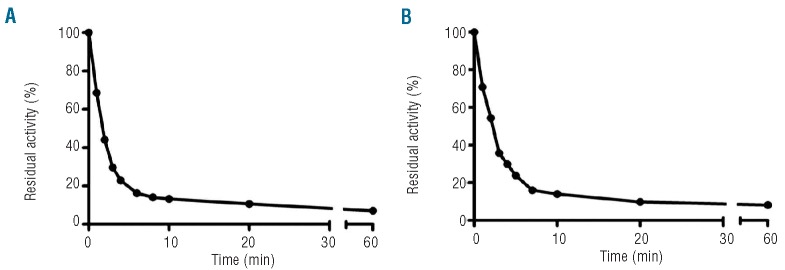
Considering GDXa inhibition by TFPI and antithrombin, we evaluated the surviving activity after spiking plasma with 50 nM GDXa or FXa at 37°C. As shown in Figure 4, the activity in plasma decreased rapidly, with a half-life of approximately 90 sec, and reached a plateau at 20 min for GDXa or FXa, confirming results previously found for FXa.26 Nevertheless, the effect on thrombin generation was maintained over time, because, at 1 h, when the residual activity of GDXa was roughly 10% of its initial activity (Figure 4B), the restoration of thrombin generation was maintained. After 1 min of incubation, endogenous thrombin potential increased from 0 to 610 nM and remained at 478 nM after 60 min (Table 4). A similar correction was also observed for the maximal peak height (75 nM and 38 nM at 1 and 60 min, respectively) as well as for the lag and peak times (Table 4).
Figure 4.
Determination of the pharmacokinetic properties of GDXa and FXa in plasma. Normal plasma was spiked with 50 nM FXa (A) or GDXa (B), and incubated at 37°C. Aliquots were removed at different times and immediately diluted 25 times in buffer A for determination of residual activity. Half-lives were 1.4±0.1 min for FXa and 1.8±0.1 min for GDXa. Data are the mean of two different experiments.
Table 4.
Effect of GDXa after 1 min and 1 h of incubation at 37°C in a severe hemophilic plasma. A severe hemophilia A plasma was spiked with 50 nM GDXa and incubated at 37°C for 1 h. Aliquots were removed both immediately and after 1 h for testing in thrombin generation assays. By definition, all values were set at zero for hemophilic plasma because no thrombin was generated.

Influence of Gla-domainless activated factor X on thrombin generation in plasma from patients with severe hemophilia A with and without inhibitor and from a patient with severe hemophilia B
Finally, we evaluated the generation of thrombin in plasma samples from five different subjects with severe hemophilia A, including one with an inhibitor titrated at 50 BU, and from one individual with severe hemophilia B. Thrombin generation was almost undetectable in all six plasma samples when coagulation was triggered by 1 pM TF, whereas it was restored in the presence of 20 and 50 nM GDXa. Table 2 shows that corrections were observed, at different degrees, for all plasma samples and for all parameters related to thrombin generation. In the presence of 20 nM GDXa, we observed an endogenous thrombin potential of 374±128 nM and a peak height of 22±11 nM. The lag time and peak time decreased to 5.0±1.5 and 13.9±3.8 min, respectively.
Furthermore, a dose effect was observed, as these values rose to 533±132 nM for endogenous thrombin potential and 46±20 nM for peak height when 50 nM GDXa was added. The lag time and peak time decreased to 2.8±0.7 and 9.2±2.7 min, respectively.
Discussion
The latest major advance in hemophilia treatment is the availability of vast amounts of clotting factors, which became possible through the production of recombinant clotting factors. However, the subsequent appearance of inhibitors has become the new challenge to be addressed in hemophilia treatment. New approaches to the treatment of this severe bleeding diathesis would, therefore, be welcomed. Targeting a negative regulator of the blood clotting cascade is a radically new strategy in this arena. This strategy may be more efficient when aimed at the first step of the cascade and its inhibitor, TFPI, as shown by the pioneering work of Nordfang and Erhardtsen.14,15 These authors first suggested the potential efficacy of this tactic when using antibodies to block TFPI. More recently, two different kinds of TFPI inhibitors have been investigated: sulfated polysaccharides16,19 and aptamers.17,18 Our study, using a strategy to inhibit TFPI that differs from the three previously published methods, confirms the potential interest of this target with regards to restoring blood clotting in hemophilia patients with or without inhibitors.
We used a truncated form of a normal partner of the quaternary inhibition complex of the extrinsic pathway of coagulation: GDXa. In contrast to FXa, GDXa, which does not bind to procoagulant phospholipids, is unable to activate blood coagulation in hemophilia plasma by itself (Figure 1B), as it is almost completely devoid of any intrinsic capacity to activate prothrombin into thrombin, as previously described.20 Furthermore, in the absence of procoagulant properties, GDXa may have a safer therapeutic margin compared to activated fractions/factors.11 Moreover, the strict necessity of the presence of TF (Figure 1B) to normalize thrombin generation by GDXa in hemophilia plasma would restrict its activity to sites of active bleeding, whereas the effect of activated fractions/factors may be more widespread.
GDXa normalized thrombin generation in plasma from hemophilia patients at a concentration lower than required when rFVIIa is used for this purpose. As shown in Figure 1E and 1F, 10 nM GDXa was slightly more efficient than 200 nM rFVIIa for thrombin generation, suggesting that GDXa is almost 20 times as potent as rFVIIa under these conditions. In addition to TFPI, GDXa also interacts with antithrombin, and this may explain the very short half-life (close to 2 min) that was observed using chromogenic substrates. However, there was a difference between the pharmacokinetics and pharmacodynamics of GDXa in plasma because 60 min after incubation, the thrombin generation signal was still corrected for all parameters. Indeed, endogenous thrombin potential was almost 80% of that observed at 1 min, whereas peak time and lag time were still shorter than those of the control. This may be explained by persistence of the GDXa-TFPI complex, which therefore sustains the correction of thrombin generation. Moreover, as the affinity of GDXa for TFPI is lower than that of FXa (Figure 3D), as previously demonstrated,28 the generation of FXa in the presence of GDXa would result in the accumulation of sufficient FXa to reverse the formation of the GDXa-TFPI complex and ultimately restore the inhibition of coagulation activation.
Finally, the use of a fragment of a protein naturally present in the plasma of hemophilia patients may be less immunogenic than using non-physiological molecules that may be naturally immunogenic or become immunogenic when coupled to a protein, such as is observed in heparin-induced thrombocytopenia.
The proposed mechanisms of action for GDXa and TFPI are schematically represented in Figure 5. In a healthy person, the extrinsic tenase (TF-FVIIa) is inhibited by TFPI when FXa is produced and binds to it (Figure 5A). The quaternary complex formed (FXa-TFPI-FVIIa-TF) then prevents further activation of factors IX and X. This is physiologically compensated for by the intrinsic tenase (FVIIIa-FIXa). In hemophilia patients, the intrinsic tenase is blocked. Initiation of coagulation is normal, but the propagation step, depending on the intrinsic tenase, is blocked. Inhibition of TFPI by GDXa may allow prolonged generation of FXa by the extrinsic tenase and, therefore, normalization of thrombin generation.
Figure 5.
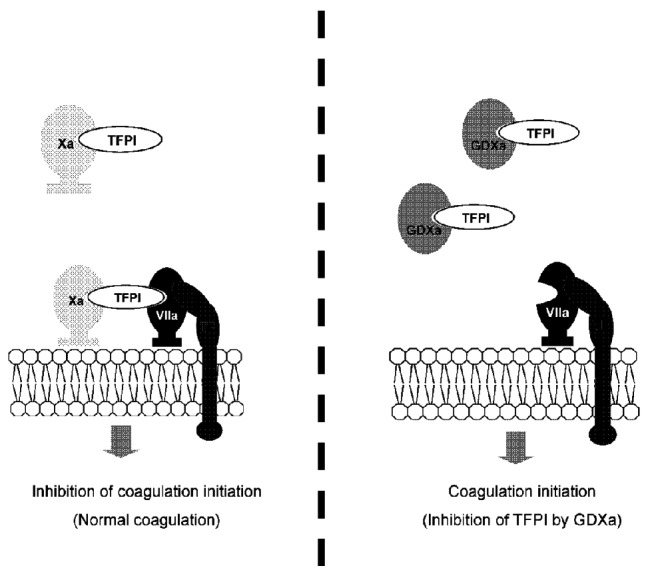
Working hypothesis for the mechanism of action of GDXa. (A) The Xa-TFPI complex is the physiological inhibitor of the TF/FVIIa coagulation initiation complex. (B) Exogenous GDXa competes with FXa for binding to TFPI, preventing the formation of the FXa-TFPI-FVIIa-TF complex and restoring thrombin generation.
Similarly to anti-TFPI antibodies, GDXa corrected the thrombin generation assay. In contrast, the profile of the thrombin generation assay observed following the administration of antibodies against antithrombin suggests that the mechanism of action of GDXa is not through inhibition of antithrombin. This suggestion is reinforced by the fact that the GDXa concentrations used (20 and 50 nM) are two orders of magnitude below the plasma antithrombin concentration (≈ 5 μM). Conversely, TFPI plasma concentration (≈ 3 nM) is compatible with TFPI saturation by GDXa and, therefore, supports the mechanism depicted in Figure 5B.
Collectively, our results suggest that GDXa may be an efficient and safe alternative to currently used bypassing agents in the treatment of hemophilia with or without inhibitor.
Footnotes
Funding: the authors are indebted to the Laboratoire Français du Fractionnement et des Biotechnologies (LFB) for supporting this study.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Bolton-Maggs PHB, Pasi KJ. Haemophilias A and B. Lancet. 2003;361(9371):1801–9. doi: 10.1016/S0140-6736(03)13405-8. [DOI] [PubMed] [Google Scholar]
- 2.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–44. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 3.Gringeri A, Mantovani LG, Scalone L, Mannucci PM, Cocis SG. Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood. 2003;102(7):2358–63. doi: 10.1182/blood-2003-03-0941. [DOI] [PubMed] [Google Scholar]
- 4.Iorio A, Halimeh S, Holzhauer S, Goldenberg N, Marchesini E, Marcucci M, et al. Rate of inhibitor development in previously untreated hemophilia A patients treated with plasma-derived or recombinant factor VIII concentrates: a systematic review. J Thromb Haemost. 2010;8(6):1256–65. doi: 10.1111/j.1538-7836.2010.03823.x. [DOI] [PubMed] [Google Scholar]
- 5.DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007;138(3):305–15. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 6.Kempton CL, White GC., II How we treat a hemophilia A patient with a factor VIII inhibitor. Blood. 2009;113(1):11–7. doi: 10.1182/blood-2008-06-160432. [DOI] [PubMed] [Google Scholar]
- 7.Tellier Z, Andre MH, Polack B. Management of haemophilia A-inhibitor patients: clinical and regulatory perspectives. Clin Rev Allergy Immunol. 2009;37(2):125–34. doi: 10.1007/s12016-009-8115-4. [DOI] [PubMed] [Google Scholar]
- 8.Barthels M. Clinical efficacy of prothrombin complex concentrates and recombinant factor VIIa in the treatment of bleeding episodes in patients with factor VIII and IX inhibitors. Thromb Res. 1999;95(4):S31–8. doi: 10.1016/s0049-3848(99)00082-1. [DOI] [PubMed] [Google Scholar]
- 9.Ingerslev J. Efficacy and safety of recombinant factor VIIa in the prophylaxis of bleeding in various surgical procedures in hemophilic patients with factor VIII and factor IX inhibitors. Semin Thromb Hemost. 2000;26(4):425–32. doi: 10.1055/s-2000-8463. [DOI] [PubMed] [Google Scholar]
- 10.Astermark J, Donfield SM, DiMichele DM, Gringeri A, Gilbert SA, Waters J, et al. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) study. Blood. 2007;109(2):546–51. doi: 10.1182/blood-2006-04-017988. [DOI] [PubMed] [Google Scholar]
- 11.Aledort LM. Comparative thrombotic event incidence after infusion of recombinant factor VIIa versus factor VIII inhibitor bypass activity. J Thromb Haemost. 2004;2(10):1700–9. doi: 10.1111/j.1538-7836.2004.00944.x. [DOI] [PubMed] [Google Scholar]
- 12.Tuddenham EGD. Ways to bypass a blocked tenase complex. Thromb Haemost. 2006;95(1):1–2. [PubMed] [Google Scholar]
- 13.Hrachovinova I, Cambien B, Hafezi-Moghadam A, Kappelmayer J, Camphausen RT, Widom A, et al. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat Med. 2003;9(8):1020–5. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 14.Nordfang O, Valentin S, Beck TC, Hedner U. Inhibition of extrinsic pathway inhibitor shortens the coagulation time of normal plasma and of hemophilia plasma. Thromb Haemost. 1991;66(4):464–7. [PubMed] [Google Scholar]
- 15.Erhardtsen E, Ezban M, Madsen MT, Diness V, Glazer S, Hedner U, et al. Blocking of tissue factor pathway inhibitor (TFPI) shortens the bleeding time in rabbits with antibody induced haemophilia A. Blood Coagul Fibrinolysis. 1995;6(5):388–94. doi: 10.1097/00001721-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Prasad S, Lillicrap D, Labelle A, Knappe S, Keller T, Burnett E, et al. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood. 2008;111(2):672–9. doi: 10.1182/blood-2007-07-098913. [DOI] [PubMed] [Google Scholar]
- 17.Parunov LA, Fadeeva OA, Balandina AN, Soshitova NP, Kopylov KG, Kumskova MA, et al. Improvement of spatial fibrin formation by the anti-TFPI aptamer BAX499: changing clot size by targeting extrinsic pathway initiation. J Thromb Haemost. 2011;9(9):1825–34. doi: 10.1111/j.1538-7836.2011.04412.x. [DOI] [PubMed] [Google Scholar]
- 18.Waters EK, Genga RM, Schwartz MC, Nelson JA, Schaub RG, Olson KA, et al. Aptamer ARC19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood. 2011;117(20):5514–22. doi: 10.1182/blood-2010-10-311936. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Scallan CD, Broze GJ, Jr, Patarroyo-White S, Pierce GF, Johnson KW. Improved coagulation in bleeding disorders by non-anticoagulant sulfated polysaccharides (NASP) Thromb Haemost. 2006;95(1):68–76. [PubMed] [Google Scholar]
- 20.Skogen WF, Esmon CT, Cox AC. Comparison of coagulation factor Xa and des-(1-44)factor Xa in the assembly of prothrombinase. J Biol Chem. 1984;259(4):2306–10. [PubMed] [Google Scholar]
- 21.Broze GJ, Warren L, Novotny W, Higuchi D, Girard J, Miletich J. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988;71(2):335–43. [PubMed] [Google Scholar]
- 22.Kazama Y. The importance of the binding of factor Xa to phospholipids in the inhibitory mechanism of tissue factor pathway inhibitor: the transmembrane and cytoplasmic domains of tissue factor are not essential for the inhibitory action of tissue factor pathway inhibitor. Thromb Haemost. 1997;77(3):492–7. [PubMed] [Google Scholar]
- 23.Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost. 2006;96(5):553–61. [PubMed] [Google Scholar]
- 24.van Veen JJ, Gatt A, Cooper PC, Kitchen S, Bowyer AE, Makris M. Corn trypsin inhibitor in fluorogenic thrombin-generation measurements is only necessary at low tissue factor concentrations and influences the relationship between factor VIII coagulant activity and thrombogram parameters. Blood Coagul Fibrinolysis. 2008;19(3):183–9. doi: 10.1097/MBC.0b013e3282f4bb47. [DOI] [PubMed] [Google Scholar]
- 25.De Smedt E. Advanced thrombinoscopy: PhD thesis. University Maastricht; 2007. [Google Scholar]
- 26.Bunce MW, Toso R, Camire RM. Zymogen-like factor Xa variants restore thrombin generation and effectively bypass the intrinsic pathway in vitro. Blood. 2011;117(1):290–8. doi: 10.1182/blood-2010-08-300756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baugh RJ, Broze GJ, Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273(8):4378–86. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 28.Lockett JM, Mast AE. Contribution of regions distal to glycine-160 to the anticoagulant activity of tissue factor pathway inhibitor. Biochemistry. 2002;41(15):4989–97. doi: 10.1021/bi016058n. [DOI] [PubMed] [Google Scholar]
- 29.Lindhout T, Willems G, Blezer R, Hemker HC. Kinetics of the inhibition of human factor Xa by full-length and truncated recombinant tissue factor pathway inhibitor. Biochem J. 1994;297(Pt 1):131–6. doi: 10.1042/bj2970131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesselschmidt R, Likert K, Girard T, Wun T, Broze GJ. Tissue factor pathway inhibitor: the carboxy-terminus is required for optimal inhibition of factor Xa. Blood. 1992;79(8):2004–10. [PubMed] [Google Scholar]