Abstract
Injection of the seaweed toxin kainic acid (KA) in rats induces a severe status epilepticus initiating complex neuropathological changes in limbic brain areas and subsequently spontaneous recurrent seizures. Although neuropathological changes have been intensively investigated in the hippocampus proper and the dentate gyrus in various seizure models, much less is known about changes in parahippocampal areas. We now established telemetric EEG recordings combined with continuous video monitoring to characterize the development of spontaneous seizures after KA-induced status epilepticus, and investigated associated neurodegenerative changes, astrocyte and microglia proliferation in the subiculum and other parahippocampal brain areas. The onset of spontaneous seizures was heterogeneous, with an average latency of 15 ± 1.4 days (range 3–36 days) to the initial status epilepticus. The frequency of late spontaneous seizures was higher in rats in which the initial status epilepticus was recurrent after its interruption with diazepam compared to rats in which this treatment was more efficient. Seizure-induced neuropathological changes were assessed in the subiculum by losses in NeuN-positive neurons and by Fluoro-Jade C staining of degenerating neurons. Neuronal loss was already prominent 24 h after KA injection and only modestly progressed at the later intervals. It was most severe in the proximal subiculum and in layer III of the medial entorhinal cortex and distinct Fluoro-Jade C labeling was observed there in 75% of rats even after 3 months. Glutamatergic neurons, labeled by in situ hybridization for the vesicular glutamate transporter 1 followed a similar pattern of cell losses, except for the medial entorhinal cortex and the proximal subiculum that appeared more vulnerable. Glutamate decarboxylase65 (GAD65) mRNA expressing neurons were generally less vulnerable than glutamate neurons. Reactive astrocytes and microglia were present after 24 h, however, became prominent only after 8 days and remained high after 30 days. In the proximal subiculum, parasubiculum and entorhinal cortex the number of microglia cells was highest after 30 days. Although numbers of reactive astrocytes and microglia were reduced again after 3 months, they were still present in most rats. The time course of astrocyte and microglia proliferation parallels that of epileptogenesis.
Keywords: Temporal lobe epilepsy, Subiculum, Entorhinal cortex, Animal epilepsy model, EEG, Epileptogenesis
Abbreviations: DG, dentate gyrus; FJ-C, Fluoro Jade C; GAD65 and GAD67, glutamate decarboxylase 65 and 67; GFAP, glial fibrillary acidic protein; KA, kainic acid; NeuN, neuron-specific nuclear protein; SE, status epilepticus; Sub, subiculum; TLE, temporal lobe epilepsy; VGLUT1, vesicular glutamate transporter 1
Highlights
► The onset of spontaneous seizures was highly variable (3–36 days) with an average latency of 15 days. ► Massive neurodegeneration was already present 24 h after KA-induced seizures. ► Neurons of the proximal subiculum and EC layer III preferentially degenerate. ► Distribution of reactive gliosis roughly matches the pattern of neurodegeneration. ► Time course of reactive gliosis parallels that of epileptogenesis.
1. Introduction
A single episode of sustained status epilepticus (SE) can be the cause for mesial temporal lobe epilepsy (TLE), both in patients and in experimental animals (Lothman and Bertram, 1993). In patients, a seizure-free period, often lasting for years, follows the initial SE and precedes the occurrence of the first spontaneous seizures and thus manifestation of epilepsy (Falconer et al., 1964; Sagar and Oxbury, 1987). It is assumed that during this “silent period” neuropathological changes develop making the brain susceptible to recurrent seizures. In rats, electrical stimulation or injections of toxins like kainic acid (KA) or pilocarpine are widely used as animal models to induce an SE and associated changes. For investigating neuropathological changes leading to spontaneous seizures and thus manifestation of epilepsy, the assessment of the onset of spontaneous seizures is crucial. So far, chronic electrographic recordings through multiple permanent brain electrodes have been used in epilepsy models and varying results have been obtained for the onset of acute seizures in different animal models (Bertram et al., 1990; Goffin et al., 2007; Pitkanen et al., 2002; Vezzani et al., 1999b; Williams et al., 2007). A drawback of this approach is that the animals have to be permanently connected to the electrode cables and therefore are not accessible to more detailed behavioral investigations. We now established telemetric EEG recordings combined with continuous video monitoring in freely moving rats and characterized the development of spontaneous seizures after intraperitoneal injection of KA.
Neuropathological changes associated with KA-induced SE have been well characterized in the dentate gyrus and hippocampus proper. They include fast development of brain edema, local bleeding (Sperk et al., 1983), break down of the blood–brain barrier (Lassmann et al., 1984; Seiffert et al., 2004; Sokrab et al., 1989; van Vliet et al., 2007) followed by severe neuronal damage in sectors CA3 and CA1 of the hippocampus proper, the dentate gyrus and associated limbic brain areas such as the amygdala, the piriform and entorhinal cortices and the thalamus (Ben-Ari et al., 1980; Du et al., 1995; Nadler, 1981; Schwob et al., 1980; Sperk et al., 1983). In recent years a crucial role of the subiculum and of other parahippocampal regions was recognized in epileptogenesis (Palma et al., 2006; Wozny et al., 2005a,b) and network hyperexcitability was demonstrated in the subiculum in animal models and in human TLE (de Guzman et al., 2006; Huberfeld et al., 2011; Knopp et al., 2005). Interestingly, in human TLE the subiculum remains largely preserved (Alonso-Nanclares et al., 2011; Bratz, 1899; Furtinger et al., 2001) whereas parvalbumin and glutamate decarboxylase 65 (GAD65) positive interneurons of the subiculum are lost after pilocarpine- and KA-induced seizures (Drexel et al., 2011; Knopp et al., 2008). In the present study, we therefore investigated neurodegeneration and changes in the expression of reactive astrocytes and microglia in the proximal and distal parts of the subiculum, the pre- and parasubiculum and the entorhinal cortex.
2. Experimental procedures
2.1. Animals
Male Sprague–Dawley rats (210–260 g, Forschungsinstitut für Versuchstierzucht, Himberg, Austria) were housed in individually ventilated cages at a temperature of 22–23 °C, a relative humidity of 50–60%, and a 12 h light/dark cycle. They had free access to food and water. All animal experiments were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, the European Communities Council Directive of 24 November 1986 (86/609/EEC). They were approved by the Committee for Animal Protection of the Austrian Ministry of Science. Care was taken to minimize suffering of the rats. Seventy-five rats with KA-induced seizures and 33 controls were included in the study (in total 108 rats). Thirty-five of the KA treated rats and 14 controls were monitored by EEG-telemetry and video recording.
2.2. Implantation of EEG-transmitters
For implantation of EEG-transmitters we followed a protocol established by Pitsch et al. (2007) used in mice. Rats were given analgesic treatment (5 mg/kg carprofen s.c., Rimadyl, Pfizer, USA) 60 min prior to surgery. Anesthesia was initiated by i.p. injection of 60 mg/kg thiopental (Sandoz, Kundl, Austria) and was maintained by application of sevoflurane (Sevorane, Abbott, Vienna, Austria) through a veterinary anesthesia mask (VIP 3000, Matrx by Midmark, USA).
The skin on the head and the back between the shoulders was shaved and swabbed with 70% ethanol. After placing the rat in the stereotactic apparatus (David Kopf Instruments, Tujunga, USA), its eyes were moistened with 0.9% NaCl and xylocaine-spray (lidocaine-hydrochloride, AstraZeneca, Vienna, Austria) was applied locally at the anticipated incision sites. An incision was set at the back between the shoulders and the biopotential transmitter (TA10EA-F20, Data Sciences International, Arden Hills, USA) was inserted creating a subcutaneous pocket. Then, a second incision was cut rostrocaudally in the skin over the skull and the periosteum was removed. Two holes were drilled into the skull (4.0 mm posterior and 3.0 mm left and right to the bregma) and the electrode leads were attached to the skull bone with stainless steel screws (M1*2, Hummer und Rieß GmbH, Nürnberg, Germany) in an epidural position and fixed with dental cement (Paladur – Heraeus Kulzer, Henry Schein, Austria). Incisions on skull and back were closed and the rats were placed under an infrared lamp until recovery of consciousness. The rats were given additional pain relief (5 mg/kg carprofen) once a day for three days and were allowed to recover for one week.
2.3. Kainic acid injection
In total, 45 transmitter-implanted rats were injected with 10 mg/kg KA each (5 mg/ml in saline, pH 7.0, i.p.) and 14 rats with saline. Two rats did not respond and 8 died. In 14 rats the electrodes became loose after 3–8 weeks. Thus, 19 KA-injected rats (stage 3 and 4) were monitored (EEG- and video recordings) for the entire three months. Rats that lost their electrodes earlier than 8 weeks after KA, were used for determining the onset of spontaneous seizures and, when reasonable, for neuropathological evaluation. For investigating neurodegeneration and gliosis at earlier intervals we treated in addition 52 rats with KA without monitoring EEG.
2.4. Evaluation of status epilepticus
Upon injection of KA, development of acute seizures and SE were monitored for at least 3 h and rated as described previously (Sperk et al., 1983). Briefly, a 5-stage rating scale based on behavioral changes was used. Rats without any obvious behavioral changes were rated as stage 0; rats exposing only wet dog shakes as stage 1; rats with chewing, head bobbing and forelimb cloni as stage 2; rats with generalized seizures and rearing as stage 3; rats with generalized seizures, rearing and falling (loss of postural tone) as stage 4, and rats that died during SE were rated as stage 5. To reduce mortality and severity of the neuropathological changes, rats were treated with diazepam (10 mg/kg i.p., Gewacalm, Nycomed Austria GmbH, Linz, Austria) 2 h after the first generalized seizure.
2.5. Telemetric EEG and video monitoring
Transmitter-implanted rats were single-housed in individually ventilated cages (l: 34 cm, w: 27 cm, h: 20 cm) and EEGs were recorded using an EEG-telemetry system (Dataquest A.R.T. Data Acquisition 4.0 for telemetry systems, Data Sciences International, Arden Hills, USA) with concomitant video monitoring for three days before and up to three months after KA injection. Transmitter emitted EEG signals were recorded by receiver plates located below the cages, transferred to a PC system by a data exchange matrix, and saved to an external hard disk drive. Axis 221 network cameras (Axis communications AB, Lund, Sweden) were used for video monitoring. During darkness, illumination was established using an infrared spotlight (Conrad Electronic GmbH, Wels, Austria). The video cameras were synchronized with the telemetric EEG system.
EEG activity was recorded at a sampling rate of 1000 Hz with no a priori filter cut-off. For detecting spontaneous recurrent seizures EEG traces were evaluated by two independent observers. Seizures were defined as EEG segments with continuous synchronous high-frequency and high-amplitude oscillations (>2 × baseline amplitude) with a minimum duration of 10 s. Additionally, prolonged periods of increased EEG activity were also screened for the presence of a period of post-ictal depression of the EEG signal (below baseline EEG activity). Duration of single spontaneous seizures was determined in the EEG trace. Seizures identified by EEG were then investigated for a behavioral correlate by inspecting the synchronized video recordings and were classified as subclinical (EEG seizures only) or clinical seizures (EEG and motor seizures).
For automatic detection of spike events during SE, raw EEG traces were filtered (high-pass at 10 Hz) to eliminate movement-related artifacts. For each rat, an artifact-free 10 min period of awake EEG was used as baseline EEG. For automated spike detection, threshold amplitude was set 5 standard deviations above and below the mean of the baseline signal and each positive or negative baseline crossing was counted (SIGVIEW 2.4, SignalLab, http://www.sigview.com).
2.6. Preparation of tissue and of sections
For subsequent immunohistochemistry and Nissl staining, rats were killed either 24 h (n = 9), 8 days (n = 9), 1 month (n = 13), or 3 months (n = 15) after the initial KA-induced SE. They were deeply anesthetized by i.p. injection of thiopental (150 mg/kg, Sandoz GmbH, Kundl, Austria) and subsequently perfused transcardially with phosphate buffered saline pH 7.4 (40 ml) followed by 100 ml of ice-cold 4% paraformaldehyde in 50 mM phosphate buffer, pH 7.4. Brains were carefully removed from the skulls and post-fixed in ice-cold paraformaldehyde for 90 min, immersed in ice-cold 20% sucrose in 50 mM phosphate buffer for 24 h, snap-frozen in −70 °C isopentane (Merck, Darmstadt, Germany, 3 min) and stored at −70 °C. Horizontal 30 μm sections were cut on a cryostat-microtome (Carl Zeiss AG, Vienna, Austria), collected in 50 μM Tris–buffered saline (TBS, pH 7.2) containing 0.1% NaN3 (Merck, Darmstadt, Germany) and stored at 4 °C. Nissl staining (cresyl violet) was performed in every 11th section for collecting series of slides at the same anatomical level from the different rats for subsequent concomitant histochemical labeling. Brains from 20 saline-injected rats were processed accordingly and used as controls for immunohistochemistry.
For in situ hybridization, additional rats were killed by exposure to CO2 24 h (n = 5), 8 days (n = 6), 1 month (n = 5) and 3 months (n = 13) after KA-induced SE. Thirteen saline-injected rats were used as controls. After exposure to CO2, rats were decapitated and the brains were quickly removed and snap-frozen in isopentane (−70 °C). Horizontal 20 μm sections were cut, collected on glass slides and stored at −70 °C.
2.7. Fluoro-Jade C labeling
To identify degenerating neurons, we performed Fluoro Jade C staining (FJ-C, Chemicon, USA) according to Schmued et al. (2005).
2.8. Indirect immunohistochemistry
Immunohistochemistry for neuron-specific nuclear protein (NeuN) was performed on free-floating sections. Sections were incubated in 50 mM Tris–buffered saline with 0.4% Triton X-100 (TBS-Triton, Sigma, St. Louis, USA) for 30 min and then in blocking serum (10% normal rabbit serum in TBS-Triton, 90 min). They were incubated over night at room temperature with a monoclonal antibody for NeuN (1:10,000; MAB 377, Chemicon, USA) in 10% blocking serum. After rinsing in TBS-Triton (3 times, 5 min), the sections were incubated with a horseradish peroxidase-coupled secondary antibody (P260 rabbit anti mouse antibody, DAKO, Glostrup, Denmark) 1:200 in 10% blocking serum/TBS-Triton (150 min, room temperature). After washing in TBS, the sections were reacted with 0.001% of diaminobenzidine tetrahydrochloride dihydrate (DAB, Fluka, Sigma-Aldrich Handels GmbH, Vienna, Austria) in TBS-buffer containing 0.005% H2O2 (30%, Merck, Darmstadt, Germany). They were mounted on gelatin-coated glass slides in 55% ethanol, allowed to dry overnight in ethanol, cleared in butyl acetate and cover-slipped with Eukitt mounting medium (O. Kindler GmbH, Freiburg, Germany). Omission of the primary antisera eliminated all specific immunoreactivity.
2.9. Ox-42 immunohistochemistry by the avidin-biotin method
To label type 3 complement receptor CR3 (Ox-42) of reactive microglia the ABC-immunohistochemistry was performed in free-floating sections. They were incubated in 0.4% Triton in TBS (TBS-Triton) for 30 min and then in blocking serum (10% normal horse serum in TBS-Triton; 90 min). The sections were then incubated with primary antibody (MCA275G, Serotec, UK, 1:30,000 in 10% blocking serum with 0.1% NaN3) over night and then rinsed in TBS-Triton (three times for 5 min). They were then incubated with the biotinylated secondary antibody (1:200 in 10% blocking serum, Vectastain ABC-KIT, Vector Laboratories, Burlingame, USA) for 30 min, rinsed with TBS-Triton (5 min) and incubated with the avidin-biotin peroxidase complex (1:100 in 10% blocking serum; Vectastain ABC-KIT, Vector Laboratories, Burlingame, USA) for 30 min. After rinsing the sections in TBS-buffer, they were reacted with 0.001% of DAB and 0.005% of H2O2 in TBS-buffer for 4 min and transferred to TBS. The sections were mounted on gelatin-coated glass slides and cover-slipped as described above.
2.10. Immunofluorescence for glial fibrillary acidic protein (GFAP)
Native and reactive astrocytes were labeled for glial fibrillary acidic protein (GFAP) using a monoclonal primary antibody conjugated to the fluorescent cyanine dye Cy3. To reduce background labeling, sections were incubated in sodium borohydride (1% in TBS) for 30 min. After two 15 min washing steps in TBS, sections were incubated in 20% normal goat serum (in 0.4% TBS-Triton) at room temperature for 1 h. They were then incubated with the primary antibody (GFAP/Cy3 C9205, Sigma–Aldrich, Vienna, Austria, 1:1000 in 2% normal goat serum and 0.4% TBS-Triton with 0.1% NaN3) at 4 °C in the dark for 72 h. After three washing steps with TBS (10 min each), the sections were mounted in gelatin-KCr(SO4)2 (Merck, Darmstadt, Germany) and cover-slipped using Vectashield mounting medium (Vector laboratories, Burlingame, USA). The sections were analyzed using a Cy3 filter-set (MQ Cy3, F41-007, Zeiss, Vienna, Austria).
2.11. In situ hybridization
Custom-synthesized oligonucleotides (Microsynth, Balgach, Switzerland) complementary to the mRNAs for the vesicular glutamate transporter VGLUT1 and for glutamate decarboxylase 65 (GAD65) were used as probes: VGLUT1: 5′-GTA GTG CAG GTG ACT CGT AGG AGA CAA GCA ACC AGA ACA GGT ACC-3′, bases 866–910 (GenBank ID: NM_053859.1; Ni et al., 1994); GAD65: 5′-CTC CTT CAC AGG CTG GCA GCA GGT CTG TTG CGT GGA G-3′, bases 348–384 (GenBank ID: NM_012563.1; Chang and Gottlieb, 1988). The oligonucleotides (2.5 pmol) were labeled at the 3′-end with [35S] α-thio-dATP (1300 Ci/mmol; New England Nuclear, Boston, USA) by reaction with terminal deoxynucleotidyltransferase (Roche Austria GmbH, Vienna, Austria) and in situ hybridization was performed as described previously (Furtinger et al., 2001). After exposure to BioMax MR films for 7 (VGLUT1) or 20 days (GAD65), sections were dipped at 42 °C in photosensitive emulsion (NTB-2, Kodak, Rochester, USA, diluted 1:1) with distilled water, air dried, and exposed for 10 (VGLUT1) or 30 days (GAD65). Dipped sections and BioMax MR films were developed using Kodak D19 developer (Kodak, Rochester, USA). After counterstaining with cresyl violet, photoemulsion-dipped sections were dehydrated, cleared in butyl acetate, and cover-slipped with Eukitt.
2.12. Evaluation of neuronal loss and tissue shrinkage
We estimated the extent of neuronal loss in the ventral subiculum, pre- and parasubiculum, and entorhinal cortex by determining neuron density in NeuN-labeled sections (three sections per rat). Grayscale images (8 bit) of the parahippocampal region were taken at 50-fold magnification using a Zeiss Axiophot microscope equipped with a Zeiss AxioCam digital camera (both Zeiss, Vienna, Austria) and imported into the image processing software ImageJ (ImageJ 1.43c, National Institutes of Health, USA). A threshold (automatic threshold) was set resulting in a black and white image. Using the ImageJ tool “Polygon selections”, the areas of interest were outlined manually and the function “Analyze particles” was used to measure the total area of the outlined region and the percentage of area that was occupied by labeled neuronal cell bodies. For each rat, the % areas covered by cells (area fraction values) of left and right side of the respective parahippocampal areas of three sections were averaged. We defined and evaluated the following subregions: The proximal and distal part of the pyramidal cell layer of the subiculum, the principal cell layers of the pre- and parasubiculum, and layers II, III and V–VI of the medial and lateral EC. To investigate tissue shrinkage in regions with severe neurodegeneration 3 months after KA injection, photomicrographs of NeuN-labeled sections at the same anatomical level (2 sections per rat) were taken at 25-fold magnification and imported in ImageJ. Subregions of the parahippocampal region (proximal subiculum, presubiculum, parasubiculum and layer III of the EC) were outlined manually and the areas of the respective regions were measured.
2.13. Quantification of VGLUT1 and of GAD65 mRNA expressing neurons
The numbers of VGLUT1- and GAD65 mRNA expressing neurons were assessed on bright field photomicrographs (200-fold magnification) of 20 μm thick photoemulsion-dipped sections after radioactive in situ hybridization. Cell counts were performed in the pyramidal cell layer of the proximal and distal parts of the subiculum, and in layer III of the medial and lateral EC. In brief, photomicrographs were imported into the image processing software ImageJ and a circle with a diameter of 320 μm (area 80,425 μm2) was placed above the respective cell layers. Neurons inside the circle expressing VGLUT1- or GAD65 mRNA (displaying silver grains around the soma) were counted and neuronal density (neurons per area) was calculated. Values from both hemispheres were averaged.
2.14. Quantification of reactive astrocytes and microglia
To quantify the extent of glial activation, reactive microglial cells and astrocytes were counted in 30 μm sections (2 sections per rat) reacted for Ox-42 or GFAP immunoreactivities, respectively. Microglial cells and astrocytes were defined as “reactive” when they presented strongly increased Ox-42- or GFAP-labeling and altered morphology (e.g. thicker filopodia) compared to resting microglia or astrocytes in controls. Counting was performed using an ocular grid covering an area of 62,500 μm2 at 400-fold magnification and cell density (cells/mm2) was calculated. Principal cell layers in the following subregions of the parahippocampal region were investigated: proximal and distal parts of the subiculum, presubiculum, parasubiculum, layers III and V–VI of the medial and lateral EC, and layers II–III of the perirhinal cortex. Values from both hemispheres were averaged.
2.15. Statistical analyses
Statistical analyses were carried out using the GraphPad Prism 5.0a software for Macintosh (GraphPad Software, San Diego, CA, USA). For comparison of mean values of two groups, the Student’s t-test was used. For determining between-group differences among multiple sets of data compared to controls, ANOVA with Dunnett’s multiple comparison post hoc test was used. Linear regression analysis was performed using GraphPad Prism for investigating relationships between measured variables. Data are presented as mean ± SEM.
3. Results
3.1. Status epilepticus and recurrent seizures increased EEG-activity
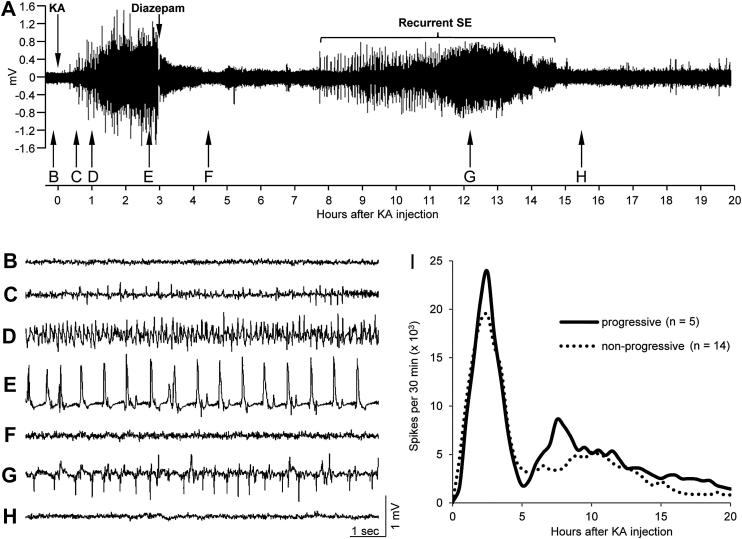
Forty-three of 45 transmitter-implanted rats developed SE after KA injection. Two rats showed stage 2 seizures, 20 rats stage 3 seizures, and 13 rats developed stage 4 seizures. Two rats had no seizures and 8 rats died during SE. The mean latency to onset of EEG seizures in rats with rating 3 and 4 was 15.5 ± 1.95 min and that to the first generalized (stage 3) motor seizure 81.1 ± 5.52 min after KA injection (range 20–145 min). The frequency of generalized motor seizures during the SE was 6.8 ± 0.79 seizures per hour. Fig. 1A shows a typical EEG recording covering 20 h after induction of SE by KA. Injection of diazepam (10–20 mg/kg 120 min after first stage 3 seizure) temporarily reduced EEG activity (see trace in Fig. 1F). However, high amplitude EEG activity (without major motor signs, Fig. 1G) recurred 4 ± 1.1 h after diazepam injection in all rats with initial rating 3 to 4 and persisted for 22 ± 2.4 h. Spike numbers assessed by spike counting then reached close to baseline levels in about 50% of the rats (Fig. 1I). In five rats high spike frequency recurred several hrs after diazepam treatment (Fig. 1I). They showed significantly higher numbers of EEG spikes from 3 to 6 h after diazepam injection compared to the remaining 14 rats (42,638 ± 6161 vs. 24,546 ± 4444 spikes/3 h; P = 0.0445). These rats with recurrent high spike frequency had clear progression of spontaneous seizures (Fig. 2D) in contrast to most rats in which diazepam treatment was more effective (Fig. 2C).
Fig. 1.
Status epilepticus (SE) assessed by telemetric EEG- and video recording. Panel A shows a typical EEG-recording covering 20 h after KA injection (10 mg/kg). Mean latency to increased EEG activity was 15.5 ± 1.95 min after KA injection, whereas mean latency to behavioral SE was 83 ± 9.1 min. Injections of diazepam (10–20 mg/kg 120 min after first stage 3 seizure) reduced EEG activity but failed to completely stop SE, and increased EEG-activity reappeared again after 4 ± 1.1 h in average and persisted for 22 ± 2.4 h. Panels B to H show 10 s EEG traces as indicated by arrows in A. Panel B, baseline before KA injection; C, at the onset of subclinical seizures (EEG seizures only); D, at the onset of motor symptoms of SE (1 h after KA-injection); E, trace during SE (note EEG-spikes at a frequency of ∼1.6 Hz); F, reduction of EEG amplitude close to baseline after diazepam injection; G, EEG activity during the recurrent SE (around 9 h after diazepam and 12 h after KA injection), and H, activity after seizing of the recurrent SE (around 12.5 h after diazepam and 15.5 h after KA injection). Panel I depicts the development of EEG-spiking during the first 20 h after KA injection in two subpopulations of rats that experienced either few (<80; dotted line) or frequent (>140) and progressive (solid line) spontaneous recurrent seizures during the subsequent three months (see Fig. 2C and D). Note that increased spike frequency recurred in the group with subsequent progressive seizures.
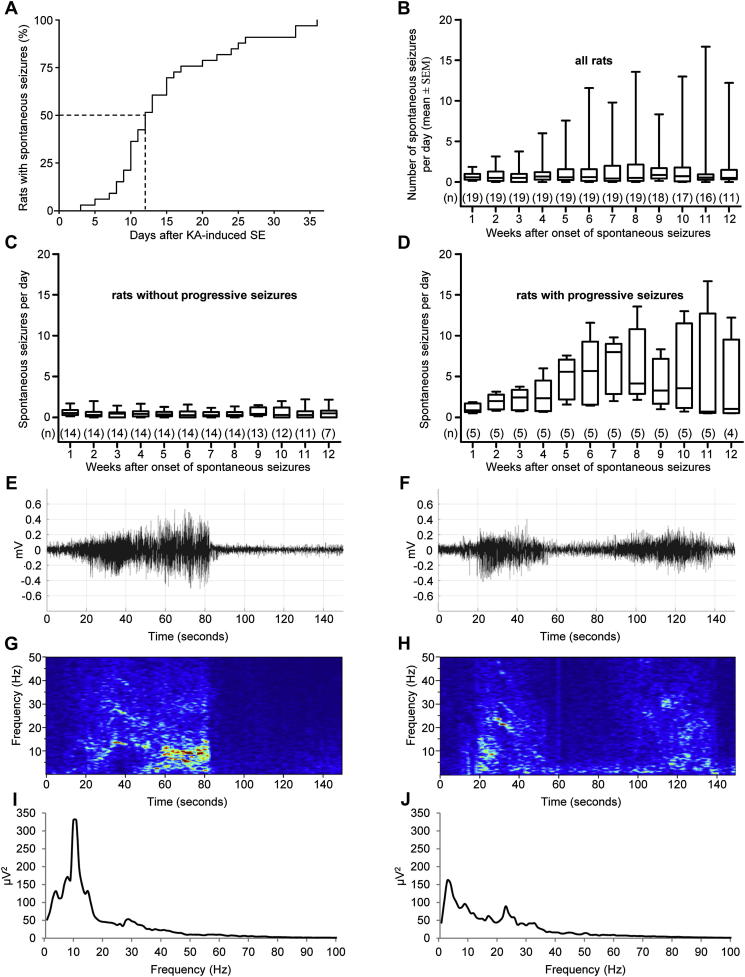
Fig. 2.
Development of spontaneously recurrent seizures. Panel A depicts the summative occurrence of the first spontaneous seizure in rats with initial rating 3 and 4. Three days after SE the first spontaneous EEG seizure was observed in the first rat and in 50% of rats after 12 days. After 36 days, all rats (n = 33) showed spontaneous recurrent seizures. In 77% of rats, the first spontaneous seizure was detectable in EEG only and not accompanied by motor signs. Seizures with a behavioral correlate (clinical seizures) in addition to EEG seizures were detected in average 6.4 ± 1.61 days later. B, Numbers of spontaneous seizures per day in all rats after the first spontaneous seizure (n = 19) shown as box plots. Spontaneous seizures occurred in clusters (not shown) and their frequency increased over time in some rats. Panels C and D illustrate the numbers of seizures in rats without progression of subsequent spontaneous seizures (C) and in rats with seizure progression (D), respectively. Panels E and F show 150 s of raw EEG traces of the two dominant seizure types (both seizures were spontaneous generalized stage 3 seizures). The respective spectrograms and power spectra of two representative examples of the same seizure types are shown in panels G to J. Seizure type I (E, G, I) was predominantly (69% of EEG-seizures) detected in all rats and consisted of a single period of increased EEG amplitude. Seizure type II (F, H, J) consisted of two phases of high EEG-amplitude separated by a short phase of low amplitude and accounted for 19% of all spontaneous seizures.
3.2. Manifestation of spontaneous seizures
Telemetric recordings of the EEG and video monitoring were performed continuously for 3 months. In the two rats with stage 2 SE rating the onsets of spontaneous seizures were 6 and 27 days after KA injection, respectively, and these rats experienced 12 and 2 spontaneous seizures during the observation period. In rats with rating 3 to 4 during SE (n = 33), the first spontaneous EEG seizures were detected between 3 and 36 days after KA injection (mean 14.9 ± 1.43 days; Fig. 2A). Fifty percent of these rats had developed at least one spontaneous seizure after 12 days. In 77% of rats the first spontaneous EEG seizure was not accompanied by a behavioral correlate and the first spontaneous motor seizures were detected in average 21.4 ± 3.62 days after the initial SE. Fifty percent of rats developed at least one motor (convulsive) seizure during the first 17 days (not shown). After the initial spontaneous seizure the rats showed 1.4 ± 0.44 seizures per day in average (Fig. 2B). In respect to seizure frequency, the 19 long-term monitored rats could be separated into two groups: i) rats showing no increase in numbers of spontaneous seizures (n = 14; 0.5 ± 0.08 seizures per day) and ii) in rats with progressing seizure frequency (n = 5; 4.2 ± 0.90 seizures per day; Fig. 2C and D). The second group had failed to respond fully to the anticonvulsive diazepam treatment during the initial SE (see under 3.1. and Fig. 1I). However, there was no significant difference in the latency to the onset of spontaneous seizures between these two groups (P = 0.9162).
The latency to the onset of spontaneous seizures was also not different between rats with behavioral seizure rating 3 and those with rating 4 in the SE (P = 0.6886). It also did not correlate with the latency to the first generalized seizure (stage 3) during SE (r2 = 0.00249, P = 0.5741). Spontaneous seizures occurred mostly in clusters and their mean duration was 46 ± 0.6 s. In 90.2% of spontaneous seizures detected by EEG recordings, also concomitant motor seizures were seen in the video monitoring. The remaining 9.8% of EEG seizures were not accompanied by motor signs (subclinical seizures). The majority of spontaneous seizures occurred during sleep or inactivity (79.9%). Only a small proportion of seizures was evoked by handling the rats, e.g. when changing the bedding (2.8%). Ten weeks after the initial onset of spontaneous seizures the total time spent in seizures was 58 ± 14.0 min. No electrical or behavioral seizures were detected in control rats.
We observed two typical EEG patterns during spontaneous recurrent seizures in all rats (Fig. 2E and F). Seizure type I (Fig. 2E) was predominantly detected (69% of all EEG-seizures). It consisted of a single period of increased EEG activity. Seizure type II consisted of two phases with increased EEG activity separated by a short period (<30 s) of low amplitude (Fig. 2F) and accounted for 19% of all spontaneous seizures. Twelve percent of all spontaneous seizures were somewhat different and did not clearly fit into these two categories. Seventy-one percent of seizure type I and 19% of seizure type II developed during sleep or inactivity. Seizure type II was most frequently associated with spontaneous seizures without behavioral correlate (51% of all subclinical seizures) whereas only 35% of type I seizures were subclinical. If seizure type II was associated with behavioral correlates the motor signs generally occurred during the second phase of increased EEG activity. Typically, ictal high-amplitude discharges were followed by a post-ictal EEG depression (Fig. 2E and F).
3.3. Neurodegeneration in parahippocampal regions
3.3.1. Loss in NeuN-positive neurons
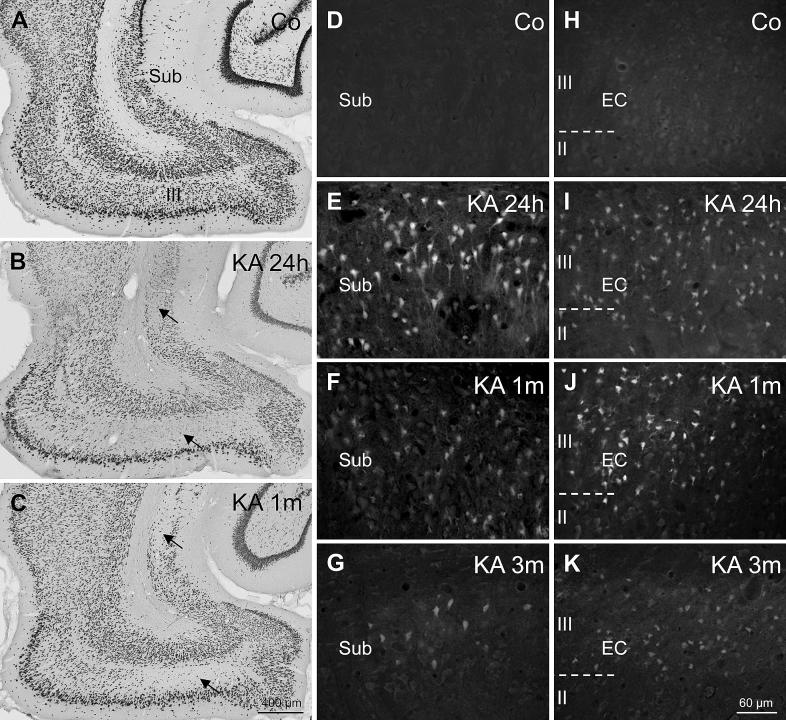
We used NeuN labeling to encompass neuronal cell losses and Fluoro-Jade C (FJ-C) staining for detecting neurons in the process of degeneration. Neuropathological changes are shown for rats with seizure rating 3 and 4 during the SE. Changes in stage 2 rats were similar. Due to the small group size, we did not include them in the analysis. As shown in Fig. 3A–C and in Table 1, losses of NeuN-positive neurons were detected throughout parahippocampal areas. They were most prominent in layer III of the medial EC (mean loss: 49.9 ± 4.76%) and in the proximal (adjacent to sector CA1) part of the subiculum (mean loss: 37.2 ± 4.26%; Fig. 3B and C; Table 1). Neurons in the distal part of the subiculum (adjacent to the presubiculum) were less vulnerable (mean loss: 19.9 ± 2.18%; Fig. 3B and C). Also in the pre- and parasubiculum, only moderate neurodegeneration was observed (mean loss of 17.7 ± 2.84% and 19.3 ± 3.04%, respectively; Table 1). In the parasubiculum mainly the area adjacent to layer II of the EC was affected. Degeneration of NeuN-positive neurons was maximal or close to maximal already early after KA injection (24 h after KA-induced SE), indicating the causal relationship with the initial SE. At the 3-months interval significantly reduced densities of NeuN-positive neurons were observed in all investigated areas.
Fig. 3.
Neurodegeneration in the parahippocampal region after KA-induced SE. NeuN-labeling in controls (A) and in rats at different time intervals after KA injection (B, C) revealed an early onset of widespread neurodegeneration in parahippocampal regions. The proximal subiculum and layer III of the medial EC were the most severely affected regions (arrows in B and C). Fluoro Jade-C (FJ-C) staining of degenerating neurons in the proximal subiculum (D–G) and layer III of the medial EC (H–K) revealed that massive neurodegeneration was present already 24 h after the initial SE (E, I). At this interval, FJ-C stained neurons, dendrites, and axons were present in virtually all subregions of the parahippocampal region and were especially dense in the proximal subiculum (E) and in layer III of the medial EC (I). In both subregions, FJ-C stained neurons were still present one (F and J) and – in 75% of rats – three months after KA-induced SE (G and K).
Table 1.
NeuN-ir neurons in parahippocampal regions after KA-induced status epilepticus. Reduced densities of NeuN-ir neurons indicate rapid neurodegeneration notably in the proximal subiculum and layer III of the medial EC. Neuronal densities were assessed on 30 μm thick sections labeled for NeuN-ir. Data are shown as % of controls (ANOVA with Dunnett’s multiple comparison post hoc test was used for group comparisons; *P < 0.05; **P < 0.01; ***P < 0.001). Tissue shrinkage was observed in regions with severe neurodegeneration 3 months after KA injection and may explain increases in density of NeuN-ir neurons at that time interval (areas in % of control: proximal subiculum: 86 ± 2.6%***; distal subiculum: 96 ± 3.6%; presubiculum: 97 ± 2.7%; parasubiculum: 92 ± 2.9%; entorhinal cortex layer III: 88 ± 3.2%*).
| Region | KA 24h (n = 9) | KA 8d (n = 9) | KA 1m (n = 13) | KA 3m (n = 15) |
|---|---|---|---|---|
| Proximal subiculum | 54 ± 5.3% ∗∗∗ | 59 ± 7.2% ∗∗∗ | 65 ± 5.2% ∗∗∗ | 77 ± 3.4% ∗∗∗ |
| Distal subiculum | 85 ± 4.1% ∗∗ | 94 ± 4.1% | 94 ± 2.3% | 77 ± 3.2% ∗∗∗ |
| Presubiculum | 90 ± 2.5% | 96 ± 1.6% | 90 ± 2.2% | 77 ± 4.9% ∗∗∗ |
| Parasubiculum | 81 ± 2.1% ∗∗ | 95 ± 2.7% | 85 ± 5.5% ∗∗ | 80 ± 3.8% ∗∗∗ |
| Medial EC | ||||
| Layer II | 83 ± 4.3% ∗∗ | 91 ± 3.5% | 86 ± 3.9% ∗∗ | 78 ± 3.8% ∗∗∗ |
| Layer III | 42 ± 6.4% ∗∗∗ | 41 ± 5.5% ∗∗∗ | 63 ± 7.6% ∗∗∗ | 50 ± 4.8% ∗∗∗ |
| Layers V–VI | 85 ± 3.7% ∗∗ | 89 ± 3.7% | 89 ± 3.5% ∗ | 81 ± 3.5% ∗∗∗ |
| Lateral EC | ||||
| Layer II | 89 ± 4.4% | 95 ± 3.8% | 87 ± 4.7% | 74 ± 4.5% ∗∗∗ |
| Layer III | 77 ± 3.6% ∗ | 84 ± 2.8% | 85 ± 3.2% | 67 ± 5.5% ∗∗∗ |
| Layers V–VI | 82 ± 4.9% ∗ | 90 ± 5.2% | 88 ± 5.5% | 79 ± 3.8% ∗∗∗ |
In the proximal subiculum, the relative density of NeuN-positive neurons appeared to recover to some extent after 3 months (Table 1). There, the area of the pyramidal cell layer was significantly smaller than in controls (86 ± 2.6% of controls, P = 0.0008). This tissue shrinkage was presumably caused by the severe neurodegeneration in this region and by the decreasing gliosis at this interval after the initial SE. Significant tissue shrinkage was also present in layer III of the EC (88 ± 3.2% of controls, P = 0.017) 3 months after KA injection. In layer III of the EC, however, the relative density of NeuN-positive neurons did not seem to recover after 3 months (Table 1). This might point to a greater extent of ongoing neurodegeneration caused by spontaneous recurrent seizures. Indeed, highest densities of FJ-C stained neurons are found in layer III of the EC at this time interval (see Fig. 3K).
Loss of NeuN-positive neurons did neither correlate with the latency (proximal subiculum, r2 = 0.0400, P = 0.5332; distal subiculum, r2 = 0.0188, P = 0.6705; presubiculum, r2 = 0.0097, P = 0.5332; parasubiculum, r2 = 0.0103, P = 0.7543; medial EC, layer III, r2 = 0.0432, P = 0.5168) nor with the number of spontaneous seizures (proximal subiculum, r2 = 0.0669, P = 0.4169; distal subiculum, r2 = 0.1016, P = 0.3126; presubiculum, r2 = 0.0464, P = 0.5014; parasubiculum, r2 = 0.0162, P = 0.6935; medial EC, layer III, r2 = 0.2162, P = 0.1277) nor with the clinical rating in the SE (not shown).
3.3.2. Loss in VGLUT1 mRNA containing neurons
For determining the portion of principal neurons degenerating we estimated the loss of VGLUT1 mRNA containing neurons. Similar as for NeuN, the extent of neurodegeneration was already maximal or close to maximal in all investigated subregions 24 h after the initial SE (Table 2). Also here, the proximal part of the subiculum (loss by 30–40%) and layer III of the medial EC (mean loss by 62%) were the most vulnerable areas, while the distal subiculum (loss by 29% in average) and layer III of the lateral EC (loss by 26%) were less affected.
Table 2.
Reduced density of VGLUT1- and GAD65-mRNA expressing neurons in the subiculum and layer III of the EC. Densities of neurons (number of neurons/mm2) were quantified in darkfield photomicrographs of photoemulsion-dipped sections after in situ hybridization and expressed as % of controls ± SEM. Data for early intervals (24 h and 8 days) and late intervals after KA-induced SE (1 and 3 months) were pooled, respectively. Below the densities of VGLUT and GAD65-mRNA expressing neurons in the respective controls are shown (ANOVA with Dunnett’s multiple post hoc test; *P < 0.05; **P < 0.01; ***P < 0.001).
| n | Proximal Sub | Distal Sub | Medial EC III | Lateral EC III | |
|---|---|---|---|---|---|
| VGLUT1 mRNA | |||||
| KA, 1–8 days (% of control) | 11 | 56 ± 6.9%∗∗∗ | 68 ± 6.4%∗∗∗ | 39 ± 8.2%∗∗∗ | 76 ± 2.7%∗∗∗ |
| KA, 1–3 months (% of control) | 18 | 58 ± 4.2%∗∗∗ | 74 ± 4.0%∗∗∗ | 38 ± 6.3%∗∗∗ | 72 ± 3.2%∗∗∗ |
| Controls (neurons/mm2) | 13 | 848 ± 32.2 | 894 ± 27.2 | 861 ± 34.3 | 779 ± 27.7 |
| GAD65 mRNA | |||||
| KA, 1–8 days (% of control) | 11 | 64 ± 6.4%∗∗∗ | 79 ± 3.1%∗∗ | 73 ± 4.0%∗∗ | 73 ± 7.5%∗ |
| KA, 1–3 months (% of control) | 18 | 67 ± 3.8%∗∗∗ | 78 ± 4.5%∗∗ | 68 ± 3.9%∗∗∗ | 86 ± 3.7% |
| Controls (neurons/mm2) | 13 | 114 ± 6.2 | 102 ± 5.9 | 82 ± 7.6 | 68 ± 7.4 |
3.3.3. Loss in GAD65 mRNA containing neurons
The total number of GAD65 mRNA positive neurons was considerably lower (up to 90%) than that of VGLUT1 mRNA containing cells. Neurodegeneration was less severe than for principal neurons. It was strongest in the proximal subiculum (loss by 35%) and somewhat weaker in the other areas (distal subiculum, medial and lateral EC, layer III), in average comprising losses by 14–32% (Table 2).
3.3.4. FluoroJade labeling of degenerating neurons
FJ-C labels neurons that are in the process of neurodegeneration (Fig. 3D–K). It revealed a pattern largely complementary to NeuN labeling and VGLUT1 in situ hybridization. In regions with decreased density of NeuN-positive or VGLUT1 expressing neurons, in particular in the proximal subiculum and in layer III of the EC (Fig. 3B and C), we observed numerous FJ-C labeled cells. The numbers of FJ-C labeled neurons were highest after 24 h (Fig. 3E and I). At later time intervals, the numbers of FJ-C labeled neurons gradually declined but residual FJ-C labeled neurons were still present e.g. in the proximal subiculum (Fig. 3G) and most notably in layer III of the EC of 75% of stage 3 and 4 rats after 3 months (Fig. 3K).
3.4. Expression of activated astrocytes
Numerous GFAP-positive astrocytes were present in all layers of the parahippocampal areas of controls (Fig. 4A). They contained long thin processes (Fig. 4F) and were most densely packed in the alveus, in the outer molecular layer of the subiculum, in the outer half of layer I of the pre- and parasubiculum and in the superficial third of layer I of the EC (Fig. 4A). In controls, no astrocytes with morphological changes typical for activated astrocytes were detected (residual astrocytes). Reactive astrocytes with increased GFAP labeling and altered morphology (thickening of their processes) were detected sporadically already 24 h after KA injection reaching approximately 13–52 cells/mm2 (Table 3). At this interval, morphology and labeling intensity of the vast majority of astrocytes was similar as observed in controls (Fig. 4B and G).
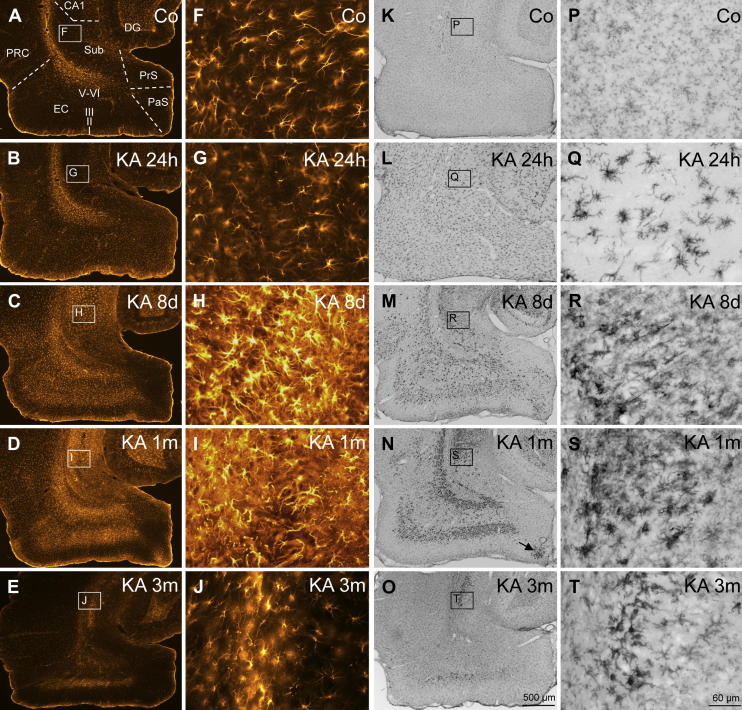
Fig. 4.
Reactive gliosis after KA-induced SE. Activation of astrocytes (A–J) occurs slower than that of microglia. Astrocytes reveal thin processes and moderate GFAP-labeling in the parahippocampal region of controls (A, F) and 24 h after KA-induced SE (B, G; resting astrocytes). Activated astrocytes with increased GFAP-labeling and altered morphology (hypertrophic processes) were detected in the subiculum, parasubiculum, entorhinal and perirhinal cortex 8 days (C, H) and 1 month after SE (D, I). The distribution of activated astrocytes and of reactive microglia was similar at the later intervals. In the subiculum and in the EC astrocytes with increased GFAP-labeling were still present three months after KA-induced SE (E, J). Panels K–T depict changes in Ox-42 (CD11b)-labeled microglia after KA-induced seizures. Resting microglial cells are almost evenly dispersed in the parahippocampal region of controls (K) and display a highly ramified morphology (P). Activated microglia with altered morphology and intense Ox-42 labeling is present already 24 h after SE (L, Q). At this interval, activated microglial cells are still evenly distributed in all parahippocampal subregions (L). At later intervals reactive microglia accumulates in layers with severe neurodegeneration (M, N, R, S; note the cluster of reactive microglia in the parasubiculum; arrow in N) and is still present 3 months after the initial SE in the proximal subiculum and in layer III of the EC (O, T). Abbreviations: CA1, hippocampal sector CA1; DG, dentate gyrus; EC, entorhinal cortex; PaS, parasubiculum; PRC, perirhinal cortex; PrS, presubiculum; Sub, subiculum.
Table 3.
Epilepsy-induced activation of astrocytes in the parahippocampal region. Reactive astrocytes were identified by their increased GFAP labeling and altered morphology (see methods section). They were counted in 30 μm thick sections immune-labeled for GFAP. Cell densities (number of cells/mm2) are presented as mean ± SEM. Controls (n = 22) revealed no reactive astrocytes. Therefore we did the statistical comparison versus the 24 h group. Statistical analysis was performed by ANOVA with Dunnett’s multiple comparison post hoc test for group comparisons (*P < 0.05; **P < 0.01; ***P < 0.001; animal numbers (n) are given in parenthesis). The highest cell densities per area are labeled in bold.
| Region | KA 24 h (n = 9) | KA 8d (n = 9) | KA 1m (n = 10) | KA 3m (n = 14) |
|---|---|---|---|---|
| Subiculum | ||||
| Proximal Sub. | 48 ± 23.5 | 801 ± 42.8∗∗∗ | 798 ± 28.8∗∗∗ | 308 ± 40.3∗∗∗ |
| Distal Sub. | 40 ± 16.2 | 534 ± 53.5∗∗∗ | 460 ± 37.2∗∗∗ | 106 ± 23.8 |
| Presubiculum | 13 ± 6.9 | 115 ± 34.9∗∗∗ | 13 ± 5.4 | 3 ± 1.6 |
| Parasubiculum | 37 ± 14.1 | 228 ± 32.8∗∗∗ | 245 ± 38.3∗∗∗ | 41 ± 11.9 |
| Medial EC | ||||
| Layer III | 36 ± 11.0 | 777 ± 43.7∗∗∗ | 640 ± 52.6∗∗∗ | 211 ± 50.7∗ |
| Layers V–VI | 30 ± 12.6 | 774 ± 32.0∗∗∗ | 729 ± 36.1∗∗∗ | 213 ± 30.0∗∗∗ |
| Lateral EC | ||||
| Layer III | 19 ± 9.5 | 497 ± 38.4∗∗∗ | 322 ± 48.3∗∗∗ | 55 ± 15.5 |
| Layers V–VI | 52 ± 21.5 | 691 ± 39.4∗∗∗ | 614 ± 22.0∗∗∗ | 266 ± 47.1∗∗∗ |
| Perirhinal cortex | 36 ± 10.6 | 491 ± 39.0∗∗∗ | 326 ± 34.2∗∗∗ | 84 ± 26.1 |
At subsequent intervals (8 d and 1 month after KA-induced SE) intensely labeled and densely packed reactive astrocytes with hypertrophic processes were present in virtually all parahippocampal areas (Fig. 4C, D, H and I). Highest densities of activated astrocytes were present in the proximal subiculum (801 ± 42.8 cells/mm2) and in layers III (777 ± 43.7 cells/mm2) and V–VI (774 ± 32.0 cells/mm2) of the medial EC. At this interval, interestingly, reactive astrocytes were more concentrated in layer III of the medial than in the lateral part of the EC (Fig. 4D, Table 3). With the exception of the presubiculum, one month after SE the number of activated astrocytes was almost as high as after 8 days (Table 3 Fig. 4C and D). In the presubiculum it declined. After 3 months the number of GFAP-positive cells became reduced in all areas, although they were still significantly increased in the proximal and distal subiculum, in layers III and V–VI of the medial EC, in layers V–VI of the lateral EC (Fig. 4E and J, Table 3).
3.5. Expression of reactive microglia
Reactive microglia was detected using an antibody for the type 3 complement receptor (Ox-42). In controls, faint labeling of microglial cells with thin processes was seen and they were roughly evenly distributed within the subiculum and the other parahippocampal regions (see Fig. 4K and P). Occasionally, single reactive microglial cells were also detected in controls (not shown) at densities of 3–8 cells/mm2. The time course of appearance of reactive microglia after the KA-induced SE was different from that of proliferating astrocytes. After 24 h, virtually all microglial cells displayed increased Ox-42 labeling intensity and thicker processes but were still evenly distributed (Fig. 4L and Q, Table 4). Their number increased to about 320–460 cells/mm2 in all parahippocampal subfields (Table 4).
Table 4.
Epilepsy-induced activation of microglia in the parahippocampal region. The numbers of reactive microglia (number of cells/mm2) were counted in 30 μm thick sections immune-labeled for Ox-42 (CD-11b). Data are presented as mean ± SEM. Statistical analysis was performed by ANOVA with Dunnett’s multiple comparison post hoc test for group comparisons (*P < 0.05; **P < 0.01; ***P < 0.001; animal numbers (n) are given in parenthesis). The highest cell densities per brain area are labeled in bold.
| Region | Controls (n = 22) | KA 24 h (n = 9) | KA 8d (n = 9) | KA 1m (n = 10) | KA 3m (n = 14) |
|---|---|---|---|---|---|
| Subiculum | |||||
| Proximal Sub. | 8.0 ± 1.49 | 455 ± 21.2∗∗∗ | 1220 ± 66.3∗∗∗ | 1602 ± 97.3∗∗∗ | 407 ± 60.4∗∗∗ |
| Distal Sub. | 4.4 ± 1.37 | 420 ± 16.8∗∗∗ | 398 ± 38.3∗∗∗ | 406 ± 70.1∗∗∗ | 114 ± 16.3∗ |
| Presubiculum | 2.9 ± 0.84 | 326 ± 25.4∗∗∗ | 100 ± 19.1∗∗∗ | 60 ± 11.5∗∗ | 33 ± 6.4 |
| Parasubiculum | 5.1 ± 1.45 | 360 ± 20.0∗∗∗ | 276 ± 40.8∗∗∗ | 468 ± 111.4∗∗∗ | 91 ± 12.5 |
| Medial EC | |||||
| Layer III | 6.2 ± 1.82 | 438 ± 34.2∗∗∗ | 754 ± 69.5∗∗∗ | 1058 ± 142.3∗∗∗ | 278 ± 57.6∗∗ |
| Layers V–VI | 7.3 ± 1.66 | 405 ± 24.4∗∗∗ | 699 ± 55.4∗∗∗ | 946 ± 118.1∗∗∗ | 241 ± 45.3∗∗ |
| Lateral EC | |||||
| Layer III | 5.5 ± 1.78 | 454 ± 22.9∗∗∗ | 593 ± 82.1∗∗∗ | 653 ± 113.9∗∗∗ | 147 ± 37.3 |
| Layers V–VI | 8.0 ± 1.66 | 437 ± 25.9∗∗∗ | 696 ± 55.3∗∗∗ | 983 ± 96.6∗∗∗ | 194 ± 33.8∗∗ |
| Perirhinal cortex | 6.5 ± 1.36 | 444 ± 32.2∗∗∗ | 589 ± 38.8∗∗∗ | 474 ± 68.1∗∗∗ | 112 ± 24.5∗ |
Eight days after KA injection reactive microglia accumulated in individual parahippocampal areas (Fig. 4M and R, Table 4), notably in the pyramidal cell layer (Fig. 4R) and in the inner molecular layer of the subiculum, in the parasubiculum, in layers I, III, V and VI of the EC, as well as in the perirhinal cortex (see Fig. 4M). On the other hand, only few strongly Ox-42 labeled cells were seen in the presubiculum (layers II–III) and in layer II of the EC. In layer II of the parasubiculum, adjacent to the EC, a cluster of reactive microglial cells was present (Fig. 4M) corresponding to the cell losses in this area. A few intensely labeled microglial cells were found in layer I of the parasubiculum where degenerating parvalbumin containing dendrites were observed recently (Drexel et al., 2011).
One month after KA injection, accumulation of reactive microglia was even more pronounced. All layers of the proximal subiculum contained strongly labeled microglial cells (Fig. 4N and S) reaching 1602 ± 97.3 cells/mm2 (Table 4). In the distal part of the subiculum Ox-42 positive cells were less numerous and mainly restricted to the pyramidal cell layer (Fig. 4N, Table 4). Numbers of reactive microglia did not further increase in the distal subiculum and the perirhinal cortex and already declined in the presubiculum where only few cells were strongly Ox-42 immunoreactive (Fig. 4N, Table 4). A cluster of reactive microglia was located in layer II of the distal parasubiculum (arrow in Fig. 4N).
Although Ox-42 positive cells were still seen 3 months after KA-induced SE in many rats, their distribution was more restricted (Fig. 4O). Intensely labeled cells were present in the pyramidal cell layer of the proximal subiculum (Fig. 4T), in layers III and V–VI of the EC, and in the perirhinal cortex (Fig. 4O). In the pre- and parasubiculum and in layers I and II of the EC only very few strongly labeled cells were present in virtually all rats of this interval. In the presubiculum the highest density of reactive microglia was seen already 24 h after KA injection (326 ± 25.4 cells/mm2, Table 4).
4. Discussion
In our present study we established video-assisted long-term telemetric recordings for characterizing the occurrence and development of spontaneous seizures in freely moving rats after KA-induced SE, and investigated the time course and regional presentation of subsequent neurodegeneration and proliferation of microglia and astrocytes in the subiculum and other parahippocampal areas. The main findings are: 1) the first spontaneous seizure was predominantly detectable only in the EEG and without behavioral correlate. The latency between SE and presentation of the first spontaneous seizure was variable ranging from 3 to 36 days. 2) Similarly, the frequency and intensity of subsequent spontaneous seizures was variable. They did not correlate with the seizure rating during the preceding SE. 3) Rats, however, with insufficient anticonvulsant treatment 2 h after onset of the SE developed more frequent spontaneous seizures than rats with efficient anticonvulsant interruption of SE. In these rats, the number of spontaneous seizures was progressing over the 3 months observation period. 4) Spontaneous seizures occurred in clusters preferentially during sleep and showed different types of manifestation. 5) The onset of seizure-induced neurodegeneration was fast and close to maximal already after 24 h. The proximal subiculum and layer III of the medial EC were most severely affected. 6) Neurodegeneration was somewhat less severe in GABA neurons than in principal neurons. 7) Reactive astrocytes and microglia were detected at the same sites already after 24 h. They became prominent, however, after 8 and 30 days, respectively. 8) Significant neurodegeneration and a cluster of accumulating reactive astrocytes and microglia were observed in the parasubiculum.
4.1. Status epilepticus and manifestation of spontaneous seizures
Induction of SE by systemic injection of KA was highly reproducible and in agreement with previous studies (Ben-Ari et al., 1979; Fuller and Olney, 1981; Sperk et al., 1983). The majority of KA-injected rats (73%) developed an SE exhibiting generalized seizures of rating 3 or 4. The interruption of SE by diazepam effectively reduced the mortality (to less than 18%) and the severity of histopathology without preventing subsequent development of spontaneous recurrent seizures.
Combined video- and EEG monitoring was valuable for defining the length of the latent period before the onset of spontaneous seizures in the freely moving rats and allowed to detect and discriminate subclinical seizures (without behavioral correlate) and clinical seizures (with motor seizures detected in video monitoring). Although almost all rats experienced similarly severe motor seizures during the SE, the onset of spontaneous seizures was heterogeneous ranging from 3 to 36 days and did not correlate to behavioral seizure rating in the SE or with the onset of first stage 3 seizures in SE. Interestingly, using cortical electrodes, also for the pilocarpine model a similar mean latency (7.2 days) with a high variability (range: 5–17 days) was observed (Goffin et al., 2007). In this study, however, only 77% of rats developed spontaneous seizures during an observation period of 23 days. Also in a model based on hourly systemic injections of low doses of KA to trigger the SE and recording with chronic electrodes, a similarly long latency (mean: 11 days) and broad range (7–37 days) of the onset of spontaneous seizures was observed (Williams et al., 2009). Similar to our study, clustering of spontaneous seizures was also reported for the pilocarpine and the low-dosing KA model (Goffin et al., 2007; Grabenstatter et al., 2005; Williams et al., 2009).
In rats showing increased recurrent spike activity 3–6 h after diazepam treatment subsequent spontaneous seizures were significantly more frequent and progressed. This observation indicates a direct priming of the late seizures by the recurring initial SE. The mechanism for this is unclear. It could be due to an additional kindling effect.
The fact that 80% of the spontaneous seizures were presented during sleep or inactivity suggests a prominent role of the vigilance state on brain excitability. It is consistent with observations in other animal models and in human TLE demonstrating an increased propensity to generate epileptiform activity during sleep or inactivity (see Dinner, 2002 for review; Hellier and Dudek, 1999).
Two different types of spontaneous seizures were typically observed: i) one single seizure period or ii) a pair of seizures interrupted by an about 30 s period of low amplitude. The relevance of these apparent differences is not clear. Since we were using epidural and not depth electrodes we cannot exclude that different sites of seizure generation may be the cause. Interestingly, in those seizures where two periods of high amplitude were separated by a short phase of low amplitude (seizure type II), behavioral correlates were usually only seen during the second period of high amplitude, indicating generalization of an initial subclinical seizure.
4.2. Neurodegeneration: NeuN-ir and FluoroJade
Neurodegeneration was assessed by the loss of NeuN-positive and by FJ-C-labeling of degenerating neurons. In addition we assessed GAD65 and VGLUT1 mRNAs for determining specific losses in GABA and glutamate neurons, respectively. The most prominent neuronal losses developed in the proximal subiculum and in layer III of the medial EC (see Du et al., 1995). Although significant, they were less pronounced in the pre- and parasubiculum. Taken from cell counts of NeuN-positive neurons, neurodegeneration appeared to be almost maximal already after 24 h in all investigated areas (subiculum, parasubiculum, all layers of the EC) except for the presubiculum. In subregions with the most severe neurodegeneration (proximal subiculum, layer III of the medial EC) the density of surviving neurons, presumably due to tissue shrinkage, apparently increased again at later intervals (1 and 3 months).
FJ-C labeling, however, was still detectable in 75% of rats after 3 months indicating residual neurodegeneration. A 35% reduction of neuronal density in the proximal to intermediate subiculum has been reported in the pilocarpine model of TLE although this study did not discriminate between changes in the proximal and distal subiculum (Knopp et al., 2005). Strikingly, the subiculum is almost entirely preserved in most TLE patients in spite of severe damage of the hippocampus proper (Furtinger et al., 2001; Thom et al., 2005).
The restriction of neurodegeneration to the proximal subiculum may be explained by its excitatory innervations originating in layer III of the EC (temporo-ammonic path). Whereas the medial EC (layer III) projects mainly to the distal subiculum, projections of the lateral EC primarily innervate the proximal subiculum. Thus the rapid loss of layer III pyramidal neurons in the medial EC may protect the respective target neurons in the distal subiculum due to a loss in excitatory drive.
In the EC, high vulnerability of neurons in layer III of the medial EC have been well documented already 24 h after inducing SE by KA (Du et al., 1995), pilocarpine (Kobayashi et al., 2003; Wozny et al., 2005a), lithium-pilocarpine (Du et al., 1995) or electrical stimulation (Du et al., 1995; van Vliet et al., 2004). Our study revealed neuronal losses ranging from 37% to 59% in layer III of the medial EC. The extent of neurodegeneration was similar between rats killed either 24 h or 8 days after KA-induced SE. At later intervals, however, neuronal densities even seemed to recover somewhat, which is probably attributable to the observed tissue shrinkage. This suggests that the observed neurodegeneration is mainly a consequence of the initial SE and not of the subsequent spontaneous seizures. On the other hand, FJ-C labeling at the late intervals indicates neurodegenerative processes ongoing even 3 months after the initial SE.
The high vulnerability of layer III neurons in the medial EC may be related to its connectivity. The medial, but not the lateral part of layer III of the EC receives a specific and dense, mostly glutamatergic projection from principal neurons of the presubiculum (Caballero-Bleda and Witter, 1993; Wouterlood et al., 2004). These neurons largely survive the initial SE. Thus, seizure-induced over-excitation of principal neurons in the presubiculum may significantly contribute to the neuronal death at their target site in layer III of the medial EC (Eid et al., 1996). Indeed, lesion of the presubiculum by ibotenic acid injection partially protected neurons in layer III of the EC from KA-induced neurodegeneration (Eid et al., 2001).
4.3. Neurodegeneration: VGLUT1 and GAD65mRNA
Due to different fixation procedures required, the loss in NeuN-ir neurons and that in VGLUT1 and GAD65 mRNA positive neurons was examined in different groups of animals. Never the less, there is a good correlation between the general loss of neurons labeled for NeuN and those labeled for the markers of GABA and glutamate neurons, respectively. Taken the three months interval, loss of VGLUT1 mRNA containing neurons exceeds that of NeuN-positive cells in the proximal but not in the distal subiculum and in layer III of the medial but not of the lateral EC. In contrast, GAD65 mRNA expressing neurons are, except for the lateral EC, less vulnerable than the whole neuronal population labeled for NeuN. Accordingly losses in VGLUT1 mRNA containing neurons are somewhat higher than those of GAD65 mRNA containing neurons. Only in the medial EC losses in VGLUT1 mRNA containing neurons almost double those of GAD65 mRNA containing cells. In general this may indicate that principal neurons are more densely innervated by excitatory projections than interneurons in these areas. Since GAD67 and GAD65 are generally expressed together (Soghomonian and Martin, 1998) GAD65 mRNA labels all GABA neurons. Subpopulations of these may differ in vulnerability to seizure-induced damage. Thus, losses in parvalbumin containing neurons are more severe than those in GAD65 mRNA containing ones (Drexel et al., 2011). In the lateral EC layer III the number of GAD65 mRNA positive neurons appeared to recover somewhat (statistically not significant) at the 1–3 months interval. Explanations may be tissue shrinkage or better detection due to upregulation of GAD65 mRNA in surviving GABA neurons. Also proliferation of neurons may be considered although this seems to be mainly restricted to the dentate gyrus and the olfactory pathway (Parent, 2007).
4.4. Reactive gliosis: a chronic inflammatory state in the subiculum and EC
Activated astrocytes and reactive microglia, characterized by increased expression of GFAP and Ox-42, respectively, were already present 24 h after KA-induced SE. General activation of astrocytes became manifest after 24 h and further markedly increased after 8 days and 1 month (being somewhat higher after 8 days in most areas). After 3 months it became significantly reduced but was still present in most areas. Presentation of reactive microglia was somewhat different. After 24 h about 10 times more reactive microglial cells than activated astrocytes were present. Their number increased after 8 days, but in most areas reached its maximum only after 1 month (except for the presubiculum, distal subiculum and perirhinal cortex) and reactive microglia was still present in the subiculum and EC after 3 months.
Development of spontaneous seizures took place between 3 and 36 days after the initial status epilepticus. Although residual neurodegeneration was still seen at late intervals tissue damage was mainly concluded prior to the first spontaneous seizures observed. Reactive astrocytes and microglia were expressed during the period of upcoming initial spontaneous seizures. Their pattern and time course of presentation may indicate that they develop as a consequence of neurodegeneration. Interestingly numbers of astrocytes initially activated at the 24 h interval were low and their distribution more or less equal in all parahippocampal areas, presumably as a consequence of the generalized seizures. Thereafter activated microglia and astrocytes follow the pattern of neurodegeneration and presumably most severe excitatory activity. Although the time course of activation was somewhat different, the regional distribution patterns of reactive microglia and astrocytes were similar. Areas with highest neurodegeneration, such as the proximal subiculum and layer III of the medial EC showed also highest numbers of reactive astrocytes and microglia indicating a relationship between neurodegeneration and glia proliferation. Both events may be driven by excitatory activity within the respective circuitries during the initial SE or by subsequent spontaneous seizures. They may, however, be also critically involved in the development of spontaneous seizures.
Recent studies revealed an important role of inflammatory processes in the manifestation of seizures. Pro-inflammatory cytokines such as interleukin1-β (IL-1β) or tumor necrosis factor α (TNF-α), presumably released from activated astrocytes and microglia increase network excitability (Akassoglou et al., 1997; Campbell et al., 1993; De Simoni et al., 2000; Vezzani et al., 1999a). Furthermore, activated astrocytes likely contribute to increased extracellular glutamate levels seen in epileptic foci (see Glass and Dragunow, 1995 for review) either by increased release of glutamate (Angulo et al., 2004; Jabs et al., 2008) or by reduced uptake of extracellular glutamate via the astrocytic glutamate transporter GLT-1 (Mathern et al., 1999; Rothstein et al., 1996; Tanaka et al., 1997) although Takahashi et al. (2010) did not detect a change in GLT-1 expression 7 days after KA-induced SE. Furthermore, impaired K+ buffering caused by a reduced expression of Kir channels in astrocytes has been implicated in enhanced seizure susceptibility of sclerotic hippocampal tissue (Seifert et al., 2010).
Thus, the persisting state of glial proliferation in the proximal subiculum and in the EC may contribute to the increased excitability of these regions reported in the recent years (Cohen et al., 2002; de Guzman et al., 2008; Knopp et al., 2005; Kobayashi et al., 2003) and may have a role in the generation and manifestation of spontaneous recurrent seizures.
5. Conclusions
In conclusion, video-assisted telemetric EEG recording is a valid tool for monitoring subclinical and clinical seizures in freely moving rats. The latency of onset of spontaneous seizures after KA-induced SE is similar as that seen in other animal models of TLE and using depth electrodes for EEG monitoring. KA-induced seizures cause a widespread but specific pattern of neurodegeneration in parahippocampal areas that is mostly followed by astrocytic and microglia proliferation in the same areas. The time course of glial proliferation, but not that of neurodegeneration, correlates with the time range of onset of spontaneous seizures, supporting a relationship of astrocyte and microglia proliferation in epileptogenesis.
Acknowledgements
We thank Dr. Albert J. Becker and Dr. Julika Pitsch for advice and help in establishing video-assisted telemetry. We also thank Anna Wieselthaler-Hölzl and Elisabeth Gasser for excellent technical assistance. This research was supported by the Austrian Research Funds (grant number P19464) and by the European Union Grant FP6 EPICURE (LSH-CT-2006-037315).
Contributor Information
Meinrad Drexel, Email: meinrad.drexel@i-med.ac.at.
Günther Sperk, Email: guenther.sperk@i-med.ac.at.
References
- Akassoglou K., Probert L., Kontogeorgos G., Kollias G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J. Immunol. 1997;158:438–445. [PubMed] [Google Scholar]
- Alonso-Nanclares L., Kastanauskaite A., Rodriguez J.R., Gonzalez-Soriano J., Defelipe J. A stereological study of synapse number in the epileptic human hippocampus. Front. Neuroanat. 2011;5:8. doi: 10.3389/fnana.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo M.C., Kozlov A.S., Charpak S., Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Tremblay E., Ottersen O.P., Naquet R. Evidence suggesting secondary epileptogenic lesion after kainic acid: pre treatment with diazepam reduces distant but not local brain damage. Brain Res. 1979;165:362–365. doi: 10.1016/0006-8993(79)90571-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Tremblay E., Ottersen O.P., Meldrum B.S. The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res. 1980;191:79–97. doi: 10.1016/0006-8993(80)90316-9. [DOI] [PubMed] [Google Scholar]
- Bertram E.H., Lothman E.W., Lenn N.J. The hippocampus in experimental chronic epilepsy: a morphometric analysis. Ann. Neurol. 1990;27:43–48. doi: 10.1002/ana.410270108. [DOI] [PubMed] [Google Scholar]
- Bratz E. Ammonshornbefunde bei Epileptikern. Arch. Psychiatr. Nervenkrankh. 1899;32:820–835. [Google Scholar]
- Caballero-Bleda M., Witter M.P. Regional and laminar organization of projections from the presubiculum and parasubiculum to the entorhinal cortex: an anterograde tracing study in the rat. J. Comp. Neurol. 1993;328:115–129. doi: 10.1002/cne.903280109. [DOI] [PubMed] [Google Scholar]
- Campbell I.L., Abraham C.R., Masliah E., Kemper P., Inglis J.D., Oldstone M.B., Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.C., Gottlieb D.I. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J. Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Navarro V., Clemenceau S., Baulac M., Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- de Guzman P., Inaba Y., Biagini G., Baldelli E., Mollinari C., Merlo D., Avoli M. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus. 2006;16:843–860. doi: 10.1002/hipo.20215. [DOI] [PubMed] [Google Scholar]
- de Guzman P., Inaba Y., Baldelli E., de Curtis M., Biagini G., Avoli M. Network hyperexcitability within the deep layers of the pilocarpine-treated rat entorhinal cortex. J. Physiol. 2008;586:1867–1883. doi: 10.1113/jphysiol.2007.146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni M.G., Perego C., Ravizza T., Moneta D., Conti M., Marchesi F., De Luigi A., Garattini S., Vezzani A. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur. J. Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- Dinner D.S. Effect of sleep on epilepsy. J. Clin. Neurophysiol. 2002;19:504–513. doi: 10.1097/00004691-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Drexel M., Preidt A.P., Kirchmair E., Sperk G. Parvalbumin interneurons and calretinin fibers arising from the thalamic nucleus reuniens degenerate in the subiculum after kainic acid-induced seizures. Neuroscience. 2011;189:316–329. doi: 10.1016/j.neuroscience.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F., Eid T., Lothman E.W., Kohler C., Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J. Neurosci. 1995;15:6301–6313. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T., Jorritsma-Byham B., Schwarcz R., Witter M.P. Afferents to the seizure-sensitive neurons in layer III of the medial entorhinal area: a tracing study in the rat. Exp. Brain Res. 1996;109:209–218. doi: 10.1007/BF00231782. [DOI] [PubMed] [Google Scholar]
- Eid T., Du F., Schwarcz R. Ibotenate injections into the pre- and parasubiculum provide partial protection against kainate-induced epileptic damage in layer III of rat entorhinal cortex. Epilepsia. 2001;42:817–824. doi: 10.1046/j.1528-1157.2001.042007817.x. [DOI] [PubMed] [Google Scholar]
- Falconer M.A., Serafetinides E.A., Corsellis J.A. Etiology and Pathogenesis of temporal lobe epilepsy. Arch. Neurol. 1964;10:233–248. doi: 10.1001/archneur.1964.00460150003001. [DOI] [PubMed] [Google Scholar]
- Fuller T.A., Olney J.W. Only certain anticonvulsants protect against kainate neurotoxicity. Neurobehav. Toxicol. Teratol. 1981;3:355–361. [PubMed] [Google Scholar]
- Furtinger S., Pirker S., Czech T., Baumgartner C., Ransmayr G., Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J. Neurosci. 2001;21:5804–5812. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M., Dragunow M. Neurochemical and morphological changes associated with human epilepsy. Brain Res. Brain Res. Rev. 1995;21:29–41. doi: 10.1016/0165-0173(95)00005-n. [DOI] [PubMed] [Google Scholar]
- Goffin K., Nissinen J., Van Laere K., Pitkanen A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp. Neurol. 2007;205:501–505. doi: 10.1016/j.expneurol.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Grabenstatter H.L., Ferraro D.J., Williams P.A., Chapman P.L., Dudek F.E. Use of chronic epilepsy models in antiepileptic drug discovery: the effect of topiramate on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2005;46:8–14. doi: 10.1111/j.0013-9580.2005.13404.x. [DOI] [PubMed] [Google Scholar]
- Hellier J.L., Dudek F.E. Spontaneous motor seizures of rats with kainate-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999;35:47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- Huberfeld G., Menendez de la Prida L., Pallud J., Cohen I., Le Van Quyen M., Adam C., Clemenceau S., Baulac M., Miles R. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat. Neurosci. 2011;14:627–634. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- Jabs R., Seifert G., Steinhauser C. Astrocytic function and its alteration in the epileptic brain. Epilepsia. 2008;49(Suppl. 2):3–12. doi: 10.1111/j.1528-1167.2008.01488.x. [DOI] [PubMed] [Google Scholar]
- Knopp A., Kivi A., Wozny C., Heinemann U., Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J. Comp. Neurol. 2005;483:476–488. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- Knopp A., Frahm C., Fidzinski P., Witte O.W., Behr J. Loss of GABAergic neurons in the subiculum and its functional implications in temporal lobe epilepsy. Brain. 2008;131:1516–1527. doi: 10.1093/brain/awn095. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Wen X., Buckmaster P.S. Reduced inhibition and increased output of layer II neurons in the medial entorhinal cortex in a model of temporal lobe epilepsy. J. Neurosci. 2003;23:8471–8479. doi: 10.1523/JNEUROSCI.23-24-08471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H., Petsche U., Kitz K., Baran H., Sperk G., Seitelberger F., Hornykiewicz O. The role of brain edema in epileptic brain damage induced by systemic kainic acid injection. Neuroscience. 1984;13:691–704. doi: 10.1016/0306-4522(84)90089-7. [DOI] [PubMed] [Google Scholar]
- Lothman E.W., Bertram E.H., 3rd Epileptogenic effects of status epilepticus. Epilepsia. 1993;34(Suppl. 1):S59–S70. doi: 10.1111/j.1528-1157.1993.tb05907.x. [DOI] [PubMed] [Google Scholar]
- Mathern G.W., Mendoza D., Lozada A., Pretorius J.K., Dehnes Y., Danbolt N.C., Nelson N., Leite J.P., Chimelli L., Born D.E., Sakamoto A.C., Assirati J.A., Fried I., Peacock W.J., Ojemann G.A., Adelson P.D. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52:453–472. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- Nadler J.V. Minireview. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- Ni B., Rosteck P.R., Jr., Nadi N.S., Paul S.M. Cloning and expression of a cDNA encoding a brain-specific Na(+)-dependent inorganic phosphate cotransporter. Proc. Natl. Acad. Sci. U.S.A. 1994;91:5607–5611. doi: 10.1073/pnas.91.12.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E., Amici M., Sobrero F., Spinelli G., Di Angelantonio S., Ragozzino D., Mascia A., Scoppetta C., Esposito V., Miledi R., Eusebi F. Anomalous levels of Cl- transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8465–8468. doi: 10.1073/pnas.0602979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent J.M. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog. Brain Res. 2007;163:529–540. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- Pitkanen A., Nissinen J., Nairismagi J., Lukasiuk K., Grohn O.H., Miettinen R., Kauppinen R. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog. Brain Res. 2002;135:67–83. doi: 10.1016/S0079-6123(02)35008-8. [DOI] [PubMed] [Google Scholar]
- Pitsch J., Schoch S., Gueler N., Flor P.J., van der Putten H., Becker A.J. Functional role of mGluR1 and mGluR4 in pilocarpine-induced temporal lobe epilepsy. Neurobiol. Dis. 2007;26:623–633. doi: 10.1016/j.nbd.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Rothstein J.D., Dykes-Hoberg M., Pardo C.A., Bristol L.A., Jin L., Kuncl R.W., Kanai Y., Hediger M.A., Wang Y., Schielke J.P., Welty D.F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Sagar H.J., Oxbury J.M. Hippocampal neuron loss in temporal lobe epilepsy: correlation with early childhood convulsions. Ann. Neurol. 1987;22:334–340. doi: 10.1002/ana.410220309. [DOI] [PubMed] [Google Scholar]
- Schmued L.C., Stowers C.C., Scallet A.C., Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Schwob J.E., Fuller T., Price J.L., Olney J.W. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience. 1980;5:991–1014. doi: 10.1016/0306-4522(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Seifert G., Carmignoto G., Steinhauser C. Astrocyte dysfunction in epilepsy. Brain Res. Rev. 2010;63:212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Seiffert E., Dreier J.P., Ivens S., Bechmann I., Tomkins O., Heinemann U., Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian J.J., Martin D.L. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol. Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Sokrab T.E., Kalimo H., Johansson B.B. Endogenous serum albumin content in brain after short-lasting epileptic seizures. Brain Res. 1989;489:231–236. doi: 10.1016/0006-8993(89)90855-x. [DOI] [PubMed] [Google Scholar]
- Sperk G., Lassmann H., Baran H., Kish S.J., Seitelberger F., Hornykiewicz O. Kainic acid induced seizures: neurochemical and histopathological changes. Neuroscience. 1983;10:1301–1315. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- Takahashi D.K., Vargas J.R., Wilcox K.S. Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol. Dis. 2010;40:573–585. doi: 10.1016/j.nbd.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Watase K., Manabe T., Yamada K., Watanabe M., Takahashi K., Iwama H., Nishikawa T., Ichihara N., Kikuchi T., Okuyama S., Kawashima N., Hori S., Takimoto M., Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Thom M., Zhou J., Martinian L., Sisodiya S. Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain. 2005;128:1344–1357. doi: 10.1093/brain/awh475. [DOI] [PubMed] [Google Scholar]
- van Vliet E.A., Aronica E., Tolner E.A., Lopes da Silva F.H., Gorter J.A. Progression of temporal lobe epilepsy in the rat is associated with immunocytochemical changes in inhibitory interneurons in specific regions of the hippocampal formation. Exp. Neurol. 2004;187:367–379. doi: 10.1016/j.expneurol.2004.01.016. [DOI] [PubMed] [Google Scholar]
- van Vliet E.A., da Costa Araujo S., Redeker S., van Schaik R., Aronica E., Gorter J.A. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Vezzani A., Conti M., De Luigi A., Ravizza T., Moneta D., Marchesi F., De Simoni M.G. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J. Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A., Ravizza T., Moneta D., Conti M., Borroni A., Rizzi M., Samanin R., Maj R. Brain-derived neurotrophic factor immunoreactivity in the limbic system of rats after acute seizures and during spontaneous convulsions: temporal evolution of changes as compared to neuropeptide Y. Neuroscience. 1999;90:1445–1461. doi: 10.1016/s0306-4522(98)00553-3. [DOI] [PubMed] [Google Scholar]
- Williams P.A., Hellier J.L., White A.M., Staley K.J., Dudek F.E. Development of spontaneous seizures after experimental status epilepticus: implications for understanding epileptogenesis. Epilepsia. 2007;48(Suppl 5):157–163. doi: 10.1111/j.1528-1167.2007.01304.x. [DOI] [PubMed] [Google Scholar]
- Williams P.A., White A.M., Clark S., Ferraro D.J., Swiercz W., Staley K.J., Dudek F.E. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J. Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood F.G., Van Haeften T., Eijkhoudt M., Baks-Te-Bulte L., Goede P.H., Witter M.P. Input from the presubiculum to dendrites of layer-V neurons of the medial entorhinal cortex of the rat. Brain Res. 2004;1013:1–12. doi: 10.1016/j.brainres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Wozny C., Gabriel S., Jandova K., Schulze K., Heinemann U., Behr J. Entorhinal cortex entrains epileptiform activity in CA1 in pilocarpine-treated rats. Neurobiol. Dis. 2005;19:451–460. doi: 10.1016/j.nbd.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Wozny C., Knopp A., Lehmann T.N., Heinemann U., Behr J. The subiculum: a potential site of ictogenesis in human temporal lobe epilepsy. Epilepsia. 2005;46(Suppl. 5):17–21. doi: 10.1111/j.1528-1167.2005.01066.x. [DOI] [PubMed] [Google Scholar]