Abstract
Although exercise training (ExT) is an important therapeutic strategy for improving quality of life in patients with chronic heart failure (CHF), the central mechanisms by which ExT is beneficial are not well understood. The angiotensin II type 1 receptor (AT1R) plays a pivotal role in the development of CHF, and is upregulated in a number of tissues due in part to transcription factor nuclear factor kappa B (NF-κB). In addition, AT1R is marked for internalization and recycling via G protein coupled receptor kinase (GRK) phosphorylation. Because previous studies have shown that the beneficial effects of ExT in CHF rely on a reduction in Ang II, we hypothesized ExT would decrease AT1R, GRK5 and NF-κB protein expression in the paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM) of CHF rats. Following infarction by coronary artery ligation, animals were exercised four weeks post-surgery on a treadmill at a final speed of 25 m/min for 60 minutes, 5 days/week for 6 weeks. Western blot analysis of PVN and RVLM micropunches revealed an upregulation of AT1R, GRK5 and NF-κB in the infarcted group that was reversed by ExT. Furthermore, the relative expression of phosphorylated AT1R and AT1R/GRK5 physical association was increased in the CHF sedentary group, and reversed by ExT. Overexpression of GRK5 in cultured CATH.a neurons blunted angiotensin II-mediated upregulation of AT1R and NF-κB; conversely, silencing of GRK5 exacerbated angiotensin II-mediated AT1R and NF-κB upregulation. Taken together, increased GRK5 may regulate AT1R expression in CHF, and ExT mitigates AT1R and its pathway components.
Keywords: angiotensin II, GRK5, NF-κB, receptor turnover
Introduction
A hallmark of the chronic heart failure (CHF) state is increased sympathetic outflow, which can be correlated to both disease severity and mortality1. However, the mechanisms underlying the increased sympathoexcitation are not completely understood. A good deal of work has focused on the role of the angiotensin II (Ang II) type 1 receptor (AT1R) regulation in the development of CHF and as a driver of sympathoexcitation2–4.
The paraventricular nucleus (PVN) of the hypothalamus is an integrative center for endocrine, hormonal, and neural control. The PVN contains separate neuronal cell populations: the parvocellular subgroup, which projects to the rostral ventrolateral medulla (RVLM) and the intermediolateral cell column (IML) of the spinal cord, influencing sympathetic nerve activity; and magnocellular neurons, which project to the posterior pituitary to release vasopressin 5, 6. Neurons from the RVLM, in turn, project to the sympathetic preganglionic motor neurons, whose activity is responsible for blood pressure regulation and baroreflex function and are the primary drivers of sympathetic tone7. Previous data from this laboratory and others suggest that in rats with CHF the neurons in these nuclei are hyperactive 8–10.
Exercise training (ExT) in humans with CHF was originally contra-indicated due to fear of worsening cardiac function combined with the assumption that patients with CHF would have limited capacity for ExT and increased risk for exercise-related mortality11. It is becoming increasingly accepted that ExT in CHF is safe and can increase survival, decrease complications, and abrogate increases in muscle sympathetic nerve activity 12–15. However, the mechanism by which ExT exerts these beneficial effects in the CHF state and its effect on AT1R expression and signaling are not well understood.
Upon binding of Ang II to the AT1R, a G protein coupled receptor, the Gαq signaling cascade is activated leading to a wide range of downstream consequences including increased intracellular calcium, inositol trisphosphate, phospholipase A2, increased NAD(P)H oxidase activity, janus kinase (JAK), c-jun N-terminal kinase (JNK), extracellular signal related kinase (ERK) and protein kinase C activity16. In CHF, increased circulating Ang II can lead to long-term activation of many of these signaling events via the AT1R. In addition, similar to other enhanced sympathoexcitatory disease conditions like hypertension, the AT1R is upregulated in both central and peripheral tissues17–19. Recent data from our laboratory has also shown that in a neuronal cell line, Ang II mediated upregulation of AT1R is, in part, driven by nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1), two transcription factors that regulate the expression of many genes during CHF and other clinical pathologies20.
Upon ligand binding, most G protein-coupled receptors exhibit a rapid loss of responsiveness following activation, leading to receptor internalization. This desensitization is mediated, in part, by G protein coupled receptor kinases (GRKs), which phosphorylate the active receptor 21. The phosphorylated receptor is then bound by β-arrestin and sequestered into endosomal vesicles for either recycling or degradation 22. Previous work has shown that the AT1R can be phosphorylated by both GRK5 and/or GRK2 (βARK1) in a tissue-specific manner. GRK2, or βARK1, not only plays a role in promoting β-arrestin binding to GPCRs, but it also phosphorylates non-GPCR substrates, and is implicated in signal transduction 23. GRK5 is a membrane associated kinase, and is robustly expressed in heart and lung tissue, smooth and skeletal muscle and brain 24–27. GRK5 is upregulated in an Ang II and calcium-dependent manner in cultured vascular smooth muscle cells and in hypertensive rat aorta 26. However, the role that GRK5 plays in AT1R regulation in the brain has not yet been elucidated. In the current study, we hypothesized that central GRK5 and p65 NF-κB, two proteins involved in AT1R expression, are also increased during CHF and normalized by ExT.
Methods
Animals
Male Sprague-Dawley rats weighing 220 to 280 g (Sasco Breeding Laboratories, Omaha, NE) were fed and housed according to institutional guidelines. Protocols were approved by the University of Nebraska Institutional Animal Care and Use Committee and were in accordance with the American Physiological Society’s Guiding Principles in the Care and Use of Laboratory Animals. Rats were given rat chow and water ad libitum and were housed in a room with a 12-h light-dark cycle. Rats were allowed to acclimatize for one week prior to cardiac surgery.
Induction of heart failure
Rats were randomly assigned to either the sham-operated control group or the CHF group. CHF was induced by ligation of the left coronary artery as has been described previously 8. The expanded methods are available in the Online Supplement.
Metabolic Cage Assessment and Measurement of Urinary Norepinephrine Excretion
Please see the online supplement for metabolic cage assessment and measurement of urinary norepinephrine excretion.
Exercise Training Protocol
Four weeks following coronary artery ligation surgery, rats were randomly assigned to either ExT or Sedentary (Sed) groups to produce four total experimental groups: Sham Sed, HF Sed, Sham ExT, and HF ExT. ExT was carried out on a motor-driven treadmill (Columbus Instruments, Columbus, OH) at a final speed of 20–25 m/min, 60 min/day and 5–10% incline for a period of up to six weeks according to a protocol modified from Musch and Terrell 28. Additional protocol details can be found in the online supplement.
Micropunch of the PVN and RVLM and isolation of protein for Western blot measurements
Micropunches of the PVN and RVLM were isolated as described previously29. Additional protocol details can be found in the online supplement. Cell Culture and Maintenance. CATH.a catecholaminergic neurons were maintained in RPMI-1640 with 15% horse serum and 5% fetal bovine serum and an antibiotic cocktail. 48–72 hours prior to experimentation, cells were differentiated by serum starvation as performed previously20. Please see the online supplement for additional details. Western blot measurement of AT1R and GRK5 proteins. Western blot measurements are detailed within the online supplement.
Statistical Analysis
Data are presented as mean ± SE. The data were subjected to one-way ANOVA followed by comparison for individual group differences using the Newman-Keuls test or Bonferroni correction 30. Statistical significance is indicated by a value of P<0.05.
Results
Heart weight data and echocardiographic values are shown in the online supplemental Table S1. Briefly, there was a significant decrease in ejection fraction (EF) and fractional shortening (FS), and a significant increase in cardiac dimensions in the CHF animals compared to sham. However, there was no effect of ExT on any of these parameters, consistent with previous finidngs31. To confirm an ExT effect, the soleus of some animals was removed and analyzed for citrate synthase activity. As expected, citrate synthase levels were significantly elevated in both sham and CHF ExT groups (Sham ExT: 13.0 ± 1.7 μmol/g/min; CHF ExT: 12.3 ± 0.9 μmol/g/min compared to the Sed groups: Sham Sed: 8.3 ± 1.5 μmol/g/min; CHF Sed: 8.4 ± 0.87 μmol/g/min; p<0.03 comparing Sed with ExT for each group), demonstrating a significant effect of ExT.
To assess the effects of ExT on a more global index of sympathetic tone we assessed urinary NE excretion in the four groups of animals. Compared to Sham Sed animals, CHF Sed animals excreted significantly more NE (1.95 ± 0.57 vs 4.11 ± 1.40 μg NE/24 hrs; p<0.05). On the other hand, CHF ExT animals excreted significantly less NE compared to HF Sed animals (0.55 ± 0.04 vs 4.11 ± 1.40 μg NE/24 hrs; p<0.05). Sham ExT animals excreted approximately the same NE as Sham Sed animals (1.94 ± 0.46, 1.95 ± 0.57, respectively). Taken together, these data suggest that ExT decreases sympathoexcitation in rats with CHF.
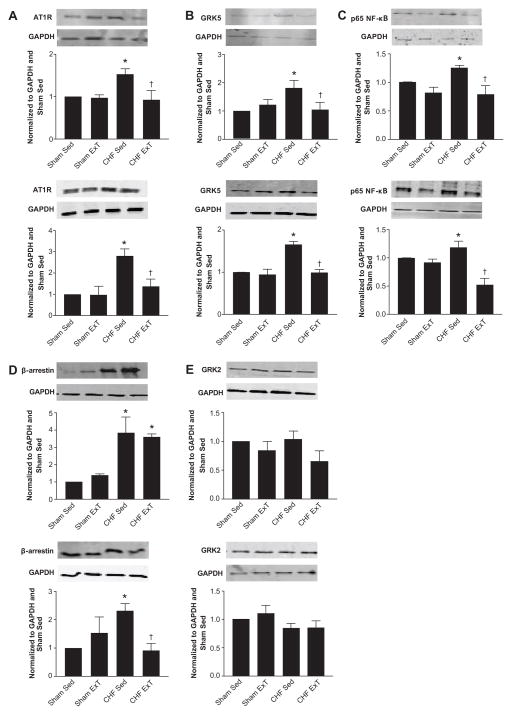
Given that one of the hallmarks of CHF is increased circulating Ang II concentrations 32, we hypothesized that this would lead to an increase in AT1R expression in the PVN and RVLM of CHF Sed animals and a subsequent decrease in AT1R protein following ExT. We performed Western blot analysis on lysed PVN and RVLM micropunches and probed for AT1R expression (Figure 1A). There was a significant increase in AT1R protein expression in the CHF Sed animals compared to the sham groups in both nuclei. Following ExT, AT1R expression in CHF animals was decreased to sham levels.
Figure 1.
AT1R, GRK5, p65 NF-κB and β-arrestin are increased in the PVN (solid bars) and RVLM (hatched bars) of CHF animals and normalized by ExT. GRK2, another kinase implicated in regulating AT1R expression, is unchanged in the PVN during both CHF and ExT. * p<0.05 vs Sham Sed; † p<0.05 vs CHF Sed; n= 5–7.
Because AT1R expression is altered in CHF animals, we next considered the role of GRK2 and GRK5 in the regulation of AT1R during CHF and its modulation by ExT. We performed Western blotting on the aforementioned micropunch lysates and immunoblotted for GRK5 and GRK2 (Figure 1B, Figure 1E). GRK5 protein was increased in CHF Sed animals by 50% compared to Sham Sed animals. This elevated GRK5 protein was normalized by ExT, thereby following the same trend observed for AT1R protein. These fluctuations in GRK5 and AT1R were also mimicked by changes in β-arrestin in the PVN (Figure 1D). Although β-arrestin was also significantly greater in the CHF Sed animals compared to sham in the RVLM, ExT did not normalize β-arrestin expression. In contrast, GRK2 was unchanged across animal groups (Figure 1E).
Given that NF-κB activation has been implicated in the upregulation of a number of genes in the CHF state 33, 34, and that previous studies from our laboratory have shown that in a mouse catecholaminergic CATH.a neuronal cell line, Ang II stimulation leads to increased NF-κB levels 20, we also investigated changes in the phosphorylated form of NF-κB in CHF and ExT animals. Examination of PVN and RVLM micropunch lysates for the p65 subunit of NF-κB indicate that this transcription factor is elevated in CHF animals, and is normalized following ExT (Figure 1C). Interestingly, in the PVN of CHF ExT animals, there was significantly lower p65 NF-κB protein expression compared to Sham Sed animals. These data imply a relationship between GRK5 and AT1R, and potential involvement of NF-κB.
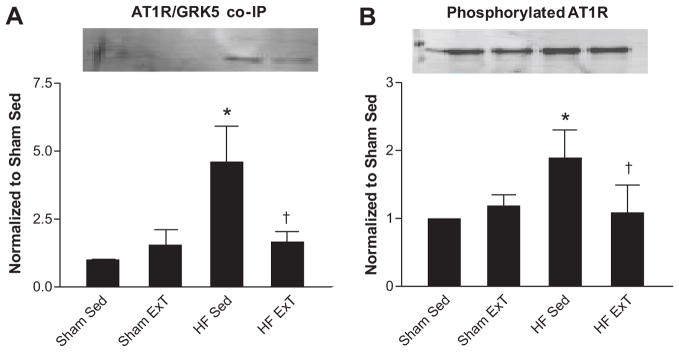
To further examine the relationship between GRK5 and AT1R, we performed co-immunoprecipitation experiments in the same PVN micropunch total cell lysates (Figure 2A). We hypothesized there would be increased GRK5/AT1R interaction in the CHF Sed group compared to sham animals and that this interaction would be decreased in the CHF ExT group. Indeed, there was a four-fold increase in GRK5/AT1R association in CHF Sed animals compared to sham groups which was normalized by ExT. To confirm a functional effect of GRK5 on AT1R, we examined the amount of phosphorylated AT1R by immunoprecipitating using an anti-AT1R antibody, and immunoblotting with an anti-phosphothreonine antibody (a site of GRK5 action) (Figure 2B). There was a 100% increase in phosphorylated AT1R in CHF Sed animals compared to sham sed. ExT in CHF animals exhibited a degree of phosphorylated AT1R that was similar to sham sed animals. Because GRK2 levels do not change in the face of either CHF or ExT, we wanted to confirm the GRK5-AT1R interaction is specific by repeating co-immunoprecipitation experiments in these same lysates immunoblotting for GRK2. As shown in Supplemental Figure 2A, GRK2 and AT1R do not associate. As a control for any non-specific protein-protein interaction, we performed experiments in the same lysates pulling down for the α2C adrenergic receptor (α2CAR), which has been shown previously to not be modified by GRK535 (Supplemental Figure 2B). Indeed, in all lysates tested, there was no interaction between the α2CAR and GRK5, but we were able to detect both GRK5 and GAPDH in the total lysates on the same blot.
Figure 2.
AT1R/GRK5 association and phosphorylated AT1R increase in the PVN total lysates during CHF. A. GRK5 and AT1R association. B. Changes in phosphorylated AT1R determined by co-immunoprecipitation using an anti-AT1R antibody, and immunoblotting using a phosphothreonine antibody. * p<0.05 vs Sham; † p<0.05 vs CHF Sed; n= 5–7.
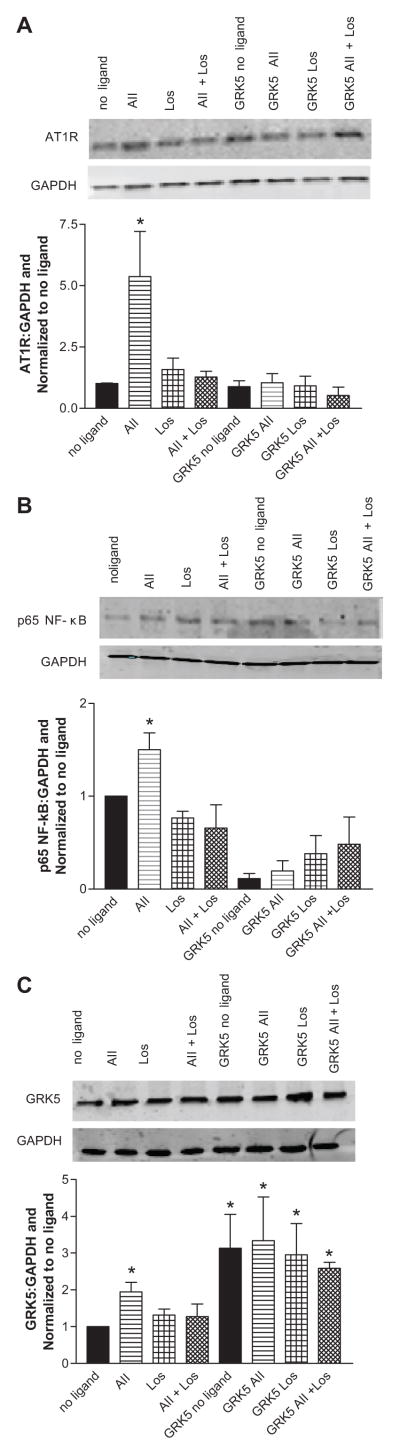
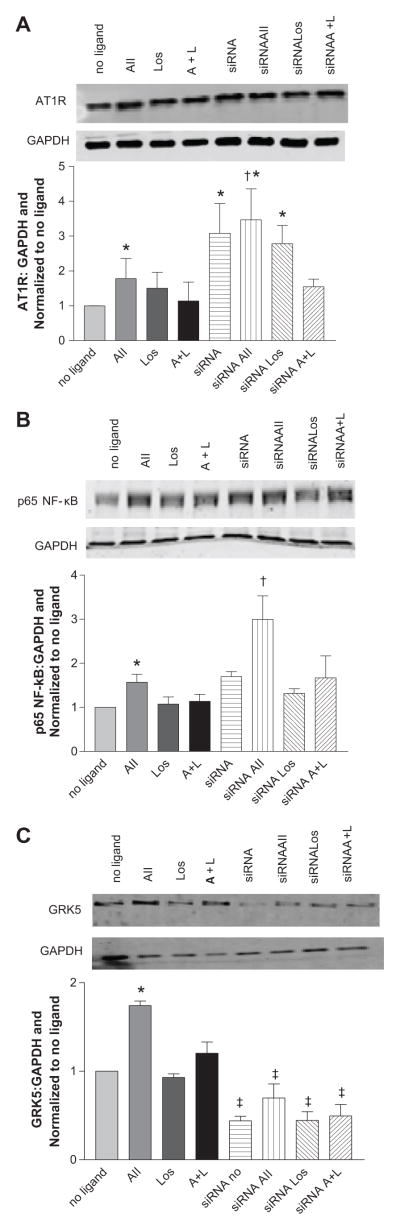
To further explore the interaction between AT1R and GRK5, we next used the CATH.a neuronal cell line to examine changes in AT1R expression following manipulation of GRK5 either by overexpression or knockdown. We first characterized the changes in AT1R, GRK5 and p65 NF-κB protein expression, following stimulation with 100 nM Ang II for 4 hours (Figures 3A–3C). As expected, stimulation with Ang II led to an upregulation of all proteins; conversely, stimulation with 100 nM losartan (Los), an AT1R antagonist, or co-stimulation with Ang II plus Los did not provoke any changes in protein abundance. In addition, these experiments were repeated at 1nM Ang II which evoked similar results (see supplemental Figure S3). Importantly, the upregulation of AT1R and p65 NF-κB following Ang II stimulation was completely prevented following transient transfection of GRK5 plasmid (Figures 3A–3C). These data further suggest that GRK5 plays a role in the regulation of AT1R, potentially via modulation of NF-κB. To confirm this finding, we performed the inverse experiments in which we silenced GRK5 expression in CATH.a neurons with siRNA knockdown (Figures 4A–4C). Knockdown of GRK5 led to a 3 fold increase in AT1R protein levels prior to stimulation, and following addition of Ang II, AT1R was upregulated further.
Figure 3.
GRK5 overexpression normalizes AT1R and p65 NF-κB protein levels following stimulation with Ang II. Values are expressed as a ratio of protein to GAPDH and normalized to no ligand. A. AT1R, B. p65 NF-κB, C. GRK5. * p<0.05 vs no ligand; n= 5–7.
Figure 4.
GRK5 knockdown with siRNA leads to greater increases in AT1R and p65 NF-κB protein following Ang II stimulation. Values are expressed as a ratio of protein to GAPDH and normalized to no ligand. A. AT1R, B. p65 NF-κB, C. GRK5. * p<0.05 vs no ligand, † p<0.05 vs non-siRNA Ang II stimulation, ‡ vs non-siRNA; n=4–6.
Discussion
The main finding in the current study was that the expression of AT1R, GRK5 and p65 NF-κB were augmented in the PVN and RVLM of animals with CHF, and this augmentation was abrogated following a regimen of ExT for six weeks. Furthermore, we demonstrated that ExT is associated with normalization in AT1R protein expression in the PVN. Importantly, this normalization was concomitant with a decrease in GRK5, a protein responsible for marking the AT1R for internalization and degradation21, and NF-κB, a transcription factor involved in the upregulation of AT1R 20.
The observation that ExT reduces AT1R expression is consistent with the idea that this maneuver is capable of impacting protein expression to this and potentially other GPCRs 32, 36. This and other laboratories have shown that central AT1R expression is upregulated in CHF and hypertension 32, 37. We have previously described a transcriptional pathway by which the AT1R is regulated in CHF and in response to exogenous Ang II38.
It is still unclear if ExT impacts all of the downstream proteins that are involved in this pathway. Unfortunately, almost nothing is known about AT1R regulation outside of its transcription in the brain and the control of autonomic outflow in disease states following interventions such as ExT. We sought to explore the role of the G protein regulatory pathway in these observations. In this regard, an interesting aspect of the current study was that GRK5 was also upregulated in CHF Sed animals, and this increase was normalized by ExT. Functionally, our data suggest that GRK5 and AT1R are interacting, and that GRK5 may play a role in the regulation of AT1R (i.e. increase in receptor phosphorylation; Figure 2). Subsequent studies in the CATH.a neurons indicated that silencing of GRK5 exacerbates AT1R and p65 NF-κB upregulation following Ang II stimulation and that overexpression of GRK5 blunts any Ang II-mediated AT1R and p65 NF-κB increase. Taken together, we propose that the ancillary increase in GRK5 in the PVN and RVLM is a compensatory mechanism responding to the primary increase in AT1R seen in CHF. The AT1R behaves in a non-classical manner as compared to other GPCRs; instead of a ligand-induced receptor desensitization and internalization, AT1R expression increases with continued Ang II presence. Although initially counterintuitive that GRK5, a regulatory kinase would also be increased with AT1R and p65 NF-κB, our data would suggest that, in fact, GRK5 upregulation seen either in CHF Sed animals or in CATH.a neurons stimulated with Ang II is a means by which the cell prevents even further increases in Ang II. In essence, GRK5 is serving as a “brake” for the Ang II/AT1R feed-forward axis. This scenario is certainly not unprecedented. For instance, a similar example also seen in CHF is the response of Atrial Natriuretic Peptide (ANP). One would expect that the increase in ANP secretion would evoke a natriuresis as a way of normalizing volume in CHF. However, this does not happen because of the many antinatriuretic factors that are activated in CHF 39. Similarly, we propose here that GRK5 is upregulated to counterbalance, even further, increases in AT1R expression. Work by Ishizaka et al. has shown that GRK5 is upregulated in vascular smooth muscle cells in an Ang II-dependent manner, and is also upregulated in the aorta of hypertensive animals 26. We hypothesize that in the absence of GRK5, the upregulation of AT1R seen in CHF would be further exacerbated. GRKs have previously been suggested as potential therapeutic targets for the treatment of CHF40,41. In a study by Sorriento et al., an adenovirus encoding the amino terminus of GRK5 was injected into the cardiac wall of spontaneously hypertensive rats (SHRs), and decreased left ventricular hypertrophy via a kinase-independent stabilization of IκB 42. In light of our previous data in the CATH.a neuronal cell line that indicated NF-κB protein was upregulated in an Ang II-dependent manner20, it is possible that there are physical and functional interactions of central GRK5, NF-κB and IκB in both normal and CHF animals. Whether ExT has an effect on the stabilization of the GRK5/IκB/NF-κB complex and if there is a protective effect of GRK5 overexpression in the brain of animals with increased sympatho-excitation is not clear at the present time. It is of note that p65 NF-κB protein decreased in CHF ExT animals to levels lower than that of sham. Because p65 NF-κB is a transcription factor upon which a number of signaling cascades besides AT1R converge, it is likely that some of the other beneficial effects of ExT in CHF animals that we did not examine in this study are also being altered, thereby leading to a redundant (or additional) decrease in p65 NF-κB. GRK5 can also translocate to the nucleus of cardiac myocytes and serve as a Histone Deacetylase Kinase (HDAC kinase) 43. It will be important to investigate this novel aspect of GRK5 signaling in the brain.
The role of ExT as a useful therapeutic paradigm in the setting of CHF has been examined in several clinical trials. Bellardinelli et al. clearly demonstrated that a supervised ExT regimen not only reduced cardiovascular events but also increased survival 11. This study was carried out in a relatively small cohort and neurohormonal data were not provided. A larger multi-center clinical trial is on-going to evaluate the effects of ExT in CHF patients. The HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial has so far indicated an enhanced quality of life and a reduction in hospitalization as well as a reduction in all cause mortality for CHF ExT patients 44.
Unfortunately, these clinical studies do not address mechanisms. In a study by Adams et al. it was clearly shown that ExT enhanced endothelial function in the forearm vasculature by an NO dependent mechanism 45. It is not clear if this contributes to central alterations as well. In a seminal study by Roveda et al. 14, direct recording of muscle sympathetic nerve activity (MSNA) in patients with CHF provided strong support for the inhibition of peripheral sympathetic outflow following ExT, which is consistent with that observed in the current study46. In fact, our data suggest a more global reduction in sympathetic outflow based on the decrease in NE excretion.
Perspectives
We show here that ExT normalizes the upregulated AT1R, GRK5, and p65 NF-κB protein seen in the PVN and RVLM of CHF animals. We speculate that GRK5 upregulation in the CHF state may be one mechanism to prevent further increases in AT1R expression. These data suggest that ExT and central RAS modulation may be highly interactive in various disease states characterized by sympathoexcitation. Although the question of how ExT mediates beneficial effects in CHF remains unclear, the correlative decreases in AT1R and p65 NF-κB may be one potential mechanism. It is possible that targeting GRK5 as an alternate means of downregulating AT1R in the CHF state may provide a novel strategy for potential therapy applicable to a variety of sympathoexcitatory states including hypertension.
Supplementary Material
Novelty and Significance.
What Is New?
There is upregulation in angiotensin II type 1 receptor (AT1R), G protein coupled receptor kinase 5 (GRK5), and p65 nuclear factor kappa b (p65 NF-κB) in the rostral ventral lateral medulla (RVLM) and paraventricular nucleus (PVN) of heart failure animals.
The expression of all of these proteins is normalized by Exercise Training.
In vitro studies strongly suggest the concomitant increase in GRK5 is a means by which central AT1R’s are regulated.
What Is Relevant?
Exercise training decreases central AT1R, p65 NF-κB and GRK5.
The response of GRK5 and AT1R in response to Ang II in vitro uncovered a new potential target for modulation of Ang II signaling.
Summary
Exercise training decreases central AT1R and intermediate proteins involved in its regulation.
Acknowledgments
The technical assistance of Ms. Pamela Curry and Ms. Johnnie F. Hackley are greatly appreciated.
Sources of Funding
This work was supported by NIH grant PO1 HL62222 and a supplement to KKVH.
Footnotes
Conflict of Interest/Disclosures
None.
References
- 1.Packer M. Neurohormonal Interactions and Adaptations in Congestive Heart Failure. Circulation. 1988;77:721–730. doi: 10.1161/01.cir.77.4.721. [DOI] [PubMed] [Google Scholar]
- 2.Kleiber AC, Zheng H, Sharma NM, Patel KP. Chronic At1 Receptor Blockade Normalizes Nmda-Mediated Changes in Renal Sympathetic Nerve Activity and Nr1 Expression within the Pvn in Rats with Heart Failure. Am J Physiol Heart Circ Physiol. 2010;298:H1546–1555. doi: 10.1152/ajpheart.01006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang HJ, Zhang F, Zhang Y, Gao XY, Wang W, Zhu GQ. At1 Receptor in Paraventricular Nucleus Mediates the Enhanced Cardiac Sympathetic Afferent Reflex in Rats with Chronic Heart Failure. Auton Neurosci. 2005;121:56–63. doi: 10.1016/j.autneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Interaction between Cardiac Sympathetic Afferent Reflex and Chemoreflex Is Mediated by the Nts At1 Receptors in Heart Failure. Am J Physiol Heart Circ Physiol. 2008;295:H1216–H1226. doi: 10.1152/ajpheart.00557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson LW, Sawchenko PE. Hypothalamic Integration: Organization of the Paraventricular and Supraoptic Nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 6.Shafton AD, Ryan A, Badoer E. Neurons in the Hypothalamic Paraventricular Nucleus Send Collaterals to the Spinal Cord and to the Rostral Ventrolateral Medulla in the Rat. Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 7.Badoer E, Merolli J. Neurons in the Hypothalamic Paraventricular Nucleus That Project to the Rostral Ventrolateral Medulla Are Activated by Haemorrhage. Brain Res. 1998;791:317–320. doi: 10.1016/s0006-8993(98)00140-1. [DOI] [PubMed] [Google Scholar]
- 8.Patel KP, Zhang PL, Krukoff TL. Alterations in Brain Hexokinase Activity Associated with Heart Failure in Rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R923–R928. doi: 10.1152/ajpregu.1993.265.4.R923. [DOI] [PubMed] [Google Scholar]
- 9.Vahid-Ansari F, Leenen FH. Pattern of Neuronal Activation in Rats with Chf after Myocardial Infarction. Am J Physiol. 1998;275:H2140–2146. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZH, Francis J, Weiss RM, Felder RB. The Renin-Angiotensin-Aldosterone System Excites Hypothalamic Paraventricular Nucleus Neurons in Heart Failure. Am J Physiol Heart Circ Physiol. 2002;283:H423–433. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]
- 11.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, Controlled Trial of Long-Term Moderate Exercise Training in Chronic Heart Failure: Effects on Functional Capacity, Quality of Life, and Clinical Outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 12.Conn EH, Williams RS, Wallace AG. Exercise Responses before and after Physical Conditioning in Patients with Severely Depressed Left Ventricular Function. Am J Cardiol. 1982;49:296–300. doi: 10.1016/0002-9149(82)90504-5. [DOI] [PubMed] [Google Scholar]
- 13.Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of Moderate Exercise Training on Thallium Uptake and Contractile Response to Low-Dose Dobutamine of Dysfunctional Myocardium in Patients with Ischemic Cardiomyopathy. Circulation. 1998;97:553–561. doi: 10.1161/01.cir.97.6.553. [DOI] [PubMed] [Google Scholar]
- 14.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The Effects of Exercise Training on Sympathetic Neural Activation in Advanced Heart Failure: A Randomized Controlled Trial. J Am Coll Cardiol. 2003;42:854–860. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 15.Keteyian SJ. Exercise in the Management of Patients with Chronic Heart Failure. Curr Heart Fail Rep. 2010;7:35–41. doi: 10.1007/s11897-010-0002-z. [DOI] [PubMed] [Google Scholar]
- 16.Kaschina E, Unger T. Angiotensin At1/At2 Receptors: Regulation, Signalling and Function. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Neuronal Angiotensin Ii Type 1 Receptor Upregulation in Heart Failure: Activation of Activator Protein 1 and Jun N-Terminal Kinase. Circ Res. 2006;99:1004–1011. doi: 10.1161/01.RES.0000247066.19878.93. [DOI] [PubMed] [Google Scholar]
- 18.Billet S, Aguilar F, Baudry C, Clauser E. Role of Angiotensin Ii At1 Receptor Activation in Cardiovascular Diseases. Kidney Int. 2008;74:1379–1384. doi: 10.1038/ki.2008.358. [DOI] [PubMed] [Google Scholar]
- 19.Guyenet PG. The Sympathetic Control of Blood Pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 20.Mitra AK, Gao L, Zucker IH. Angiotensin Ii-Induced Upregulation of at(1) Receptor Expression: Sequential Activation of Nf-Kappab and Elk-1 in Neurons. Am J Physiol Cell Physiol. 2010;299:C561–569. doi: 10.1152/ajpcell.00127.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Palczewski K, Benovic JL. G-Protein-Coupled Receptor Kinases. Trends Biochem Sci. 1991;16:387–391. doi: 10.1016/0968-0004(91)90157-q. [DOI] [PubMed] [Google Scholar]
- 22.O’Dowd BF, Lefkowitz RJ, Caron MG. Structure of the Adrenergic and Related Receptors. Annu Rev Neurosci. 1989;12:67–83. doi: 10.1146/annurev.ne.12.030189.000435. [DOI] [PubMed] [Google Scholar]
- 23.Penela P, Murga C, Ribas C, Lafarga V, Mayor F., Jr The Complex G Protein-Coupled Receptor Kinase 2 (Grk2) Interactome Unveils New Physiopathological Targets. Br J Pharmacol. 2010;160:821–832. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker JK, Gainetdinov RR, Feldman DS, McFawn PK, Caron MG, Lefkowitz RJ, Premont RT, Fisher JT. G Protein-Coupled Receptor Kinase 5 Regulates Airway Responses Induced by Muscarinic Receptor Activation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L312–319. doi: 10.1152/ajplung.00255.2003. [DOI] [PubMed] [Google Scholar]
- 25.Jones SW, Baker DJ, Greenhaff PL. G Protein-Coupled Receptor Kinases 2 and 5 Are Differentially Expressed in Rat Skeletal Muscle and Remain Unchanged Following Beta2-Agonist Administration. Exp Physiol. 2003;88:277–284. doi: 10.1113/eph8802472. [DOI] [PubMed] [Google Scholar]
- 26.Ishizaka N, Alexander RW, Laursen JB, Kai H, Fukui T, Oppermann M, Lefkowitz RJ, Lyons PR, Griendling KK. G Protein-Coupled Receptor Kinase 5 in Cultured Vascular Smooth Muscle Cells and Rat Aorta. Regulation by Angiotensin Ii and Hypertension. J Biol Chem. 1997;272:32482–32488. doi: 10.1074/jbc.272.51.32482. [DOI] [PubMed] [Google Scholar]
- 27.Gainetdinov RR, Bohn LM, Walker JK, Laporte SA, Macrae AD, Caron MG, Lefkowitz RJ, Premont RT. Muscarinic Supersensitivity and Impaired Receptor Desensitization in G Protein-Coupled Receptor Kinase 5-Deficient Mice. Neuron. 1999;24:1029–1036. doi: 10.1016/s0896-6273(00)81048-x. [DOI] [PubMed] [Google Scholar]
- 28.Musch TI, Terrell JA. Skeletal Muscle Blood Flow Abnormalities in Rats with a Chronic Myocardial Infarction: Rest and Exercise. Am J Physiol. 1992;262:H411–H419. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- 29.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise Training Normalizes Enhanced Glutamate-Mediated Sympathetic Activation from the Pvn in Heart Failure. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1863–1872. doi: 10.1152/ajpregu.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawson-Saunders B, Trapp RG. Basic and Clinical Biostatistics. Norwalk, CT: Appleton & Lange; 1994. [Google Scholar]
- 31.Xu X, Wan W, Powers AS, Li J, Ji LL, Lao S, Wilson B, Erikson JM, Zhang JQ. Effects of Exercise Training on Cardiac Function and Myocardial Remodeling in Post Myocardial Infarction Rats. J Mol Cell Cardiol. 2008;44:114–122. doi: 10.1016/j.yjmcc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zucker IH, Schultz HD, Patel KP, Wang W, Gao L. Regulation of Central Angiotensin Type 1 Receptors and Sympathetic Outflow in Heart Failure. Am J Physiol Heart Circ Physiol. 2009;297:H1557–1566. doi: 10.1152/ajpheart.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Brasier AR. Angiotensinogen Gene Activation by Angiotensin Ii Is Mediated by the Rel a (Nuclear Factor-Kappab P65) Transcription Factor: One Mechanism for the Renin Angiotensin System Positive Feedback Loop in Hepatocytes. Mol Endocrinol. 1996;10:252–264. doi: 10.1210/mend.10.3.8833654. [DOI] [PubMed] [Google Scholar]
- 34.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain Nuclear Factor-Kappa B Activation Contributes to Neurohumoral Excitation in Angiotensin Ii-Induced Hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jewell-Motz EA, Liggett SB. G Protein-Coupled Receptor Kinase Specificity for Phosphorylation and Desensitization of Alpha2-Adrenergic Receptor Subtypes. J Biol Chem. 1996;271:18082–18087. doi: 10.1074/jbc.271.30.18082. [DOI] [PubMed] [Google Scholar]
- 36.MacDonnell SM, Kubo H, Crabbe DL, Renna BF, Reger PO, Mohara J, Smithwick LA, Koch WJ, Houser SR, Libonati JR. Improved Myocardial Beta-Adrenergic Responsiveness and Signaling with Exercise Training in Hypertension. Circulation. 2005;111:3420–3428. doi: 10.1161/CIRCULATIONAHA.104.505784. [DOI] [PubMed] [Google Scholar]
- 37.Reja V, Goodchild AK, Phillips JK, Pilowsky PM. Upregulation of Angiotensin At1 Receptor and Intracellular Kinase Gene Expression in Hypertensive Rats. Clin Exp Pharmacol Physiol. 2006;33:690–695. doi: 10.1111/j.1440-1681.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Role of Oxidant Stress on At1 Receptor Expression in Neurons of Rabbits with Heart Failure and in Cultured Neurons. Circ Res. 2008;103:186–193. doi: 10.1161/CIRCRESAHA.108.179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura M, Yasue H, Okumura K, Ogawa H, Jougasaki M, Mukoyama M, Nakao K, Imura H. Different Secretion Patterns of Atrial Natriuretic Peptide and Brain Natriuretic Peptide in Patients with Congestive Heart Failure. Circulation. 1993;87:464–469. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 40.Rengo G, Lymperopoulos A, Leosco D, Koch WJ. Grk2 as a Novel Gene Therapy Target in Heart Failure. J Mol Cell Cardiol. 2011;50:785–792. doi: 10.1016/j.yjmcc.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorn GW., 2nd Grk Mythology: G-Protein Receptor Kinases in Cardiovascular Disease. J Mol Med. 2009;87:455–463. doi: 10.1007/s00109-009-0450-7. [DOI] [PubMed] [Google Scholar]
- 42.Sorriento D, Ciccarelli M, Santulli G, Campanile A, Altobelli GG, Cimini V, Galasso G, Astone D, Piscione F, Pastore L, Trimarco B, Iaccarino G. The G-Protein-Coupled Receptor Kinase 5 Inhibits Nfkappab Transcriptional Activity by Inducing Nuclear Accumulation of Ikappab Alpha. Proc Natl Acad Sci U S A. 2008;105:17818–17823. doi: 10.1073/pnas.0804446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martini JS, Raake P, Vinge LE, DeGeorge BR, Jr, Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ. Uncovering G Protein-Coupled Receptor Kinase-5 as a Histone Deacetylase Kinase in the Nucleus of Cardiomyocytes. Proc Natl Acad Sci U S A. 2008;105:12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP. Effects of Exercise Training on Health Status in Patients with Chronic Heart Failure: Hf-Action Randomized Controlled Trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of Regular Physical Activity on the Nad(P)H Oxidase and Angiotensin Receptor System in Patients with Coronary Artery Disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 46.Martinez DG, Nicolau JC, Lage RL, Toschi-Dias E, de Matos LD, Alves MJ, Trombetta IC, Dias da Silva VJ, Middlekauff HR, Negrao CE, Rondon MU. Effects of Long-Term Exercise Training on Autonomic Control in Myocardial Infarction Patients. Hypertension. 2012;58:1049–1056. doi: 10.1161/HYPERTENSIONAHA.111.176644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.