Abstract
Background/Aim:
To evaluate the immunohistochemical expression of proliferating cell nuclear antigen (PCNA) and CD34 in colorectal adenomas and carcinomas, and to correlate this expression with different clinicopathologic parameters.
Materials and Methods:
The study was retrospectively designed. A total of 86 tissue samples, including 33 paraffin blocks from patients with colorectal adenomas, 33 paraffin blocks from patients with colorectal adenocarcinomas, and a control group of 20 samples of nontumerous colonic tissue, were included in the study. From each block, 3 sections of 5 ΅m thickness were taken, 1 section was stained with hematoxylin and eosin (H and E) and the other 2 sections were stained immunohistochemically for PCNA and CD34. Scoring of the immunohistochemical staining was performed using a specified automated cellular image analysis system (Digimizer).
Results:
PCNA expression was significantly increased in a sequence of normal mucosa–adenoma–carcinoma. It was significantly higher in adenomas ≥ 1 cm and those with severe dysplasia, and it showed a significant positive correlation with grade and lymph node involvement in colorectal carcinoma. CD34 showed significantly higher expression in carcinoma than adenoma and in adenoma than in the control group. CD34 expression showed a significant correlation with adenomas carrying severe dysplasia and large-sized adenomas (≥1cm). It was significantly correlated with tumor grade, lymphovascular invasion, and lymph node involvement in colorectal carcinoma.
Conclusion:
PCNA plays an important role in colorectal neoplastic progression and can be utilized as ancillary marker for the risk of malignant transformation in colorectal adenomas as it correlates with high grade dysplasia and size. Intratumoral quantification of the mean (A and N) of CD34 in colorectal carcinoma reflects the grade of tumors and can predict lymph node involvement and lymphovascular invasion, to make a useful additional prognostic factor.
Keywords: CD34, colorectal adenoma, colorectal carcinoma, PCNA
Colorectal cancer (CRC) is a major public health problem and there is renewed interest in understanding the basic principles of its molecular biology.[1] Currently, outcome of colon cancer has been improved through the early detection and excision of precancerous polyps. These clinical strategies have been formed by our understanding of the molecular pathology underpinning colorectal carcinogenesis.[2] Proliferating cell nuclear antigen (PCNA) is a 36-kDa DNA polymerase delta auxiliary protein that complexes with cyclin D and cyclin-dependent kinases. It is involved in the proliferation of neoplastic as well as non-neoplastic cells and it is specifically expressed in proliferating cell nuclei. This specific antibody recognizes PCNA protein, which is at the maximum level in the late G1 and S phase of proliferating cells.[3] PCNA immunohistochemistry can be used as a reliable marker of the proliferative compartment in both normal and neoplastic colonic mucosae.[4] It had been reported that PCNA-labeling index (PCNA-LI) is increased in the following order: normal mucosa of the large intestine, hyperplastic polyp, tubular adenoma with low-grade atypia, and tubular adenoma with high-grade atypia and adenocarcinoma. PCNA-LI of epithelial tumor cells is significantly increased in adenomas with high grade of dysplasia irrespective of the histological type or size of the tumor.[5] Because of its direct relationship with cell proliferation, PCNA is considered to be an important factor in the prognosis of colorectal carcinoma.[6] Previous studies showed that there was a significant relationship between PCNA-LI and grade of the tumor, vessel invasion, distant metastasis, and prognosis.[7,8]
Most studies have reported angiogenesis once the invasive carcinoma has been established. However, even in a premalignant stage, epithelial cells have increased proliferation (as a manifestation of the “field effect”) and therefore would be expected to require increased blood supply. Angiogenesis has previously been shown as early as small adenomatous polyp or even the aberrant crypt foci (ACF) stage.[9] In a study of Moreira et al., analysis of immunohistochemical expression of CD31 and CD34 in colorectal cancer, colorectal adenomas, and colorectal non-neoplastic lesions by microvessel density (MVD) assessment and by measurement of total vascular area using computer image analysis system showed progressive increase in the vessel counts from non-neoplastic tissue to carcinoma. CD34 expression in the inner portion of CRC was correlated with recurrence/metastasis and survival, and this might represent a prognostically relevant parameter in colorectal cancer.[10]
The aim of the present study is to evaluate the immunohistochemical expression of PCNA and CD34 in colorectal adenomas and adenocarcinomas and to correlate this expression with different clinicopathologic parameters.
MATERIALS AND METHODS
The study was retrospectively designed. A total of 86 tissue samples (paraffin block and tissue biopsies) were included in the study. An informed consent was taken from patients and relatives of control (autopsy) cases.
Out of the total number of tissue samples, 66 paraffin blocks from patients with colorectal tumors were collected from Gastroenterology and Hepatology Center, Gastroenterology Unit at Al-Khadhmiya Teaching Hospital and private laboratories for the period 2006–2010, including 33 blocks from patients with colorectal adenoma and 33 blocks from patients with colorectal adenocarcinoma. The control group included 20 samples of nontumorous colonic tissue taken from autopsy cases from the Institute of Forensic Medicine. These specimens were processed and paraffin embedded in the same center. The clinicopathologic parameters were obtained from patients’ admission case sheets and pathology reports. An absolute confidentiality of the patients’ vital information was maintained for ethical purposes and an ethical approval was obtained from institutions in which the study was carried out.
From each block, 3 sections of 5 μm thickness were taken and the other 2 sections were stained immunohistochemically using three steps—indirect streptavidin method for Monoclonal Mouse Anti-Rat PCNA, Clone PC10 (DAKO, Denmark) and Monoclonal Mouse Anti-Human CD34, clone QBEnd-10 (DAKO, Denmark). Brown nuclear staining of PCNA is considered as a positive reaction. Positive control is the germinal center in reactive follicular hyperplasia in lymphoid tissue. Brown cytoplasmic staining of endothelial cells of CD34 is considered as a positive reaction. Positive control is lymph node tissue. Technical negative control for both markers was obtained by omission of primary antibody.
For colorectal adenocarcinoma, H and E slides were revised for the type, grade, and stage (according to Astler–Coller staging system). Additional histopathologic features were studied including: intratumoral lymphocytic infiltration and lymphovascular invasion. For colorectal adenoma, H and E slides were revised for the type and grade of dysplasia.
Scoring of immunohistochemical staining
Scoring of immunohistochemical staining was performed using specified automated cellular image analysis system, Digimizer software, version 3.7.0.[11] Each immunohistochemically stained slide was scanned by a light microscope (Proway, China) for the positive brown immunostaining and 3 fields that reflect the best of the overall immunostaining were chosen and captured using a Sony digital camera (digital still camera DSH-H55).
Determination of staining intensity cutoff value
To obtain a cutoff value for intensity of immunostaining, photomicrographs presented at the website of NordiCQ showing different grades of brown color intensity(strong (+3) for dark brown, moderate (+2) for brown, weak (+1) for light brown to yellow) were analyzed by Digimizer software, and the digital value of intensity was classed into 3 categories: weak, moderate, and strong, taking into consideration that the digital value of intensity of staining is inversely proportional to digital number in the Digimizer color scale [Table 1].[11]
Table 1.
Determination of staining intensity cutoff value[11]

Statistical analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS program) version 16 and Microsoft Office Excel 2007. Numeric data were expressed as mean ± standard error of mean, frequency was used to express discrete data. Analysis of variance was used to analyze numeric data, whereas Chi-square test was used to analyze discrete data, and Bonferroni test was used for multiple comparisons. Scattered graph was used to show the relationship between various markers. P value of less than 0.05 was considered significant. For Digimizer software, the integrated statistics window displays statistics (n, mean of area, mean of average intensity, standard deviation, minimum, and maximum) of the measurements in the measurements list; these measurements were saved as an Excel 2007 spreadsheet file.
RESULTS
Clinicopathologic parameters
The results concerning clinicopathologic parameters assessed in patients studied are shown in Table 2.
Table 2.
Clinicopathologic parameters of patients studied
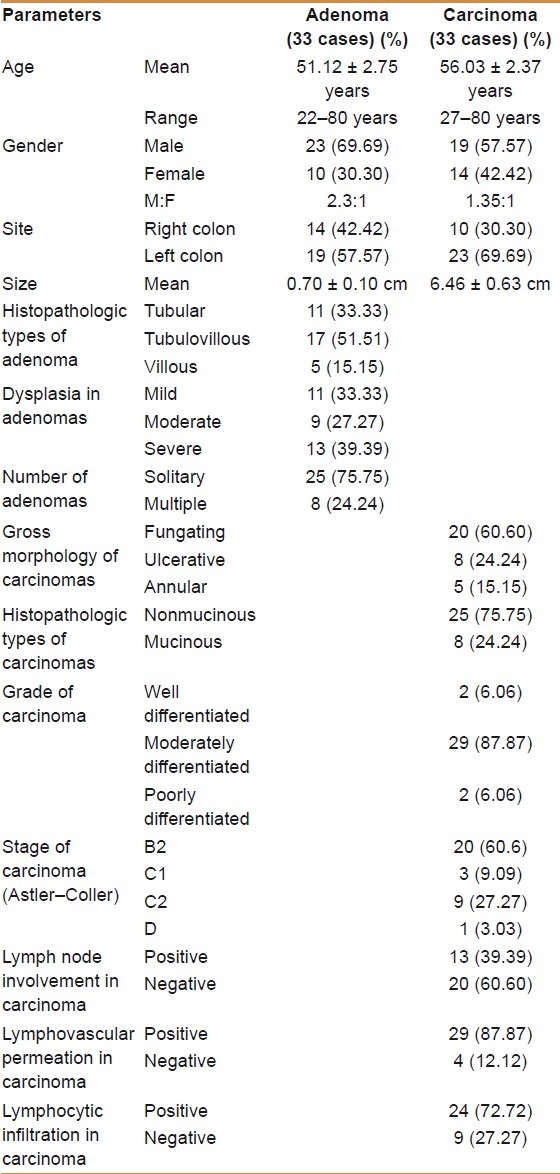
Comparison of PCNA and CD34 immunohistochemical expressions in colorectal carcinoma, adenoma, and control group
The classification of the PCNA- and CD34-positive cases of carcinoma, adenoma, and control groups into different grades of intensity (negative, weak, moderate, and strong) according to the tabulated values of NordiCQ laboratories showed that strong PCNA staining was mainly seen in carcinoma cases 18 (54.54%) in comparison with adenoma 11 (33.33%) and control groups 5 (15.15%). Strong CD34 staining was mainly seen in carcinoma cases 16 (48.48%) in comparison with adenoma 15 (45.45%) and control groups 7 (35%) [Table 3].
Table 3.
Classification of marker intensity into (negative, weak, moderate, and strong) according to the tabulated values of NORDICQ laboratories and its association with the study groups (control, adenoma, and carcinoma)
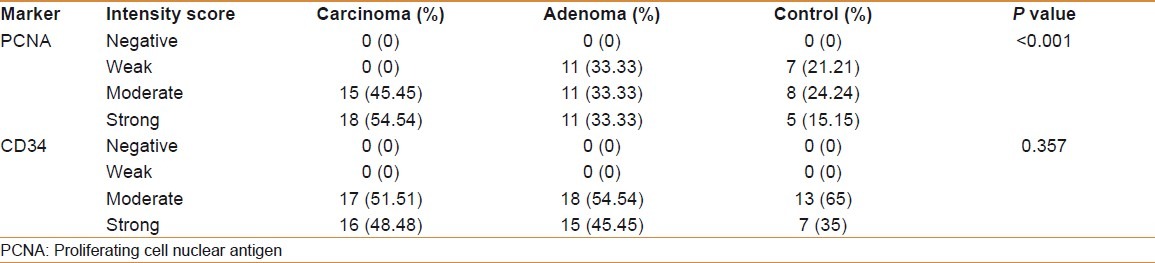
The mean of the 3 digital parameters of PCNA immunohistochemical expression [Area (A), number of objects (N), and intensity (I)] and the mean of (A and N) of CD34 were significantly increased in a sequence of normal mucosa–adenoma–carcinoma. There was no significant difference in the intensity (I) of expression of CD34 among carcinoma, adenoma, and control groups [Table 4] [Figures 1–6].
Table 4.
Comparison of digimizer parameters (A, N, I) of PCNA and CD34 expression among patients and control groups
Figure 1.
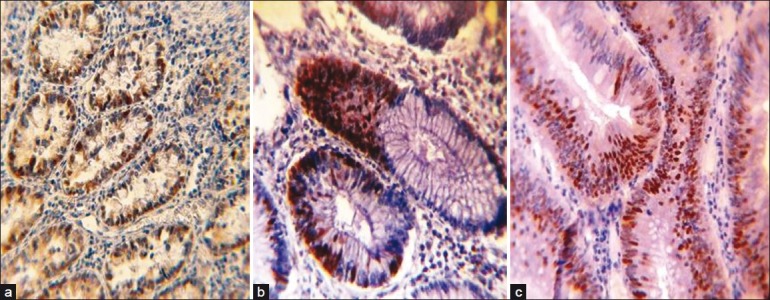
Proliferating cell nuclear antigen (PCNA) immunohistochemical expression in: (a) normal colonic tissue showing PCNA-positive brown nuclear staining (40×); (b) tubular colonic adenoma with mild dysplasia showing PCNA-positive brown nuclear staining, note the increasing no. of PCNA-positive nuclei in comparison with a (40×); (c) tubulovillous colonic adenoma with moderate dysplasia showing increasing no. of PCNA-positive brown stained nuclei in comparison with a and b (40×)
Figure 6.
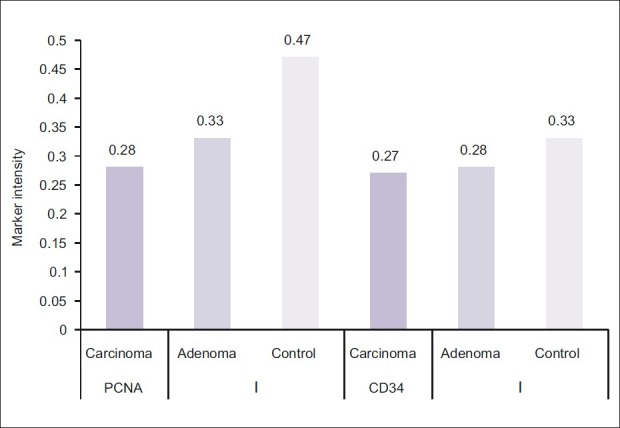
Comparison of Digimizer parameter [Intensity (I)] of proliferating cell nuclear antigen and CD34 expression among patients and control groups (digital value of intensity of staining is inversely proportional to digital number in the Digimizer color scale)
Figure 2.
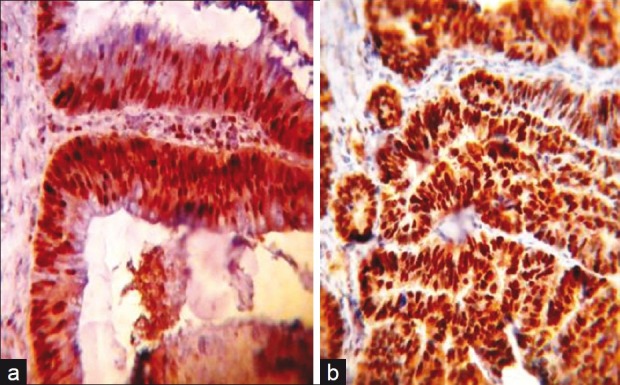
Proliferating cell nuclear antigen (PCNA) immunohistochemical expression in: (a) tubulovillous colonic adenoma with severe dysplasia showing large no. of PCNA-positive brown stained nuclei (40×); (b) moderately differentiated colonic adenocarcinoma showing larger no. of PCNA-positive brown stained nuclei in comparison with a (40×). Note the increasing no. of stained nuclei in carcinoma in comparison with adenoma with no much difference in the staining intensity
Figure 3.
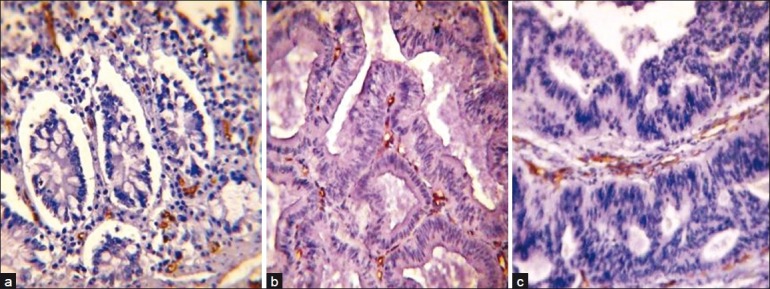
CD34-positive immunohistochemical brown endothelial staining in: (a) normal colonic tissue (40×); (b) tubulovillous colonic adenoma with severe dysplasia (40×); (c) moderately differentiated colonic adenocarcinoma (40×). Note the increase in the staining area of CD34 in a sequence of normal mucosa–adenoma–carcinoma with no difference in the staining intensity
Figure 4.
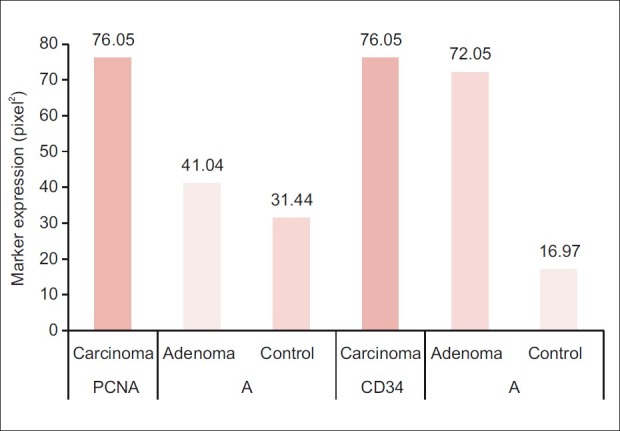
Comparison of Digimizer parameter (area (A)) of proliferating cell nuclear antigen and CD34 expression among patients and control groups
Figure 5.
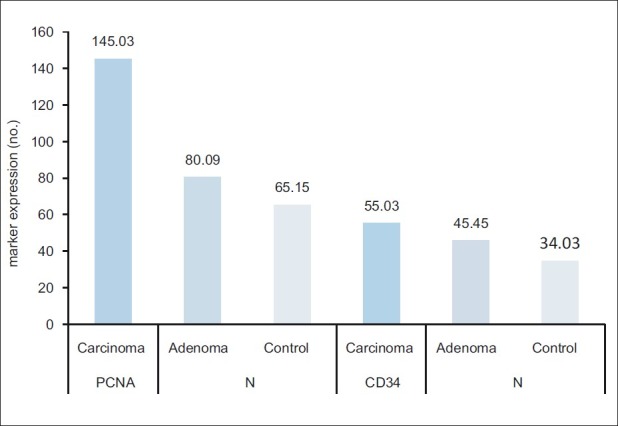
Comparison of Digimizer parameter [no. of objects (N)] of proliferating cell nuclear antigen and CD34 expression among patients and control groups
Correlation of PCNA and CD34 immunohistochemical expression with different clinicopathologic parameters in colorectal adenomas and carcinomas
There was no significant correlation between age, gender, site, number and histopathologic types of adenomas with the mean of (A, N, and I) of PCNA and (A, N, and I) of CD34 [Table 5].
Table 5.
Correlations of PCNA and CD34 immunohistochemical expression with different clinicopathologic parameters in patients with colorectal adenomas
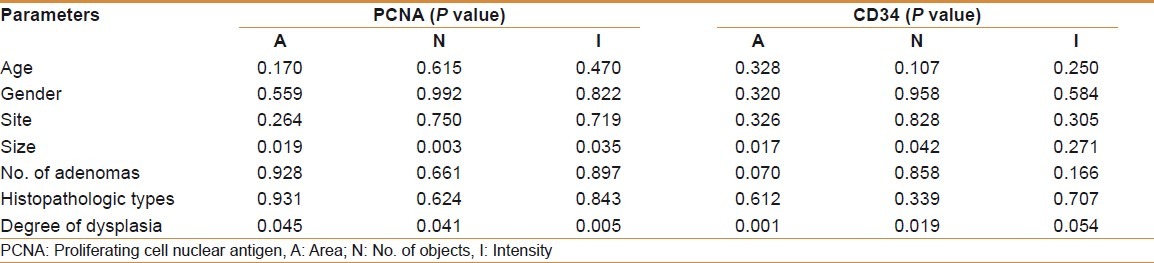
The mean (A, N, and I) of PCNA and the mean (A and N) of CD34 were significantly higher in large-sized adenoma (≥1 cm) and in adenomas with severe dysplasia than those with mild or moderate dysplasia [Table 5].
There was no significant correlation between age, gender, site, size, gross morphology, histopatholgic types, stage, and lymphocytic infiltration with the mean (A, N, and I) of PCNA and the mean (A, N, and I) of CD34 [Table 6].
Table 6.
Correlations of PCNA and CD34 immunohistochemical expression with different clinicopathologic parameters in patients with colorectal carcinomas
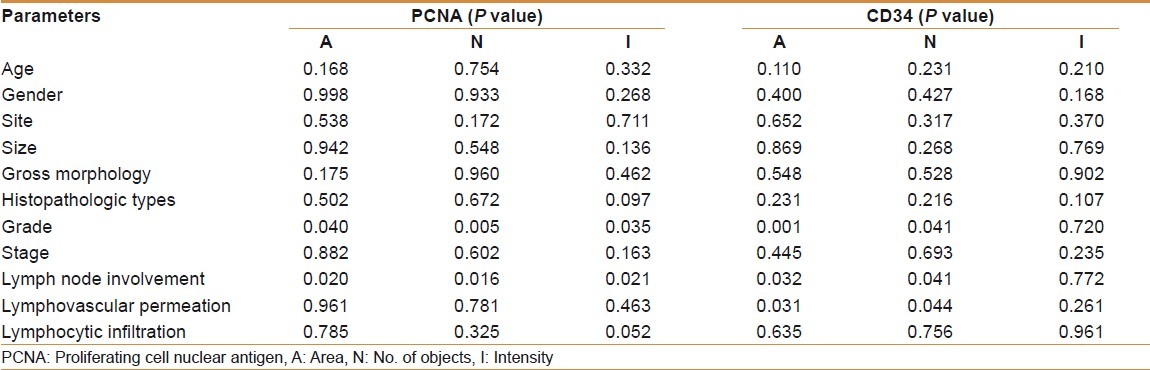
There was a significant positive correlation between grade and lymph node involvement in colorectal carcinoma with the mean (A, N, I) of PCNA and the mean (A and N) of CD34. Lymphovascular permeation in colorectal carcinoma was significantly correlated with the mean (A and N) of CD34 with no significant correlation with the mean (A, N, I) of PCNA [Table 6].
DISCUSSION
The present study showed that positive nuclear immunohistochemical staining for PCNA was seen in all cases of colorectal carcinoma, adenoma, and control group. This can be illustrated by the fact that PCNA is expressed in all proliferating cells keeping in mind that intestinal mucosal cells are in continuous proliferation and shedding. Additionally, PCNA is also involved in the DNA repair and it is known that the PCNA immunostaining may appear in cases where DNA repairs occur. Moreover, unlike Ki-67, PCNA may continue to be expressed in cells that have left the cell cycle.[3]
In terms of staining intensity, the present study showed that carcinoma cases recorded significantly higher frequency of cases with strong intensity of staining followed by adenoma then control groups (54.54%, 33.33%, 15.15% respectively, P < 0.001) and when comparing the three digital parameters of Digimizer software (area “A,” number of objects “N,” and intensity “I”), the results revealed that all these parameters were significantly increased in a sequence of normal mucosa–adenoma–carcinoma. In normal mucosa, PCNA-labeled cells were observed in the colonic crypts. The stained cells had a uniform arrangement in the lower half of the crypts (the active proliferative zone) in contrast to staining within the tumor nuclei (in adenoma and carcinoma), which was diffuse (predominantly) or granular and involving mostly the whole neoplastic glands reflecting a more intense proliferative activity being higher in carcinoma.
The current work found that PCNA overexpression was significantly correlated with size and increasing grade of dysplasia of colorectal adenoma while it had nonsignificant correlation with other clinicopathologic parameters, including age and gender of patients, site, villous histology, and number of adenomas.
Several studies are in accordance with the present study. Yan-Fang et al. showed that the positive rate of PCNA expression increases in a sequence of normal mucosa–adenoma–carcinoma.[12] Zi-Jian and Li demonstrated that positive expression of PCNA is significantly increased during transformation from colorectal adenoma to carcinoma.[13] Yu-Qin et al. had measured the PCNA-LI by visual inspection and the PCNA area rate (PCNA-AR), determined with the newly developed image processor for analytical pathology (IPAP), in tissue samples obtained by endoscopy and concluded that the PCNA-AR and PCNA-LI increased significantly in the following order: normal mucosa of the large intestine, hyperplastic polyp, tubular adenoma with low-grade dysplasia, and tubular adenoma with high-grade dysplasia and adenocarcinoma.[5] Shpitz et al. and Masahiro et al. reported that PCNA-LI was increased in proportion to the degree of dysplasia and size of the adenoma.[14,15] Bielicki et al.[16] demonstrated that the mean PCNA-LI was significantly correlated with high-grade dysplasia irrespective of histologic type or size of adenoma, in accordance with the present data except for size of adenoma. Yang et al. showed that carcinoma cells had a significantly higher PCNA index than adenomas or control specimens and PCNA was overexpressed in the villous, moderate, or severe dysplastic, and larger adenomas in keeping with this study. In addition, the transitional mucosa neighboring carcinoma showed an elevation of the mean PCNA index.[17]
From the above findings, it is obvious that PCNA expression plays an important role in colorectal carcinogenesis as it is sequentially increased during neoplastic progression from normal colonic tissue into adenoma then carcinoma and can be utilized as a marker of risk of malignant transformation in colorectal adenomas, being highly correlated with high grade of dysplasia and increasing size.
Considering colorectal carcinoma, the current study revealed that PCNA expression was significantly correlated with increasing grade and lymph node involvement, meanwhile no significant correlation was seen between age, gender, site, histopathologic types, gross morphology, stage, lymphovascular invasion, lymphocytic infiltration, and PCNA expression. The stepwise increase of PCNA expression with the dedifferentiation of the colorectal adenocarcinomas indicates a cell hyperproliferation in poorly differentiated adenocarcinomas.
Several studies are in keeping with the current one. Georgescu et al. investigated the proliferative activity of colorectal adenocarcinoma and stated that the tumor proliferative activity increased with the decrease in the degree of cell differentiation. The differences between grade 1, 2, and 3 adenocarcinomas were statistically significant.[3] Guzinska-Ustymowicz et al. (2009) revealed that there was no association between the expressions of PCNA and histologic type of tumor, distant metastasis, gender, or age of patients. However, a correlation of PCNA with lymph node metastases was observed.[18] Likewise, Guzineska-Ustymowicz et al. (2008) concluded that the expressions of PCNA were found to correlate with the presence of lymph node metastases, but not with patient age or tumor location.[19] Zi-Jian and Li, and Shao-Xin also demonstrated that overexpression of PCNA was associated with lymph node metastasis in colorectal carcinoma.[13,20] Yan-Fang et al. concluded that expression of PCNA is correlated with the degree of differentiation and lymph node metastasis in colorectal carcinoma.[12] Kang and Park found nonsignificant correlation of PCNA expression with stage of colorectal cancer.[21]
Limited number of studies including the present one performed analysis of angiogenesis across all stages of the adenoma–carcinoma sequence. Previous studies have suggested that the angiogenic switch occurs simultaneously with tumor invasion;[22] however, the present data demonstrated a significant increase of (area “A” and number of objects “N”) of CD34 immunohistochemical expression in carcinoma compared with adenoma and in adenoma compared with control group and found a significant correlation between CD34 immunohistochemical expression and grade of dysplasia and large-sized adenoma (≥1 cm). These data suggest that angiogenesis, induced by the deregulated angiogenic factors, is stimulated early in colorectal tumorigenesis and reaches a maximum level in adenocarcinomas. New blood vessel formation is maintained thereafter.
In keeping with these findings, Najim, in Iraq, investigated the correlation of MVD and staining area of CD34, using computerized image analysis system, with various clinicopathologic variables in colorectal carcinogenesis and found that there was a significant difference in staining area of CD34 between adenoma and carcinoma of the colon being higher in carcinoma, suggesting that vascular density and remodeling of pre-existing vascular network around benign colorectal lesion may serve as a possible tool to assist diagnosis, to predict the risk of malignant transformation of premalignant lesions and to assign prognosis of early malignant process in the colon.[23] Staton et al. also reported a significant increase in MVD across the adenoma–carcinoma sequence with the greatest increase in MVD being related to the onset of dysplasia.[24] Other studies also reported that MVD of colorectal cancers was significantly higher than colorectal adenomas and MVD in colorectal adenomas was significantly higher than in normal colorectal mucosa.[10,25–27] Tarta et al. evaluated 3-dimensional parameters and bidimensional microvascular quantification in colorectal adenomas and recorded that microvascular quantification, volume, and microvascular length estimate increased gradually with high-grade dysplasia as compared with the low grade ones.[28] Aotake et al. also demonstrated a gradual increment of MVD during the progression from low-grade dysplasia to high-grade dysplasia and cancer.[29] Moreira et al. determined MVD and total vascular area by computer image analysis and reported that there was no significant difference of MVD or total vascular area counts among histologic types of adenoma (tubular, tubulovillous, and villous) in agreement with the present work.[30]
It had been reported previously that angiogenesis plays an important role in metastasis and that MVD correlates significantly with LN metastasis of submucosal CRC.[31] The present work showed that the mean (A and N) of CD34 was significantly correlated with grade, lymphovascular permeation, and lymph node involvement in colorectal adenocarcinoma with nonsignificant correlation with age, gender, site, size, gross morphology, histopathologic type, stage, and lymphocytic infiltration. These findings suggest that intratumoral quantification of the mean (A and N) of CD34 in colorectal carcinoma reflects the grade of tumors and can predict lymph node involvement and lymphovascular invasion so that it may be a useful additional prognostic factor.
In agreement to such findings, Najim, in Iraq, found that MVD and the staining area of CD34 using computerized image analysis system was significantly correlated with grade of colorectal adenocarcinoma and lymph node metastasis with nonsignificant correlation with age, sex, size, site, histopathologic type, and tumor stage.[23] Sharifi et al. in Iran concluded also that tumor-induced angiogenesis of colorectal carcinoma significantly correlated with histologic tumor grade and there was no significant correlation between intratumoral MVD and sex and age of patients, localization, and stage and histologic tumor type.[32] Similarly, Elagoz et al. found that intratumoral MVD was positively correlated with grade, stage, lymph node metastasis, and lymphovascular invasion,[33] which is in accordance with the present work except for the stage. Liang et al. also demonstrated that greater MVD, using CD34 immunohistochemical staining, was significantly associated with vascular invasion of colorectal cancer cells.[34] Kaneko et al. also stated that lymph node metastasis in colorectal cancer was significantly high in cases with high MVD using CD34.[31] Staton et al. reported that there was a significant correlation of MVD with Dukes′ stage and lymph node involvement.[24] The positive correlation with lymph node involvement goes with the present study. When stage is taken into consideration, the results of the two studies are discordant.
In other studies, MVD was evaluated using other markers of angiogenesis. Saad et al. and Gurzu et al. observed that both CD31 and CD105 microvessel counts were correlated with lymph node metastases independent of tumor stage.[35,36] Ahmed and Mohammed in Iraq assessed the intratumoral MVD in colorectal carcinoma using PECAM-1(CD31) and factor VIII [von Willebrand factor (vWF)], immunostaining and recorded significant correlation of MVD with lymph node metastasis and tumor size (≥3 mm) and a nonsignificant correlation with grade and stage.[37]
The discrepancies in the clinicopathologic significance of MVD can be caused by methodologic differences between studies using different antibodies (factor VIII, CD31 or CD34). In addition, some authors have chosen patients with tumors in all Dukes’ stages; others have only studied certain stages. In addition to limited number of cases and the subjectivity in the selection of areas with high vessel count (hot spot) and multiple independent observers to score the results accurately.
It is important to clarify the advantages of computerized method, which is applied in the present study. First, the assessment of angiogenesis by means of a computerized method is objective and reproducible. Second, the greater rapidity of assessing tumoral angiogenesis provided by the computerized method for analyzing histologic images may make it possible to apply this in clinical practice, considering that the slowness of the conventional method has been deemed an obstacle. Third, endothelial area by the computerized method was particularly useful in the evaluation of tumors with high vessel density, in which the presence of microvessels very close to each other makes manual counting difficult and laborious. Finally, since measurement of the endothelial area represents the total quantity of vascular endothelium on the histologic thin section, there is no need to separately identify each vessel. The measurement of the endothelial area was made only when endothelial wall was identified by brown stain.[23,30,38]
In conclusion, PCNA plays an important role in colorectal neoplastic progression and can be utilized as ancillary marker for the risk of malignant transformation in colorectal adenomas being highly correlated with high-grade dysplasia and increasing size. CD34 immunohistochemical expression is correlated with grade of dysplasia and large-sized adenoma (≥1 cm). Intratumoral quantification of the mean (A and N) of CD34 in colorectal carcinoma reflects the grade of tumors and can predict lymph node involvement and lymphovascular permeation so that it may be a useful additional prognostic factor.
Footnotes
Source of Support: College of Medicine /Al-Nahrain University Especially the Department of Pathology and Forensic Medicine, and Teaching Laboratories at Al-Khadhmiya Teaching Hospital
Conflict of Interest: None declared.
REFERENCES
- 1.Morán A, Ortega P, de Juan C, Fernández-Marcelo T, Frías C, Sánchez-Pernaute A, et al. Differential colorectal carcinogenesis: Molecular basis and clinical relevance. World J Gastrointest Oncol. 2010;2:151–8. doi: 10.4251/wjgo.v2.i3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worthley DL, Leggett BA. Colorectal Cancer: Molecular features and clinical opportunities. Clin Biochem Rev. 2010;31:31–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Georgescu CV, Sâftoiu A, Georgescu CC, Ciurea R, Ciurea T. Correlations of proliferation markers, p53 expression and histological findings in colorectal carcinoma. J Gastrointestin Liver Dis. 2007;16:133–9. [PubMed] [Google Scholar]
- 4.Jin W, Gao MQ, Lin ZW, Yang DX. Quantitative study of multiple biomarkers of colorectal tumor with diagnostic discrimination model. World J Gastroenterol. 2004;10:439–42. doi: 10.3748/wjg.v10.i3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo YQ, Ma LS, Zhao YL, Wu KC, Pan BR, Zhang XY. Expression of proliferating cell nuclear antigen in polyps from large intestine. World J Gastroenterol. 1999;5:160–4. doi: 10.3748/wjg.v5.i2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JC, Wang ZR, Cheng YJ, Yang DZ, Shi JS, Liang AL, et al. Expression of proliferating cell nuclear antigen and CD44 variant exon 6 in primary tumors and corresponding lymph node metastases of colorectal carcinoma with Dukes’ stage C or D. World J Gastroenterol. 2003;9:1482–6. doi: 10.3748/wjg.v9.i7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Poznak C, Tan L, Panageas KS, Arroyo CD, Hudis C, Norton L, et al. Assessment of molecular markers of clinical sensitivity to single-agent taxane therapy for metastatic breast cancer. J Clin Oncol. 2002;20:2319–26. doi: 10.1200/JCO.2002.08.125. [DOI] [PubMed] [Google Scholar]
- 8.Yue H, Na YL, Feng XL, Ma SR, Song FL, Yang B. Expression of p57(kip2), Rb protein and PCNA and their relationships with clinicopathology in human pancreatic cancer. World J Gastroenterol. 2003;9:377–80. doi: 10.3748/wjg.v9.i2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wali RK, Roy HK, Kim YL, Liu Y, Koetsier JL, Kunte DP, et al. Increased microvascular blood content is an early event in colon carcinogenesis. Gut. 2005;54:654–60. doi: 10.1136/gut.2004.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreira LR, Schenka AA, Latuf-Filho P, Penná AL, Lima CS, Soares FA, et al. Immunohistochemical analysis of vascular density and area in colorectal carcinoma using different markers and comparison with clinicopathologic prognostic factors. Tumour Biol. 2011;32:527–34. doi: 10.1007/s13277-010-0147-0. [DOI] [PubMed] [Google Scholar]
- 11.Qasim BJ, Ali HH, Hussein AG. Immunohistochemical expression of estrogen and progesterone receptors in human colorectal adenoma and carcinoma using specified automated cellular image analysis system: A clinicopathological study. Oman Med J. 2011;26:307–14. doi: 10.5001/omj.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan-Fang A, Yong M, Jing-Hua L. The expressions of PCNA and Bcl-2 in colorectal adenoma and carcinoma and their clinicopathological and prognostic significance. Acta Acad Med Xuzhou. 2006;6:11–17. [Google Scholar]
- 13.Zi-Jian T, Li D. The Expression of p53and PCNA and their significance in colorectal neoplasm. J Basic Clin Oncol. 2001;6:40–8. [Google Scholar]
- 14.Shpitz B, Bomstein Y, Mekori Y, Cohen R, Kaufman Z, Grankin M, et al. Proliferating cell nuclear antigen as a marker of cell kinetics in aberrant crypt foci, hyperplastic polyps, adenomas, and adenocarcinomas of the human colon. Am J Surg. 1997;174:425–30. doi: 10.1016/s0002-9610(97)00122-0. [DOI] [PubMed] [Google Scholar]
- 15.Masahiro K, Yasuyuki S, Katsuyuki K, Shigetoyo S, Sho W, Kei W. Significance of cell proliferation and expression of mutant p53 protein for carcinogenesis of colorectal adenoma by immunohistochemical examination. J Jpn Soc Colo-Proctol. 1999;52:193–9. [Google Scholar]
- 16.Bielicki D, Markiewski M, Wielondek M, Chosia M, Domagala W. PCNA defined proliferative activity of epithelial tumor cells in adenomas of the colon. Pol J Pathol. 1995;46:151–4. [PubMed] [Google Scholar]
- 17.Yang HB, Hsu PI, Chan SH, Lee JC, Shin JS, Chow NH. Growth kinetics of colorectal adenoma-carcinoma sequence: An immunohistochemical study of proliferating cell nuclear antigen expression. Hum Pathol. 1996;27:1071–6. doi: 10.1016/s0046-8177(96)90286-5. [DOI] [PubMed] [Google Scholar]
- 18.Guzinska-Ustymowicz K, Pryczynicz A, Kemona A, Czyzewska J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Res. 2009;29:3049–52. [PubMed] [Google Scholar]
- 19.Guzineska-Ustymowicz K, Stepiene E, Kemona A. MCM-2, Ki-67 and PCNA protein expressions in pT3G2 colorectal cancer indicated lymph node involvement. Anticancer Res. 2008;28:451–7. [PubMed] [Google Scholar]
- 20.Shao-Xin W. Expression of E-cad and PCNA in colorectal cancer and its significance. Mod Med Health. 2009;18:18–21. [Google Scholar]
- 21.Kang G, Park CJ. Clinical significance of PCNA and nm23 expression in invasive colorectal carcinoma. J Korean Soc Coloproctol. 2001;17:47–52. [Google Scholar]
- 22.Takahashi Y, Ellis LM, Mai M. The angiogenic switch of human colon cancer occurs simultaneous to initiation of invasion. Oncol Rep. 2003;10:9–13. [PubMed] [Google Scholar]
- 23.Salmo N, Hussain AG, Najim MM. A study of angiogenesis in human colorectal tumors by using anti-CD34 antibody (assessment by light microscope and computer-aided image analysis system) Fac Med Baghdad. 2007;49:425–33. [Google Scholar]
- 24.Staton CA, Chetwood AS, Cameron IC, Cross SS, Brown NJ, Reed MW. The angiogenic switch occurs at the adenoma stage of the adenoma carcinoma sequence in colorectal cancer. Gut. 2007;56:1426–32. doi: 10.1136/gut.2007.125286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang TJ, Kim JR, Bae HI. Microvessel quantification, expression of p53 protein and MIB-1 in colorectal adenoma and carcinoma. Korean J Pathol. 1997;31:40–50. [Google Scholar]
- 26.Weichang C, Qiang L, Rui L. Clinical study of microvessel density and vascular endothelial growth factor in colorectal cancers. Jiangsu Med J. 2002;7:4–8. [Google Scholar]
- 27.Yu Z, Lei G, Wen-Qing Z, Xiao-Mei S, Feng T. Expression of Ki67,P53 microvessel density in human colorectal tumor. Fudan Univ J Med Sci. 2003;6:21–8. [Google Scholar]
- 28.Tarta C, da Silva VD, Teixeira CR, Prolla JC, Meurer L, Neto CC, et al. Digital image analysis and stereology of angiogenesis in polypoid and nonpolypoid colorectal adenomas. Anal Quant Cytol Histol. 2004;26:201–6. [PubMed] [Google Scholar]
- 29.Aotake T, Lu CD, Chiba Y, Muraoka R, Tanigawa N. Changes of angiogenesis and tumor cell apoptosis during colorectal carcinogenesis. Clin Cancer Res. 1999;5:135–42. [PubMed] [Google Scholar]
- 30.Moreira LR, Schenka AA, Filho PL, Lima CS, Trevisan MA, Vassallo J. Comparison of blood neoangiogenesis and lymphatic vascularization in colorectal adenomas from patients with and without concomitant colorectal cancer. Braz J Med Biol Res. 2009;42:593–8. doi: 10.1590/s0100-879x2009005000004. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko I, Tanaka S, Oka S, Yoshida S, Hiyama T, Arihiro K, et al. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol. 2007;13:3829–35. doi: 10.3748/wjg.v13.i28.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharifi N, Ghaffarzadegan K, Ayatollahi H, Shakeri MT, Sadeghian MH, Azari JB. Evaluation of angiogenesis in colorectal carcinoma by CD34 immunohistochemistry method and its correlation with clinicopathologic parameters. Acta Med Iran. 2009;47:161–4. [Google Scholar]
- 33.Elagoz S, Egilmez R, Koyuncu A, Muslehiddinoglu A, Arici S. The intratumoral microvessel density and expression of bFGF and nm23-Hl in colorectal cancer. Pathol Oncol Res. 2006;12:21–7. doi: 10.1007/BF02893427. [DOI] [PubMed] [Google Scholar]
- 34.Liang JT, Huang KC, Jeng YM, Lee PH, Lai HS, Hsu HC. Microvessel density, cyclo-oxygenase 2 expression, K-ras mutation and p53 overexpression in colonic cancer. Br J Surg. 2004;9:355–61. doi: 10.1002/bjs.4447. [DOI] [PubMed] [Google Scholar]
- 35.Saad RS, Liu YL, Nathan G, Celebrezze J, Medich D, Silverman JF. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in colorectal cancer. Mod Pathol. 2004;17:197–203. doi: 10.1038/modpathol.3800034. [DOI] [PubMed] [Google Scholar]
- 36.Gurzu S, Jung J, Azamfirei L, Mezei T, Cîmpean AM, Szentirmay Z. The angiogenesis in colorectal carcinomas with and without lymph node metastases. Rom J Morphol Embryol. 2008;49:149–52. [PubMed] [Google Scholar]
- 37.Ahmed MM, Mohammed SH. Significance of intratumoral microvessel density quantification based on immunohistochemical detection of PECAM-1 and vWF in colorectal carcinoma from Iraqi patient. Indian J Pathol Microbiol. 2010;53:439–46. doi: 10.4103/0377-4929.68268. [DOI] [PubMed] [Google Scholar]
- 38.Leme MB, Waitzberg AF, Artigiani Neto R, Linhares MM, Matos D. Assessment of angiogenesis expression and its relationship with prognosis of colorectal cancer by conventional and computer-assisted histopathological image analysis. Acta Cir Bras. 2006;21:392–7. doi: 10.1590/s0102-86502006000600007. [DOI] [PubMed] [Google Scholar]


