Abstract
Neuronal exocytosis is catalyzed by the SNARE protein syntaxin-1A1. Syntaxin-1A is clustered in the plasma membrane at sites where synaptic vesicles undergo exocytosis2,3. However, how syntaxin-1A is sequestered is unknown. Here, we show that syntaxin clustering is mediated by electrostatic interactions with the strongly anionic lipid phosphatidylinositol-4,5-bisphosphate (PIP2). We found with super-resolution STED microscopy on the plasma membrane of PC12 cells that PIP2 is the dominant inner-leaflet lipid in ~73 nm-sized microdomains. This high accumulation of PIP2 was required for syntaxin-1A sequestering, as destruction of PIP2 by the phosphatase synaptojanin-1 reduced syntaxin-1A clustering. Furthermore, co-reconstitution of PIP2 and the C-terminal part of syntaxin-1A in artificial giant unilamellar vesicles resulted in segregation of PIP2 and syntaxin-1A into distinct domains even when cholesterol was absent. Our results demonstrate that electrostatic protein-lipid interactions can result in the formation of microdomains independent of cholesterol or lipid phases.
Phosphoinositides are lipids that contain an inositol headgroup conjugated to 1–3 phosphate groups. With ~1% of total lipids in the inner leaflet of the plasma membrane4, PIP2 is the most abundant phosphoinositide. Earlier studies identified PIP2 as a second messenger in the phospholipase-C pathway. However, the list of cellular functions of PIP2 is rapidly growing, and PIP2 is also involved in membrane targeting, cytoskeletal attachment, endocytosis and exocytosis4. PIP2 interacts with many different proteins, either via unstructured basic residue-rich regions or via more structured domains4,5.
Neuronal exocytosis requires plasma membrane PIP22,6-8. PIP2 levels at the plasma membrane determine the rates of vesicle priming, the size of the readily releasable pool, and the rates of sustained exocytosis in stimulated cells2,6,8. This regulation is probably mediated by interactions of PIP2 with proteins involved in docking and fusion such as rabphilin, CAPS, synaptotagmin, SCAMP2 and Mints6,9. In docking, PIP2 clusters may act as molecular ‘beacons’ that target synaptic vesicles to the fusion sites. Indeed, PIP2 is locally enriched at the sites of docked vesicles and colocalizes with at least 5–10% of the microdomains of syntaxin-1A (Supp. Fig. 1)2,3,9, the membrane-anchored t-SNARE of neuronal exocytosis1.
The amount of PIP2 at the sites of membrane fusion in PC12 cells has been estimated at 3–6% PIP2 of surface area (Supp. Fig. 2)9. In these experiments, membrane sheets were specifically stained for PIP2 with the PH domain of protein lipase C delta fused to GFP9 or citrine (a YFP analog10; PHPLCδ-citrine, Supp. Fig. 1–2), and the fluorescence from the punctuated PIP2-microdomains was quantified. However, as explained in reference9, this approach underestimates the fraction of PIP2 if the size of the PHPLCδ-microdomains is smaller than the ~200 nm diffraction limited resolution of conventional fluorescence microscopy. To obtain a more accurate estimate, we re-analyzed PC12 membrane sheets labeled with PHPLCδ-citrine or an antibody raised against PIP2 using super resolution STED (stimulation emission depletion) microscopy11 (Fig. 1a–c). These experiments revealed that the PIP2 stained clusters are much smaller than anticipated, with an average diameter of only 73 ± 42 nm (s.d.). Although this is still a higher estimate since it represents the microdomain size convoluted with the resolution of the STED microscope (~60 nm), it is in good agreement with the size of the syntaxin-1A microdomains12. Using this value, we re-calculated the surface density of PIP2 (Supp. Methods). For this calculation, we first estimated the total amount of PIP2 in a microdomain when sampled with the diffraction limited resolution of our epi-fluorescence microscope (Supp. Fig. 2; Fig. 1d, black curve). We then calculated the peak concentration when this PIP2 was concentrated into 73 nm microdomains (Fig. 1d, red curve). Here, we assumed a Gaussian distribution of PIP2 in the microdomains. A peak surface density of 82% PIP2 was obtained (Fig. 1d). It needs to be kept in mind that (i) at these high PIP2 concentrations, molecular crowding might hinder binding of PHPLCδ-citrine, (ii) relatively small errors in microdomain size and resolution of the microscopes result in substantial errors, and (iii) PHPLCδ-citrine and antibody binding may alter PIP2 localization and is only indicative of PIP2 microdomains. Nevertheless, our values are much higher than any previous estimate and it seems safe to conclude that PIP2 is the dominant inner-leaflet lipid in the microdomains. The question then arises by which molecular mechanism such high concentrations of PIP2 are achieved.
Figure 1.
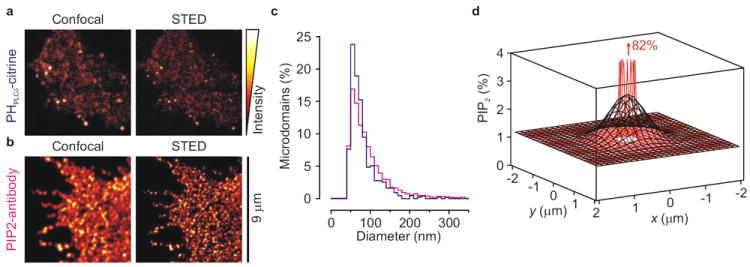
PIP2 is the predominant inner-leaflet lipid in roughly 73 nm-sized microdomains. (a) Confocal and corresponding nanoscale-resolution STED image of a PHPLCδ-citrine stained membrane sheet of PC12 cells. Note the increase in resolution. (b) Same as a, but now immunostained with a monoclonal PIP2-antibody and a secondary antibody labeled with Alexa Fluor 488. (c) Size distribution of microdomains with PHPLCδ-citrine (blue; n = 433, 24 sheets, 2 independent preparations) and PIP2-antibody (pink; n = 2,959, 22 sheets, 2 independent preparations). The average diameter (full width at half maximum) was 73 ± 42 nm (s.d.) of PHPLCδ-citrine and 87 ± 62 nm (s.d.) for PIP2-antibody. (d) Spatial distribution of PIP2. Black: the PIP2 distribution when sampled at too low (377 nm) diffraction limited resolution (from Supp. Fig. 2). Red: approximation of the PIP2 distribution in the ~73 nm microdomains. PIP2 was accumulated at ~82% of total surface area. See Supp. Methods for details.
PIP2 has a net negative charge of -3–54 and interacts with polybasic stretches of amino acids4,5,13,14. Proteins with such stretches can sequester PIP2 even in excess of monovalent anionic lipids, such as MARCKS, spermine and even pentalysine (Lys5)5,14. Similar to these proteins, syntaxin-1A also possesses a stretch of basic amino acids. These residues are adjacent to the transmembrane domain and are in contact with the head-groups of the phospholipids (Supp. Fig. 3a)15,16. Indeed, it is well established that this conserved stretch with 5 positive residues (260-KARRKK) interacts with PIP29,15-17. Removal of charge diminishes this interaction (Supp. Fig. 3b–c), but syntaxin-1A remains capable of fusing membranes even upon removal of all 5 charges9,16. Because PIP2 colocalizes with at least a fraction of syntaxin-1A microdomains (Supp. Fig. 1)2, we speculated that their interaction might drive domain formation similar to various soluble lipid binding proteins5,14. Two independent approaches were used to test this hypothesis: (i) reconstitution in giant unilamellar vesicles (GUVs)18, and (ii) hydrolysis of PIP2 in PC12 cells using a membrane-targeted variant of the PIP2 phosphatase synaptojanin-17.
Murray and Tamm17 showed syntaxin-1A clustered in a non-raft way in neutral cholesterol:phosphatidylcholine (PC) membranes. Here, cholesterol clusters syntaxin-1A by competing for solvation by PC. Indeed, a synthetic C-terminal peptide of syntaxin-1A (residues 257–288; 3 mol%; Fig. 2, Supp. Fig. 4a) clustered in domains in > 50% of GUVs composed of DOPC (1,2-dioleoyl-sn-glycero-3-PC) with 20 mol% cholesterol. This peptide contained both the polybasic juxtamembrane linker and transmembrane region and was N-terminally labeled with either rhodamine red or Atto647N. Analysis of fluorescence showed a 1.6 ± 0.2 (s.d.; n = 18) fold enrichment of syntaxin-1A257–288 in these clusters, but this lower estimate is limited by the optics. Negatively charged PIP2 or DOPS (1,2-dioleoyl-sn-glycero-3-phosphatidylserine) dispersed these clusters (Fig. 2)17. Thus, while cholesterol competition might explain syntaxin-1A clusters that are not enriched in PIP2 (Supp. Fig. 1), they cannot explain the high accumulation of PIP2 at the sites of docked vesicles. However, 1.5 mol% (total lipids) PIP2 also clustered syntaxin-1A in 1–10 μm domains in 1–5% of the GUVs (Fig. 2, Supp. Fig. 4b–c). These domains did not depend on cholesterol or DOPS. In these domains, PIP2 was 1.9 ± 0.2 (s.d.; n = 13; Supp. Fig. 5) and syntaxin-1A257–288 5.5 ± 1.4 (s.d.; n = 27) fold enriched based on fluorescence. Importantly, no domains were observed without peptide or when the PIP2 concentration exceeded 5 mol%. Divalent cations can act as bridges between two adjacent lipids and induce aggregation of PIP2 into clusters19-21, but even 1 mM Ca2+ was not sufficient to compete syntaxin-1A away from the microdomains. Domains were present with both synthetic dioleoyl-PIP2 and with PIP2 extracted from pig brain (Supp. Fig. 4b). Thus, syntaxin-1A can be clustered in the membrane both by cholesterol and PIP2.
Figure 2.
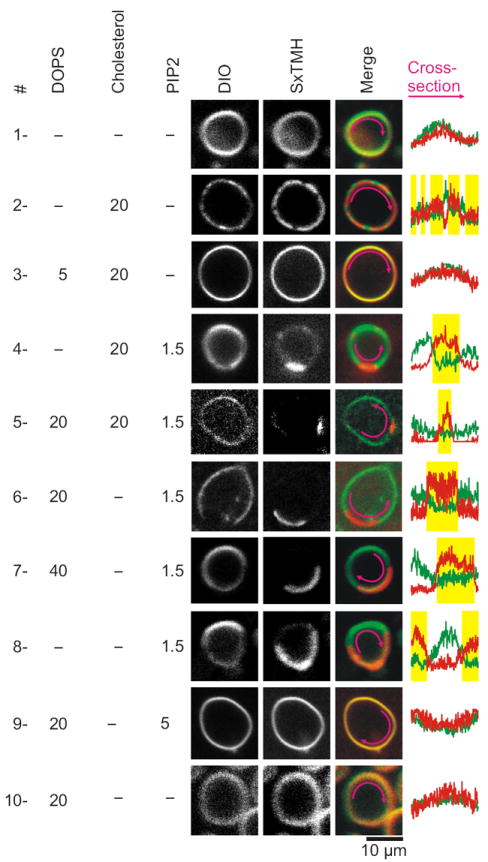
Confocal microscopy of syntaxin-1A domains in artificial membranes. Syntaxin-1A257–288 labeled with Atto647N (SxTMH; red) was reconstituted in GUVs. The membranes were composed of DOPC with 1.5 mol% of the fluorescent lipid analog DiO (3,3’-dioctadecyloxacarbocyanine; green) and the percentages DOPS, cholesterol and PIP2 indicated in the figure. In absence of anionic phospholipids, 20% cholesterol clustered syntaxin-1A in many small clusters (condition #2), as predicted by Murray and Tamm17. Inclusion of > 5% anionic DOPS dispersed these clusters (#3). 1.5% PIP2 partitioned SxTMH in 1–10 μm-sized domains regardless of cholesterol or DOPS (#4–8). These clusters were no longer observed with 5% PIP2 (#9). The pink arrows show the part of the membrane used for cross-sections. Yellow bars indicate the position of the domains. More data is presented in Supp. Fig. 4–9.
These cholesterol- and PIP2-mediated clusters both differ from ‘rafts’. They also differ from each other. First, PIP2-domains are always round and only 1–2 per vesicle, whereas cholesterol generally (but not always) induces many small domains (Supp. Fig. 4). Second, fluorescence recovery after photobleaching showed that syntaxin-1A remained mobile in the PIP2-domains while syntaxin-1A was essentially immobile in the cholesterol-dependent clusters (Supp. Fig. 6). Syntaxin-1A thus diffuses in the PIP2-domains and forms large circular domains for minimizing boundary energy21. Third, 6-dodecanoyl-2-dimethylaminonaphthalene (Laurdan)22 showed a high hydration of the PIP2-domains, whereas the cholesterol domains were much denser packed (Supp. Fig. 7). Fourth, phase contrast microscopy showed a thickening of the cholesterol-dependent clusters, but not of the PIP2-domains (Supp. Fig. 8). Thus, even though no saturated lipids are present, the cholesterol-dependent domains show behavior that essentially resembles the Lo phase. In contrast, the PIP2-domains seem much more disordered and resemble the Ld phase. Ca2+ demixing of polyanionic amphiphiles showed that electrostatic interactions can indeed lead to liquid-like domains21.
The transmembrane helix of syntaxin-1A has been reported to homodimerize. However, introducing the M267A C271A I279A mutations that prevent homodimerization of the syntaxin-1A peptides23 did not prevent cholesterol or PIP2 mediated clustering (Supp. Fig. 9). In contrast, no PIP2-domains were observed when two charges (K264A K265A) from the polybasic linker were removed, but cholesterol-dependent clusters were still observed (Supp. Fig. 5). Overexpression of the C-terminal part of syntaxin-1A fused to GFP24 in PC12 cells also showed 4–8-fold loss of clustering of the K264A K265A mutant (Supp. Fig. 10–11). These data show that electrostatic interactions between PIP2 and the juxtamembrane helix of syntaxin-1A are sufficient for domain formation.
We then set out to investigate to what extent PIP2 is required for syntaxin-1A clustering in PC12 cells. For this purpose, we expressed a RFP-tagged construct containing the phosphatase domain of synaptojanin-1 fused to a CAAX-box, resulting in its targeting to the plasma membrane7. Synaptojanin-1 is a polyphosphoinositide 5-phosphatase, and the expression of the construct completely removed PIP2 from the plasma membrane (Supp. Fig. 12)6,7. Importantly, synaptojanin-1 expression 3.7-fold reduced the punctuate distribution of endogenous syntaxin-1A (Fig. 3; Supp. Fig. 13). Thus, this provides evidence that PIP2 is indeed required for at least part of syntaxin-1A microdomain formation.
Figure 3.
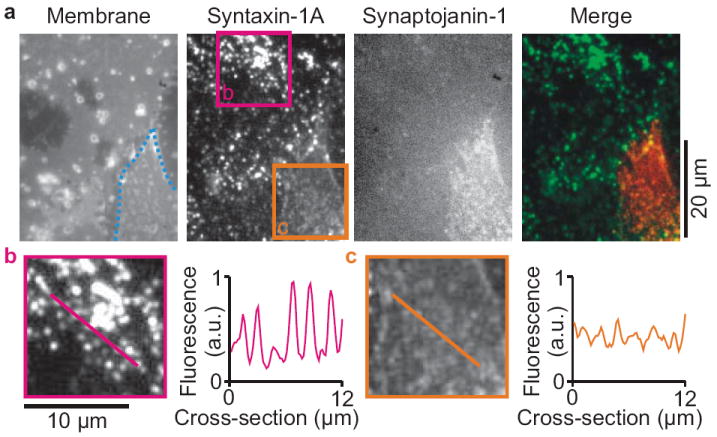
Removal of PIP2 reduces syntaxin-1A clustering in PC12 cells (a) Membrane sheets of PC12 cells stained with TMA-DPH (1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene)12,24. Immunostaining with a monoclonal antibody raised against syntaxin-1A and a secondary antibody labeled with DyLight649 showed that endogenous syntaxin-1A clustered in microdomains (region b; pink)2,3,9,12,24. Overexpressing the RFP-tagged and membrane-targeted catalytic region of synaptojanin-1 (residues 498–901; cell outlined in blue)7 reduced this syntaxin-1A clustering 3.7-fold (region c; orange; see Supp. Fig. 13). Synaptojanin-1 is the 5-phosphatase of PIP2 and overexpression of the construct completely removes PIP2 from the membrane (Supp. Fig. 12). (b–c) Magnification of the regions of interest from a and cross-sections to indicate the clustering.
We performed molecular dynamics simulations to gain insight in the precise conformation of the PIP2-syntaxin-1A microdomains. In these coarse-grained simulations, several atoms are represented by one simulation bead25,26 (Supp. Fig. 14). This allowed for simulations of relatively large lipid bilayers of ~2,500 copies of a 4:1 molar ratio of DOPC:DOPS and 40–64 copies of syntaxin-1A257–288 and PIP2. Within 10 μs simulation time, up to 10 copies of syntaxin-1A257–288 clustered with PIP2 into microdomains (Supp. Fig. 15). Equal amounts of PIP2 and syntaxin-1A were present in the bulk-phase of those domains, while more PIP2 and DOPS associated transiently to the periphery. We used this information to construct a domain with 64 copies of syntaxin-1A (Fig. 4, Supp. Movie 1), which is comparable to the syntaxin-1A content in the microdomains in PC12 cells12. These domains were stable over 6 μs simulation time and contained <10% residual DOPC or DOPS. Together, we conclude that syntaxin-1A and PIP2 can form dynamic, amorphous networks with PIP2 acting as a ‘charge-bridge’ and spanning the distance between the various syntaxin-1A molecules (Fig. 4c).
Figure 4.
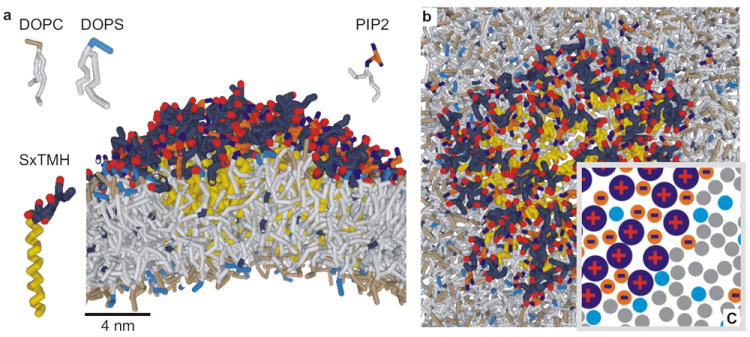
Simulations of the dynamic and amorphous PIP2-syntaxin-1A microdomains (a) Side and (b) top-view of a coarse-grained molecular dynamics simulation. 64 copies of syntaxin-1A257–288 (SxTMH) and 64 copies of PIP2 were incorporated in a bilayer composed of a 4:1 molar ratio DOPC:DOPS. PIP2 was only present in the membrane leaflet facing the N-terminus of syntaxin-1A257–288. Simulations were performed with 150 mM NaCl. See Supp. Methods for details. White: alkyl chains of the lipids. Cyan: DOPS headgroup. Grey: DOPC headgroup. Yellow: transmembrane region of syntaxin-1A257–288 (residues 266–288). Blue-red: polybasic linker region (residues 257–265, charges in red). Orange-blue: anionic PIP2 headgroup (charges in blue). The domains were stable over 6 μs simulation time; see supplementary movie 1. (c) Simplified scheme of the cluster.
In summary, our findings show that electrostatic interactions between the membrane lipid PIP2 and the SNARE syntaxin-1A suffice to induce membrane sequestering and microdomain formation without the need for high local PIP2 production or a (complex) ‘molecular fence’ restricting PIP2 and protein diffusion27. This does not exclude an additional role for protein-protein interactions between either transmembrane helices or soluble domains. In fact, these seem essential for segregation of proteins in similar structure and size, such as syntaxin-1A and syntaxin-4 (both have polybasic regions and cluster separately)12,24. The mutual enrichment of syntaxin-1A and PIP2 at the fusion sites by electrostatic interactions has clear advantages. First, accumulation of syntaxin-1A may facilitate SNARE interactions and thereby increase the membrane fusion efficiency3,28. Second, the lipid environment modulates the energetic requirements for fusion13,16. Third, both PIP2 and syntaxin-1A function as molecular docking sites and facilitate assembly of the complete fusion machinery1,2,6-9. Our findings that electrostatic protein-lipid interactions are sufficient for membrane sequestering constitute a novel mechanism for the formation of protein microdomains in the membrane that is clearly distinct from the well-established lipid phases18,29.
Methods Summary
PHPLCδ-citrine was expressed in Escherichia coli and purified with his-tag affinity purification. PC12 cells were maintained and propagated as described3,24. PC12 cells were transfected using Lipofectamine LTX (Invitrogen). Membrane sheets were prepared by rupturing the cells with probe sonication as described24. Immunostaining24 and microscopy11,24 were performed as described. The peptides were synthesized via microwave-assisted Fmoc solid phase synthesis. Peptides were mixed with lipids in organic solvent and GUVs were formed by the drying rehydration procedure. The molecular dynamics simulations were performed with the GROMACS simulation package and the MARTINI coarse-grained model25,26. See Supp. Methods for details.
Supplementary Material
Acknowledgments
We thank Matthew Holt, Gertrude Bunt, Fred S. Wouters and Christian Eggeling for advice and Volker Haucke and Seong Joo (Freie Universität Berlin, Germany) for the RFP-synaptojanin-1 construct. G.v.d.B is financed by the Human Frontier Science Program. This work was supported by the US National Institutes of Health (P01 GM072694, to R.J.) and the Deutsche Forschungsgemeinschaft SFB803 (to K.M., J.H.R., U.D., H.G. and R.J.).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions
G.v.d.B and R.J. designed the experiments and wrote the paper. K.M., B.E.H. and U.D. synthesized the peptides. J.H.R. and H.G. performed the simulations. H.A. performed the TIRF and K.I.W. and S.W.H the STED microscopy. M.D. contributed to the protein purification and immunofluorescence. G.v.d.B. performed all other experiments. All authors contributed to the manuscript.
The authors declare no competing financial interests.
References
- 1.Jahn R, Scheller RH. SNAREs-engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi K, et al. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- 3.Lang T, et al. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 6.Wen PJ, Osborne SL, Meunier FA. Dynamic control of neuroexocytosis by phosphoinositides in health and disease. Prog Lipid Res. 2011;50:52–61. doi: 10.1016/j.plipres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Milosevic I, et al. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay JC, Martin TF. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- 9.James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 11.Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett. 1994;19:780–782. doi: 10.1364/ol.19.000780. [DOI] [PubMed] [Google Scholar]
- 12.Sieber JJ, et al. Anatomy and dynamics of a supramolecular membrane protein cluster. Science. 2007;317:1072–1076. doi: 10.1126/science.1141727. [DOI] [PubMed] [Google Scholar]
- 13.Williams D, Vicôgne J, Zaitseva I, McLaughlin S, Pessin JE. Evidence that electrostatic interactions between vesicle-associated membrane protein 2 and acidic phospholipids may modulate the fusion of transport vesicles with the plasma membrane. Mol Biol Cell. 2009;20:4910–4919. doi: 10.1091/mbc.E09-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denisov G, Wanaski S, Luan P, Glaser M, McLaughlin S. Binding of basic peptides to membranes produces lateral domains enriched in the acidic lipids phosphatidylserine and phosphatidylinositol 4,5-bisphosphate: an electrostatic model and experimental results. Biophys J. 1998;74:731–744. doi: 10.1016/S0006-3495(98)73998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kweon DH, Kim CS, Shin YK. The membrane-dipped neuronal SNARE complex: a site-directed spin labeling electron paramagnetic resonance study. Biochemistry. 2002;41:9264–9268. doi: 10.1021/bi025934+. [DOI] [PubMed] [Google Scholar]
- 16.Lam AD, Tryoen-Toth P, Tsai B, Vitale N, Stuenkel EL. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Mol Biol Cell. 2008;19:485–497. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray DH, Tamm LK. Clustering of syntaxin-1A in model membranes is modulated by phosphatidylinositol 4,5-bisphosphate and cholesterol. Biochemistry. 2009;48:4617–4625. doi: 10.1021/bi9003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacia K, Schuette CG, Kahya N, Jahn R, Schwille P. SNAREs prefer liquid-disordered over “raft” (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J Biol Chem. 2004;279:37951–37955. doi: 10.1074/jbc.M407020200. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho K, Ramos L, Roy C, Picart C. Giant unilamellar vesicles containing phosphatidylinositol(4,5)bisphosphate: characterization and functionality. Biophys J. 2008;95:4348–4360. doi: 10.1529/biophysj.107.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levental I, et al. Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers. Biochemistry. 2009;48:8241–8248. doi: 10.1021/bi9007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christian DA, et al. Spotted vesicles, striped micelles and Janus assemblies induced by ligand binding. Nat Mater. 2009;8:843–849. doi: 10.1038/nmat2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser HJ, et al. Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci USA. 2009;106:16645–16650. doi: 10.1073/pnas.0908987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laage R, Rohde J, Brosig B, Langosch D. A conserved membrane-spanning amino acid motif drives homomeric and supports heteromeric assembly of presynaptic SNARE proteins. J Biol Chem. 2000;275:17481–17487. doi: 10.1074/jbc.M910092199. [DOI] [PubMed] [Google Scholar]
- 24.Sieber JJ, Willig KI, Heintzmann R, Hell SW, Lang T. The SNARE motif is essential for the formation of syntaxin clusters in the plasma membrane. Biophys J. 2006;90:2843–2851. doi: 10.1529/biophysj.105.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries AH. The MARTINI forcefield: coarse grained model for biomolecular simulations. J Phys Chem B. 2007;111:7812–7824. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- 26.Yesylevskyy S, Schafer LV, Sengupta D, Marrink SJ. Polarizable water model for the coarse-grained Martini force field. PLoS Comp Biol. 2010;6:e1000810. doi: 10.1371/journal.pcbi.1000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol. 2002;157:1071–1081. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Bogaart G, Jahn R. Counting the SNAREs needed for membrane fusion. J Mol Cell Biol. 2011;3:204–205. doi: 10.1093/jmcb/mjr004. [DOI] [PubMed] [Google Scholar]
- 29.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


